Abstract
Cold-water carbonate bioconstructions are the product of complex interactions between calcifying organisms and the surrounding environment, and deeply contribute in affecting the evolution of the submarine landscape in space and time. Important variables contributing to their development, growth and/or demise include sedimentary dynamics, food supply, physical and chemical characteristics of water masses and local hydrodynamic regimes. Geomorphological studies of bioconstructions are therefore critical in deciphering the physical and biological processes contributing to their development. The aim of this chapter is to summarise the state of the art of geomorphic studies on temperate coralligenous bioconstructions and cold-water coral reefs/mound systems, both representing the two most important and largest carbonate bioconstructions in temperate and deep-sea waters. The importance of these biogenic constructions covers several aspects. They can represent important carbonate factories of the deep sea, archives of past climate and oceanographic conditions and they support increased species diversity and complex biotic interactions with respect to the surrounding seafloor. Because of their remote location (from hundreds to thousands of meters below sea level), only since the last tens of years, technological advances are allowing fine-scale physical and ecological investigations of these bioconstructions, containing ecosystem engineers able to strongly modify the landscape heterogeneity. An increased awareness of their potential extension and significance as natural resource, is now steering scientific efforts towards the need to overcome the gaps in knowledge and contribute to prevent their vulnerability to an increasing human pressure.
The original version of this chapter was revised: For detailed information please see Erratum. The erratum to this chapter is available at https://doi.org/10.1007/978-3-319-57852-1_28
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Many marine organisms can contribute to the creation, development and maintenance of complex biogenic three dimensional structures, commonly named bioconstructions, which develop through the accretion or accumulation of organogenic deposits, and are able to affect the evolution of the submarine seascape in space and time. From a semantic point of view, “bioconstruction” is a term having a landform connotation as well as a process meaning. Bioconstruction, bioerosion and bioprotection represent biologically mediated components of earth surface processes and constitute the main subjects of bio-geomorphological studies (Naylor 2005). Within the marine realm, bioconstructions are produced all over the submerged environment, from coastal areas to the deep-sea, by different organisms belonging to extremely diverse taxa (bacteria, algae, numerous species of metazoans). Bioconstructions can form through biologically-induced or biologically-controlled calcification, or may result from material bind from other sources (e.g. using organic cement or sediment baffling) or develop from a combination of the two processes (Lowenstam 1981; Riding 2002; Naylor 2005). Plants and animals generally having a calcareous composition create a new hard substrate by skeletal deposition followed by superposition and overgrowing, or adhere to pre-existent hard substrates, and persist as solid elements after their death. Within time, the accumulation of these limy remains may lead to the development of solid and complex constructions which may give rise to relevant landforms, with living communities generating new frameworks over a build-up of cemented skeletal remains of dead organisms (Wood 1999; Riding 2002). The growing dynamics and ecological functioning of building organisms clearly affect the morphological variability of bioconstructions. Sedimentary dynamics, physical and chemical characteristics of water masses and local hydrodynamic regimes (waves and currents) are in most cases critical variables exerting a major control on growth and demise of bioconstructions in space and time. The study of the origin, configuration and evolution of landforms created by bioconstructors requires therefore a strongly multidisciplinary approach to comprehensively understand the links between biotic and physical processes involved in their formation. Furthermore, geomorphological studies are revealing to be critical in deciphering the physical and biological processes that contributed to the development and distribution of bioconstructions. A rich and diverse terminology has been adopted to describe the calcareous biogenic landforms created by sessile organisms. Some of the most adopted terminology does not specifically refer to the natural processes involved in their formation, neither to their composition or structure. This is the case of the terms “concretion” or “build-up” (see Riding 2002 for a deeper analysis and a comprehensive list of references). Others debated definitions are aimed to address the sedimentological, geomorphological and ecological characteristics contributing to the formation of bioconstructions. This is the case of the term reef. Riding (2002) presented a comprehensive review of the composition and structure of biogenic reefs, occurring at a large variety of scales and depths, drawing the attention to the high diversity and variable sedimentary composition of the reef-forming deposits. Despite classical common definitions of reefs generally being based on the dominant constituent organisms (e.g.: microbial, algal, archaeocyath, stromatoporoid, coral, rudist, etc.—see Table 1 in Cocito 2004), these generally lack a focus on their geomorphic aspect. The term reef refers here to a persistent bioconstructed morphologic framework (generally formed by several interacting organisms) with a clear three dimensional expression, which influences the local sedimentary dynamics and oceanography and supports living benthic communities. Other terms which refer to biogenic-mediated geomorphic features with a distinct shape are banks, originally indicating biogenetic accumulations resulting from baffling and binding processes of benthic organisms (Lowenstam 1950; Klement 1967; Riding 2002), but now largely adopted to generically indicate sub-horizontal biogenic frameworks surrounded by marked breaks of slope, and mounds (Toomey and Finks 1969; Wilson 1975; James and Bourque 1992; Bosence and Bridges 1995; Riding 2002; Roberts et al. 2006; Foubert and Henriet 2009; Rodríguez-Martínez 2011), indicating positive reliefs, from few to hundreds of meters tall, and generally displaying rounded, semicircular, ellipsoid or elongated shapes. Mounds can be composed of a variety of dominant organisms capable of trapping or baffling fine sediments (e.g. cold water corals, bryozoans, polychaetes) and therefore occurring in a variably-composed sedimentary matrix. Mound structures have been recognized in several geological records (Hebbeln and Samankassou 2015), with the oldest features being of early Palaeozoic age (Monty 1995). A rigorous nomenclature explaining the different origin, composition and structure of banks and mounds is in any case still missing, as confirmed by the rich sequence of adopted adjectives (mud-mounds, detritial mounds, carbonate mounds, carbonate mud mound, lime mud mound, microbial mounds, mud-banks, reef mounds, stromatactis mounds, etc.) (see Table 4.2 in Roberts et al. 2009).
Bioconstructions of coastal and shelf seas have been the subject of comprehensive studies since more than a century. Tropical coral reefs, for example, are the most studied bioconstructions on a global scale, mainly owing to the ecological relevance of these large ecosystems, considered among the most important hotspots of marine biodiversity. Tropical corals are able to create entire bio-constructed coastal landforms, several hundreds of kilometres long and hundreds of meters across, whose extension is easily visible in satellite images. These reefs are widely addressed in all basic texts on marine biology, coastal geomorphology and stratigraphy (Wood 1999; Montaggioni and Braithwaite 2009; Woodroff and Webster 2015). Other widely studied coastal and shallow water bioconstructions are found in temperate seas (James and Clarke 1997; Pedley and Carannante 2006). These concretions create complex structures in moderate to strong hydrodynamic regimes, from the coastal intertidal environment to the infralittoral setting, whose deepest limit is marked by a strong decrease of light, insufficient to support the photosynthetic rate of photophilous plants. The dominant coastal temperate bioconstructors are coralline algae such as Lithophyllum byssoides (Lamarck) Foslie, vermetid gastropods, other species of coralline algae and several bryozoans, polychaetes and oysters, able to develop solid and few meters thick calcareous rims, sub-horizontal benches and sporadic reefs along the coastline (Laborel 1987; Relini 2009; Kružić 2013).
The extension and the geomorphological characteristics of important submerged bioconstructions, able to form large reefs and mound structures in partially unexplored deeper environments, have been revealed only over the last 30 years thanks to the availability of seafloor acoustic mapping techniques and the development of scientific Remotely Operated Vehicles (ROV). This is the case for the coralligenous formations and cold-water coral reefs and mounds, the latter developing in a wide depth range, from tens up to a few thousands of meters. The importance of these biogenic constructions covers several aspects. They can represent important carbonate factories of the deep sea (Titschack et al. 2008), and archives of past climate and oceanographic conditions (Frantz et al. 2005; Montagna et al. 2006; Ries 2006; Kamenos et al. 2012; Thierens et al. 2013). They support increased species diversity and complex biotic interactions with respect to the surrounding seafloor.
The aim of this chapter is to summarise the state of the art on geomorphology, distribution and main composition of the temperate coralligenous bioconstructions and cold-water coral reefs/mound systems, representing the two most important and largest carbonate bioconstructions in the deep ocean.
2 Coralligenous Bioconstructions
Coralligenous (C) bioconstructions represent calcareous build-ups of biogenic origin (i.e.: organic frame-reefs; Riding 2002), formed since the Holocene transgression in the Mediterranean, through the multi-stratified accretion in dim light condition of encrusting coralline algae and invertebrate associations (Sartoretto et al. 1996; Ballesteros 2006). They are generally distributed from a few meters (Sarà 1971) down to about 120–150 m water depth, the deepest C occurrence corresponding to the oligotrophic, transparent waters of the Eastern Mediterranean basin (Laubier 1966; Hong 1982; Ballesteros 2006). C produces distinct biogenic reef-like frameworks from few to tens of meters large and up to 4–6 m thick on sub-horizontal seafloors across different depths, or covering hard and complex surfaces along the lower limit of submerged rocky-coast and circalittoral slopes, displaying variable lateral continuity and thickness. C thrives with low to moderate level of nutrient inputs, temperatures from 10 to 23 °C and low to moderate hydrodynamics (Relini 2009), promoting the development of the biogenic framework and limiting the deposition of terrigenous sediments (Morganti et al. 2001; Balata et al. 2006). Low light conditions represent the key parameter that controls the growth of coralline algae in C (Relini 2009). The depth-range of C is therefore variable through the photic zone, since a number of environmental parameters and physical settings determine the total illumination reaching the seafloor. Submarine topography for instance has a significant effect on modulating the seafloor illumination, since seafloor irregularities (escarpments, overhanging walls, caves, etc.) can create a number of favourable low-light conditions even at shallow depths.
The term Coralligenous (or Coralligène, the original French name) refers to a complex of biocoenoses (C complex) and to the habitat it represents (Laborel 1961). The main dominating communities correspond to calcareous algae (shallower sectors of the bioconstruction), suspension feeders (deeper sectors, cavities and overhangs), borers (inner sectors) and even soft-bottom fauna (within sediments deposited in cavities, depressions and holes) (Fig. 1). The volumetrically most important framework builders in C are calcareous red algae Corallinophycidae (CCA), bryozoans, polychaetes and cnidarians (Laubier 1966; Di Geronimo et al. 2002; Novosel et al. 2004; Cocito 2004; Basso et al. 2007; Ballesteros 2006; Giaccone et al. 2009; Rosso and Sanfilippo 2009). The interplay of biotic and abiotic factors (mainly: light penetration, availability of trophic resources, substrate exposition, sedimentation rate, freshwater influence, biotic interactions) produces several C facies, unevenly distributed along geographical and depth gradients (Pérès 1982; Bellan-Santini et al. 1994, Sartoretto 1994; Canals and Ballesteros 1997; Casellato and Stefanon 2008; Bonacorsi et al. 2012). Ballesteros (2006) provided a first quantification of the number of species (about 1670) forming the coralligenous communities and identified an algal-dominated assemblage in the relatively shallow C, contrasting with an animal-dominated C in the deep. An overview of the environmental factors influencing their development and distribution, illustrating most of the complex biotic relationships and ecological processes that create and destroy the calcareous frameworks was also provided (Ballesteros 2006).
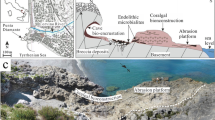
Characteristic organisms of coralligenous formations. a Pink layered/foliose encrusting calcareous red algae growing at the surface of a biogenic substrate (coralligenous). Serpulid tubes also occur; b Fan-shaped red to yellow branching colonies of Octocorallia (Paramuricea clavata) growing at the top of a coralligenous build-up. The colonies are typically oriented on a plane that is normal to the direction of the main current; c Sub-horizontal coralligenous substrate encrusted by various species of purple coralline algae and orange sponges. Note the small branching colonies of the Octocorallia Eunicella sp. and the large sea urchin Echinus melo feeding on algae; d Branching colony of the bryozoan Pentapora fascialiss growing on the flank of a coralligenous build-up, where several species of encrusting sponges also occur. One Halocynthia papillosa ascidian (sea-squirt) is attached below the Pentapora colony and tiny tube-dwelling serpulids grow at its base; e Cerianthus membranaceus (Anthozoa) on coralligenous substrate covered by coralline red algae and Peyssonnelia spp.; f Overhanging surface of coralligenous substrate bordering a submarine cave, encrusted by numerous solitary corals (the yellow Scleractinia Leptopsammia pruvoti), the ascidians H. papillosa, sponges and algae. Photos courtesy of F. Mastrotoraro
Significant literature has been also produced on the physical/geological characterization of the reef-like structures formed by the coralligenous biocoenoses, including their fossil counterparts (Bosence and Pedley 1982; Carannante and Simone 1996; Rasser 2000; Nelson et al. 2001; Rasser and Piller 2004; Nalin et al. 2006; Bracchi et al. 2016).
First pioneer studies carried out by Johannes Walther (1860–1937, see Ginsburg et al. 1994) and Abel (1961) interestingly outlined a number of similarities between Mediterranean C and Red Sea coral reefs, in terms of structural complexity. As for coral-reefs (and most of bioconstructions), C are indeed complex features in terms of their physical structure, associated biota and the ecological processes they generate (Laubier 1966). Their morphology can reach a high degree of complexity, with rugged surfaces and many cavities and overhangs due to irregular growth of the benthic calcifiers, erosional processes, boring organisms, or a combination of these factors. In these dark crevices and under overhangs the shade-loving assemblages develop, including sponges and, in some cases, the precious red coral Corallium rubrum, whose occurrence suggested the name Coralligène (= generating the coral, Marion 1883; Basso et al. 2007; Virgilio et al. 2006; Relini 2009). Space competition among encrusting calcifiers, differential growth rates, cementation, episodes of sedimentation and partial erosion generates through time a significant structural complexity and environmental heterogeneity (Jones et al. 1994), which in turn controls the distribution and abundance of coexisting species, and increasing the C habitat biodiversity.
2.1 Geomorphology of Coralligenous Bioconstructions
The extent to which the C biota can modify the submarine environment, affecting the evolution of submerged landforms is extremely variable. C can cover and drape quite homogenously the pre-existing hard substrate enhancing its topographic complexity, or produce new distinct geomorphic features on the seafloor, of variable shapes and dimensions, that characterize the seascape in wide areas of the continental shelf. A precise description of their 3D morphological development and extension is however often overlooked. In literature C formations are generally indicated as reefs, concretions, frameworks, build-ups, pillars, outcrops, but they still lack a detailed systematic classification of morphotypes and associated processes, since the drivers that control their differentiation have been poorly investigated (Bracchi et al. 2015, 2016; Doxa et al. 2016).
A first attempt of showing the relevance of C in marine geomorphological research has been provided by Laborel (1961), with the description of five coralligenous types: cave and overhang concretions, wall concretions, concretions at the base of submarine walls, concretions over flat rocky surfaces and platform coralligenous assemblages. These types were distinguished according to their position and exposure to light penetration along an almost steep coastal belt and submerged shelf and the type of substrate on which they grow. Pérès and Picard (1951, 1964) introduced the term “coralligène de plateau” to describe C settled on a coarse bioclastic sub-horizontal bottom and originated from the coalescence of algal concretions (rhodoliths; Bosellini and Ginsburg 1971; Nalin et al. 2006), distinguishing this type of C from the one growing on sloping and complex rocky surfaces. Following Pérès and Picard (1964), most authors indicate C as banks when they develop on flat bottoms (e.g.: coralligène de plateau), whereas the term rim is used to refer to those lips and rims growing on sub-vertical substrates (e.g.: cliffs or slopes in the circalittoral), and also occurring at the opening of submarine caves (Laborel 1987; Ballesteros 2006). These authors thus introduced a first “geomorphological” classification, trying to merge two different criteria to distinguish C: the “morphology” and the “genesis”, this latter related to the type of substrate on which C develops. However, in several cases the genesis of banks is not related to the coralligène de plateau, and on the other hand, a vertical C structure could develop with no need of an underlying cliff (Basso et al. 2007; Bracchi et al. 2015).
Both coralligenous rims and coralligenous banks vary considerably in their 3D morphological development, showing a wide range of morphotypes (Fig. 2). Rims may be poorly developed or grow to extreme sizes reaching widths of more than 3 m (Laborel 1987; Ballesteros 2006). They can be single or multiple features and may set up at regular or irregular intervals. Rocky vertical or sub-vertical walls, which can be found along the open shelf or in submerged caves, represent the typical settings for the development of coralligenous rims (Laborel 1987).
Multibeam point data acquired along different continental shelf regions of the Mediterranean. a Large continuous coralligenous bioconstructions showing an almost flat top with a typical bumpy aspect; b Edge of a large coralligenous bioconstruction, rising almost 5 m from a flat seafloor, with a rugged and pitted surface and vertical to stepped, irregular boundaries; c Single isolated coralligenous bioconstructions (smaller are roughly 1 m large and no more than 1 m high), distributed along a flat seafloor. Larger coralligenous bioconstructions with distinct boundaries are visible at the end of the multibeam line; d Large (ca. 40 m wide) coralligenous bioconstructions rising from 1 to 2 m from the seafloor, with a flat surface and distinct vertical boundaries. Minor isolated coralligenous bioconstructions (ca. 1 m large and no more than 1 m hight) are also visible on the right; e Large coralligenous bioconstructions (more than 40 m wide and from 1 to 2 m high) with indistinct boundaries and separated by a channel. Note the subaqueous dunes documenting the action of unidirectional bottom currents; f Coralligenous bioconstructions with internal sediment patches where subaqueous dunes are visible
When forming banks, C generally occurs in groups rather than as isolated structures. A rich descriptive terminology (e.g.: heads, blocks, vertical pillars, columns, ridges etc.) has been used to refer to the variety of morphotypes they can develop and since the availability of advanced hydro-acoustic mapping systems (such as MB swath bathymetry) calibrated by visual systems such as ROV and drop cameras, research on the geomorphology of submerged bioconstructions has advanced (Figs. 1 and 2). Bracchi et al. (2015) described the morphological diversity across and between individual C structures, mainly featured in columns and ridges and covering more than 436 km2 of the whole Apulian continental shelf, in the southern-central Mediterranean (Campiani et al. 2014). The proposed classification can be considered descriptive, since it is based on the C morphological aspects, whereas the interpretation of genetic relationships and processes between C and substrata are on purpose not explored. According to Bracchi et al. (2015) ridges can reach lateral extensions of tens of meters with heights ranging between 2 and 5 m. Columns represent more distinct features on the seafloor, which may occur in clusters or be isolated, often in association with Posidonia oceanica meadows, or with coarse detritic sediments. In most cases, the action of bottom currents and low sedimentation rates seem to represent favourable conditions, which promote coralligenous development instead of the deposition of terrigenous sediments. Bedforms documenting the action of unidirectional bottom currents are often mapped along the edges of bioconstructions and between them, where sediments and detritic sediment accumulate within the more depressed areas (Fig. 2). Coralligenous atolls, with a radius ranging from 20 to 30 m, have been recently mapped offshore northern Cap Corse (Bonacorsi et al. 2012), northern Mediterranean, between 106 and 112 m water depth. These features form a circular crown structure (made up of coralligenous bioconstructions combined with praline rhodoliths and many invertebrates) with a central core formed by a rounded C formation up to 3 m wide and 0.5 m high (Bonacorsi et al. 2012) surrounded by soft sediments. Similar morphologies have never been observed in other locations, likely owing to the lack of extensive high-resolution acoustic mapping of the (Mediterranean) seafloor, specifically below 50 m of water depth. The shape of these unique and peculiar features has been related to external inherited processes responsible for the initial creation of hard substrates (i.e. gas venting associated to pockmark formation—see Chapter “Cold Seep systems”).
Despite the lack of a comprehensive investigation on the role of Quaternary geomorphic processes on the C distribution and growing processes, the inherited geomorphic landscape of the seafloor likely plays a fundamental role affecting the morphological diversity of C. A quite good documentation exists on the relevance of some major environmental parameters such as slope, current exposure, water transparency, temperature and sedimentation in controlling the differentiation in coralligenous biocoenoses. Nevertheless, most research lacks the investigation of the relationships between the variability of the framework morphologies and changes in the associated biological community and parameters. Some authors documented how the observed differentiation in shape and dimension of C is often inherited from the morphology of the hard substrates that allowed the settlement and growth of coralline algae. Most of them are represented by continental shelf rocky outcrops (Bracchi et al. 2015, 2016) and/or modified relic morphologies originated during previous low-stand or transgressive stages of the sea level (e.g. beach-rocks, sediment bars, etc. Navone et al. 1992) and located in areas subject to low sedimentation rate. After the initial settlement of coralline algae and during the time of coralligenous development, rising in sea-level caused changes in hydrodynamic regime, sediment input and light penetration. The interplay of these ecological controls influenced different growth rates of benthic calcifiers and the development dynamics of C, in turn affecting its 3D morphology (Pérès and Picard 1951, 1964; Sartoretto et al. 1996; Di Geronimo et al. 2005; Nalin et al. 2006; Bracchi et al. 2016).
C formations were thus affected by Mediterranean sea-level oscillations during the Quaternary. Since the present-day C depth distribution coarsely corresponds to that portion of seafloor that was emerged during the last glacial maximum, the build-up of C must have occurred during the last transgression. Investigations in the north-western Mediterranean provided evidence that circalittoral C formed mostly in the early Holocene (from about 8.5 kyrs BP) at a paleodepth not greater than 10–15 m and with a maximum accumulation rate (about 0.83 mm y−1) between 5 and 8 kyrs BP (Sartoretto et al. 1996). After that period a decrease in accumulation rate (about 0.006 mm y−1 or nil) has been documented for C deeper than 50 m of water depth, suggesting that significant accumulation rates presently occur only at shallow water depth (Sartoretto 1994; Sartoretto et al. 1996; Ballesteros 2006).
2.2 Coralligenous Distribution
C is common in the whole Mediterranean Sea, although the bioconstructions seem to be more abundant in the Adriatic, Aegean and Tyrrhenian Seas and in the Algero-Provençal Basin. A much lower number of occurrences have been reported for the Levantine Sea and Tunisian Plateau/Gulf of Sidra (Martin et al. 2014), although lack of reports of C at any particular site does not necessarily imply their absence, especially for the deep circalittoral, which has not been fully investigated by acoustic mapping and ground truthing (i.e.: video inspections). For the same reason, most of the C occurrences reported in literature are located between 10 and 50 water depth, while less information exists for the deeper sites (50–150 m). Several efforts have been promoted in order to fill these gaps (Campiani et al. 2014), although insufficient data are still available on the regional distribution of C across the whole Mediterranean Sea. The only available quantitative information on C extension actually corresponds to the Apulian continental shelf, where a total surface of 436 km2 of seafloor is covered by coralligenous habitats (Campiani et al. 2014).
A tentative large-scale assessment of the predicted distribution of C on a basin scale has been modelled on the basis of existing records of a total 2763.4 km2 of Mediterranean C (Martin et al. 2014). Bathymetry, slope of the seafloor and nutrient input were the three main contributors to the model (combined contribution of 84.1%), whilst the remaining three predictors (euphotic depth, phosphate concentration and geostrophic velocity of sea surface current) had a minor contribution. Unfortunately, the resulting model has a weak support by direct observations and published data. Considering the available polygon data and their associated surface areas, it is estimated that as much as 95% of coralligenous habitat may still need to be mapped across the Mediterranean basin, especially in deeper areas (Martin et al. 2014).
3 Cold-Water Coral Reefs and Carbonate Mounds
3.1 Cold Water Corals and Physical Habitats
Corals are marine animals belonging to the phylum Cnidaria, a large and complex taxonomic group. According to Cairns (2007) the generic term “coral” includes all cnidarians belonging to the classes Anthozoa and Hydrozoa that produce calcium carbonate secretions. This definition encompasses over 5000 colonial and solitary species, ranging from stony corals (or true corals), provided with continuous carbonate exo- or endoskeletons (e.g. Scleractinia, Stylasteridae, Coralliidae), to soft corals (most Alcyonacea) formed by organic tissues and secondarily by microscopic biomineralised sclerites. Among the stony corals, able to build a hard carbonatic skeleton, there are some species known as “framework building” corals, owing to their ability to form complex three-dimensional carbonate structures, providing shelter and resources for a wide range of organisms. Most framework-building corals belong to the order Scleractinia and produce aragonite skeletons. Over 95% of them (around 765 species) consists of zooxanthellate species (Roberts et al. 2009) that live in warm, shallow and oligotrophic waters and contribute to the formation of colourful and diverse tropical reefs through photosynthetic processes of symbiotic algae. Very few species of framework building corals are azooxanthellate (18), i.e. they lack symbiotic dynoflagellates, 17 of them thriving in cold and generally deep waters. The latter are generally defined as stony “cold-water corals” (CWC) and, unlike tropical corals, they do not require the occurrence of light neither of warm and shallow waters. They cover a wide range of depths (39–3000 m) and latitudes (70°N–60°S) and feed on organic particles and organisms advected by water masses. CWC bioconstructions therefore develop where oceanography plays an important role in food transport processes (Mienis et al. 2014). Recent studies suggest a coupling between the CWC reef fauna and the surface productivity in relation to the local hydrography and sedimentary dynamics (Mienis et al. 2014; Mohn et al. 2014; Moreno Navas et al. 2014).
The most common framework building cold water corals are Lophelia pertusa and Madrepora oculata, observed worldwide, with larger occurrence in the North Atlantic and Mediterranean waters (Roberts et al. 2009). L. pertusa and M. oculata are most commonly observed in waters between 7 and 10 °C although they are able to tolerate a wider temperature range, between 4 and 15 °C (Brooke et al. 2013; Naumann et al. 2014) (Fig. 3). These species have been found on a depth range from tens of meters up to 1500/2000 m (Zibrowius 1980), and along different physiographic and geomorphologic settings. Other CWC species able to create bioconstructions are Solenosmilia variabilis, Enallopsammia profunda, Oculina varicosa and Goniocorella dumosa (Roberts et al. 2006; Hebbeln et al. 2016).
The occurrence of hard substrates on which coral colonies can start to settle is probably the most relevant pre-requisite for CWC bioconstructions to grow. However, oceanographic forcing and food input also represent key environmental variables for their survival (Duineveld et al. 2004, 2007; Van Oevelen et al. 2009). Suitable environments for cold-water coral settlement are regions where the interaction between large scale seafloor morphology and water mass dynamics (bottom currents, internal waves controlled by tides or density interfaces) create moderate to strong hydrodynamics and turbulent mixing, coupled with reduced terrigenous input and a constant delivery of organic matter. These settings generally coincide with outer shelf/upper slope regions (Thiem et al. 2006; Dullo et al. 2008), fjords (Freiwald et al. 2004; Försterra et al. 2005), seamounts (De Vogelaere et al., 2005; Tracey et al., 2011), landslides (De Mol et al. 2009; Lo Iacono et al. 2014a; Savini et al. 2016), canyons (Orejas et al. 2009; Huvenne et al. 2011; Gori et al. 2013) and ocean gateways (Lo Iacono et al. 2014b; Lo Iacono et al. 2016). CWC are often observed along outer shelves and fjords of sub-polar and high latitude regions, settling on iceberg plough-marks and other erosive features produced during the last glacial cycle (Freiwald et al. 1999, 2002; Lindberg et al. 2007). Along the open slope, substrates providing suitable habitats for CWC settlement generally coincide with abrupt geomorphologies inherited from previous geologic events, such as landslides or fault scarps (Fosså et al. 2002; Colman et al. 2005; De Mol et al. 2009; Lo Iacono et al. 2014a; Savini et al. 2016). Following a feedback mechanism, the complex terrain of these settings enhances moderate to strong hydrodynamics, in turn maintaining exposed morphologies and low sedimentation rates along the CWC reefs. Steep or vertical walls of submarine canyons flushing organic-rich sediments to the deep-sea are also suitable habitats for CWC. The abrupt morphology and complex terrains of canyons naturally protect benthic ecosystems from the impact of destructive trawl fishing, taking a strategic role in designing new strategies for the conservation of natural resources (Orejas et al. 2009; Huvenne et al. 2011). Finally, seamounts and volcanic banks are prominent topographic highs, able to increase the upwelling of nutrient-rich waters resulting in a remarkable diversity and richness of fish and benthic fauna, including CWC (Genin et al. 1986; Duineveld et al. 2004; Rogers et al. 2007).
3.2 Cold Water Coral Reefs
If suitable environmental conditions (temperature, salinity, dissolved oxygen and pH values, turbulent current regimes and food availability) persist in time, framework building CWC are able to form extensive and complex carbonate reefs. CWC reefs develop in a delicate interplay between coral growth and bioerosion processes, giving rise to a complex ecosystem consisting of live coral colonies trapping and baffling suspended fine sediments, interspersed with dead reef framework, coral rubble and bioturbated sandy muds (Freiwald et al. 2004; Roberts et al. 2009), where other organisms (gorgonians, anthipatarians, sponges, polychaetes, molluscs, crustaceans, bryozoans, echinoderms, cirripeds and brachiopods) also contribute to the carbonate buid-up (Fig. 4).
Examples of accompanying species in habitat forming CWC reefs. a Dendrophyllia cornigera. b Sea urchin Cidaris cidras and an unidentified sponge, c Sponge Pachastrella molinifera and living colonies of M. oculata. d The black coral Leiopathes glaberrima and the gorgonian Acanthogorgia hirsuta (along the right side). e The sponge Asconema setubalense, the fish Helicolenus dactylopterus, the crab Bathynectes sp. f The black coral Parantipathes larix and the sponges Asconema setubalense. g The gorgonian Acanthogorgia hirsuta, an unidentified sponge and living colonies of Madrepora oculata. h The back coral Leopathes glaberrima. i The gorgonian Villogorgia bebrycoides and the sponge Asconema setubalense. j The cnidarian Anthomastus grandiflorus. Photos © Oceana
When unidirectional bottom currents prevail, the most pronounced geomorphic features along reefs generally correspond to ridge-like elongated reliefs, or “cigar shaped” reefs, few to hundreds of meters long, whose orientation is generally controlled by the main current patterns and where different sectors generally correspond to distinct habitats (Freiwald et al. 2002; Buhl-Mortensen and Lepland 2007; Buhl-Mortensen et al. 2016a). The specific dominance of living coral communities decreases from the head to the tail of the reef along the current direction, in parallel with an increase of dead framework and coral rubble supporting higher biodiversity (Fig. 6 in Buhl-Mortensen and Lepland 2007 and Fig. 8 in Freiwald et al. 2004). The generally asymmetric geomorphology of the reef and the orientation of the living colonies facing the bottom currents along its front may indicate a migration of the reef against the direction of local currents, which generally range between 5 and 20 cm s−1 (Buhl-Mortensen et al. 2016a), suggesting these features as dynamic geomorphic elements developing under an interacting mechanism between coral growth rates and hydrodynamic regimes.
The most thriving reefs mapped until the present are located along the western Norwegian (Sula and Røst reefs) and Scottish margins (Mingulay reef) within a depth range of 30–450 m, and are mainly dominated by Lophelia pertusa communities (Freiwald et al. 2002; Roberts et al. 2005, 2009). More than 370 species associated to CWC bioconstructions are reported from the Mingulay reef and more than 1100 from the Norwegian reefs (Freiwald et al. 2002). The Norwegian Røst Reef, the world’s largest known cold-water coral reef, has a length of about 35 km, a width of up to 2.8 km and is around 35 m thick (Fosså et al. 2005), probably developed through merging processes of several single reefs. Uranium/Thorium dating inicates that many of the Norwegian reefs started to develop around 9 kyr BP, during the sea level rise of the last glacioeustatic cycle (Rokoengen and Østmo 1985; Buhl-Mortensen 2000). Morphometric analyses suggest a linear relationship between reef height and width, with their width being two to five times larger than their height (Buhl-Mortensen et al. 2001). Despite possible hiatuses and demise stages, vertical reef accretion rates for the Norwegian margin are estimated to be around 1.2 mm y−1 (Buhl-Mortensen 2000).
3.3 Development of Cold Water Coral Reefs and Mounds
A key characteristic for reef development both in space and time is the cyclic alternation between reef growth, bioerosion and sediment accumulation. Corals can gradually form coalescing thickets (Squires 1964) starting from small isolated colonies, owing to their capability to form extensive skeletal carbonate structures. Subsequently, passing through the stage of coral coppice (Squires 1964), they may give rise to CWC reefs, displaying a complex 3D structure and reaching dimension of up to hundreds of meters. Wilson (1979) presented a pioneering work on the spatial development of a reef. According to this model, different stages of coral growth give rise to a progressive outwards expansion of the bioconstruction, where colonies are spatially organized in concentric rings, named “Wilson rings” (Fig. 3 in Wilson 1979). This process of reef generation starts when small and adjacent coral patches nucleate around an initial coral colony (“ticket stage” of the reef development). During the following coral coppice stage, an increased production of coral rubble through bioerosion processes provides new available substrate for the growth of young colonies along the external border and within the innermost regions of the bioconstruction, which gradually become a complex 3D reef structure, where the amount of dead corals and coral debris by far exceeds the number of living colonies (Wilson 1979; Roberts et al. 2009). Actual models consider the cyclic alternation of distinct environmental stages as the most important factor for reef development in time. Changing environmental conditions induce crucial shifts in the dominating processes along the reef including oceanographic and sedimentary regimes, physical/chemical processes, biodiversity and species composition (Roberts et al. 2009; Douarin et al. 2009). After an initial settlement of colonies on hard substrates, a following expansion of coral frameworks coincides with suitable environmental conditions persisting in time, during which sediments trapped and baffled by thriving corals constitute an important structural component of the reef (Dorschel et al. 2007; Frank et al. 2009; Douarin et al. 2009). A general demise stage of living reefs occurs during the transition towards less suitable environmental conditions (e.g.: changing temperature, decreased food supply and productivity), bringing the start of coral collapse and increased production of coral debris and associated fauna. A consequent decrease of the reef seafloor complexity reduces the hydrodynamic regimes at the bottom and may increase the hemipelagic sediment accumulation (Douarin et al. 2009).
Within tens to hundreds of thousands of years, the alternation of suitable and unsuitable environmental conditions along reefs generally corresponds to cyclic interchanges between dominance of coral growth (thriving stages of the reefs) and fine sediment accumulation (demise stages). These long scale variations, generally related to the Quaternary glacioeustatic oscillations and associated changes in oceanographic conditions (Dorschel et al. 2005; Roberts et al. 2006, 2009), can give origin to pronounced morphologies defined as Cold-Water Coral mounds, forming through several cycles of reef development and representing sensitive carbonatic archives of past climatic and oceanographic changes (Kano et al. 2007; Raddatz et al. 2011; Fink et al. 2013; Thierens et al. 2013). CWC mounds correspond to positive reliefs generally occurring in clusters or as isolated features, distributed across a depth range of 70–1200 m and whose geomorphology corresponds to semi-circular, conical, tear-drop or linear ridge-like shapes (Kenyon et al. 2003; Masson et al. 2003; Van Rooij et al. 2003; van Weering et al. 2003; Huvenne et al. 2009; Lo Iacono et al. 2014a, b) (Fig. 5).
a MB 3D model showing the two clusters of the West Melilla CWC mounds (max 45 m tall), Alboran Sea, Western Mediterranean. The larger cluster developed on a buried submarine landslide, as suggested by its lobular shape (see Lo Iacono et al. 2014a); b side-scan snar mosaic showing the elliptic Darwin CWC mounds (Eastern Atlantic). The inset highlights the sharp and contrasting acoustic facies associated to living CWC colonies (see Huvenne et al. 2009; Victorero-Gonzalez et al. 2016); c MB 3D model showing the linear CWC Cabliers mound system, Alboran Sea, Western Mediterranean, developed on a volcanic substrate. Note the deep incised erosive moat along the base of the mound (see also Lo Iacono et al. 2014b); d MB 3D model showing the circular Magellan mounds, eastern Atlantic (data from GSI—INFOMAR Project)
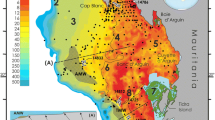
The height of cold-water coral mounds can vary from a few meters to up to hundreds of meters whereas the width of their base can reach hundreds of meters for sub-circular shapes to up to few tens of kilometres for linear features. Geo-acoustic mapping of such important geomorphic features has unveiled hundreds of CWC mound fields, mainly spread along the NE and NW Atlantic margins and the central-western Mediterranean (Fig. 6), where they deeply affect the present-day seascape configuration. The largest mounds, observed along the North East region of the Atlantic Sea (Irish Margin, Porcupine Seabight and Rockall Trough) are also defined as Giant Mounds. These features can reach dimensions of up to 350 m high and few kilometres wide (Kenyon et al. 2003; Mienis et al. 2007) and correspond to carbonate factories reaching back to Pliocene times (2.6 Ma; Kano et al. 2007). However, recent studies revealing the existence of new large mound fields along the western African margin reinforce the need for further mapping expedition along less known areas. Interestingly, the large-scale distribution patterns of mounds coincide in most cases with the boundaries between prominent water masses in the Atlantic and developed close to prominent density interfaces, once more suggesting the primary role of the oceanography in the development and maintenance processes of CWC ecosystems.
Distribution map of the largest CWC mounds and living reefs along the Atlantic margins and the Mediterranean Sea. 1 Campos Basin Mounds—Lopes and Hajdu (2014), 2 Campeche Mounds—Hebbeln et al. (2014), 3 Gulf of Mexico Mounds—Georgian et al. (2016), 4 Visca Knoll Mounds—Brooke and Schroeder (2007), 5 West Florida Mounds—Neumann et al. (1977), 6 Florida Mounds—Grasmueck et al. (2006), 7 Cape Lookout Mounds—Mienis et al. (2014), 8 Angola Mini Mounds—Le Guillox et al. (2009), 9 Ghana Mounds—Buhl-Mortensen et al. (2016b), 10 Banda Mounds—Eisele et al. (2014), 11 Eugen Seibold Mounds—Glogowski et al. (2015), 12 Pen Duick Mounds—Van Roij et al. (2011), 13 West Melilla Mounds—Lo Iacono et al. (2014a), 14 East Melilla Mounds—Fink et al. (2013), 15 Cabliers Mound—Lo Iacono et al. (2014b), 16 Chella Mound—Lo Iacono et al. (2018), 17 Tuscan Archipelago Mounds—Remia and Taviani (2005), 18 Pantelleria Mounds—Martorelli et al. (2011), 19 Santa Maria di Leuca Mounds—Savini et al. (2014), 20 Breoghan Mounds—Somoza et al. (2014), 21 Ferrol and Coruña Canyon mini Mounds—unpublished, 22 Penmarc’h and Guilvinec mini Mounds—De Mol et al. (2011), 23 Explorer and Dangeard mini Mounds—Stewart et al. (2014), 24 Arc Mounds—Mohn et al. (2014), 25 Porcupine Bank Canyon Mounds—Mazzini et al. (2012), 26 Hovland Mounds—Hovland et al. (1994), 27 Magellan Mounds—Hovland et al. (1994), 28 Viking Mounds—Foubert et al. (2011), 29 Galway Mounds—Dorschel et al. (2007), 30 Therese Mounds—De Mol et al. (2007), 31 Challenger Mound—Kano et al. (2007), 32 Macnas Mounds—Wilson et al. (2007), 33 Enya Mounds—Van Rooij et al. (2009), 34 Pelagia Mounds—Kenyon et al. (2003), 35 Logachev Mounds—Kenyon et al. (2003), 36 Francken Mounds—Wienberg et al. (2008), 37 Mingulay Mounds——Roberts et al. (2005), 38 Darwin Mounds—Masson et al. (2003), 39 Tisler Reef—Wild et al. (2009), 40 Sula Reef—Freiwald et al. (2002), 41 Traena Reef/Mounds—Lindberg (2004), 42 Røst Reef—Freiwald et al. (2004), 43 Stjernsund Reef/Mounds—Freiwald et al. (1997). MMP Magellan Mound Province (Hovland et al. 1994); HMP Hovland Mound Province (Hovland et al. 1994); BMP Belgica Mound Province (Henriet et al. 1998)
Living reefs and coral assemblages are generally observed along and around the top of the mounds, coinciding with increased rugosity values of the seafloor (Guinan et al. 2009). The flanks of the mounds, that can reach steep slope gradients, are smoother and commonly characterized by dead coral framework, coral rubble and fine sediments, sometimes transported to deeper depths through the action of gravitational processes, reflected in small down flank organogenic sedimentary trails and lobes (Roberts et al. 2005). The elevated morphology of mounds, protruding into the water column, creates topographic obstacles, which steer the intensity and direction of bottom currents, in turn contributing in maintaining and enhancing their shapes. Fine grained deposits can be observed along the lee sides of mounds, sheltered from dominant bottom currents and extending from few to hundreds of meters, whereas large sediment wave fields composed of coarse coral debris can be found along the inter-mound areas, more exposed to the action of vigorous hydrodynamics (Kenyon et al. 2003; Huvenne et al. 2009). However, these processes are still poorly understood at small spatial scales (meters to tens of meters), mainly owing to the paucity of high resolution morpho-sedimentary models and oceanographic records, allowing to observe these interactions at the spatial scales they occur.
Elongated mounds extending for up to tens of kilometres have often been observed above tectonic trusts or linear volcanic ridges (Lo Iacono et al. 2014b) and related to the action of unidirectional bottom currents (Messing et al. 1990; Correa et al. 2012; Hebbeln et al. 2014). Unidirectional currents flowing along linear geomorphic features (tectonic ridges, shelf margins) probably contribute in merging adjacent sub-circular isolated mounds in longer linear coalescent systems (Eisele et al. 2014; Buhl-Mortensen et al. 2016b). Supporting this observation, a global pyramidal distribution of mound dimensions shows several small mounds compared to few larger features (Huvenne et al. 2003; Roberts et al. 2009).
The erosive action of strong bottom currents, enhanced by the morphology of the mound itself, can generate tens of meters deep and hundreds of meters wide linear scoured depressions along the bases of mounds, defined as “moats” Esentia et al. (2018). These erosive features are generally associated with “contourite drifts”, corresponding to elongated sedimentary deposits with variable size and thickness developing under moderate to strong persistent current regimes (Van Rooij et al. 2003; Hebbeln et al. 2016; Esentia et al. 2018). The side by side development of contouritic systems and CWC mounds suggests a complex interaction between transport of organic and clastic sediments under moderate to strong hydrodynamic regimes and the development of these prominent bioconstructions. As for the CWC mounds, the sedimentary records of contouritic drifts are controlled by regional changes of sedimentary regimes controlled by past glacioeustatic changes (Hernández-Molina et al. 2014).
4 Biodiversity of Deep-Sea Bioconstructions: Environmental Issues, Management Strategies and Future Perspectives
CWC reefs and mounds and coralligenous bioconstructions are considered hotspots of biodiversity in the deep marine realm, contributing to regulate the dynamics of natural resources in the ocean, including fish stocks of economic importance (Guidetti et al. 2002, Salomidi et al. 2012, Paoli et al. 2016, Tribot et al. 2016 Roberts et al. 2009 and references therein). They have been declared as sensitive and vulnerable marine ecosystems by national and international legislations and are actually included in the list of endangered habitats by governmental organizations and conservation agencies (OSPAR 2008) (red list habitats).
CWC reefs for instance, serve as important spawning, nursery, feeding and refuge areas for a multitude of fishes and invertebrates (Fosså et al. 2002; Husebø et al. 2002; Colman et al. 2005; Stone 2006; Ross and Quattrini 2007; Baillon et al. 2012; Henry et al. 2013). Diversity within these reefs has been found to be orders of magnitude higher than the surrounding seafloor, in some case rivalling shallow-water reefs (Rogers 1999; Henry and Roberts 2007). Their rough morphology, made up of crevices cavities and small build-ups, provides habitats for several other species. Norwegian authorities and EU have introduced regulations to ban destructive bottom trawling activities along such vulnerable ecosystems (Fosså and Skjoldal 2010), assigning to Norway the largest European deep-sea area protected from the threat of industrial activities.
Despite their relevant role in marine ecosystems and ocean biodiversity, the existence and conservation of these benthic communities is being seriously compromised by human-related activities. The physico-chemical perturbations caused by the increasing emissions of CO2, such as the increasing temperature and the decreasing pH (i.e. ocean acidification), have a negative impact on the calcification of the skeleton of calcareous algae and invertebrates (Rodolfo-Metalpa et al. 2010; Basso 2012). It is expected that ocean acidification will enhance both bioerosion and chemical dissolution of carbonates in the future, with undersaturation expected to affect especially the deep, cold-water carbonates (Guinotte et al. 2006; Tribollet et al. 2009; Lunden et al. 2013; Murray et al. 2016; Wisshak et al. 2014). The presence of commercial fisheries in these habitats induced destructive bottom trawling which strongly threatened the habitat complexity and biodiversity of deep-sea bioconstructions. Trawling over deep coral areas can be compared to land deforestation for the relevant loss of biodiversity that both processes involve (Watling and Norse 1997). Conservation groups are making scientific and political communities aware of the vulnerability of deep coral habitats to such massive exploitation in several Exclusive Economic Zones around the world. In an effort to protect these unique resources, researchers are refining acoustic mapping techniques and statistical models that are able to perform solid prediction of their distribution (Tittensor et al. 2009; Ross and Howell 2012; Robert et al. 2015, 2016). The Barcelona Convention’s Action plan adopted in 2008 for the conservation of coralligenous outcrops and other calcareous bio-concretions declared the need of legal measures for their protection. Deep-sea bioconstructions are also considered with particular attention in the EU’s Habitats Directive (under 1170 “Reefs”), and in the Bern Convention. More recently, the Marine Strategy Framework Directive (MSFD; European Parliament and Council of the European Union 2008; European Commission 2010), aimed at achieving the “Good Environmental Status” (GES) of all marine waters by 2020, required each Member State to develop a strategy of knowledge-based sustainable management for its marine waters. On the basis of the Barcelona Convention and other international initiatives for the environmental protection of deep-sea ecosystems (UNEP-MAPRAC/SPA 2008, 2010; UNEP-MAP 2011), a group of habitat types, among which C ad CWC, has been identified as of special scientific or biodiversity interest (MSFD, Annex III, Table 1). The implementation of appropriate monitoring measures is indeed instrumental to guarantee a sustainable management of C and CWC., Although an extensive array of increasing high quality data on CWCs exists for the northeast Atlantic margin, there are still several gaps in understanding the functioning and evolution of these complex habitats, even regarding any potential relationship between their ecological dynamics and the resulting geomorphic expression. Furthermore, the lack of full coverage high-resolution seafloor maps in the deep-sea may introduce a bias in the recorded extension of these relevant geomorphic features. For instance, recent oceanographic expedition along the western African and South American margins are revealing the existence of new CWC mound fields, where living reefs are supporting a high biodiversity (Le Guillox et al. 2009; Eisele et al. 2014; Lopes and Hajdu 2014; Glogowski et al., 2015; Buhl-Mortensen et al. 2016b).
New data on less explored areas may be of great significance, both regionally and globally, for:
-
(1)
refining the assessment of biodiversity at a global scale
-
(2)
better define the resilience of deep-sea habitats to human induced climatic forcing
-
(3)
better estimating the contribution to the ocean carbon budget of cool-water carbonatic systems
-
(4)
better constraining on a basin scale the main paleoceanographic events in recent time, including high-resolution focus on late Pleistocene and Holocene rapid climate events.
References
Abel EF (1961) Uber die Beziehung mariner Fische zu Hartbodenstrukturen. Sper Osterr Akad Wiss (Math Nat Kl Abt A) 170:223–263
Baillon S, Hamel JF, Wareham VE, Mercier A (2012) Deep cold-water corals as nurseries for fish larvae. Front Ecol Environ 10(7):351–356
Balata D, Acunto S, Cinelli F (2006) Spatio–temporal variability and vertical distribution of a low rocky subtidal assemblage in the north-west Mediterranean. Estuar Coast Shelf Sci 67:553–561. doi:10.1016/j.ecss.2005.12.009
Ballesteros E (2006) Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr Mar Biol Annu Rev 44:23–195
Basso D (2012) Carbonate production by calcareous red algae and global change. In: Basso D, Granier B (eds) Calcareous algae and global change: from identification to quantification. Geodiversitas 34:13–33
Basso D, Nalin R, Massari F (2007) Genesis and composition of the Pleistocene Coralligène de plateau of the Cutro Terrace (Calabria, Southern Italy). N Jb Geol Paläont 244(2):73–182
Bellan-Santini D, Lacaze JC, Poizat C (1994) Les biocénoses marines et littorales de Méditerranée, synthèse, menaces et perspectives. Collection Patrimoines Naturels. Secrétariat de la Faune et de la Flore/M.N.H.N. 19:1–246
Ben Haj S, Boero F, Cebrian D, De Juan S, Limam A, Lleonart J, Torchia G, Rais C (eds), RAC/SPA, Tunis, pp 100
Bonacorsi M, Pergent-Martini C, Clabaut P, Pergent G (2012) Coralligenous ‘‘atolls’’: discovery of a new morphotype in the Western Mediterranean Sea. C R Biol 335:668–672
Bosellini A, Ginsburg NR (1971) Form and internal structure of recent algal nodules (Rhodolites) from Bermuda. J Geol 79:669–682
Bosence DWJ, Pedley HM (1982) Sedimentology and palaeoecology of a Miocene coralline algal biostrome from the Maltese Islands. Palaeogeogr Palaeoclimatol Palaeoecol 38:9–43. 10.1016/0031-0182(82)90062-1
Bosence DWJ, Bridges PH (1995) A review of the origin and evolution of carbonate mud-mounds. In: Monty CLV, Bosence DWJ, Bridges PH, Pratt BR (eds) Carbonate mud-mounds, their origin and evolution. Special Publications International Association Sedimentologists, vol 23. Blackwell, Oxford, pp 3–9
Bracchi VA, Savini A, Marchese F, Palamara S, Basso D, Corselli C (2015) Coralligenous habitat in the Mediterranean Sea: a geomorphological description from remote data. Ital J Geosci 134(1):32–40. doi:10.3301/IJG.2014.16
Bracchi VA, Nalin R, Basso D (2016) Morpho-structural heterogeneity of shallow-water coralligenous in a Pleistocene marine terrace (Le Castella, Italy). Pal Pal Pal 454:101–112
Brooke S, Schroeder WW (2007) State of deep coral ecosystems in the Gulf of Mexico Region: Texas to the Florida straits. In: Lumsden SE, Hourigan TF, Bruckner AW, Dorr G (eds) The state of deep coral ecosystems of the United States. NOAA Technical Memorandum CRCP-3, Silver Spring, MD, pp 271–306
Brooke S, Ross SW, Bane JM, Seim HE, Young CM (2013) Temperature tolerance of the deep-sea coral Lophelia pertusa from the southeastern United States. Deep-Sea Res II 92:240–248
Buhl-Mortensen PB (2000) Lophelia pertusa (Scleractinia) in Norwegian waters. Distribution, growth, and associated fauna. Dr. Scient thesis, Department of Fisheries and Marine Biology, University of Bergen, Norway
Buhl-Mortensen PB, Lepland A (2007) Ecological consequences of exploration drilling on coral reefs. Fiskenog Havet 7:123
Buhl-Mortensen PB, Hovland MT, Fosså JH, Furevik DM (2001) Distribution, abundance and size of Lophelia pertusa coral reefs in mid-Norway in relation to seabed characteristics. J Mar Biol Assoc UK 81:581–597
Buhl-Mortensen P, Buhl-Mortensen L, Purser A (2016a) Trophic ecology and habitat provision in cold-water coral ecosystems. In: Rossi S, Bramanti L, Gori A, Orejas Saco del Valle C (eds) Marine animal forests, the ecology of benthic biodiversity hotspots, Springer International Publishing, Switzerland, pp 2–23
Buhl-Mortensen L, Serigstad B, Buhl-Mortensen P, Olsen MN, Ostrowski M, Błażewicz-Paszkowycz M, Appoh E (2016b) First observations of the structure and megafaunal community of a large Lophelia reef on the Ivorian-Ghanaian margin (the Gulf of Guinea). Deep-Sea Res Part II. 10.1016/j.dsr2.2016.06.007
Cairns S (2007) Deep-water corals: an overview with special reference to diversity and distribution of deep-water scleractinian corals. Bull Mar Sci 81(3):311–322
Campiani E, Foglini F, Fraschetti S, Savini A, Angeletti L (2014) Conservation and management of coralligenous habitat: experience form the BIOMAP project. GEOHAB meeting 2014, Lorne, Australia, 6–9 May, Abstract Volume
Canals M, Ballesteros E (1997) Production of carbonate particles by phytobenthic communities on the Mallorca-Menorca shelf, Northwestern Mediterranean Sea. Deep-Sea Res II Top Stud Oceanogr 44:611–629. doi:10.1016/S0967-0645(96)00095-1
Carannante G, Simone L (1996) Rhodolith facies in the central–southern Appenines Mountains, Italy. In: Franseen EK, Esteban M, Ward WC, Rouchy JM (eds) Models for carbonate stratigraphy from miocene reef complexes of Mediterranean regions. SEPM concepts in sedimentology and palaeontology, vol 5, pp 261–275
Casellato S, Stefanon A (2008) Coralligenous habitat in the northern Adriatic Sea: an overview. Mar Ecol 29:321–341
Cocito S (2004) Bioconstruction and biodiversity: their mutual influence. Sci Mar 68(1):137–144
Colman JG, Gordon DM, Lane AP, Forde MJ, Fitz PJJ (2005) Carbonate mounds off Mauritania, Northwest Africa: status of deep-water corals and implications for management of fishing and oil exploration activities. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 417–441
Correa TBS, Eberli GP, Grasmueck M, Reed JK, Correa AMS (2012) Genesis and morphology of cold-water coral ridges in a unidirectional current regime. Mar Geol 326–328:14–27
De Mol B, Kozachenko M, Wheeler A, Alvares H, Henriet JP, Le Roy O (2007) Thérèse Mound: a case study of coral bank development in the Belgica Mound Province, Porcupine Seabight. Int J Earth Sci 96:103–120
De Mol B, Huvenne VAI, Canals M (2009) Cold-water coral banks and submarine landslides: a review. Int J Earth Sci 98(4):885–899
De Mol L, Van Rooij D, Pirlet H, Greinert J, Frank N, Quemmerais F, Henriet JP (2011) Cold-water coral habitats in the Penmarc’h and Guilvinec Canyons (Bay of Biscay): Deep-water versus shallow-water settings. Mar Geol 282:40–52
De Vogelaere A, Burton EJ, Trejo T, King CE, Clague DA, Tamburri MN, Cailliet GM, Kochevar RE, Douros WJ (2005) Deep-sea corals and resource protection at the Davidson Seamount, California, U.S.A. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 1189–1198
Di Geronimo I, Di Geronimo R, Rosso A, Sanfilippo R (2002) Structural and taphonomic analysis of a columnar coralline algal build-up from SE Sicily. Géobios 24:86–95
Di Geronimo I, Messina C, Rosso A, Sanfilippo R, Sciuto F, Vertino A (2005) Enhanced biodiversity in the deep: early pleistocene coral communities from southern Italy. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 61–86
Dorschel B, Hebbeln D, Foubert A, White M, Wheeler AJ (2007) Hydrodynamics and cold-water coral facies distribution related to recent sedimentary processes at Galway Mound west of Ireland. Mar Geol 244:184–195
Dorschel B, Hebbeln D, Ruggeberg A, Dullo W, Freiwald A (2005) Growth and erosion of a cold-water coral covered carbonate mound in the Northeast Atlantic during the Late Pleistocene and Holocene. Earth Planet Sc Lett 233(1–2):33–44
Douarin M, Sinclair DJ, Elliot M, Henry LA, Long D, Mitchison F, Roberts JM (2009) Changes in fossil assemblage in sediment cores from Mingulay Reef Complex (NE Atlantic): implications for coral reef build-up. Deep Sea Res Part II 99:286–296
Doxa A, Holon F, Deter J, Villeger S, Boissery P, Mouquet (2016) Mapping biodiversity in three-dimensions challenges marineconservation strategies: the example of coralligenous assemblages in North-Western Mediterranean Sea. Ecol Ind 61:1042–1054
Duineveld GCA, Lavaleye MSS, Berghuis EM (2004) Particle flux and food supply to a seamount cold-water coral community Galicia Bank, NW Spain. Mar Ecol Prog Ser 277:13–23
Duineveld GCA, Lavaleye MSS, Bergman MJN, De Stigter H, Mienis F (2007) Trophic structure of a cold-water coral mound community (Rockall Bank, NE Atlantic) in relation to the near-bottom particle supply and current regime. Bull Mar Sci 81(3):449–467
Dullo WC, Flögel S, Rüggeberg A (2008) Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar Ecol Prog Ser 371:165–176
Eisele M, Frank N, Wienberg C, Titschack J, Mienis F, Beuck L, Tisnerat-Laborde N, Hebbeln D (2014) Sedimentation patterns on a cold-water coral mound off Mauritania. Deep-Sea Res II 99:307–315
Esentia I, Stow D, Smillie Z (2018) Contourite drifts and associated bedforms. In: Micallef A, Krastel S, Savini A (eds) (2018) Submarine Geomorphology. Springer
European Commission (2010) Commission decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters. Off J Eur Union L232/14
European Parliament, Council of the European Union (2008) Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off J Eur Union L164/19
Fink HG, Wienberg C, Hebbeln D, McGregor HV, Schmiedl G, Taviani M, Freiwald A (2013) Oxygen control on Holocene cold-water coral development in the eastern Mediterranean Sea. Deep-Sea Res-I 62:89–96
Försterra G, Beuck L, Häussermann V, Freiwald A (2005) Shallow-water Desmophyllum dianthus (Scleractinia) from Chile: characteristics of the biocoenoses, the bioeroding community, heterotrophic interactions and (paleo)-bathymetric implications. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 937–977
Fosså JH, Skjoldal HR, (2010) Conservation of cold water coral reefs in Norway. In Grafton RQ Hilborn R, Squires D, Tait M, Williams M (eds) Handbook of marine fisheries conservation and management. Oxford University Press, New York, pp 215–230
Fosså JH, Mortensen PB, Furevik DM (2002) The deep-water coral Lophelia pertusa in Norwegian waters: distribution and fishery impacts. Hydrobiologia 471:1–12
Fosså JH, Lindberg B, Christensen O, Lundälv T, Svellingen I et al (2005) Mapping of Lophelia reefs in Norway: experiences and survey methods. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 359–391
Foubert A, Henriet JP (2009) Nature and significance of the recent carbonate mound record: the mound challenger code. Lecture notes in earth sciences, vol 126. Springer, 298 pp. ISBN: 978-3-642-00289-2
Foubert A, Huvenne VAI, Wheeler A, Kozachenko M, Opderbecke J, Henriet JP (2011) The Moira Mounds, small cold-water coral mounds in the Porcupine Seabight, NE Atlantic: Part B—Evaluating the impact of sediment dynamics through high-resolution ROV-borne bathymetric mapping. Mar Geol 282:65–78
Frank N, Ricard E, Lutringer-Paquet A, Van Der Land C, Colin C, Blamart D, Foubert A, Van Rooij D, Henriet J-P, De Haas H, Van Weering T (2009) The Holocene occurrence of cold water corals in the NE Atlantic: implications for coral carbonate mound evolution. Mar Geol 266:129–142
Frantz BR, Foster MS, Riosmena-Rodriguez R (2005) Clathromorphum nereostratum (Coral- linales, Rhodophyta): the oldest alga? J Phycol 41:770–773
Freiwald A, Henrich R, Pätzold J (1997) Anatomy of a deep-water coral reef mound from Stjernsund, West-Finnmark, northern Norway. Cool-water carbonates. In: James NP (ed) SEPM Special Publication, 56, pp 141–161
Freiwald A, Wilson JB, Henrich R (1999) Grounding pleistocene icebergs shape recent deep-water coral reefs. Sed Geol 125:1–8
Freiwald A, Huhnerbach V, Lindberg B, Wilson JB, Campbell J (2002) The Sula Reef complex, Norwegian Shelf. Facies 47:179–200
Freiwald A, Fosså JH, Grehan A, Koslow T, Roberts JM (2004) Cold-water coral reefs. UNEP-WCMC, Cambridge, UK
Genin A, Dayton PK, Lonsdale PF, Spiess FN (1986) Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 322:59–61
Georgian SE, DeLeo D, Durkin A, Gomez CE, Kurman M, Lunden JJ, Cordes EE (2016) Oceanographic patterns and carbonate chemistry in the vicinity of cold-water coral reefs in the Gulf of Mexico: implications for resilience in a changing ocean. Limnol Oceanogr 61:648–665
Giaccone G, Giaccone T, Catra M (2009) Association with Laminaria rodriguezii on a detritic bottom and on rocks: cystoseiretum zosteroidis Giaccone 1973 subass. Laminarietosum rodriguezii Giaccone 1973. In: Priority habitats according to the SPA/BIO protocol (Barcelona Convention) present in Italy. Identification sheets 16:204–208
Ginsburg RN, Gischler E, Schlager W (1994) Johannes Walther on reefs. Geological milestones, vol II. Comparative Sedimentology Laboratory, Rosential School of Marine and Atmospheric Science, University of Miami, 141 pp
Glogowski S, Dullo WC, Feldens P, Liebetrau V, Von Reumont J, Hühnerbach V, Krastel S, Wynn RB, Flögel S (2015) The Eugen Seibold coral mounds offshore western Morocco: oceanographic and bathymetric boundary conditions of a newly discovered cold-water coral province. Geo-Mar Lett 35:257–269
Gori A, Orejas C, Madurell T, Bramanti L, Martin M, Quintanilla E, Marti-Puig P, Lo Iacono C, Puig P, Requena S, Greeacre M, Gili JM (2013) Bathymetrical distribution and size structure of coldwater coral populations in the Cap de Creus and Lacaze-Duthiers canyons (northwestern Mediterranean). Biogeosciences 10:2049–2060
Grasmueck M, Eberli GP, Viggiano DA, Correa T, Rathwell G, Luo J (2006) Autonomous underwater vehicle (AUV) mapping reveals coral mound distribution, morphology, and oceanography in deep water of the Straits of Florida. Geophys Res Lett 33:L23616. doi:10.1029/2006GL027734
Guidetti P, Terlizzi A, Fraschetti S, Boero F (2002) Spatio-temporal variability in fish assemblages associated with coralligenous formations in south eastern Apulia (SE Italy). Ital J Zool 69:325–331
Guinan J, Grehan AJ, Dolan MFJ, Brown C (2009) Quantifying relationships between video observations of cold-water coral cover and seafloor features in Rockall Trough, west of Ireland. MEPS 375:125–138
Guinotte JM, Orr J, Cairns S, Freiwald A, Morgan L, George R (2006) Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front Ecol Environ 4:141–146
Hebbeln D, Samankassou E (2015) Where did ancient carbonate mounds grow—in bathyal depths or in shallow shelf waters? Earth Sci Rev 145:56–65
Hebbeln D, Wienberg C, Wintersteller P, Freiwald A, Becker M, Beuck L, Dullo C, Eberli GP, Glogowski S, Matos L, Forster N, Reyes-Bonilla H, Taviani M, The MSM 20-4 Shipboard Scientific Party (2014) Environmental forcing of the Campeche cold-water coral province, southern Gulf of Mexico. Biogeosciences 11:1799–1815
Hebbeln D, Van Rooij D, Wienberg C (2016) Good neighbours shaped by vigorous currents: cold-water coral mounds and contourites in the North Atlantic. Mar Geol 378:171–185
Henriet J-P, De Mol B, Pillen S, Vanneste M, Van Rooij D, Versteeg W, Croker PF, Shannon PM, Unnithan V, Bouriak S, Chachkine P, Porcupine-Belgica 97 Shipboard Party (1998) Gas hydrate crystals may help build reefs. Nature 391:648–649
Henry LA, Roberts JM (2007) Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep Sea Res Part I Oceanogr Res 54(4):654–672
Henry LA, Navas JM, Roberts JM (2013) Multi-scale interactions between local hydrography, seabed topography, and community assembly on cold-water coral reefs. Biogeosciences 10(4):2737–2746
Hernández-Molina FJ, Llave E, Preu B, Ercilla G, Fontan A, Bruno M, Serra N, Gomiz JJ, Brackenridge RE, Sierro FJ, Stow DAV, García M, Juan C, Sandoval N, Arnaiz A (2014) Contourite processes associated with the Mediterranean outflow water after its exit from the Strait of Gibraltar: global and conceptual implications. Geology 42:227–230
Hong JS (1982) Contribution à l’étude des peuplements d’un fond coralligène dans la région marseillaise en Méditerranée Nord-Occidentale. Bull KORDI 4:27–51
Hovland M, Croker PF, Martin M (1994) Fault-associated seabed mounds (carbonate knolls?) off western Ireland and northwest Australia. Mar Pet Geol 11:232–246
Husebø Å, Nøttestad L, Fosså JH, Furevik DM, Jørgensen SB (2002) Distribution and abundance of fish in deep-sea coral habitats. Hyrdobiologia 471:91–99
Huvenne VAI, De Mol B, Henriet JP (2003) A 3D seismic study of the morphology and spatial distribution of buried coral banks in the Porcupine Basin, SW of Ireland. Mar Geol 198:5–25
Huvenne VAI, Masson DG, Wheeler AJ (2009) Sediment dynamics of a sandy contourite: the sedimentary context of the Darwin cold-water coral mounds, Northern Rockall Trough. Int J Earth Sci (Geol Rundsch) 98:865–884
Huvenne VAI, Tyler PA, Masson DG, Fisher EH, Hauton C, Hühnerbach V, Le Bas T, Wolff GA (2011) A picture on the wall: innovative mapping reveals cold-water coral refuge in submarine Canyon. PLoS ONE 6(12):e28755. doi:10.1371/journal.pone.0028755
James NP, Bourque P-A (1992) Reefs and mounds. In: Walker RG, James NP (eds) Facies Models, response to sea level change. Geological Association of Canada, Geotext (1), pp 323–347
James NP, Clarke JDA (eds) (1997) Cool-water carbonates. SEPM Special Publication 56, Tulsa, OK, pp 1–20
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Kamenos NA, Hoey TB, Nienow P, Fallick AE, Clavere T (2012) Reconstructing greenland ice sheet runoff using coralline algae. Geology 40(12):1095–1098
Kano A, Ferdelman TG, Williams T, Henriet JP, Ishikawa T, Kawagoe N, Takashima, C, Kakizaki Y, Abe K, Sakai S, Browning E, Li X, The IODP Expedition 307 Scientists (2007) Age constraints on the origin and growth history of a deep-water coral mound in the northeast Atlantic drilled during integrated ocean drilling program expedition 307. Geology 35(11):1051–1054
Kenyon NH, Akhmetzhanov AM, Wheeler AJ, Van Weering TCE, De Haas H, Ivanov MK (2003) Giant carbonate mud mounds in the southern Rockall Trough. Mar Geol 195:5–30
Klement KW (1967) Practical classification of reefs and banks, bioherms and biostromes. Am Assoc Pet Geol Bull 51:167–168
Kružić P (2013) Bioconstructions in the Mediterranean: present and future. In: Goffredo S, Dubinsky Z (eds) The Mediterranean Sea: its history and present challenges, Springer, Berlin, pp 435–447
Laborel J (1961) Le concrétionnement algal ‘Coralligène’ et son importance géomorphologique en Méditerranée. Recueils Trav Stat Mar Endoume 23:37–60
Laborel J (1987) Marine biogenic constructions in the Mediterranean. a review. Sci Rep Port-Cros Natl Park 13:97–126
Laubier L (1966) Le coralligène des Albères. Monographie biocénotique. Annales Institut Océanographique, Paris, 43(2):137–316
Le Guilloux E, Olu K, Bourillet JF, Savoye B, Iglesias FP, Sibuet M (2009) First observations of deep-sea coral reefs along the Angola margin. Deep-Sea Res II 56:2394–2403
Lindberg B (2004) Cold-water coral reefs on the norwegian shelf—acoustic signature, geological, geomorphological and environmental setting. Ph.D. thesis. Department of Geology, University of Tromsø
Lindberg B, Berndt C, Mienert J (2007) The Fugløy Reef at 70°N; acoustic signature, geologic, geomorphologic and oceanographic setting. Int J Earth Sci 96–1, Special volume: carbonate mounds on the NW European margin: a window into earth history
Lo Iacono C, Gracia, E, Ranero C, Emelianov M, Huvenne V, Bartolome R, Booth-Rea G, Prades J, MELCOR Cruise Party (2014a) The West Melilla cold water coral mounds, Eastern Alboran Sea: morphological characterization and environmental context. Deep-Sea Res II 99:316–326
Lo Iacono C, Huvenne VAI, Gonzalez LV, Vertino A, Van Rooij D, Gràcia E, Ranero CR, the GATEWAYS Cruise Party (2016) Living reefs and CWC mounds in the Alboran Sea (Western Mediterranean). Holocene evolution and present-day conditions. 6th ISDSC, 11–16 September, Boston, USA
Lo Iacono C, Savini A, Huvenne V, Gràcia E, 2018. Habitat mapping of cold-water corals in the Mediterranean Sea. In: Orejas C, Jimenez C (eds) Past, present and future: Mediterranean cold-water corals. Springer, Berlin
Lo Iacono C, Victorero Gonzalez L, Huvenne VAI, Van Roji D, Gràcia E, Ranero C, The GATEWAYS Cruise Party (2014b) Morphology and shallow stratigraphy of the West Melilla and Cabliers CWC Mounds (Alboran Sea). Preliminary insights from the GATEWAYS MD194 Cruise. Second Deep-Water Circulation Congress, Ghent (Belgium), Sept 2014
Lopes D, Hajdu D (2014) Carnivorous sponges from deep-sea coral mounds in the Campos Basin (SW Atlantic), with the description of six new species (Cladorhizidae, Poecilosclerida, Demospongiae). Mar Biol Res 10(4):329–356
Lowenstam HA (1950) Niagaran reefs of the Great Lakes area. J Geol 58:431–487
Lowenstam HA (1981) Minerals formed by organisms. Science 211:1126–1131
Lunden JJ, Georgian SE, Cordes EE (2013) Aragonite saturation states at cold-water coral reefs structured by Lophelia pertusa in the northern Gulf of Mexico. Limnol Oceanogr 58:354–362
Marion F (1883) Esquisse d’une topographie zoologique du Golfe de Marseille. Annales Muséum Histoire Naturelle Marseille 1(1):1–108
Martin CS, Giannoulaki M, De Leo F, Scardi M, Salomidi M, Knitweiss L, Pace ML, Garofalo G, Gristina M, Ballesteros E, Bavestrello G, Belluscio A, Cebrian E, Gerakaris V, Pergent G, Pergent-Martini C, Schembri PJ, Terribile K, Rizzo L, Ben Souissi J, Bonacorsi M, Guarnieri G, Krzelj M, Macic V, Punzo E, Valavanis V, Fraschetti S (2014) Coralligenous and maerl habitats: predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci Rep 4:5073. doi:10.1038/srep05073
Martorelli E, Petroni G, Chiocci FL, Party Pantelleria Scientific (2011) Contourites offshore Pantelleria Island (Sicily Channel, Mediterranean Sea): depositional, erosional and biogenic elements. Geo-Mar Lett 31:481–493
Masson DG, Bett BJ, Billett DSM, Jacobs CL, Wheeler AJ, Wynn RB (2003) The origin of deep-water, coral-topped mounds in the northern Rockall Trough, Northeast Atlantic. Mar Geol 194:159–180
Mazzini A, Akhmetzhanov A, Monteys X, Ivanov M (2012) The Porcupine Bank Canyon coral mounds: oceanographic and topographic steering of deep-water carbonate mound development and associated phosphatic deposition. Geo-Mar Lett 32(3):205–225
Messing CG, Neumann AC, Lang JC (1990) Biozonation of deep-water lithoherms and associated hardgrounds in the northeastern Straits of Florida. Palaios 5:15–33
Mienis F, De Stigter HC, White M, Duineveld G, De Haas H, Van Weering TCE (2007) Hydrodynamic controls on cold-water coral growth and carbonate-mound development at the SW and SE Rockall Trough Margin, NE Atlantic Ocean. Deep Sea Res Part I 54(9):1655–1674
Mienis F, Duineveld GCA, Davies AJ, Lavaleye MMS, Ross SW, Seim H, Bane J, Van Haren H, Bergman MJN, De Haas H, Brooke S, Van Weering TCE (2014) Cold-water coral growth under extreme environmental conditions, the Cape Lookout area, NW Atlantic. Biogeosciences 11:2543–2560
Mohn C, Rengstorf A, White M, Duineveld G, Mienis F, Soetaert K, Grehan A (2014) Linking benthic hydrodynamics and cold-water coral occurrences: a high-resolution model study at three cold-water coral provinces in the NE Atlantic. Prog Oceanogr 122:92–104
Montaggioni LF, Braithwaite CJR (2009) Quaternary coral reef systems. History, development processes and controlling factors. Developments in marine geology 5, Elsevier, 550 pp
Montagna P, McCulloch M, Taviani M, Mazzoli C, Vendrell B (2006) Phosphorus in cold-water corals as a proxy for seawater nutrient chemistry. Science 312(5781):1788–1791
Monty CLV (1995) The rise and nature of carbonate mud-mounds: an introductory actualistic approach. In: Monty CLV, Bosence DWJ, Bridges PH, Pratt BR (eds) Carbonate mud-mounds: their origin and evolution. IAS Special Publication 23, p 11–48
Moreno Navas J, Miller PL, Henry LA, Hennige SJ, Roberts JM, (2014) Ecohydrodynamics of cold-water coral reefs: a case study of the mingulay reef complex (Western Scotland). PLoS-ONE 9–5
Morganti C, Cocito S, Sgorbini S (2001) Contribution of biocostructor to coralligenous assemblages exposed to sediment deposition. Biol Mar Mediterr 8:283–286
Murray RJ, Murray F, Anagnostou E, Hennige S, Gori A, Henry L-A, Fox A, Kamenos N, Foster GL (2016) Cold-water corals in an era of rapid global change: are these the deep ocean’s most vulnerable ecosystems? In: Goffredo S, Dubinsky Z (eds) The cnidaria, past, present and future, Part VIII, pp 593–606
Nalin R, Basso D, Massari F (2006): Pleistocene coralline algal build-ups (coralligène de plateau) and associated bioclastic deposits in the sedimentary cover of Cutro marine terrace (Calabria, Southern Italy) In: Pedley HM, Carannante G (eds) Coolwater carbonates: depositional systems and palaeoenvironmental control. London Geological Society Special Publications 255, pp 11–22
Naumann MS, Orejas C, Ferrier-Pagès C (2014) Species-specific physiological response by the cold-water corals Lophelia pertusa and Madrepora oculata to variations within their natural temperature range. Deep-Sea Res II 99:36–41
Navone A, Bianchi CN, Orrù P, Ulzega A (1992) Saggio di cartografia geomorfologica e bionomica nel parco marino di Tavolare – Capo Coda Cavallo (Sardegna Nord-orientale). Oebalia (suppl XVII):469–478
Naylor LA (2005) The contribution of biogeomorphology to the emerging field of geobiology. Pal Pal Pal 219(1–2):35–51
Nelson CS, Freiwald A, Titschack J, List S (2001) Lithostratigraphy and sequence architecture of temperate mixed siliciclastic-carbonate facies in a new Plio-Pleistocene section at Plimiri, Rhodes Island (Greece). Occas Rep 25:1–50 (Department of Earth Sciences, University of Waikato)
Neumann AC, Kofoed JW, Keller GH (1977) Lithoherms in the Straits of Florida. Geology 5(1):4–10
Novosel M, Olujic G, Cocito S, Pozar-Domac A (2004) Submarine freshwater springs in the Adriatic Sea: a unique habitat for the nryozoan Pentapora fascialis. In: Moyano HI, Cancino JM, WyseJackson PN (eds) Bryozoan studies, A.A. Balkerma Publisher, Lisse
Orejas C, Gori A, Lo Iacono C, Puig P, Gili JM (2009) Distribution of the deep corals Madrepora oculata, Lophelia pertusa, Dendrophyllia cornigera, and quantification of anthropogenic impact in the Cap de Creus Canyon (North Western Mediterranean). MEPS—Marine Ecology Progress Series 397, pp 37–51
OSPAR Commission (2008) Descriptions of habitats on the OSPAR list of threatened and/or declining species and habitats. Reference number 2008–7
Paoli C, Montefalcone M, Morri C, Vassallo P, Bianchi CN (2016) Ecosystem functions and services of the marine animal forests. In: Rossi S, Bramanti L, Gori A, Saco del Valle CO (eds) Marine animal forests the ecology of benthic biodiversity hotspots, Springer, Switzerland, ISBN: 978-3-319-17001-5
Pedley HM, Carannante G (2006) Cool-water carbonates: depositional systems and palaeoenvironmental controls. Geological Society London Special Publications, vol 255
Pérès JM (1982) Structure and dynamics of assemblages in the benthal. Mar Ecol 5(1):119–185. In: Kinne O (ed) Marine ecology: a comprehensive, integrated treatise on life in oceans and coastal waters: 5. Ocean management, vol 1, pp 119–185
Pérès JM, Picard J (1951) Note sur les fonds coralligènes de la région de Marseille. Archives de zoologie expérimentale et générale 88:24–38
Pérès JM, Picard J (1964) Nouveau manuel de bionomie benthique de la Mer Méditerranée. Rec Tr St Mar Endoume 31(47):1–137
Raddatz J et al (2011) Paleoenvironmental reconstruction of Challenger Mound initiation in the Porcupine Seabight, NE Atlantic. Mar Geol 282:79–90
Rasser MW (2000) Coralline red algal limestones of the late Eocene alpine Foreland Basin in upper Austria: component analysis, facies and paleoecology. Facies 42(1):59–92. doi:10.1007/BF02562567
Rasser MW, Piller WE (2004) Crustose algal frameworks from the Eocene Alpine Foreland. Pal Pal Pal 206:21–39
Relini G (Ed) (2009) Marine bioconstructions—nature’s architectural seascapes. Italian habitats 22. Italian Ministry of the Environment and Territorial Protection, Friuli Museum of Natural History, Comune di Udine, 87 p
Remia A, Taviani M (2005) Shallow-buried Pleistocene Madrepora-dominated coral mounds on a muddy continental slope, Tuscan Archipelago, NE Tyrrhenian Sea. Facies 50:419–425
Riding R (2002) Structure and composition of organic reefs and carbonate mud mounds: concepts and categories. Earth Sci Rev 58:163–231
Ries JB (2006) Aragonite production in calcite seas: effect of seawater Mg/Ca ratio on the calcification and growth of the calcareous alga Penicillus capitatus. Paleobiology 31(3):445–458
Robert K, Jones D, Tyler P, Van Rooij D, Huvenne V (2015) Finding the hotspots within a biodiversity hotspot: fine-scale biological predictions within a submarine canyon using high-resolution acoustic mapping techniques. Mar Ecol 36:1256–1276
Robert K, Jones D, Roberts M, Huvenne V (2016) Improving predictive mapping of deep-water habitats: considering multiple model outputs and ensemble techniques. Deep Sea Res Part I Oceanogr Res Pap 80–89
Roberts JM, Brown CJ, Long D, Bates CR (2005) Acoustic mapping using a multibeam echosounder reveals cold-water coral reefs and surrounding habitats. Coral Reefs 24:654–669
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Roberts JM, Wheeler AJ, Freiwald A, Cairns S (2009) Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge University Press, Cambridge, United Kingdom
Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso JP (2010) Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7:289–300
Rodríguez-Martínez M (2011) Mud mounds. In: Encyclopedia of geobiology, pp667–675
Rogers AD (1999) The Biology of (L 1758) and other deep-water reef-forming corals and impacts from human activities. Int Rev Hydrobiol 84(4):315–406
Rogers AD, Baco A, Griffi H, Hart T, Hall-Spencer JM (2007) Corals on seamounts. In: Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS (eds) Seamounts: ecology, fisheries & conservation, Wiley-Blackwell, pp 141–169
Rokoengen K, Østmo SR (1985) Shallow geology off Fedje western Norway. IKU report. 24.1459/01/85
Ross SW, Quattrini AM (2007) The fish fauna associated with deep coral banks off the southeastern United States. Deep Sea Res Part I 54–6:975–1007
Ross RE, Howell KL (2012) Use of predictive habitat modelling to assess the distribution and extent of the current protection of ‘listed’ deep-sea habitats. Divers Distrib 19:433–445
Rosso A, Sanfilippo R (2009). The contribution of bryozoans and serpuloideans to coralligenous concretions from SE Sicily. In: UNEP-MAP-RAC/SPA, Proceedings of the first symposium on the coralligenous and other calcareous bio-concretions of the Mediterranean Sea, Tabarka, 15–16 Jan 2009, pp 123–128
Salomidi M, Katsanevakis S, Borja Á, Braeckman U, Damalas D, Galparsoro I, Mifsud R, Mirto S, Pascual M, Pipitone C, Rabaut M, Todorova V, Vassilopoulou V, Vega Fernández T (2012) Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: a stepping stone towards ecosystem-based marine spatial management. Mediteranean Mar Sci 13(1):49–88
Sarà M (1971) Un biotopo da proteggere: il coralligeno pugliese. Atti I Simp Naz Conserv Nat 1–25:145–151
Sartoretto S (1994) Structure et dynamique d’un nouveau type de bioconstruction à Mesophyllum lichenoides (Ellis) Lemoine (Corallinales, Rhodophyta). Comptes Rendus de l’Académie des Sciences de Paris, Sciences de la Vie 317:156–160
Sartoretto S, Verlaque M, Laborel J (1996) Age of settlement and accumulation rate of submarine “coralligène” (−10 to −60 m) of the north Western Mediterranean Sea; relation to Holocene rise in sea level. Mar Geol 130:317–331
Savini A, Marchese F, Verdicchio G, Vertino A (2016) Submarine slide topography and the distribution of vulnerable marine ecosystems: a case study in the Ionian Sea (Eastern Mediterranean). In: Lamarche G, et al (eds) Submarine mass movements and their consequences, advances in natural and technological hazards research, vol 41. Springer, Dordrecht, pp 163–170
Savini A, Vertino A, Marchese F, Beuck L, Freiwald A, Roberts JM (2014) Mapping cold-water coral habitats at different scales within the Northern Ionian Sea (Central Mediterranean): an assessment of coral coverage and associated vulnerability. PloS ONE 9(1):e87108
Somoza L, Ercilla G, Urgorri V, León L, Medialdea T, Paredes M, Gonzaleza FJ, Nombelae MA (2014) Detection and mapping of cold-water coral mounds and living Lophelia reefs in the Galicia Bank, Atlantic NW Iberia margin. Mar Geol 349:73–90
Squires DF (1964) Fossil coral thickets in Wairarapa, New Zealand. J Palaeontology 38:904–915
Stewart HA, Davies JS, Guinan J, Howell KL (2014) The dangeard and explorer canyons, South Western approaches UK: geology, sedimentology and newly discovered cold-water coral mini-mounds. Deep-Sea Res II 104:230–244
Stone RP (2006) Coral habitat in the Aleutian Islands of Alaska: depth distribution, fine-scale species associations, and fisheries interactions. Coral Reefs 25(2):229–238
Thiem O, Ravagnan E, Fosså JH, Berntsen J (2006) Food supply mechanisms for cold-water corals along a continental shelf edge. J Mar Syst 60:207–219
Thierens M, Browning E, Pirlet H, Loutre MF, Dorschel B, Huvenne VAI, Titschack J, Colin C, Foubert A, Wheeler AJ (2013) Cold-water coral carbonate mounds as unique palaeo-archives: the plio-pleistocene challenger mound record (NE Atlantic). Quatern Sci Rev 73:14–30
Titschack J, Nelson CS, Beck T, Freiwald A, Radtke U (2008) Sedimentary evolution of a late pleistocene temperate red algal reef (Coralligène) on Rhodes, Greece: correlation with global sea–level fluctuations. Sedimentology 55:1747–1776
Tittensor DP, Baco AR, Brewin PE, Clark MR, Consalvey M, Hall-Spencer J, Rowden AA, Schlacher T, Stocks KI, Rogers AD (2009) Predicting global habitat suitability for stony corals on seamounts. J Biogeogr 36:1111–1128
Toomey DF, Finks RM (1969) Middle Ordovician (Chazyan) mounds, southern Quebec, Canada: a summary report. New York State Geological Association, 41st annual meeting, Guidebook to field excursions. Plattsburgh, New York, pp 121–134
Tracey DM, Rowden AA, Mackay KA, Compton T (2011) Habitat-forming cold-water corals show affinity for seamounts in the New Zealand region. Mar Ecol Prog Ser 430:1–22
Tribollet A, Godinot C, Atkinson M, Langdon C (2009) Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Global Biogeochem Cycles 23:3
Tribot A-S, Mouquet N, Villéger S, Raymond M, Hoff F, Boissery P, Holon F, Deter J (2016) Taxonomic and functional diversity increase the aesthetic value of coralligenous reefs. Sci Rep 6:34229. doi:10.1038/srep34229
UNEP-MAP (2011) Convention for the protection of the marine environment and the coastal region of the mediterranean and its protocols. MAP Special Publications http://195.97.36.231/dbases/MAPpublications/BCP_Eng.pdf. Accessed 24 July 2015. Monitoring deep Mediterranean rhodolith beds. Available from: https://www.researchgate.net/publication/287760121_Monitoring_deep_Mediterranean_rhodolith_beds. Accessed Jan 11 2017
UNEP-MAP-RAC/SPA (2008) Action plan for the conservation of the coralligenous and other calcareous bio-concretions in the Mediterranean Sea. Ed. RAC/SPA Tunis, 21 pp
UNEP-MAP-RAC/SPA (2010) The Mediterranean Sea Biodiversity: state of the ecosystems, pressures, impacts and future priorities. In: Bazairi H, Ben Haj S, Boero F, Cebrian D, De Juan S, Limam A, Lleonart J, Torchia G, Rais C (eds) RAC/SPA, Tunis, pp 100
Van Oevelen D, Duineveld GCA, Lavaleye MSS, Mienis F, Soetaert K, Heipa CHR (2009) The cold-water coral community as a hot spot for carbon cycling on continental margins: a food-web analysis from Rockall Bank (northeast Atlantic). Limnol Oceanogr 54(6):1829–1844
Van Rooij D, De Mol B, Huvenne V, Ivanov M, Henriet JP (2003) Seismic evidence of current-controlled sedimentation in the Belgica mound province, upper Porcupine slope, southwest of Ireland. Mar Geol 195:31–53
Van Rooij D, Huvenne VAI, Blamart D, Henriet JP, Wheeler A, De Haas H (2009) The Enya mounds: a lost mound-drift competition. Int J Earth Sci (Geol Rundsch) 98:849–863
Van Rooij D, Blamart D, De Mol L, Mienis F, Pirlet H, Wehrmann LM, Barbieri R, Maignien L, Templer SP, De Haas H, Hebbeln D, Frank N, Larmagnat S, Stadnitskaia A, Stivaletta N, Van Weering T, Zhang Y, Hamoumi N, Cnudde V, Duyck P, Henriet JP (2011) Cold-water coral mounds on the Pen Duick Escarpment, Gulf of Cadiz: the microsystems project approach. Mar Geol 282:102–117
Van Weering TCE, De Haas H, De Stigter HC, Lykke-Andersen H, Kouvaev I (2003) Structure and development of giant carbonate mounds at the SW and SE Rockall Trough margins, NE Atlantic Ocean. Mar Geol 198:67–81
Victorero L, Blamart D, Pons-Branchu E, Mavrogordato MN, Huvenne VAI (2016) Reconstruction of the formation history of the Darwin Mounds, N Rockall Trough: how the dynamics of a sandy contourite affected cold-water coral growth. Mar Geol 378:186–195
Virgilio M, Airoldi L, Abbiati M (2006) Spatial and temporal variations of assemblages in a Mediterranean coralligenous reef and relationships with surface orientation. Coral Reefs 25:265–272
Watling L, Norse EA (1997) Disturbance of the seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv Biol 12–6:1180–1197
Wienberg C, Beuck L, Heidkamp S, Hebbeln D, Freiwald A, Pfannkuche O, Monteys X (2008) Franken Mound-facies and biocoenosis mapping of a newly-discovered ‘carbonate mound’ at the West Rockall Bank, NE-Atlantic. Facies 54:1–24
Wild C, Wehrmann LM, Mayr C, Schöttner SI, Allers E, Lundälv T (2009) Microbial degradation of cold-water coral-derived organic matter: potential implication for organic C cycling in the water column above Tisler Reef. Aquat Biol 7:71–80
Wilson JL (1975) Carbonate facies in geologic history. Springer, New York 471 pp
Wilson JB (1979) ‘Patch’ development of the deep-water coral Lophelia pertusa (L.) on Rockall Bank. J Mar Biol Assoc UK 59:165–177
Wilson MFJ, O’Connell B, Brown C, Guinan JC, Grehan AJ (2007) Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Mar Geodesy 30:3–35
Wisshak M, Schönberg CHL, Form A, Freiwald A (2014) Sponge bioerosion accelerated by ocean acidification across species and latitudes? Helgol Mar Res 201468:385
Wood RA (1999) Reef evolution. Oxford University Press, UK, 414 pp
Woodroff CD, Webster JM (2015) Coral reefs and sea-level change. Mar Geol 352:248–267
Zibrowius H (1980) Les Scléractiniaires de la Méditerranée et de l’Atlantique nordoriental. Mémoires de l’Institut Océanographique vol. 11. Mémoires de l’Institut Océanographique, Monaco
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Lo Iacono, C., Savini, A., Basso, D. (2018). Cold-Water Carbonate Bioconstructions. In: Micallef, A., Krastel, S., Savini, A. (eds) Submarine Geomorphology. Springer Geology. Springer, Cham. https://doi.org/10.1007/978-3-319-57852-1_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-57852-1_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57851-4
Online ISBN: 978-3-319-57852-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)