Abstract
Brain-Computer Interfaces (BCIs) are powerful tools for enabling communication between people and the surrounding world by directly utilizing brain activity and avoiding motor pathways. Before moving into invasive implantation of BCIs, a key issue must be resolved—localization of the areas for implantation, which might vary depending on the chosen BCI type as well as on the individual person’s characteristics. In this study, we aimed to evaluate the possibility of non-invasive navigation of subdural electrode implantation for P300 speller BCI by using magnetoencephalogaphy (MEG). The accuracy of subdural P300 speller performance based on the sites identified with MEG was comparable with the performance based on the sites identified from subdural electrode grids—80% and 90% averaged accuracy, respectively. Our study demonstrates the feasibility of using MEG as a non-invasive tool for navigating electrode implantation required for high accuracy invasive P300 speller control.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain computer interface (BCI)
- Electrocorticography (ECoG)
- Magnetoencephalography (MEG)
- P300 response
- P300 speller
1 Introduction
Brain-Computer Interfaces (BCIs) have a strong potential to significantly contribute towards improving quality of life in people with motor system-related disabilities. Indeed, BCIs may provide these people with the much needed possibility of communication by utilizing activity from the central nervous system and bypassing compromised motor pathways. There is evidence to suggest that, for severely disabled patients (such as those with advanced stages of amyotrophic lateral sclerosis—ALS, locked-in syndrome, tetraplegia or severe impairments after stroke), the surgically-implanted intracranial BCIs might be more efficient than scalp-based BCIs [1, 2]. However, before the chronic BCI implantation can be considered as a viable option for these patients, an important issue needs to be addressed—localization of intracranial electrode implantation sites, which might vary depending on the BCI approach used, as well as on each individual person’s characteristics.
In our previous studies [2, 3], we have concluded that specific approaches must be developed to identify and extract the data of interest that is necessary to achieve desired performance of chronically implanted BCIs. Among several suggested approaches, the most promising approach for future work with BCIs has been the use of non-invasive technology, such as magnetoencephalography (MEG) [3]. The MEG allows recording of neuromagnetic brain activity with precise temporal resolution. Moreover, when combined with the structural information about the brain, derived from magnetic resonance imaging (MRI), the MEG also provides excellent spatial resolution. It is routinely used for mapping of functionally significant cortical brain areas, such as motor, auditory, visual, and language during pre-surgical evaluation of patients with pharmacoresistant epilepsy [4]. Therefore, it can potentially be utilized for identifying the cortex involved in generation of signals for targeted BCI use.
The aim of our current study was to evaluate feasibility of non-invasive navigation for subdural electrode selection with MEG needed for high accuracy performance of P300 speller.
2 Methods
The study was performed in a 17-year-old right-handed female patient with intractable epilepsy, undergoing evaluation for epilepsy surgery. Two main approaches were utilized to select channels to test invasive P300 speller performance: (1) Protocol #1 (MEG-based) used MEG source localization to navigate the choice of P300 speller sites; and (2) A comparison Protocol #2 (ECoG-based) utilized statistical ECoG signal analysis.
2.1 MEG-Based Approach
2.1.1 Non-invasive Localization of P300 Generators with MEG
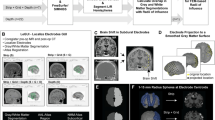
During this test, visual evoked fields were recorded in response to two letter stimuli (O and X) that were presented in an “odd-ball” paradigm manner, with 76% of NON-TARGET (letter “O”) and 24% of TARGET stimuli (letter “X”) (Fig. 1a). Altogether, 76 frequent and 24 deviant stimuli were presented. Both target and non-target stimuli had durations of 100 ms. The inter-trial interval (ITI) was randomized between 2000 and 3000 ms. The patient was instructed to keep her eyes on the fixation point in the middle of the screen and count all infrequent stimuli that appeared. The MEG signal was subjected to SPSS filtering and averaging off-line. The sources of P300 speller was localized by using equivalent current dipole (ECD) approach. Main sources responsible for P300 generation have been localized in the right central and parieto-occipital areas, as well as left frontal areas.
2.1.2 Merging Information from Subdural Electrode Location and MEG Results
After the patient was implanted with subdural electrodes, the 3-D-rendered map of the patient’s cortical surface was created, co-registered and overlaid on subdural electrodes. The localized P300 ECDs were overlaid with the 3-D-rendered cortical and grid map (Fig. 2). Eight electrodes in close proximity with localized P300 sources were selected for on-line P300 speller testing protocol. The P300 responses recorded from sites selected with the MEG-based approach and ECoG electrodes during P300 speller performance are presented in Fig. 3a.
3D-rendered cortical surface of patient’s brain with overlaid subdural grids, P300 dipoles localized with MEG, as well as locations of 8 electrodes selected by using MEG and 8 electrodes selected based on ECoG data. Note that the asymmetric bilateral electrode placement is based entirely on the clinical decision required for successful completion of the pre-surgical patient’s evaluation. During the non-invasive phase of pre-surgical evaluation for epilepsy surgery, the patient exhibited ictal activities originating from left hemisphere. However, possible right hemisphere involvement was not completely ruled out. Therefore, the overall completeness of the evaluation could have been biased without adding any additional right hemisphere grids during the second (invasive) phase of pre-surgical evaluation
Significance plots for P300 speller electrode locations for P300 speller BCI selected by: a MEG-based source localization approach; and b ECoG-based subdural electrodes selection approach. The response to the TARGET stimuli is presented in blue, and the response to NON-TARGET stimuli is presented in green. The figures contain the significance values of TARGET versus NON-TARGET (p < 0.05): The yellow plots indicate a significant negative difference; green plots indicate a significant positive difference. The variation of the color represents the level of significance (light green and light yellow: p < 0.05; dark green and dark yellow: p < 0.01)
2.2 ECoG-Based Approach
2.2.1 Screening of All Grid Electrodes
P300 responses were recorded from 158 electrodes implanted for the purpose of evaluation for epilepsy surgery during presentation of a P300 speller grid with flashing letters of the alphabet and symbols (Fig. 1b). The patient was instructed to concentrate on 5 consecutively presented letters comprising the word. Two words were presented. Each letter was highlighted in 15 columns and 15 rows. Neural activity from the subdural grids was recorded with a g.USBamp, g.tec, Austria (sampling frequency 256 Hz).
2.2.2 Selection of the Best 8 Responses Among All 158 Electrodes by Using EcoG-Based Approach
P300 responses were analyzed in a following manner: A Butterworth filter (4th order, 0.1–30 Hz) was applied and data was triggered into TARGET and NON-TARGET trials. Trials with artifacts were visually identified and removed. A Kruskal-Wallis test was used to test if TARGET and NON-TARGET samples originated from the same distribution, and led to a p-value for each sample and channel. The best channels were selected according to the longest period of significant difference (p < 0.05) between TARGET and NON-TARGET trials. The eight most significant differences were found in channels 59, 82, 94, 118, 120, 60, 61, 92 (channels 1–8 respectively on Fig. 3b). These channels were located in left frontal, front-central, central and temporo-parietal regions.
3 On-Line Testing
For online classification of the P300 speller results obtained from 8 grid electrodes selected by using MEG-based approach (Fig. 3a) and 8 grid electrodes by using ECoG-based approach (Fig. 3b), a linear classifier was computed based on a temporal feature vector from each of eight selected channels within a linear discriminant analysis (LDA). The features were extracted from the raw data, which was acquired with a sampling rate of 256 Hz. In the first pre-processing step, the raw data was 58–62 Hz notch filtered and 0.1–30 Hz band-pass filtered. After triggering the data into 800 ms trials and down-sampling by factor 12, the baseline corrected trials of all channels were combined to one feature vector containing 120 samples.
4 Results
The accuracy of the subdural P300 speller was compared for 8 electrodes identified with MEG (Protocol #1) and for 8 electrodes identified with ECoG data analysis (Protocol #2) after creating a classifier with 10 letter phrases. The accuracy of the subdural P300 speller for MEG-identified sites was 80%, whereas for ECoG-identified sites, accuracy reached 90%. Figure 4 demonstrates the accuracy of the 8 selected channels after the ECoG screening using a classifier computed from 150 TARGET and 150 NONTARGET trials, and then tested on 5 characters. Our data suggest that MEG has a potential to serve as a non-invasive tool for navigating electrode implantation of P300 speller-based BCI.
5 Discussion and Future Perspectives
BCI technology enables communication between its users and the surrounding world by bypassing any muscle activity and utilizing direct brain signal instead (for review, see [5]). The surgically-implanted invasive BCIs (based on the ECoG signal recording) can be more advantageous when compared to non-invasive ones (based on the recordings of EEG signals) [6]. The implantable BCIs might be particularly beneficial for severely disabled patients, such as those with advanced stages of neuromuscular disorders, for example, ALS [7], locked-in syndrome [8], tetraplegia [1] and others. Patients with these severe disabilities report increased willingness to use chronically implanted BCIs [9, 10]. For instance, a telephone survey of people with ALS conveyed by Huggins and colleagues [9] has demonstrated that 72% of survey participants were willing to obtain a BCI by undergoing outpatient surgery and 41% by undergoing surgery with a short hospital stay.
Some of the reasons for patients’ interest in obtaining an implantable BCI is a function of convenience and the possibility of uninterrupted access to this technology. For instance, there is no need to reapply electrodes if chronically implanted BCIs are used. Current technological advancements in the development of implantable devices, such as their flexibility due to silicon nanoribbon material [11, 12], their wireless power supply transmitted by using radiofrequencies [13], and a number of other innovative features, make it possible to forecast that implantable BCIs may become a part of people’s life in the not too distant future. Importantly, the feasibility of small surgical implantations with microelectrodes has been recently demonstrated [14,15,16], showing promise for minimally-invasive BCI surgeries [17]. Importantly, such chronic in vivo BCI implantation would allow uninterrupted 24/7 connection between the brain and the external environment for people in need.
Recent reports show that the quality of the signal, as well as ability of pattern recognition and classification in home-based non-invasive BCIs systems with patient-users, both suffer significantly when compared to the same systems used in a controlled laboratory environment [18]. Utilization of invasive BCIs at patients’ homes would contribute to the improvement of the recorded brain signal quality and, as a consequence, would lead to better BCI performance in home-based settings. Notably, invasive recordings, such as ECoG, offer higher signal-to-noise ratio than EEG [for review, see 19]. For example, Ball, Kern [20] has demonstrated that the ECoG signal recorded from subdural electrodes had a signal quality twenty to one hundred times higher than that of EEG signal recorded with scalp electrodes. In addition, the ECoG signal offers vastly superior spatial resolution and is much less susceptible to artifacts [for more information, see review by 21]. Spatial resolution of ECoG recording can be further improved by using microelectrodes. For example, high resolution ECoG (HR-ECoG) recorded with the help of microelectrodes can have 400 times higher resolution than the conventional ECoG [22]. This is of particular importance when aiming at identification of language-related brain areas/classification of spoken words [23, 24], representing a potential use for future language-related BCI systems.
However, the question about the areas of implantation for chronic BCI devices still remains open. Minimal invasiveness dictates precise a priori knowledge of implantation sites and targeted areas of implantation during the surgery. These areas may vary dependent on the targeted BCI type, as well as on each person’s individual anatomical and physiological brain characteristics. Indeed, Speier, Fried [6] demonstrated that the location of evoked responses may affect BCI performance, specifically P300 speller bit rate. In our previous study [2], we concluded that specific approaches must be developed to identify and extract the data of interest from ECoG signal recordings in order to achieve desired BCI performance. Among several proposed approaches, the most promising for future work with BCIs, in our opinion, the use of MEG [25, 26]. Although it is feasible to use MEG for BCI purposes [27], it cannot be applied for BCI control directly in everyday settings, because of its current need for a massive shielding from the external electromagnetic activity. However, it can be extremely helpful in estimating the sources of evoked responses used for BCI control. As a consequence, the MEG represents an excellent candidate for guiding desired electrode implantation intended for chronic BCI use.
The “P300 speller” paradigm is the most frequently utilized approach that allows subjects to spell words or phrases by direct brain-controlled selection from material presented on a computer screen [28]. In our current study, we have demonstrated that it is feasible to use MEG for non-invasive determination of intracranial P300 speller recoding sites. We have achieved, on average, 80% P300 speller performance accuracy with MEG-identified electrode sites. Further studies should aim at improving MEG-navigated selection sites to achieve greater BCI performance accuracy. This can be accomplished by tuning various aspects of P300 response localization, including the selection of the P300 response range, it components (P3a and P3b), as well as the selection of P300 response source localization algorithm (e.g., distributed model versus single equivalent current dipole model).
Interestingly, in our current study, the P300 generation sites derived with MEG differed from those derived from ECoG electrodes. For example, whereas MEG-derived P300 sources were localized in both right and left hemispheres (specifically, right central and parieto-occipital areas as well as left frontal areas), the main ECoG-derived P300 response generation sites were found in the left hemisphere only (specifically, in the left frontal, fronto-central, central and temporo-parietal regions). Moreover, while both approaches have identified concordant regions generating P300 responses within the left frontal lobe, the ECoG-based approach has demonstrated additional P300 responses in the left temporo-parietal region that have not been identified with MEG. The diversity of P300 generation sites identified in our study is not surprising. Indeed, multiple studies have demonstrated P300 generation in widespread areas of the frontal [29, 30], temporal [31, 32] and parietal [33] cortices, including temporo-parietal junction [34], as well as parieto-occipital cortex [35]. Therefore, all P300 generation sites identified with both MEG and ECoG in our study are consistent with previously described sites of P300 generation. The reason for only partial overlap between MEG- and ECoG-identified P300 sites in our study can be explained by different approaches utilized for P300 source/response detection, respectively. Future studies should address the issue of differences in these approaches. Finally, the approaches leading to localization of the P300 generation sites that provide with the highest P300 speller performance accuracy need to be isolated and utilized.
In summary, in our current study, the MEG proved to be a reliable navigation tool for selection of ECoG-based P300 speller sites. Several locations from different brain regions responsible for P300 generation have been selected with MEG to drive ECoG-based P300 speller BCI with reliable performance accuracy. In order to achieve future goal of using minimally invasive chronic BCIs, it is important to identify single P300 generation sites that provide the maximum BCI performance accuracy. An improvement of P300 response source localization approaches with MEG is required to achieve higher ECoG-based P300 speller accuracy. Future studies aiming at attaining these goals are underway.
References
W. Wang et al., An electrocorticographic brain interface in an individual with tetraplegia. PLoS ONE 8(2), e55344 (2013)
M. Korostenskaja et al., Non-invasive versus invasive brain-computer interfaces. Abstracts from the Fifth International Brain-Computer Interface Meeting 2013 (Asilomar Conference Center, Pacific Grove, CA, USA, 2013)
M. Korostenskaja et al., Improving ECoG-based P300 speller accuracy. Proceedings of the 6th International Brain-Computer Interface Conference 2014, vol. 088, (2014) p. 1–4
E. Pataraia et al., Magnetoencephalography in presurgical epilepsy evaluation. Neurosurg. Rev. 25(3), 141–59; discussion 160-1 (2002)
J.R. Wolpaw, Brain-computer interfaces as new brain output pathways. J. Physiol. 579(Pt 3), 3–9 (2007)
W. Speier, I. Fried, N. Pouratian, Improved P300 speller performance using electrocorticography, spectral features, and natural language processing. Clin. Neurophysiol. 124(7), 1–8 (2013)
S. Silvoni et al., Amyotrophic lateral sclerosis progression and stability of brain-computer interface communication. Amyotroph Lateral Scler Frontotemporal Degener 14(5–6), 3–6 (2013)
Z.R. Lugo et al., A vibrotactile p300-based brain-computer interface for consciousness detection and communication. Clin. EEG Neurosci. 45(1), 14–21 (2014)
J.E. Huggins, P.A. Wren, K.L. Gruis, What would brain-computer interface users want? Opinions and priorities of potential users with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12(5), 18–24 (2011)
J.L. Collinger et al., Functional priorities, assistive technology, and brain-computer interfaces after spinal cord injury. J. Rehabil. Res. Dev. 50(2), 45–60 (2013)
J. Viventi, J.A. Blanco, Development of high resolution, multiplexed electrode arrays: opportunities and challenges. 2012 IEEE Conference on Proceedings of Engineering in Medicine and Biology Soceity (2012), p. 1394–1396
J. Viventi et al., Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14(12), 599–605 (2011)
Y. Zhao et al., Implanted miniaturized antenna for brain computer interface applications: analysis and design. PLoS ONE 9(7), e103945 (2014)
B. Rubehn et al., A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 6(3), 036003 (2009)
C. Henle et al., First long term in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed. Microdevices 13(1), 59–68 (2011)
H. Toda et al., Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. Neuroimage 54(1), 3–12 (2011)
E.C. Leuthardt et al., Microscale recording from human motor cortex: implications for minimally invasive electrocorticographic brain-computer interfaces. Neurosurg. Focus 27(1), E10 (2009)
C.W. Anderson et al., A comparison of EEG systems for use with brain computer interfaces in home environments. Psychophysiology, 2013. 50 (Issue Supplement S1), p. S6
N.E. Crone, A. Sinai, A. Korzeniewska, High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog. Brain Res. 159, 75–95 (2006)
T. Ball et al., Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 46(3), 8–16 (2009)
N.J. Hill et al., Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J. Vis. Exp. 64 (2012)
T. Kim et al., Spatiotemporal compression for efficient storage and transmission of high-resolution electrocorticography data. 2012 IEEE Conference on Proceedings of Engineering in Medicine and Biology Soceity (2012), p. 1012–1015
S. Kellis et al., Classification of spoken words using surface local field potentials. 2010 IEEE Conference on Proceedings of Engineering in Medicine and Biology Soceity (2012), p. 3827–3830
S. Kellis et al., Decoding spoken words using local field potentials recorded from the cortical surface. J. Neural Eng. 7(5), 056007 (2010)
J. Xiang et al., Noninvasive localization of epileptogenic zones with ictal high-frequency neuromagnetic signals. J Neurosurg Pediatr 5(1), 13–22 (2010)
M.S. Hamalainen et al., Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev. Mod. Phys. 65, 13–97 (1993)
J. Mellinger et al., An MEG-based brain-computer interface (BCI). Neuroimage 36(3), 81–93 (2007)
L.A. Farwell, E. Donchin, Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr. Clin. Neurophysiol. 70(6), 10–23 (1988)
U. Volpe et al., The cortical generators of P3a and P3b: a LORETA study. Brain Res. Bull. 73(4–6), 20–30 (2007)
P. Baudena et al., Intracerebral potentials to rare target and distractor auditory and visual stimuli III. Frontal cortex. Electroencephalogr. Clin. Neurophysiol. 94(4), 51–64 (1995)
E. Halgren et al., Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr. Clin. Neurophysiol. 94(4), 29–50 (1995)
E. Halgren et al., Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr. Clin. Neurophysiol. 94(3), 191–220 (1995)
M.E. Smith et al., The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalogr. Clin. Neurophysiol. 76(3), 35–48 (1990)
C. Mulert et al., The neural basis of the P300 potential. Focus on the time-course of the underlying cortical generators. Eur. Arch. Psychiatry Clin. Neurosci. 254(3), 1–8 (2004)
I. Kiss, R.M. Dashieff, P. Lordeon, A parieto-occipital generator for P300: evidence from human intracranial recordings. Int. J. Neurosci. 49(1–2), 3–9 (1989)
Acknowledgements
Authors want to express their gratitude to Dr. Brendan Allison for his valuable editorial suggestions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Korostenskaja, M. et al. (2017). Estimation of Intracranial P300 Speller Sites with Magnetoencephalography (MEG)—Perspectives for Non-invasive Navigation of Subdural Grid Implantation. In: Guger, C., Allison, B., Ushiba, J. (eds) Brain-Computer Interface Research. SpringerBriefs in Electrical and Computer Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-57132-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-57132-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57131-7
Online ISBN: 978-3-319-57132-4
eBook Packages: Computer ScienceComputer Science (R0)