Abstract
Over the past several years, our group has conceived a completely new technological approach toward BCIs aimed at reversing the maladaptive plasticity induced by musculoskeletal pain. The EEG activity patterns of participants with chronic pain (tennis elbow) were differentiated from those of healthy, age and sex matched controls during real-time movement performance. Our results showed a dominance of power in the alpha frequency range only that was significantly correlated with the intensity of pain (visual analogue scale scale—VAS). Based on this novel finding, a neurofeedback system was developed allowing real-time monitoring of alpha power during idle time and movement execution (wrist extensions). Two bars were shown to the patient on a feedback screen—one containing continuous alpha power, the other only alpha power during the preparation phase of movement execution. The goal of the participant was to maintain the alpha power below the initial baseline value during movement execution. Three patients were tested using this system and their pain intensities were monitored. All participants were successful in decreasing their alpha power across days. This was accompanied by a reduction in their perceived pain VAS scores. In summary, we have developed a neurofeedback system for musculoskeletal pain that is capable of providing rapid, accurate and reliable neurofeedback in dynamic conditions, allowing the users to train their brain to reduce the pain.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the initial proposition by Daly and colleagues [1], Brain Computer Interfaces (BCIs) have increasingly been developed for the restoration of lost motor function by inducing neuromodulation (for a recent review see [2]). Typically, the participants have suffered from a central nervous system lesion, leading to abnormal movement control. Depending on lesion type, approximately 8–80% of these patients will also present with central neuropathic pain accompanied with a specific EEG signature [3, 4] that is positively correlated with the degree of somatosensory reorganization [5, 6]. Such patients require a different approach to rehabilitation through a BCI, since e.g. spinal cord injured patients present with reduced event-related desynchronization during motor imagery and a decreased power in the resting state [3], ultimately leading to a decreased classification accuracy. We have also shown that the peak negative amplitude of the movement related cortical potential (MRCP) is enhanced in this patient group; however, classification remains around 65%, likely due to its greater variability specifically in the rebound phase [4].
Similar to neuropathic pain conditions, musculoskeletal pain originating at the periphery has a significant central component [7,8,9] leading to reorganization within the cerebral cortex [10]. Using non-invasive transcranial magnetic stimulation (TMS) to map the motor cortical (M1) representation of two wrist extensor muscles (extensor carpi radialis brevis (ECRB) and extensor digitorum), patients with chronic elbow pain (lateral epicondylalgia) presented with an increased overall excitability and a closer proximity to their respective centers of gravity. These alterations were significantly correlated with the severity of pain, indicating that they are maladaptive [10]. Human experimental pain models, which mimic chronic pain states, reveal that significant maladaptive plasticity (i.e. negative alterations in the connections within the brain) occurs in the chronic musculoskeletal pain state that may lead to unfavorable alterations in the way the central nervous system controls the musculoskeletal system [11,12,13]. In an attempt to further understand the central changes in the chronic condition, we have quantified this reorganization during the transition from acute to sustained (chronic) pain using a novel model capable of inducing progressive muscle soreness, mechanical hyperalgesia, and temporal summation of pressure pain that can last up to 14 days [14, 15]. Nerve growth factor (NGF) was administered as a bolus injection of 5 μg (0.2 mL) into the right ECRB on Days 0 and 2. Corticomotor excitability and maps were assessed on Day 0, 2, 4 and 14. We demonstrated that the cortex commences its adaptation process already at day 4 of the induced pain and more importantly, at day 14 when the pain has subsided, some of these changes persisted [16]. The above studies underline that even though musculoskeletal pain may appear as a localized event, central nervous system structures play a key role in its development and experience [17].
A recent review has highlighted several non-pharmacological treatments designed to restore normal brain function concomitantly with a reduction of chronic musculoskeletal pain [18]. These include repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS) and neurofeedback. The central idea behind restoring brain activity patterns rather than relying on pharmacological treatments that reduce the pain symptom, is to avoid maladaptive alterations that may lead to secondary problems (i.e. altered movement patterns when performing a task that will induce pain in other areas thus adding to the problem rather than relieving it). In order to retrain the brain, and induce a relearning of the correct movement patterns and thereby reverse the maladaptive cortical reorganization, the mechanisms behind learning need to be satisfied. The current belief is that appropriate induction of plasticity requires the correlated activation of the relevant neural structures (“neurons that fire together, wire together”) [19]. Treatments targeting the final output stage of the brain that activates the muscles that produce the movement (e.g. the motor cortex), need to satisfy this principle. In neurofeedback approaches, the user imagines performing a specific task (also called motor imagery (MI)) that normally produces pain (e.g. reaching movement in patients with tennis elbow) while the EEG activity is continuously monitored. The EEG signals associated with the pain are extracted in real time using mathematical algorithms, and provide continuous visual feedback to the user. In this way, the user learns to modify the brain waves to reduce the painful sensation, and an association is formed between the experience of pain and the neurofeedback.
For the user to learn to associate negative brain activity with the painful sensation and its opposite, the positive brain activity with a state of no pain, it is imperative to extract relevant signals affected during chronic musculoskeletal pain. To date, there is no clear consensus on this topic. Several studies have investigated EEG oscillations in central neuropathic pain [3, 20] and musculoskeletal pain [21], although these have been restricted to either resting state or motor imagery (MI). While both types of pain exhibit a frequency specific signature in their EEG patters, when a person is performing a motor task, the effect on the EEG waves may be different. Current neurofeedback for pain treatment seeks to reduce beta (13–35 Hz) oscillations while increasing alpha (8–12 Hz) or theta (4–7.5 Hz) oscillations [22]. However, this is heuristically determined based on previous experience rather than known mechanisms [3]. A further complication with current neurofeedback is that MI enhances pain and thus may not be as useful when treating patients with chronic pain such as tennis elbow. Performing the movement may, in these cases, be more appropriate specifically when using neurofeedback as a treatment modality.
Currently, little is known on the EEG signatures of musculoskeletal pain. Past studies have investigated alterations in the power of various frequency bands following the artificial induction of this pain using hypertonic saline injections [21]. These have been restricted to the resting state. However, it is well known that pain interacts with movement, and thus the patterns are likely different than in the resting state.
As a next step, we therefore sought to obtain a deeper understanding of this type of pain during movement performance. Since the enormous indirect socioeconomic costs due to chronic musculoskeletal pain far exceed those estimated for heart disease, cancer and diabetes [23] and new non-pharmacological treatment approaches [18] are highly desirable, our proposed neurofeedback system has the potential to be one of the new exciting approaches for BCIs in the future.
2 EEG Signatures of Musculoskeletal Pain
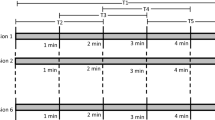
Several preliminary studies have been completed that characterize the alterations in EEG parameters induced by pain either in patients (n = 10, 38 ± 11 years) or in healthy participants (n = 19, 26 ± 4 years) prior to (HnP) and following (HwP) injections of hypertonic saline. During the experimental session, participants were seated in a chair in an upright position with the elbow joint extended at 170 ± 10°, the upper arm and shoulder fixated with Velcro tape and the forearm fully pronated. Following assessments of pressure pain thresholds (PPT) and the visual analog scale (VAS) for pain, participants had to complete four movement tasks with at least a 5-min rest interval between them, as follows: 1. Three maximum isometric voluntary contraction (MIVC) of the wrist extensors with a 1 min rest period between trials; 2. 30 index finger extensions; 3. 30 palmar grips and 4. 30 dynamic wrist extensions. Participants were asked to perform the tasks at a self-selected pace, but at a minimum frequency of 0.8 Hz and the order of tasks 2–4 was randomized. PPTs and VAS measures were repeated following each movement task. For healthy participants, all measures and tasks were performed either without pain or following a bolus injection of 5.7% hypertonic saline into the ECR of the dominant arm.
Monopolar EEG signals were recorded using an active EEG electrode system (g. GAMMAcap2, Austria) and g. USBamp amplifier (gTec, GmbH, Austria) from FP1, F3, Fz, F4, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P3, Pz and P4 according to the standard international 10–20 system. The channel selection was based on the large Laplacian with C3 or C4 (depending on the affected side) as the central channel [24]. The reference electrode was placed on Fz and the ground on the left earlobe. A single channel surface electromyography (EMG) was recorded from the extensor carpi radialis (ECR) muscle to control for the subject’s movement. All signals were sampled at a frequency of 256 Hz (16 bits accuracy) and hardware filtered from 0 to 100 Hz. Power was calculated using 1 s Hamming windows with 1 sample increment within the alpha, beta, theta and gamma band from continuous EEG (256 Hz) at all electrode locations.
Figure 1 shows Alpha power from electrode location C3 across all subjects. Alpha power was significantly increased in the pain patients compared to HnP (p < 0.05), while induced pain (HwP) showed a similar trend (Fig. 1). This was correlated with the participants’ perceived pain (VAS).
3 The Neurofeedback System
Figure 2 outlines our proposed approach for a neurofeedback system to reduce musculoskeletal pain, as well as the preliminary results from n = 1 pain patient. For the first 20 trials, participants were asked to perform wrist extensions with a light weight held in their hand. The power within the alpha frequency was subsequently calculated and served as the baseline value in the following trials, during which the alpha band power was continuously displayed to the participant in the left panel of the feedback screen. A green bar indicated a decrease in power (a desynchonization) while a red bar referred to an increase in power (a synchronization). Participants were asked to try to keep the bars green. Upon movement performance, the system fed back to the participant the power within the same band, but only for the preparation phase of the movement (right bar of the feedback screen, Fig. 2). A successful trial meant that this bar was green, and thus that power was maintained below the baseline value.
The first of three blocks of 50 trials were performed, and the percentage displayed on the screen indicated to the participant how many successful trials were completed. During the second block, the baseline values were adjusted based on those obtained for the previous block. For the third block, the baseline values were adjusted based on the second block. In this way, the task difficulty increased for each block, ensuring the participants were trained appropriately. To date, three patients have been exposed to this neurofeedback system. Results from one patient are shown in the two right hand graphs of Fig. 2. The change in sensory-motor-rhythm (SMR) during the movement execution was evident. The alpha power was decreased within a session but also across the three sessions (performed on separate days). More importantly, these decreases in power were accompanied by decreases in the VAS scale (from 5.6 after session one to 0 at the end of session three), indicating that the patient felt less pain by session number three.
4 Discussion and Long-Term Perspectives
The field of BCI has been expanding rapidly over the past decade, with researchers seeking to widen the application to a larger patient population. BCI systems designed for neuromodulation in patients suffering from a central nervous system lesion provide a prime example of such an endeavor. Here, we propose an application with even wider and deeper impact, since musculoskeletal pain affects between 13.5 and 47% of the general population. Our recent evidence has shown that this condition is accompanied by significant reorganization in cortical plasticity that even outlasts the experience of pain. The future challenge is to reverse this maladaptive process, and a BCI approach is ideally suited to meet these demands.
References
J.J. Daly, N. Hogan, E.M. Perepezko, H.I. Krebs, J.M. Rogers, K.S. Goyal, M.E. Dohring, E. Fredrickson, J. Nethery, R.L. Ruff, Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J. Rehabil. Res. Dev. 42, 723–736 (2005)
S.R. Soekadar, N. Birbaumer, M.W. Slutzky, L.G. Cohen, Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 83, 172–179 (2015)
A. Vuckovic, M.A. Hasan, M. Fraser, B. A. Conway, B. Nasseroleslami, D.B. Allan, Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain (2014)
R. Xu, N. Jiang, A. Vuckovic, M. Hasan, N. Mrachacz-Kersting, D. Allan, M. Fraser, B. Nasseroleslami, B. Conway, K. Dremstrup, D. Farina, Movement-related cortical potentials in paraplegic patients: abnormal patterns and considerations for BCI-rehabilitation. Front. Neuroeng. 7, 35 (2014)
P.J. Wrigley, S.M. Gustin, P.M. Macey, P.G. Nash, S.C. Gandevia, V.G. Macefield, P.J. Siddall, L.A. Henderson, Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex 19, 224–232 (2009)
S.M. Gustin, P.J. Wrigley, P.J. Siddall, L.A. Henderson, Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex 20, 1409–1419 (2010)
T. Graven-Nielsen, L. Arendt-Nielsen, Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr. Rheumatol. Rep. 4, 313–321 (2002)
L. Arendt-Nielsen, T. Graven-Nielsen, Muscle pain: sensory implications and interaction with motor control. Clin. J. Pain 24, 291–298 (2008)
T. Graven-Nielsen, L. Arendt-Nielsen, P. Madeleine, P. Svensson, Pain mechanisms in chronic musculoskeletal conditions. Ugeskr. Laeger 172, 1824–1827 (2010)
S.M. Schabrun, P.W. Hodges, B. Vicenzino, E. Jones, L.S. Chipchase, Novel adaptations in motor cortical maps: the relation to persistent elbow pain. Med. Sci. Sports Exerc. 47, 681–690 (2015)
S.M. Schabrun, P.W. Hodges, Muscle pain differentially modulates short interval intracortical inhibition and intracortical facilitation in primary motor cortex. J. Pain. 13, 187–194 (2012)
S.M. Schabrun, E. Jones, J. Kloster, P.W. Hodges, Temporal association between changes in primary sensory cortex and corticomotor output during muscle pain. Neuroscience 235, 159–164 (2013)
T. Graven-Nielsen, L. Arendt-Nielsen, Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat. Rev. Rheumatol. 6, 599–606 (2010)
K. Hayashi, S. Shiozawa, N. Ozaki, K. Mizumura, T. Graven-Nielsen, Repeated intramuscular injections of nerve growth factor induced progressive muscle hyperalgesia, facilitated temporal summation, and expanded pain areas. Pain 154, 2344–2352 (2013)
M.J. Bergin, R. Hirata, C. Mista, S.W. Christensen, K. Tucker, B. Vicenzino, P. Hodges, T. Graven-Nielsen, Movement evoked pain and mechanical hyperalgesia after intramuscular injection of nerve growth factor: a model of sustained elbow pain. Pain Med. 16, 2180–2191 (2015)
S. Schabrun, S.W. Christensen, N. Mrachacz-Kersting, T. Graven-Nielsen, Motor cortex reorganization and impaired function in the transition to sustained muscle pain. Cereb. Cortex (2015)
B.K. Coombes, L. Bisset, B. Vicenzino, A new integrative model of lateral epicondylalgia. Br. J. Sports Med. 43, 252–258 (2009)
M.P. Jensen, S. Hakimian, L.H. Sherlin, F. Fregni, New insights into neuromodulatory approaches for the treatment of pain. J Pain. 9, 193–199 (2008)
D.O. Hebb, The Organization of Behavior: A Neuropsychological Theory (Lawrence Erlbaum Associates Inc, Mahwah, NJ, 1949)
L. Michels, M. Moazami-Goudarzi, D. Jeanmonod, Correlations between EEG and clinical outcome in chronic neuropathic pain: surgical effects and treatment resistance. Brain Imag. Behav. 5, 329–348 (2011)
P.F. Chang, L. Arendt-Nielsen, T. Graven-Nielsen, A.C.N. Chen, Psychophysical and EEG responses to repeated experimental muscle pain in humans: pain intensity encodes EEG activity. Brain Res. Bull. 59, 533–543 (2003)
M.P. Jensen, C. Grierson, V. Tracy-Smith, S.C. Bacigalupi, S. Othmer, Neurofeedback treatment for pain associated with complex regional pain syndrome type I. J. Neurother. 11, 45–53 (2007)
J. Christensen, L. Bilde, A. Gustavsson, Socio-economic consequences of pain-intensive diseases in Denmark. (2011)
D.J. McFarland, L.M. McCane, S.V. David, J.R. Wolpaw, Spatial filter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 103, 386–394 (1997)
Acknowledgements
We wish to acknowledge our participants and all of the students from the laboratory, both past and present.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Mrachacz-Kersting, N. et al. (2017). A Brain-Computer-Interface to Combat Musculoskeletal Pain. In: Guger, C., Allison, B., Ushiba, J. (eds) Brain-Computer Interface Research. SpringerBriefs in Electrical and Computer Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-57132-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-57132-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57131-7
Online ISBN: 978-3-319-57132-4
eBook Packages: Computer ScienceComputer Science (R0)