Abstract
The effects of coastal hypoxia on fish biomass and fisheries landings in the northern Gulf of Mexico have been difficult to quantify. A main complicating factor is the fact that nutrient loading from freshwater discharge is not only the main contributor to the formation of the hypoxic zone, but also a driver of secondary productivity through bottom-up processes. Other complicating factors include food web interactions, movement of nekton to more suitable habitat, and temporal and spatial variability in hypoxic area. Through case studies using Ecopath with Ecosim and Ecospace, we show that ecosystem modeling can provide a tool to evaluate population-level effects on nekton biomass as well as changes in fisheries landings due to hypoxia. Fitting model simulations to time series (observations) in Ecosim reveals that including hypoxia improves the fit of the model to observations. These findings led to the development of a spatially and temporally dynamic Ecospace model, coupled to a physical-biological model with high skill in replicating dissolved oxygen and Chl a levels. The results of simulations with this coupled modeling approach suggest that, for most species, the positive effects of increased phytoplankton as a result of nutrient enrichment from the Mississippi River outweigh the negative effect of bottom hypoxia. Decoupling enrichment from hypoxia also showed that hypoxia does reduce biomass and landing as compared to enrichment alone, and that there are winners and losers: Some species such as red snapper decrease in biomass even with enrichment. Future directions include simulating nutrient reduction scenarios to inform management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
While the existence of the hypoxic zone off the coast of Louisiana has been well established (Rabalais et al. 2001 and earlier chapters), the effect it has on fish and fisheries has been more elusive (Rose 2000; O’Connor and Whitall 2007; Rose et al. 2009). There are several ecosystem modeling approaches that can be used to consider environmental effects on living marine resources (e.g., Ecopath with Ecosim (EwE, Christensen and Walters 2004); Trosim (Fulford et al. 2010), CASM (Bartell et al. 1999), and ATLANTIS (Kaplan et al. 2012)). In this chapter, we use the EwE modeling approach and its spatial module, Ecospace, to determine potential effects of hypoxia on fish and fisheries.
New developments in EwE described in Christensen et al. (2014a) and De Mutsert et al. (2015a) allow for the inclusion of avoidance of hypoxia by marine nekton and reduced feeding rates of organisms in response to low oxygen levels; these effects can cascade through the food web through trophic interactions. Inclusion of ports and fishing fleets allows for the simulation of the added effect of catches on the abundance of living marine resources, while simultaneously providing an estimate of landings and revenue changes during different simulation scenarios. The virtual representation allows for the decoupling of the bottom-up effect of high levels of primary production on higher trophic levels, and the negative effects of hypoxic events on these consumers related to these same high levels of primary productivity that occur in the northern Gulf of Mexico (Rabalais et al. 2002). Output of a coupled physical-biological model (Fennel et al. 2011, 2013; Laurent et al. 2012; Laurent and Fennel 2014) provides the scenarios of primary productivity on the coastal shelf with and without summer hypoxia.
In this chapter, we describe these modifications to EwE to simulate hypoxia effects in the northern Gulf of Mexico, and illustrate the modeling approach with three case studies that use simulations to evaluate effects of hypoxia on fish and fisheries. The results remind us to not underestimate the extent to which the Mississippi River outflow fuels the productivity of the northern Gulf of Mexico ecosystem, and give insight into the difficulty in finding negative empirical correlations between landings and hypoxia. Through these case studies, the rapid evolution of the capabilities of Ecopath with Ecosim and Ecospace to simulate effects of environmental drivers on fish and fisheries is highlighted.
14.1.1 The Louisiana Coastal Ecosystem
Perhaps the most characteristic feature of the Louisiana continental shelf region is the presence of two major sources of freshwater, the Mississippi and Atchafalaya rivers, that strongly influence the physics (Wiseman et al. 2004), biology (Hanson 1982; Wiseman et al. 1986), and chemistry (Ho and Barrett 1977). The freshwater runoff, which averages 1.83 × 104 m3 s−1, contributes to a low salinity, highly turbid, near shore water mass within a westward flowing coastal current, which is constrained within a steep, horizontal, salinity front on the mid-shelf about 20–50 km offshore (Crout 1983; Cochrane and Kelly 1986).
The nutrient-rich waters entering the Gulf of Mexico in this manner lead to high primary production on the coastal shelf, which in turn stimulates the secondary production of this marine ecosystem (Nixon and Buckley 2002; Livingston 2002). The Louisiana coastal area has indeed been referred to as the Fertile Fisheries Crescent (Gunter 1963); fisheries landings in Louisiana are the highest of the Gulf states and contribute significantly to the total commercial and recreational catch in the USA (Chesney et al. 2000).
High levels of primary production on the Louisiana shelf and the resulting bacterial respiration during the decay of these large amounts of organic matter, in combination with summer stratification of coastal waters, are the cause of an extensive area with hypoxic bottom waters each summer (Rabalais and Turner 2001). The areal extent of the affected region is positively related to Mississippi River discharge and has had an average size of 13,650 km2 over the past 30 years (1985–2014, http://www.gulfhypoxia.net/Overview/). This seasonal hypoxic zone affects the living marine resources of the northern Gulf of Mexico.
14.1.2 Ecological Effects of Hypoxia on the Gulf of Mexico
It has been suggested that the formation of the hypoxic zone could lead to altered food web dynamics on the Louisiana shelf (Chesney et al. 2000; Rabalais and Turner 2001). Effects may be both direct via increased mortality through prolonged exposure to low dissolved oxygen (DO) concentrations (Breitburg et al. 1999; Turner 2001; Breitburg et al. 2003) or indirect via alteration of benthic (Turner 2001) and water column (Breitburg et al. 1999; Chesney et al. 2000; Turner 2001), habitat availability, and food web structure. An example of altered food web structure would be increased abundances of gelatinous zooplankton predators that consume zooplankton and larval fish (Graham 2001; Grove and Breitburg 2005).
Several studies have described the effects of hypoxia on feeding, growth, behavior, and mortality of fishes from a variety of taxonomic groups. In particular, sublethal effects of hypoxia have been shown to result in decreased feeding (Chabot and Dutil 1999; Tallqvist et al. 1999; Pichavant et al. 2001) and growth rate (Bejda et al. 1992; Secor and Gunderson 1998; Chabot and Dutil 1999; Taylor and Miller 2001), changes in activity level (Crocker and Chech 1997; Schurmann and Steffensen 1992), and spatial distribution (Pihl et al. 1991; Breitburg et al. 1999, 2003; Keister et al. 2000; Wannamaker and Rice 2000). Studies also have demonstrated direct effects of severe or chronic hypoxia on mortality (Schurmann and Steffensen 1992; Tallqvist et al. 1999; Miller et al. 2002); specific DO levels that can elicit sublethal effects have been shown to be species-specific (see reviews by Davis 1975; USEPA 2001; Miller et al. 2002).
14.1.3 Potential Implications for Gulf of Mexico Fisheries
The hypoxic zone could have economic consequences, if hypoxia reduces production of commercially and recreationally valuable fish and shellfish (Diaz and Rosenberg 1995; Breitburg 2002; O’Connor and Whitall 2007). Aggregation near hypoxic edges has been shown for gulf shrimp and finfish, which may enhance their susceptibility to commercial shrimp trawls (Craig 2012). This “edge effect” can result in a positive correlation between hypoxia and fisheries landings, and create a false sense of increased abundances when fisheries dependent data are used to determine stock size. Positive correlations could also occur as a result of the bottom-up effect that nutrient enrichment has on higher trophic levels (Nixon and Buckley 2002; Breitburg et al. 2009).
14.1.4 Ecosystem Modeling
The concept of using ecosystem models in fisheries science and ecology is to include effects of environmental parameters, trophic interactions of multiple species, and fishing on the biomass of all species included in such models. The ecosystem models presented in this chapter have been developed with the open-source Ecopath with Ecosim modeling suite, freely available from http://www.ecopath.org. The advantage of creating a virtual representation of the northern Gulf of Mexico ecosystem is that the factors affecting living marine resources can be evaluated separately and together.
14.2 Constructing an Ecopath Model
An Ecopath model is a virtual representation of an ecosystem, with a focus on higher trophic levels. It was originally developed by Polovina (1984), and subsequently modified to replace the assumption of steady state by an assumption of mass balance over a period of time, usually a year (Christensen and Pauly 1992). Other modifications include the addition (and subsequent modification) of Ecosim, a time-dynamic module, and Ecospace, a spatially explicit module (Walters et al. 1997, 1999). These dynamic modules of EwE will be discussed in Sects. 14.3 and 14.4.
There are two master equations at the base of Ecopath. The first equation describes the production term and can be expressed as follows:
where Bi and Bj are the biomasses of the prey (i) and predators (j), respectively; (P/B)i the production/biomass ratio; EEi the ecotrophic efficiency, which is the proportion of the production that is utilized in the system; (Q/B)j the consumption/biomass ratio; DCji the fraction of prey (i) in the diet of predator (j); Yi the total fishery catch rate of (i); Ei the net migration rate (emigration–immigration); and BAi the biomass accumulation rate for (i).
The second master equation ensures energy balance within each group as follows:
To construct a model, the parameters B, P/B, Q/B, DCij, Y, E, and BA need to be specified for each group (Y only if there is a fishery), while the model solves for EE. While this is the recommended approach, the model can also solve for missing B, P/B, and Q/B parameters since Ecopath can handle solving for different and multiple unknowns while balancing the model. Missing parameters are estimated by linking the production of each group to the consumption of all groups, based on the mass-balance requirement of Eq. 14.1.
Each group in an Ecopath model can be a single species, an aggregation of multiple species of similar role in the ecosystem, (e.g., a functional group), or a single life history stage of a species with a complex trophic ontogeny (i.e., a multi-stanza group). With the multi-stanza approach, different diets, predation levels, tolerance ranges (e.g., to DO), and fisheries landings can be assigned to different life stages of a species, which increase the realism of the model. The stanzas of a species are linked with a von Bertalanffy growth model. A model like this was constructed for the Gulf of Mexico (Fig. 14.1), serving as a base model for simulations described in this chapter.
14.3 Temporal Dynamic Modeling with Ecosim
Ecosim provides temporal dynamic simulation capabilities building on the Ecopath base model. Biomass dynamics in Ecosim are expressed through a series of coupled differential equations derived from the Ecopath master equation (Eq. 14.1). The resulting differential equation for biomass is as follows:
where dB i /dt represents the growth rate during the time interval of group (i) in terms of its biomass (Bi), gi is the net growth efficiency (production to consumption ratio), \( \sum {Q_{ji} } \) the total consumption by group (i), \( \sum {Q_{ij} } \) the predation by all predators on the same group (i), Ii the immigration rate, MOi the non-predation natural mortality rate, Fi the fishing mortality rate, and ei the emigration rate.
The set of differential equations is solved in Ecosim using an Adams-Bashforth integration routine. The consumption rates are calculated based on the foraging arena theory, where the biomass of each group is divided into vulnerable and invulnerable components (Walters et al. 1997; Ahrens 2012). The transfer rate between these two components (νij) determines the relative contribution of top-down (biomass of predator impacts how much prey is consumed) and bottom-up control (being caught is a function of the prey’s productivity).
A useful feature in Ecosim is the ability to fit simulations to time series (Christensen and Walters 2011). The main purpose is to calibrate the model to observation, but can also be used to determine whether the model explains more variability with the addition of environmental drivers. This feature is demonstrated in the first case study described in Sect. 14.3.3.
14.3.1 Including Hypoxia Effects
The flow of biomass in Ecosim from prey biomass (i) to predator biomass (j) follows the function:
where aij is the rate of effective search, Vij is vulnerable prey biomass, and Pj is effective predator biomass. Pj is the group biomass for simple groups, but calculated differently when a group has ontogenetic diet splits using the multi-stanza function (see Sect. 14.2). For multi-stanza groups, Pj is the sum of biomass over ages in that group to the 2/3 power, an index of per-predator search rate.
The effective search rate aij is reduced in the model during unfavorable DO conditions using species-specific response curves. Response curves of individual fish species to environmental parameters are determined by plotting catch rate versus the environmental parameter for each group in the model, using large datasets (Fig. 14.2). This method has previously been used to include effects of salinity (De Mutsert et al. 2012). For the DO response curves in the NGOMEX Ecosim scenarios, catch rates from SEAMAP groundfish data were plotted against bottom DO values as measured during catch. The resulting parameters (the minimum DO value that is not causing limitations and the standard deviation from that point) were used to create sigmoidal curves that serve as multipliers on effective search rate (Fig. 14.2). The response (y value between 0 and 1) that corresponds with the environmental DO value (x value) is then used to multiply aij in Eq. 14.4, with DO time series data serving as the forcing function.
Oxygen response curves of five example species in the model. Reproduced from De Mutsert et al. (2015) under a creative commons license
14.3.2 Including Fisheries
Fisheries can be affected by hypoxia as well, and these effects cannot be extrapolated directly from the average biomass of living marine resources in the system (see Sect. 14.1.3). Fishing fleets can be included in the model, and the catch of each of these fleets can be simulated and form an output of the Ecosim scenarios just like group biomass. Hypoxia does not directly affect the fleets in the model, but, more realistically, effects of hypoxia on living marine resources have an indirect effect on fisheries. These indirect effects could potentially include a decrease in fisheries catches when biomass of living marine resources decreases, or an increase in fisheries catches when living marine resources congregate at the edge of the hypoxic zone (Craig 2012). The inclusion of fleets not only ensures the direct simulation of fisheries (and effects of hypoxia on fisheries) but also includes catch as a potentially major determinant of fish biomass; to determine effects of hypoxia on living marine resources, the effects of fishing cannot be ignored.
14.3.3 Case Study 1: Hypoxia in Ecosim
An Ecopath model was developed for the northern Gulf of Mexico (NGOMEX; Fig. 14.1 and Table 14.1), based on the biomass (t km−2 wet weight) of species representative of the NGOMEX using SEAMAP data (http://sero.nmfs.noaa.gov/operations_management_information_services/state_federal_liaison_branch/seamap/index.html). The model and expansions on it as described in two more case studies are referred to as the NGOMEX model. Species were divided into multiple life history stages where needed to consider ontogenetic shifts in the life history of a species. Mass balance was achieved after multiple iterations involving adjustments of input parameters, resulting in a plausible virtual representation of the northern Gulf of Mexico ecosystem.
In Ecosim, hypoxia was added, and a fishery for menhaden, shrimp, recreational, snapper/grouper, crab pots, squid, and longlines was included. Landings data used are published online by NOAA: http://www.st.nmfs.noaa.gov/st1/commercial/landings/annual_landings.html.
Two scenarios were simulated with the NGOMEX version of Ecosim in this case study: one without hypoxia as a forcing function and one with a time series of spatially averaged summer bottom hypoxia (mg/l) measured by the Louisiana Department of Wildlife and Fisheries within the area of the hypoxic zone from 1998 to 2002 as a forcing function. We ran a simulation from 1982 to 2008, which were the years for which we had SEAMAP data to which the simulations were fitted. Annual averaged biomass data derived from SEAMAP and landings documented by NOAA were used as observations to fit the predictions to. For this case study, the dissolved oxygen pattern measured from 1998 to 2002 by the Louisiana Department of Wildlife and Fisheries (LDWF) was repeated in the years of the Ecosim run from 1982 to 1997 and 2003 to 2008. Nutrient loading to the Gulf of Mexico, represented by NOx loads measured by USGS at Tarbert Landing, was kept the same in both model runs. In the first case study presented here, scenario runs with the NGOMEX model demonstrate the improvement of fit during calibration in Ecosim with the inclusion of hypoxia as an environmental factor affecting fish biomass (Fig. 14.3). The total sum of squares (SS) of the fit of the simulation to observed biomass data decreased from 780 to 670 when the hypoxia forcing function was activated. By adding hypoxia, the model explained more of the variation in the data than the model did with trophic interactions and fishing alone (Fig. 14.3).
While the Ecosim scenarios provide biomass and fisheries output, the lack of spatial dynamics in the model puts restriction on the interpretation of the outcomes. Without a spatial component, the organisms in the model do not have the ability to move away from hypoxia. In other words, hypoxia is either “on” or “off” in the model, and the only relief organisms receive from hypoxia is in time, since the DO is only reduced to hypoxic levels in the summer months. Effects of biomass aggregation at the edge of the hypoxic zone and subsequent effects on fisheries will thereby also not be simulated. A solution to this problem is provided by Ecospace, which provides spatially explicit simulations detailed in the next section. When using Ecospace, it is still useful to first use Ecosim, since model calibration by fitting simulations to time series as described in the first case study is performed in Ecosim (which notably entails incorporating density-dependent effects), and model robustness is tested in Ecosim by running Monte Carlo simulations (Christensen et al. 2004). In the above presented case study, Ecosim provides an indication that hypoxia could indeed affect biomass of living marine resources (Fig. 14.3), and that further exploration of the NGOMEX model in Ecospace is warranted.
14.4 Ecospace
14.4.1 Use of GIS in Ecospace
Up to version 6.3 of the EwE software suite, a major shortcoming of the Ecospace model has been its lack of facilities to integrate external spatial forcing data into its computations, and to deliver its outputs in geospatial data formats for model interoperability. Although the Ecopath and Ecosim models have been successfully linked to other models, the spatial model Ecospace had seen no use in this regard due to the complexity involved, including lack of capabilities to easily exchange data. Continued popularity of the EwE approach, increasing demand for the ability to use the Ecospace model in conjunction with spatial analytical tools, specialist models, and planning tools such as Marxan (Loos, 2011) resulted in increasing demand to integrate varying environmental conditions into the Ecospace model. EwE source code was migrated in 2006 to the .NET programming environment to which gave rise to the idea of a flexible spatial-temporal data framework to solve the data connectivity shortcomings of Ecospace. This spatial-temporal data framework is an abstraction layer onto the Ecospace model that facilitates the process of locating, loading, and adapting geospatial data to the Ecospace internal data formats, and facilitates the process of converting Ecospace result maps to geospatial data formats (Fig. 14.4).
Conceptual overview of the spatial-temporal data framework, which provides external GIS data to Ecospace model initialization and at runtime, and provides Ecospace results in spatial data formats when the model executes. Reproduced from Steenbeek et al. (2013) with permission
To date, the spatial-temporal framework of Ecospace has been applied in only a few case studies. One such study demonstrated that the ability of Ecospace to hindcast observed trends in species occurrences significantly improved when driven by monthly and annual SeaWiFS-derived primary production data (Steenbeek et al. 2013). Other case studies use the spatial-temporal data framework to drive the habitat foraging capacity model with altering patterns in oxygen, temperature, salinity, turbidity, substrate, or depth for exploring the impacts of environmental change on marine food webs (De Mutsert et al. 2015a, b, 2016).
14.4.2 The Habitat Capacity Model
The Ecospace model can consider cell-specific habitat descriptions or environmental parameters (level of, e.g., salinity and DO). In versions of the Ecospace model before EwE release 6.3, large-scale habitat structure with attendant impact on biomass distributions and trophic interactions was represented only by a binary habitat use pattern for each group, with each spatial cell being either suitable or not for each group. Biomass dynamics in unsuitable cells was modified by predicting higher rates of emigration, lower feeding rates, and/or higher vulnerability to predation, and there was a complex gradient calculation to modify dispersal rates so as to direct biomass toward suitable cells (Walters et al. 1999).
In the new habitat capacity model used here for hypoxia, the relative habitat capacity by group and by cell is estimated from a vector of habitat attributes—which can be made to consider any environmental parameter, e.g., DO, salinity, or temperature (Christensen et al. 2014a). The habitat capacities can be updated for each time step (in a computationally efficient manner) and can typically be obtained from output from physical or biogeochemical models.
The habitat capacities, C, are linked to trophic interactions in the foraging arena model so that it impacts the size of the foraging arena, i.e.,
The predation activity is thereby concentrated in a smaller area when C decreases which in turn impacts the vulnerable prey densities (V) more rapidly if predator density (P) increases because of a decrease in the size of the foraging arena. This in effect makes spatial patterns of biomass proportional to their habitat capacities. Furthermore, the habitat capacity model modifies spatial mixing rates so as to obtain movements toward preferred cells and avoid dispersing excessive biomass into unsuitable spatial cells.
Using the habitat capacity model, multiple layers can be added to Ecospace models. Users can define the response of organisms to each habitat attribute with response curves (shape and parameters of each response curve are user-specified), or by indicating relative habitat use. An example of the latter would be to define water cells as estuarine and offshore, and then exclude certain organisms from entering the estuaries (habitat use of the estuary would be 0, and use of offshore water cells would be 1). Habitat use can be any value between 0 and 1, and the levels of habitat could be two or more.
14.4.3 Spatial Considerations for Fishing Fleets
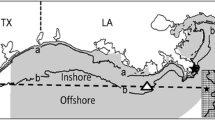
In Ecospace, the fishing mortality rates (F) are distributed using a simple “gravity model” where the proportion of the total effort (as defined in the Ecopath base model) allocated to each cell is assumed proportional to the sum over groups of the product of the biomass, and the profitability of fishing the target groups (Christensen and Walters 2004). The profitability of fishing includes the price per pound and the cell-specific cost of fishing (determined by price of gas and distance from port). The NGOMEX Ecospace model used in the second and third case studies incorporates the cost of fishing by including two ports with the highest fish landings in Louisiana: Empire-Venice and Intracoastal City (www.oceaneconomics.org; Fig. 14.5). Distance from port in addition to target species biomass in a particular cell and species-specific price per pound determines movement of fleets.
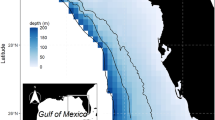
Model area of the NGOMEX (northern Gulf of Mexico) ecosystem model. Louisiana (USA) is indicated in gray, and the Mississippi River in blue. The coloration in the northern Gulf of Mexico indicates the bathymetry. The two ports with the most landings in Louisiana, Intracoastal City and empire-venice, are indicated with a black dot (source of bathymetry data: the fish and wildlife research institute). Reproduced from De Mutsert et al. (2015) under a creative commons license
14.4.4 Case Study Two: Hypoxia in Ecospace
To pursue spatial modeling with the NGOMEX Ecospace model, a base map was loaded in based on a GIS bathymetry map of coastal Louisiana. The model area of the NGOMEX Ecospace model has a 5 × 5 km grid and covers the Louisiana coastal shelf, totaling 8978 model cells (Fig. 14.5). The bathymetry of the area included in the base map has a resolution of 1 m. Bathymetry (with response curves), salinity-based habitat description (the three “levels” of habitat are fresh, brackish, and marine), and DO (with response curves) were included as habitat attributes.
The effect of hypoxia was tested in the Ecospace simulations with a stylized (and static) hypoxic zone (Fig. 14.6). Output of this case study demonstrated a decrease in biomass within the hypoxic area of organisms directly affected by low bottom oxygen (as defined through response curves) while others were unaffected or even showed an increase as an indirect effect of bottom hypoxia through trophic interactions (Fig. 14.7). Trophic interactions can result in neutral or positive effects of hypoxia on biomass of some species through release of competition for food, or release of predation pressure, as has been shown in Altieri (2008). The location of areas where biomass aggregation occurred outside the hypoxic zone was influenced by species preferences for depth and habitat in addition to hypoxia.
14.4.5 Including Spatial and Temporal Dynamic Forcing Functions
Including spatial and temporal dynamic forcing functions, which is essential to simulate the effect of summer hypoxia on living marine resources, is very data intensive. A value of bottom DO and a measure of primary production are needed for each grid cell at each time step (monthly) for the duration of the model run (typically for multiple decades). Output from hydrodynamic or physical-chemical models would provide data at this resolution, and simultaneously provide the ability to run exploratory and management-based scenarios. Generally, averaging is needed, since most hydrodynamic models provide output on shorter time steps than months, plus spatial re-averaging may be needed if the grid cells of the two models are of different size and/or shape. Whether the re-averaging of physical-chemical information makes sense ecologically needs to be determined on a case-by-case basis, and additional plug-ins can be designed to evaluate effects on shorter time steps than months if effects are known to manifest on shorter time scales (not performed in the case studies presented here, but see De Mutsert et al. 2016).
To couple the physical-biological model to the Ecospace model, a “plug-in” (i.e., a code snippet that interacts with the core model, data layers, and scientific interface) was added to the EwE source code. The plug-in reads in primary production and environmental variables (physical-biological model output) on a monthly time step, and subsequently forces the phytoplankton data with the primary productivity input, and the consumers with the environmental variables in a fashion determined by species-specific response curves (Fig. 14.2). This allowed for oxygen and Chl a to be spatially and temporally dynamic environmental drivers. Previous simulations included temporal dynamic drivers (Ecosim scenarios; Sect. 14.3.1), which would not allow for movement away from hypoxic conditions, or spatial dynamic drivers (Ecospace habitat capacity scenarios; Sect. 14.4.4), which would not allow for temporal variation of the hypoxic conditions (The stylized hypoxic zone of Fig. 14.6 was present year-round). Both situations result in an exaggeration of the effects of hypoxia. In addition to bottom DO and Chl a output of the coupled physical-biological model, species biomass and distribution are still affected by depth, habitat features, fishing, and trophic interactions. In the third case study, the dual effect of the Mississippi River plume is evaluated; nutrient enrichment, which may lead to increased secondary production, and hypoxia, which may lead to decreased secondary production are simulated together and separately.
14.4.6 Case Study 3: Hypoxia as a Spatial-Temporal Driver in Ecospace
The third case study is from De Mutsert et al. (2015a). To test effects of spatial and temporal dynamic forcing functions on the biomass of all groups in the model, bottom DO and Chl a output of a coupled physical-biological model developed in the same geographic area (Fennel et al. 2011, 2013; Laurent et al. 2012, Laurent and Fennel 2014) were used to force the NGOMEX Ecospace model. The simulated DO and Chl a output from 1990 to 2007 were first matched to the grid and time step of the NGOMEX Ecospace model (Fig. 14.8). The model output had to be extrapolated into the estuaries to provide Chl a and DO values for each of the cells in the NGOMEX model, and was extended to 2010 by repeating the last 3 years of model output. The groups in the model responded to DO as determined by the group-specific response curves (Fig. 14.2). The Chl a data were normalized and drove the biomass of phytoplankton group only with a linear function.
Example output of dissolved oxygen in mmol m−3 (top) and Chl a in mg m−3 (bottom) from Fennel et al. (2011) in a month without hypoxia (January) and a month with hypoxia (July). Monthly “maps” of this output are used as spatial-temporal forcing functions in the NGOMEX ecosystem model. Output was extrapolated in the estuaries as shown in the figure. Reproduced from De Mutsert et al. (2015) under a creative commons license
Three scenarios were tested with the NGOMEX Ecospace model with spatial-temporal dynamic drivers. In scenario 1 (“no forcing”), the coupled physical-biological model (Fennel et al. 2011) is not linked to Ecospace, which results in no phytoplankton forcing and no effects of bottom DO on species in the model. This, in a way, represents a scenario where the Mississippi River is “turned off.” In scenario 2 (“enrichment only”), the coupled physical-biological model is linked to Ecospace, but species in the model are not affected by bottom DO (i.e., not negatively affected by DO levels below normoxic conditions). In this scenario, the phytoplankton biomass is driven by the coupled physical-biological model Chl a output, but the bottom DO output has no effect on the groups in the model. In scenario 3 (“enrichment + hypoxia”), phytoplankton biomass is driven by the coupled physical–biological model, and species are affected by DO levels in a way determined by their species-specific response curve (Fig. 14.2). Some results of these simulation outputs are shown in Figs. 14.9, 14.10 and 14.11. The simulations ran from 1950 to 2010.
The results of these simulations suggest that, for most species, the positive effects of increased phytoplankton as a result of nutrient enrichment from the Mississippi River outweigh the negative effect of bottom hypoxia as a result of the same nutrient enrichment from the Mississippi River (see De Mutsert et al. (2015a) for more details). This is a result of the trophic dynamics in Ecospace (bottom-up effect of increased productivity). Similar results have been found in previous studies (Breitburg et al. 2009). A study using a similar approach in the Baltic Sea found biomass reductions when simulating nutrient reduction scenarios (Niiranen et al. 2008), although they did not consider changes to the level or area of hypoxia in response to nutrient reductions. Other mechanisms such as release from predation pressure and/or competition play a role as well (Altieri 2008), which are mechanisms well represented with the trophic dynamics in Ecospace. The bottom-up effects of nutrient enrichment plus indirect effects of trophic dynamics trickle through into the landings of the simulated fleets (Fig. 14.11).
Exceptions occur as is shown in the case of red snapper (Fig. 14.9), which was one of the least hypoxia-tolerant species in the model (Fig. 14.2), and did suffer reduced biomass with hypoxia. The red snapper simulations show an interesting case where biomass is more affected than landings, providing an example of the edge effect; fleets obtain landings at the edge of the hypoxic zone and exacerbate biomass reductions (note that there is no policy or quotas part of these simulations).
The results of model simulations as presented here can have important management implications. However, before these results are used to inform management, additional nutrient reduction scenarios need to be simulated with the model. There may be an optimum nutrient loading from the river that is lower than current levels, which needs to be identified via simulation because there is no direct linear relationship between phytoplankton biomass increase and oxygen decrease (Walker and Rabalais 2006). Simulating realistic nutrient reduction scenarios is important, because our current “no forcing” scenario is not a real-world scenario, and the comparatively small decrease in biomass caused by hypoxia as compared to the increase of enrichment (versus no forcing) may still be ecologically significant. Ongoing research is looking into these very issues.
14.4.7 Conclusions and Future Directions
In summary, the new Ecospace capabilities in combination with model output from a coupled physical-biological model can provide more realistic simulations of long-term effects of summer hypoxia on the northern Gulf ecosystem and its living marine resources. As shown in Sect. 14.4.5, connections between EwE and physical-biological models that deliver oxygen, Chl a, and other environmental drivers into the habitat foraging capacity model can be created with relative ease. True coupling of models can be accomplished on a case-by-case basis, as has recently been done in de Mutsert et al. (2015b). Other advances in Ecospace are the improved representation of the ecosystem within the model area, and the inclusion of ports, which will lead to better simulations of the fishing fleets (and socioeconomic parameters, not considered here, see, e.g., Christensen et al. 2014b) that are included in the spatial model. These tools could be very useful to inform management and can be used to simulate effects of Mississippi River nutrient reduction scenarios on living marine resources in the northern Gulf of Mexico. The spatial temporal data framework functions as a flexible data exchange engine, linking the input driver layers of the Ecospace model to external spatial-temporal data from an open-ended number of data sources and data formats. This functionality not only offers the foundation for a new way of model interoperability to EwE itself, but also aims to contribute to the discussion of standardized model interoperability for food web models in general.
Through the data access tier described in Sect. 14.4.1, bidirectional data exchange linkages to other running models can be facilitated. Data access components can forge links to other models, delivering content valid for select driver maps of the Ecospace model, and in return can deliver estimates of biomass, catch, and other typical Ecospace outputs back to a linked model. Issues such as centralized time stepping and model data conversions will have to be solved, in particular for components that are represented on both sides of a bidirectional model interoperability link.
The results of the simulations provided with the case studies presented in this chapter suggest that nutrient reductions aimed at reducing the size of the hypoxic zone may decrease secondary production, and thereby fisheries biomass and landings. Still, they also suggest that hypoxia has an effect on fisheries species, because model fits improved when dissolved oxygen was included as an environmental factor, and that effects of hypoxia are species-specific, resulting in “winners and losers.” This is another example of how trophic interactions can provide potential counterintuitive results of restorative actions (Walters et al. 2008), which can only be found when an ecosystem food web model is used to simulate potential outcomes. The results of the case studies presented here should be considered exploratory, and simulations of actual proposed nutrient reduction scenarios are needed to inform management; these efforts are underway.
References
Ahrens RN, Walters CJ, Christensen V (2012) Foraging arena theory. Fish and fisheries 13: 41–59. Altieri, A. H. 2008. Dead zones enhance key fisheries species by providing predation refuge. Ecology 89:2808–2818. doi:10.1890/07-0994.1
Bartell SM, Lefebvre G, Kaminski G, Carreau M, Campbell KR (1999) An ecosystem model for assessing ecological risks in Quebec rivers, lakes, and reservoirs. Ecol Model 124:43–67
Bejda AJ, Phelan BA, Studholme AL (1992) The effect of dissolved oxygen on the growth of young-of-the-year winter flounder, Pseudopleuronectes americanus. Environ Biol Fishes 34:321
Breitburg D (2002) Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 25:767–781
Breitburg D, Craig J, Fulford R, Rose K, Boynton W, Brady D, Ciotti BJ, Diaz R, Friedland K, Hagy J III (2009) Nutrient enrichment and fisheries exploitation: interactive effects on estuarine living resources and their management. Hydrobiologia 629:31–47
Breitburg DL, Adamack A, Rose KA, Kolesar SE, Decker B, Purcell JE, Keister JE, Cowan JH (2003) The pattern and influence of low dissolved oxygen in the Patuxent River, a seasonally hypoxic estuary. Estuaries 26:280–297
Breitburg DL, Rose KA, Cowan J (1999) Linking water quality to larval survival: predation mortality of fish larvae in an oxygen-stratified water column. Mar Ecol Prog Ser 178:39–54
Chabot D, Dutil JÄ (1999) Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J Fish Biol 55:472–491
Chesney EJ, Baltz DM, Thomas RG (2000) Louisiana estuarine and coastal fisheries and habitats: perspectives from a fish’s eye view. Ecol Appl 10:350–366
Christensen V, Coll M, Steenbeek J, Buszowski J, Chagaris D, Walters CJ (2014a). Representing variable habitat quality in a spatial food web model. Ecosystems 17:1397–1412
Christensen V, de la Puente S, Sueiro JC, Steenbeek J, Majluf P (2014b) Valuing seafood: the Peruvian fisheries sector. Mar Policy 44:302–311
Christensen V, Pauly D (1992) ECOPATH II—a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol Model 61:169–185
Christensen V, Walters CJ (2004) Ecopath with Ecosim: methods, capabilities and limitations. Ecol Model 172:109–139
Christensen V, Walters CJ (2011) Progress in the use of ecosystem modeling for fisheries management. In: Christensen V, Maclean JL (eds) Ecosystem approaches to fisheries: a global perspective. Cambridge University Press, Cambridge, pp 189–205. Cochrane J, Kelly F (1986) Low-frequency circulation on the Texas-Louisiana continental shelf. J Geophys Res Oceans (1978–2012) 91: 10645–10659
Craig JK (2012) Aggregation on the edge: effects of hypoxia avoidance on the spatial distribution of brown shrimp and demersal fishes in the Northern Gulf of Mexico. Mar Ecol Prog Ser 445:75–95
Crocker CE, Cech JJ Jr (1997) Effects of environmental hypoxia on oxygen consumption rate and swimming activity in juvenile white sturgeon, Acipenser transmontanus, in relation to temperature and life intervals. Environ Biol Fishes 50:383–389
Crout RL (1983) Wind-driven, near-bottom currents over the west Louisiana inner shelf. Louisiana State University Baton Rouge
Davis JC (1975) Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J Fish Board Can 32:2295–2332
De Mutsert K, Cowan JH Jr, Walters CJ (2012) Using Ecopath with Ecosim to explore nekton community response to freshwater diversion into a Louisiana estuary. Mar Coast Fish 4:104–116
De Mutsert K, Steenbeek J, Lewis KA, Buszowski J, Cowan JH Jr, Christensen V (2015a) Exploring effects of hypoxia on fish and fisheriges in the northern Gulf of Mexico using a dynamic spatially explicit ecosystem model. Ecol Model. doi:10.1016/j.ecolmodel.2015.10.013
De Mutsert K, Lewis KA, Buszowski J, Steenbeek J, Milroy S (2015b) 2017 coastal master plan: ecosystem outcomes, community model description (4.5). Final report. (99 pp). Baton Rouge, Louisiana: Coastal Protection and Restoration Authority
De Mutsert K, Lewis KA, Buszowski J, Steenbeek J, Milroy S (2016) Delta management fish and shellfish ecosystem model: ecopath with ecosim plus ecospace (ewe) model description. Final report. (79 pp). Baton Rouge, Louisiana: Coastal Protection and Restoration Authority
Diaz RJ, Rosenberg R (1995) Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev 33:203–245
Fennel K, Hetland R, Feng Y, DiMarco S (2011) A coupled physical-biological model of the northern Gulf of Mexico shelf: model description, validation and analysis of phytoplankton variability. Biogeosci Discuss 8
Fennel K, Hu J, Laurent A, Marta-Almeida M, Hetland R (2013) Sensitivity of hypoxia predictions for the Northern Gulf of Mexico to sediment oxygen consumption and model nesting. J Geophys Res Oceans 118:990–1002. doi:10.1002/jgrc.20077
Fulford RS, Breitburg DL, Luckenbach M, Newell RI (2010) Evaluating ecosystem response to oyster restoration and nutrient load reduction with a multispecies bioenergetics model. Ecol Appl 20:915–934
Graham W (2001) Numerical increases and distributional shifts of Chrysaora quinquecirrha (Desor) and Aurelia aurita (Linne) (Cnidaria: Scyphozoa) in the northern Gulf of Mexico. In: Jellyfish Blooms: Ecological and Societal Importance. Springer, pp 97–111
Grove M, Breitburg DL (2005) Growth and reproduction of gelatinous zooplankton exposed to low dissolved oxygen. Mar Ecol Prog Ser 301:185–198
Gunter G (1963) The fertile fisheries crescent. J Mississippi Acad Sci 9:286–290
Hanson RB (1982) Influence of the Mississippi River on the spatial distribution of micrheterotrophic activity in the Gulf of Mexico. Contrib Mar Sci 23:181–198
Ho CL, Barrett BB (1977) Distribution of nutrients in Louisiana’s coastal waters influenced by the Mississippi River. Estuar Coast Mar Sci 5:173–195
Kaplan IC, Horne PJ, Levin PS (2012) Screening California current fishery management scenarios using the Atlantis end-to-end ecosystem model. Prog Oceanogr 102:5–18
Keister JE, Houde ED, Breitburg DL (2000) Effects of bottom-layer hypoxia on abundances and depth distributions of organisms in Patuxent river Chesapeake bay. Mar Ecol Progress ser 205:43–59
Laurent A, Fennel K (2014) Simulated reduction of hypoxia in the northern Gulf of Mexico due to phosphorus limitation, Elementa 2:000022, doi:10.12952/journal.elementa.000022
Laurent A, Fennel K, Hu J, Hetland R (2012) Simulating the effects of phosphorus limitation in the Mississippi and Atchafalaya river plumes. Biogeosci 9:4707–4723. doi:10.5194/bg-9-4707-2012
Livingston SRJ (2002) Eutrophication processes in coastal systems: origin and succesion of plankton blooms and effetcs on secondary production in Gulf Coast Estuaries. CRC Press, Boca Raton, Florida
Loos SA (2011) Marxan analyses and prioritization of the central interior ecoregional assessment. J Ecosyst Manag 12
Miller D, Poucher S, Coiro L (2002) Determination of lethal dissolved oxygen levels for selected marine and estuarine fishes, crustaceans, and a bivalve. Mar Biol 140:287–296
Niiranen S, Stipa T, Pääkkönen JP, Norkko AK, Kaitala S (2008) Modelled impact of changing nutrient conditions on the Baltic Sea food web. In: Conference Proceedings of the 2008 ICES ASC
Nixon SW, Buckley BA (2002) “A strikingly rich zone”—nutrient enrichment and secondary production in coastal marine ecosystems. Estuaries 25:782–796
O’Connor T, Whitall D (2007) Linking hypoxia to shrimp catch in the northern Gulf of Mexico. Mar Pollut Bull 54:460–463
Pichavant K, Person-Le-Ruyet J, Bayon NL, Severe A, Roux AL, Boeuf G (2001) Comparative effects of long-term hypoxia on growth, feeding and oxygen consumption in juvenile turbot and European sea bass. J Fish Biol 59:875–883
Pihl L, Baden SP, Diaz RJ (1991) Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar Biol 112:349–361
Polovina JJ (1984) Model of a coral reef ecosystem. Coral Reefs 3:1–11
Rabalais NN, Turner RE (2001) Coastal hypoxia: consequences for living resources and ecosystems. Washington, DC. 2001: American Geophysical Union
Rabalais NN, Turner RE, Dortch Q, Justic D, Bierman VJ Jr, Wiseman WJ Jr (2002) Nutrient-enhanced productivity in the northern Gulf of Mexico: past, present and future. Hydrobiologia 475(476):39–63
Rabalais NN, Turner RE, Wiseman WJ (2001) Hypoxia in the Gulf of Mexico. J Environ Qual 30:320–329
Rose KA (2000) Why are quantitative relationships between environmental quality and fish populations so elusive? Ecol Appl 10:367–385
Rose KA, Adamack AT, Murphy CA, Sable SE, Kolesar SE, Craig JK, Breitburg DL, Thomas P, Brouwer MH, Cerco CF, Diamond S (2009) Does hypoxia have population-level effects on coastal fish? musings from the virtual world. J Exp Mar Biol Ecol 381(Supplement):S188–S203
Schurman H, Steffensen JF (1992) Lethal oxygen levels at different temperatures and the preferred temperature during hypoxia of the Atlantic cod Gadus morhua L. J Fish Biol 41:927–934
Secor DH, Gunderson TE (1998) Effects of hypoxia and temperature on survival, growth, and respiration of juvenile Atlantic sturgeon, Acipenser oxyrinchis. Fish Bull, 603–613
Steenbeek J, Coll M, Gurney L, Melin F, Hoepffner N, Buszowski J, Christensen V (2013) Bridging the gap between ecosystem modeling tools and geographic information systems: driving a food web model with external spatial-temporal data. Ecol Model 263:139–151
Tallqvist M, Sandberg-Kilpi E, Bonsdorff E (1999) Juvenile flounder, Platichthys flesus (L.), under hypoxia: effects on tolerance, ventilation rate and predation efficiency. J Exp Mar Biol Ecol 242:75–93
Taylor JC, Miller JM (2001) Physiological performance of juvenile southern flounder, Paralichthys lethostigma (Jordan and Gilbert, 1884), in chronic and episodic hypoxia. J Exp Mar Biol Ecol 258:195–214
Turner RE (2001) Some effects of eutrophication on pelagic and demersal food webs. In: Rabalais NN, Turner RE (eds) Coastal hypoxia: consequences for living resources and ecosystems. Washington, DC: American Geophysical Union
USEPA (2001) Ambient aquatic life water quality criteria for dissolved oxygen (saltwater); cape cod to cape hatteras. Technical report EPA-882-R-00-012. USEPA, Washington, DC
Walker ND, Rabalais NN (2006) Relationships among satellite chlorophyll a, river inputs, and hypoxia on the Louisiana continental shelf, Gulf of Mexico. Estuaries Coasts 29:1081–1093
Walters C, Christensen V, Pauly D (1997) Structuring dynamic models of exploited ecosystems from trophic mass-balance assessments. Rev Fish Biol Fish 7:139–172
Walters C, Martell SJD, Christensen V, Mahmoudi B (2008) An Ecosim model for exploring Gulf of Mexico ecosystem management options: implications of including multistanza life history models for policy predictions. Bull Mar Sci 83:251–271
Walters C, Pauly D, Christensen V (1999) Ecospace: prediction of mesoscale spatial patterns in trophic relationships of exploited ecosystems, with emphasis on the impacts of marine protected areas. Ecosystems 2:539–554
Wannamaker CM, Rice JA (2000) Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J Exp Mar Biol Ecol 249:145–163
Wiseman Jr WJ, Turner R, Justic D, Rabalais N (2004) Hypoxia and the physics of the Louisiana coastal current. In: Dying and Dead Seas Climatic Versus Anthropic Causes. Springer, pp 359–372
Wiseman WJ, Turner RE, Kelly FJ, Rouse LJ, Shaw RF (1986) Analysis of biological and chemical associations along a turbid coastal front during winter 1982. Contrib Mar Sci 29:141–151
Acknowledgements
KdM would like to acknowledge Carl Walters and Joe Buszowski for their advice and technical assistance during model development, Katja Fennel for providing output from her coupled physical-biological model, and Arnaud Laurent for preparing this output for use in the Ecospace model. We also like to thank two anonymous reviewers and the editors who improved the chapter with their thoughtful comments. This work was supported by National Oceanic and Atmospheric Administration Award NA09NOS4780233.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Mutsert, K., Steenbeek, J., Cowan, J.H., Christensen, V. (2017). Using Ecosystem Modeling to Determine Hypoxia Effects on Fish and Fisheries. In: Justic, D., Rose, K., Hetland, R., Fennel, K. (eds) Modeling Coastal Hypoxia. Springer, Cham. https://doi.org/10.1007/978-3-319-54571-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-54571-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54569-1
Online ISBN: 978-3-319-54571-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)