Abstract
In this chapter, the basic information about fuel cells (FC) has been encapsulated. The purpose is to summarize the available details about the types of FC, their design and constructions of FC, catalysts in FC, materials and the methods used for preparation of FC. Nonetheless, more emphasize is kept only on PEM for FC applications by considering the theme and contents of the book.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
A fuel cell (FC) is an energy conversion device which converts the chemical energy (stored in fuels and oxidants) into electrical energy (or electricity) through electrochemical reactions [1]. Christian Friedrich Schönbein, (professor of physics and chemistry in Basel, Switzerland, observed the first FC effect indicating the generation of water and energy from a recombination of hydrogen and oxygen. William Robert Grove (professor of physics at the London Institution) published a brief study indicating the same effect and also designed the first FC generator of electrical current. Grove used a gas feed of hydrogen and oxygen to the corresponding electrodes and demonstrated the existence of a process, which was the reverse phenomena of electrolysis, on the other hand, Schönbein’s research identified the invention of guncotton and for the discovery of ozone [2]. In the twenty-first century, FC is in demand for generation of power with environmental protection, and it has also prompted an extensive research on energy conversion technologies. Efforts have been made toward commercialization of cost-effective hydrogen-based FCs to reduce dependence on oil and decrease pollution. The major application of polymer electrolyte membrane fuel cells (PEMFC) includes transportation (lightweight vehicles, buses) because of their potential impact on the environment, i.e., the control of emission of the greenhouse gases. Other applications include stationary and portable power generation. Most of major motor manufacturing companies generally use PEM-based FCs due to their high power density and excellent dynamic characteristics as compared with other types of FCs [1–9].
2 Different Types of Fuel Cells
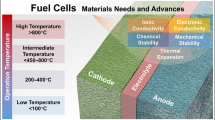
A FC basically directly converts the chemical energy into electrical energy, i.e., heat and water via a redox reaction. The basic electrochemical FC comprises of an anode, a cathode, and an electrolyte. These FCs are generally connected in series as well as parallel by using bipolar plates to provide the desired voltage or current density. The fuel is oxidized at the anode, whereas the combustive (O2) is reduced at the cathode (as an electrode). Hence, if the FC is provided with hydrogen, then FC is known as a clean energy producing device because no volatile organic compounds (VOCs) are emitted. However, the production, transportation, and distribution of hydrogen gas are not considered as clean processes. FCs are classified in different ways depending upon the nature of electrolyte to be used. The most common types of FCs are the polymer electrolyte membrane (also called the proton exchange membrane) fuel cell (PEMFC), direct methanol fuel cell (DMFC) (the same as PEMFC but instead of hydrogen, a methanol is used as the fuel), alkaline fuel cell (AFC) or alkaline anion-exchange membrane fuel cell (AEMFC), phosphoric acid fuel cell (PAFC), molten carbonate fuel cell (MCFC) and solid oxide fuel cell (SOFC), etc. These different types of FCs have various applications , i.e., PAFC, MCFC, and SOFC are used for stationary power generation, while PEMFC, DMFC, and AFC are mostly used for vehicular or portable applications. SOFCs are the other major type cells followed by PEMFC. These cells based on high-temperature O2− ion conducting oxides are best suited for larger scale stationary applications. Hydrogen in PEMFCs and methanol in DMFCs are oxidized in the catalyst layer (CL) at the anode side of the FC. The resulting protons and electrons are forced onto separate paths to the cathode, and then they recombine with oxygen to produce water. PEMFCs have become promising alternative for future energy required for clean environment, power source for automotive, and power backup applications due to certain advantages. Because PEMFCs have been benefited from the advantages such as low operating temperature, high-power density, and zero/low emission and they do not require any fuel processing equipment. However, further improvements in terms of performance, durability, and cost are necessary considerations for commercialization [1,2,3,4,5, 10, 11–14]. Figure 1 illustrates different types of FCs along with their operating conditions.
Different types of FC and their operating conditions (reprinted with permission of Ref. [4])
Table 1 summarizes the operating and applicable properties of five main types of FC.
The lifetime of a PEMFC is a function of operating conditions, component materials, and degradation mechanisms. Wu and Yuan et al. [15, 16] have reviewed and summarized different degradation mechanisms and mitigation strategies, durability tests for better performance of PEMFC [17, 18]. Costamagn et al. [9] have nicely discussed the fundamental scientific aspects of PEMFC including their science and technology covering the period of 40 years starting from the 1960s to the year 2000 [9] and Zaidi et al. [11] have reported the newer research trends took place for the development of PEMFC [11]. The use of various PEMs in water electrolysis has been reviewed by Carmo et al. [19]. There have been some alternatives to proton conductive anhydrous membranes, including heterocyclic protogenic solvents comprising polymer electrolytes for FC applications [20–22]. Anion exchange membranes have been reported as important classification for alkaline FCs [23]. Degradation mechanisms and mitigation strategies of PEMFCs’ durability have been also reviewed [15, 18], similarly test protocols [16] and stack testing [17] and bipolar plates [24] related to PEMFC were also discussed and reviewed.
3 Construction and Design of Different FC
3.1 PEMFC
Modern PEMFCs use proton-conducting PEM for power vehicular, portable, and small residential applications. The activation of kinetically hindered reactions such as oxygen reduction or methanol oxidation dissipates the energy at the electrodes. The migration of protons through the PEM and catalyst layer (CL) also dissipates the energy because of diffusion of reactants and products as well as permeation of liquid water.
Figure 2 depicts the reactions in a hydrogen-based PEMFC. The schematics of a single PEMFC and as a stack with three single cells are also presented in Fig. 2. A single PEMFC consists of an anode and a cathode (Fig. 2a) [1, 2, 4, 5, 25]. On the anode side, hydrogen flows into the flow channel through the gas diffusion layer (GDL) toward the CL, while in the anode CL, hydrogen splits into protons (hydrogen ions) and electrons. The protons pass through the membrane and flow toward the cathode CL. However, the electrons cannot pass through the membrane, but can easily pass to the cathode through an external circuit and hence it generates electricity. On the cathode side, oxygen from air flows into the flow channel through the GDL toward the CL, at the same time in cathode CL, oxygen reacts with the protons and electrons from the anode, produces water and heat. The protons transport across the membrane is basically due to the water concentration and pressure differences between the anode and cathode. This allows water to pass easily through the membrane in both directions. The reaction on the anode side indicates the splitting of hydrogen into protons and electrons, which is also known as a hydrogen oxidation reaction (HOR) [1, 2, 4, 5]:
Schematics circuits of a a single PEMFC and b a PEMFC stack with three single cells (reprinted with permission of Ref. [1])
The reaction on the cathode side indicates that oxygen, protons, and electrons combinedly form water, which is also known as an oxygen reduction reaction (ORR):
So, overall reaction suggests that simply hydrogen reacts with oxygen producing water, electrical energy, and heat:
Generally, single cells are connected in series, which form a stack to produce higher voltages. Three single cells are connected in series, as shown in Fig. 2b. The supplied gases have to be distributed into the single cells through the inlet manifolds, and the exhaust gases have to be removed through the outlet manifolds. Due to the differences in materials and in local operating conditions among the different components of PEMFC, water can be present in different states with different phase. Many researchers have attempted to understand the state of water in PEMFC, because the state and phase change of water are observed different in membrane, i.e., particularly CL, GDL, and flow channel. Figure 3 shows the schematics of the structure of each cell component in a single PEMFC for both the normal operating conditions and cold start.
Schematics of a single PEMFC with the structure of each cell component illustrated a normal operating condition; b cold start (reprinted with permission of Ref. [1])
As shown in Fig. 3a, cell temperature generally ranges from 60 to 80 °C for normal operating conditions, and water exists in both vapor and liquid forms in the flow channel as well as pore regions of GDL and CL, the ionomer (polymer electrolyte) in membrane and CL absorbs much quantities of water in liquid state or bound to H+ (e.g., H3O+). Due to this reason, PEMFC is oftenly used for automotive applications, but it should be unavoidable for vehicles driving below the freezing point of water in winter season, and also PEMFC must be able to successfully start up or “cold start” from subzero temperatures. During a “cold start” process for PEMFC, the initial cell temperature is usually equal to the surrounding temperature (usually under 0 °C in winter), and water mostly freezes [1, 6, 9]. As shown for PEMFC cold start (Fig. 3b), the formation of liquid water can be almost neglected since it freezes to ice. Therefore, water exists in vapor and ice forms but in the pore regions of GDL and CL (ice is formed if the local water vapor pressure is higher than the local water saturation pressure) for cold start of PEMFC. Since the ice can easily stick on the solid materials of CL and GDL and then difficult to move, the ice formation in flow channel might be neglected. Figure 3b also illustrates that water in the ionomer of membrane and CL may also freeze at subzero temperatures [1, 2, 4, 5, 9, 17].
As water is usually not desired in gas-supplying channels and pores then it should be quickly removed as possible because presence of water in the polymer electrolyte may serve as the proton shuttle. The water, as product, is efficiently coupled to the polymer electrolyte so it can improve the mobility of hydrogen ions that is required for the design of CL. In conventional PEM, polymeric side chains terminated by acidic hydrogen sulfonate groups are randomly tethered to hydrophobic polymeric backbones, as shown in Fig. 4 [2]. In the hydrated membrane state, mobile protons (H+ ions) are formed from the dissociation of the HSO3 groups which require a sufficient amount of water. Also water is not immobile, so proton migration is get coupled automatically to the transport of one or two water molecules in a phenomena called electro-osmotic drag.
A typical PEM. Nafion, consists of a hydrophobic fluoropolymer backbone (red) and a flexible side chain (blue) terminated by a hydrophilic sulfonate group (yellow). In the polymer electrolyte, the fibrils agglomerate and randomly self-assemble into regions that are hydrophobic (mostly red) and hydrophilic (mostly blue) (reprinted with permission of Ref. [2])
This drag alongside the proton flux shuffles water molecules toward the cathode CL. A backward flux of water molecules toward the anode CL occurs from diffusion or pressure-driven flow that opposes the water shuffling toward the cathode. It was reported [2] that the water flux in the polymer membrane depends on its morphology and current density. Hydrated state, of the polymer electrolyte’s parts even under drastic variations in operating conditions, is a key parameter of working of PEMFC. Designing PEM with reduced amount of electro-osmotic drag would be an advantage to the water management problem. This can be accomplished by partially immobilizing the water in the membrane without impairing its conductive abilities. High rates of water back flux can be achieved by using thin PEM with a higher concentration of hydrophilic regions but at the cost of less chemical stability, higher reactant permeability, and reduced mechanical robustness [2, 4, 5].
3.2 DMFC
DMFCs, prepared using PEMs, are potential alternative FCs for future cleaner energy requirements because these cells operated at relatively low temperatures and do not require any fuel processing equipment, as discussed earlier. However, limitations of DMFCs include: (i) slow kinetics of methanol oxidation at anode CL and (ii) diffusion of methanol from anode to the cathode side, across the PEM. The methanol does not only wastes fuel but also it can cause performance losses at the cathode due to the consumption of oxygen and catalyst poisoning. Thus, it is necessary to reduce the loss of methanol as fuel in PEM for improving the performance of a DMFC [3, 4, 6, 7, 26]. The working principle of DMFCs is presented in Fig. 5. New membranes with significantly reduced permeability of methanol and water transport (by diffusion or electro-osmotic drag) are required for DMFCs along with acceptable conductivity and stabilities. Diffusion of fuel (i.e., methanol in DMFC) from anode to cathode sides through the PEM may lower down the voltage efficiency and performance of FC [3, 4, 6, 7, 26].
Working principle of DMFC (reprinted with permission of Ref. [4])
DMFCs employing with PEM are one of the attractive power sources for different applications due to their stable operation at a relatively low temperatures, high energy generation yield and energy density, and simplicity. Most DMFC research has concentrated on PEMFCs that are fed directly with methanol. Methanol itself is a fuel that possesses significant electro activity, and it can be oxidized directly to generate carbon dioxide and water in a DMFC. The proton conduction through the PEM is associated with the water transport through the membrane. Methanol can also be transported to the cathode by an electro-osmotic drag (methanol crossover). This is due to the physical properties of methanol, such as its dipole moment, which consequently leads to a decrease in cell performance. It is necessary to reduce the loss of fuel across the cell for improvement in the performance of a DMFC [4, 22, 26].
The work on various PEM materials for DMFC has been reviewed by Neburchilov et al. [26]. Unlike the membranes for hydrogen fuelled PEM fuel cells, perfluorosulfonic acid-based membranes showed complete domination, but the membranes for DMFC have numerous variations. The membranes have been discussed according to various properties, included methanol crossover, proton conductivity, thermal stability, and maximum power density. Hydrocarbon and composite fluorinated membranes showed the most potential for low cost membranes with low methanol permeability and high durability [18, 26, 27, 28].
3.3 AEMFC
To overcome draw backs of DMFCs, there has been a growing interest in developing direct methanol AEMFCs using alkaline anion-exchange membranes (AEMs) as PEM [29, 30]. The electrochemical reactions in AEMFCs are expressed as follows:
In AEMFCs, OH− ions, instead of protons, are transported across the membrane. In this case, the methanol crossover and the transportation of ions are in the opposite direction. This is expected to decrease the methanol crossover of the membranes and correspondingly to decrease the poisoning of the electrode catalysts by the permeated methanol, which maintains the catalytic activity of the electrode catalysts for longer time and achieving better FC performance [29, 30].
The working principle of AEMFCs is shown in Fig. 6. The expected advantages of AEMFCs, when compared to traditional anionic FCs (AFCs), are the possibility of easy handling and stocking liquid fuels as well as the better control on carbonatation due to the solid nature of the AEMFCs [29,30,, 29, 30]).
Operating principle of AEMFCs (reprinted with permission of Ref. [30])
3.4 PAFC
In the 1960s, PAFC was preferred for the alkaline battery, where the main problem was carbonation. The PAFC was reported as the first commercialized FC. ONSI Corporation, in the United States, and Gaz de France sold 200 kW FC for providing electricity to several houses. However, the high cost as well as the tendency of phosphoric acid to solidify are major drawbacks [30].
3.5 SOFC
Like PEMFCs, SOFCs have been widely investigated, especially in the United States, Japan and Europe. These are the most energetically efficient FCs. These are generally used for stationary devices: water, is extremely hot when leaving the FC, goes through turbines to drive generators to produce electricity. However, components have to exhibit a high thermal stability [30].
3.6 MCFC
MCFCs have been developed for stationary applications and considered for promising use of reformed methane or coal gas results in low NOx emissions. The MCFC differs from other FC as it does not require any noble metal as the catalyst, but uses mainly stainless steel. As no platinum is used, the poisoning of this metal by CO gas does not occur and many fuels can thus be used, such as gasified biomass or gasified wastes. Nevertheless, the impurities contained in fuels (H2S, HCl, HF, NH3) may enhance the corrosion of the electrodes, especially the cathode, because of the growth of oxides with a poor electrical conductivity. This issue has turned out to be a lock to the development of this MCFCs [30].
4 Catalysts for Different FCs
The CLs regulate the traffic and conversion of hydrogen gas in PEMFC or methanol in DMFC at the anode and oxygen at the cathode. The water’s journey through a FC generally starts in the cathode CL where it is produced during the reduction reaction of the oxygen. Also oxygen reduction is a multistep process because it requires the migration of oxygen molecules, protons, and electrons through diffusion and conduction to the catalyst surface on which gas molecules adsorb and charge transfer occurs in the presence of water. Water as a product desorbs and diffuses away through the channels. During oxygen reduction reaction, hydrogen peroxide is an undesired intermediate which may be formed on either the anode or on the cathode; and being highly aggressive, hydrogen peroxide can degrade the PEM. The detailed stoichiometric balance of all those reaction processes can affect the overall rate of electrochemical conversion. Suitable catalysts must be stable in a hot, oxidizing, acidic, aqueous environment, while effectively driving the production of water and resisting deactivation caused by the poisoning effects of fuel contaminants and atmospheric pollutants [1, 2, 4, 5, 9, 14–16, 32–35].
Precious metals and alloys have shown the best balance of catalytic properties because catalysts should expose a large interfacial surface area so it can reduce the irreversible voltage losses during sluggish reactions such as oxygen reduction. Carbon has been preferred as supporting material because of its corrosion resistance, high conductivity and nanostructured morphology. CLs must be incorporated into a composite structure with a minimum of two distinct phases namely (i) the solid phase of carbon and platinum which can conduct electrons, (ii) Water phase filling the pore spaces in the composite which can transport protons, hydrogen and oxygen gas, and other water molecules. These kinds of two-phase thin (100–500 nm) composite layers work well for long because if they are too thick, the diffusion of dissolved reactant molecules and protons will be slow. Conversely, if they are too thin, they will be incapable to provide sufficient reaction surface. Thick layers (5–30 μm) of catalysts are typically used in PEMFCs. Solid carbon and platinum transport electrons to the catalyst, whereas a second, solid subphase, typically composed of polymer such as Nafion, provides sufficient proton conduction. To make the CL porous for creating the third percolation network, gases are allowed to diffuse readily through the catalysts. Generally, water competes with reactant gases in terms of space between the solid components and the degree to which liquid water penetrates into the CL. The ability of CL to direct water into the membrane material rather than the channel or to facilitate its evaporation affects the overall water and heat management of the cell. CL at cathode generates the maximum power density when roughly one-third of its volume is filled with proton conductor and the thickness is kept between 10 and 20 μm. A bimodal porous structures, as shown in Fig. 7, with intra- and inter-agglomerate pores, can provide the good results [2, 5, 15, 16, 33, 34]. Only 10–20% of the catalyst is utilized in three-phase composites to optimize the balance between large active areas and the transport of reactants. Thicker catalyst layers can provide larger areas to support reactions, on other side they generally impedes diffusion. Many researchers, reported proper design strives for new well-defined architectures based on nanometer scale template electrodes—using CNTs as building blocks, which can be used in the future by considering it as porous-electrode technology.
Structure and composition of CL. a Carbon (gray) coated in platinum catalysts (red) based CL in a PEMFC. b If the pores in agglomerate fill with water (blue). c Secondary pores-based agglomerates in the course of the spontaneous aggregation of carbon particles (reprinted with permission of Ref. [2])
Nanostructured thin-based CL substrates were fabricated to incorporate organic whiskers with high surface-to-volume ratios so that 100% of the catalysts can be used [2, 5]. Wanga et al. [5] reported various catalysts [Pt and several of its alloys (Pt–Co, Pt–Ni, Pt–Fe, Pt–V, Pt–Mn and Pt–Cr)] used for PEM-based FC. Non-precious metal catalysts and self-supported electrocatalysts such as metal, metal alloys are another important part in PEM of FC [14, 15, 32–42]. Various materials such as thin films of tungsten selenides [42] and phosphide-based materials [43] were used for electrocatalytic hydrogen evolution using FCs [42, 43]. The lifetime of a PEMFC is a function of operating conditions, component materials, and degradation mechanisms of electrocatalysts or CL. Wu and Yuan et al. [15, 16] reviewed and summarized different degradation mechanisms, migration strategies, and FC durability test protocols for cell components [16–18]. Support materials for PEMFC and DMFC electrocatalysts [33], phosphotungstic acid doped catalysts [44], and microstructural pore analysis of the catalyst layer in a PEMFC [37] were also reviewed and summarized, which include different NPs such as CNTs, CNFs, graphene, nanodiamonds, TiO2, TiN, TiB2, SnO2, WO3 also conducting polymers.
Sanli and Gursel et al. reported the development of graphene supported platinum (Pt/graphene) as the electrocatalysts for PEMFC [45]. They demonstrated the comprehensive overview on synthesis, characterization, and FC performance of Pt/graphene electrocatalysts. These workers prepared graphene oxide (GO) supported Pt nanoparticles for PEMFCs by several impregnation reduction methods including ethylene glycol reflux, sodium borohydride reduction, and ascorbic acid reduction. The single FC tests showed that GO has the best performance compared to graphene nanoparticles and thermally reduced GO. Catalyst coated membrane prepared by screen printing method were also used in a PEMFC [46]. The PEMFC catalysts can be electrodeposited by the use of a hydrogen depolarized anode [47].
5 Materials and Methods for Preparation of PEM for Fuel Cells
Organic–inorganic nanocomposites -based PEMFCs contain inorganic building blocks at nanoscale in organic polymer by hybridization at molecular level. Intensive researches have been carried out to develop new PEM for DMFC applications. The PEMs in DMFC serve as barrier for methanol and conduct protons. Various PEMs have been used in DMFC, which include perfluorinated ionomer Nafion, polybenzimidazole/phosphoric acid blend membranes, Nafion membrane modified with inorganic phase such as silicate and zirconium phosphate. Due to major drawbacks (high cost, loss of conductivity at high temperature, and high methanol permeability) of perfluorinated polymers restrict their applicability. Therefore, non-perfluorinated PEM based on aromatic thermoplastics, such as polyimides (PI), poly(ether sulfone) (PES), polybenzimidazole (PBI), sulfonated PEEK, have been developed which have shown excellent chemical resistance, high thermo-oxidative stability, good mechanical properties and cost effectiveness [3, 14, 26, 48].
Tripathi et al. [4] have reviewed and summarized different types of organic–inorganic nanocomposites for better understanding of molecular-level chemistry, morphology, transport behavior, and polymer degradation in comparison with Nafion. They reported recent developments about new ionomers and hybrid membranes, containing inorganic NPs to control morphology and water retention by complexation of basic polymers with different oxoacids and inorganic fillers [49]. According to recent report [4], different organic–inorganic nanocomposites were used for PEM, which include perfluorinated organic–inorganic nanocomposites (Nafion with modifiers such as Nafion-silica NPs, Nafion-ZrP, Nafion-titanium dioxide, Nafion-thiophene, Nafion-metal oxides, Nafion-zeolite, Nafion-silica-PWA, Nafion-PAni nanocomposites), partially perfluorinated organic–inorganic nanocomposites (sulfonated styrene with PTFE backbone, functionalized PVDF-silica NPs, PVDF-HFP-Nafion, PVDF-HFP-Al2O3, PVDF-PSSA-Al2O3), non-perfluorinated organic–inorganic nanocomposites (sulfonated polymers such as PEEK, PI, PES, PBI, polyphynelenes and hydrophilic polymers such as PVA and chitosan), styrene-based nanocomposites (sulfonated co-polymers of styrene, sodium styrene sulfonated grafted polyacrylonitrile, TEOS-grafted polystyrene blocks in polystyrene-b-isobutylene-styrene block copolymers, TEOS grafted PSMA-PEG composites, sulfonated PTFE and grafted with TEOS PSMA-silica hybrid, PSSA-PWA by self-assembly technique), PEEK-based nanocomposites (sulfonated PEEK-silicon dioxide-zirconium phosphate (ZrP), SPEEK–silica, SPEEK-BPO4 and PEEK-TiO2 nanocomposites), polyether sulfone (PES) based nanocomposites, polybenzimidazole (PBI) based nanocomposites (PBI-phosphoric acid-based composites, PBI-silicon dioxide), P and polyetherimide-based nanocomposites (sulfonated PI, PI-phosphosilicate gel nanocomposites, PI-polypyrrole composites), chitosan-based nanocomposites (PVA-phosphonic acid modified chitosan nanocomposites, chitosan-silica nanocomposites), PVA-based nanocomposites (PVA-chitosan composites, PVA-H2PO4 composites, PVA-silicon dioxide nanocomposites) and layered NP or nanoclay-based nanocomposites (montmorillonite-nafion), finally CNT-based polymer nanocomposites (CNT or sulfonated CNT-based Nafion composites) [4–6, 10, 11, 14, 26, 27, 48, 50].
Several polymer nanocomposites-based PEM such as sulfonated poly(ether ether ketone) (PEEK)/polyaniline composites [3, 50] were also studied. Wanga et al. [5] reviewed the technology, applications, and the need of fundamental research on PEMFC [5]. PEMFCs based on ionomers have also been developed [6]. Similarly, polyvinyl alcohol (PVA)/SiO2 hybrid based PEMs containing sulfonic acid groups were also developed for DMFC applications [7]. Organic–inorganic membranes were also prepared from polyether diamine and epoxy silane [8]. Development of PEMFC using organic–inorganic nanocomposites was reported in the literatures indicating several modifications such as: (i) improvement in the self-humidification of in membrane; (ii) reduction in the electro-osmotic drag and fuel crossover; (iii) enhancement in the mechanical and thermal strengths without deteriorating proton conductivity; (iv) increase in proton conductivity by introducing solid inorganic proton conductors; and (v) slow drying PEMs with high water retention capability [4]. Researchers have reported the different categories for the development of organic–inorganic nanocomposites based PEMFC which include (i) doping the inorganic proton conductors in PEMs; (ii) nanocomposites prepared by sol–gel method; (iii) covalently bonded inorganic segments with organic polymer chains; and (iv) acid–base PEMFC nanocomposites [4, 6].
Various polymers and their nanocomposites are in use, nowadays, for industrial FC applications [10]. PEMFC composite comprised of triblock copolymer and heteropolyacid have been also used for FC applications [51]. Organic–inorganic hybrid materials were also prepared for the development of PEMFC for separation processes [52]. PEO/KHCO3 nanocomposites were used for PEMFC applications [53]. Efforts have been made to develop PEMFC operating above 100 °C [22, 29]. Silica nanocomposites-based PEMFC systems, prepared by the sol–gel process, of polyethoxysiloxane within a sulfonated PEEK matrix have been reported [54]. Various polymer systems have been studied for applications of proton exchange membranes [27, 48].
Sulfonated polymers [3], poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety [55] and factors affecting the life of PEMFC [56] have been investigated for their PEMFC applications. An interesting study on novel polyvinyl butyl-based polymer membrane and its application in gel polymer electrolytes for lithium-ion batteries have been reported [57]. The suitability of various polymeric material-based nanocomposites membranes for PEMFC applications have examined by several workers [12, 30, 58–63]. Hybrid inorganic–organic nanocomposites made from polymer electrolytes such as PVA/SiO2 [7], graphene supported platinum nanoparticles (NPs) [45], sulfonated PEEK/silica nanocomposites [64], TiO2 nanoparticle self-assembled aromatic polyamide [64], nafion and fluorinated TiO2 for PEMFCs [65], nafion/inorganic nanocomposite [66] sulfonated PEEK/sulfonated NPs composite [67], UV polymerized 1-H-3-vinylimidazolium bis(trifluoromethanesulfonyl)imide [68], Nafion, [(ZrO2)/(HfO2)0.25] and [(SiO2)/(HfO2)0.28] NPs [69, 70], Nafion and fluorinated TiO2 NPs [71], novel polymer-coated nanoparticles dispersed-carbon micronanofibers-based air cathode [72], functionalized Al2O3 particles as additives in proton-conducting polymer [73], poly (ether-imide) [74], polytetrafluoroethylene (PTFE) [75] and high performance polymer based [76], metal-supported tubular solid oxide [77], SiO2-ceramic nanoporous substrate-reinforced sulfonated poly(arylene ether sulfone) composite [78], Poly (2,5-benzimidazole) (ABPBI)-based MEA assembly [79], poly(vinyl alcohol) (PVA) [80–82], partly fluorinated poly(arylene ether ketone sulfone) hydrophilicehydrophobic multiblock copolymers [83], multilayer-structured, SiO2/sulfonated poly(phenylsulfone) composite [84], sulfonated bisphenol-A-polysulfone-based composite PEMs containing tungstophosphoric acid [85], aligned polyaniline nanorods in situ grown on gas diffusion layer [86] were used for PEMFC applications. Various selective membranes made from polymer nanocomposites were studied for hydrogen purification [87]. A galvanostatic analysis technique was developed [88, 89] as an in situ diagnostic tool for PEMFC single cells and stacks.
6 Characterizations and Characteristic Properties of PEM for Different FC
The characteristic properties of polymer nanocomposites at the surface, interfaces, in bulk and under gas and supercritical fluid treatments have generated tremendous interest. The morphological and physicochemical properties of organic–inorganic hybrids depend on their composition, the size of the inorganic fillers and interfacial interactions. Potential characteristics such as mechanical, chemical, and thermal stability are vital for PEM. Stability of PEMs can be optimized by the degree of functionalization, interactions between organic–organic or organic–inorganic segments, controlled cross-linking, and chemical or surface modifications. Functionalization of the organic part or doping of polyelectrolytes in the matrix, are common methods, but suffer due to excessive swelling or leaching out proton carriers on prolonged use at elevated temperature [1–4, 49]. The PEEK/PAni based polymer composites were used for DMFC application considering optimum physicochemical and electrochemical properties, thermal stability as well as very low methanol permeability. Desired chemical compositions of PEM can be identified by NMR and FTIR spectroscopy. The chemical composition of PEM can also be obtained by EDX analysis. The inner structure of the PEM can be identified by TEM. Surface structure and 3D appearance of the PEM can be studied by SEM images. AFM is an effective tool for morphological characterization of PEM. Thickness of PEM can be measured by water uptake (swelling) test at particular temperature. Dimensional stability of PEM is also directly related to the water uptake, swelling properties and degree of functionalization. Organic–inorganic nanocomposites-based PEMs possess interesting route to reduce swelling by cross-linking and water storage via the inorganic segment. The surface functionalization enhances the compactness and thus reduces the swelling due to increased interactions. The hydrolytic and oxidative weight loss, obtained by refluxing the membranes under high temperature and pressure, serve as measures of chemical stability. Thus, knowledge of chemical degradation mechanisms is useful in the design of materials with improved stability and lifetime fuel cells environment. The degradation process and the thermal stability of the PEM were investigated using TGA and DSC. Data of coefficient of thermal expansion (CTE) and viscoelastic behavior of the PEM can be obtained by TMA and DMA or DMTA studies. The dynamic mechanical properties, such as storage modulus, loss modulus, and tan δ, give idea about molecular mobility transitions, such as the glass transition temperature. Ion-exchange capacity of the PEM was determined by salt splitting ion exchange method. Conductance of PEM can be measured by performing the resistance on potentiostatic two electrodes with the help of digital conductivity meter with AC mode. Proton transport number in the PEM phase can be estimated from the membrane potential measurements. Methanol permeability of the PEM can be determined using a diaphragm diffusion cell. The conductivity and methanol permeability of a PEM are crucial parameters, which give idea about their suitability for DMFC applications. To measure these properties, PEM are generally activated by chemical treatments (sulfuric acid and hydrogen peroxide) to remove impurities from the ionomer membrane and to activate sulfonic acid groups. The degree of crystallinity of nonlayered organic–ionorganic hybrid nanocomposites can be analyzed by Wide-angle X-ray scattering (WAXS) or wide-angle X-ray diffraction (WAXD)gives idea about the change in crystalline structure [3, 4, 6, 7, 58]. Various characteristics of PEMFC such as electrical properties [53], surface morphology, proton mobility [54], effective transport properties with a focus on the gas diffusion layer [34], interfacial properties [90] and bactericidal anti-fouling [64] have been extensively studied. Various models related to PEMFC were also been proposed [34, 91]. Most of proton exchange membranes [14, 92] were designed using their unit operation modeling [13, 93, 94], computational study [95], efficiency enhancement and cost reduction [96], self-passivating carbon film as bipolar plate based protective coating [97, 98], cooling flow designs [99], numerical study investigation of the effects of GDL compression and intrusion [100], PEM degradation modeling [101], improving gas diffusivity with biporous flow-field [102] and the properties effect on gas diffusivity [103] for PEMFC applications. Effects of idling temperature on high-temperature PEMFC degradation were investigated under simulated start/stop cycling conditions [104, 105].
7 Summary
The main aims and objectives behind studying about the various FCs are to give basic understanding of different types of FCs, design and constructions of FCs, materials and methods used for preparation of FCs, characterization and potential properties of FCs. Nonetheless, more emphasize is kept only on PEM for FC applications.
References
Jiao K, Li X (2011) Water transport in polymer electrolyte membrane fuel cells. Prog Energy Combus Sci 37:221–291
Eikerling M, Kornyshev AA, Kucernak AR (2006) Water in polymer electrolyte fuel cells: friend or foe? Phys Today 59(10):38–44
Nagarale RK, Gohil GS, Shahi VK (2006) Sulfonated poly(ether ether ketone)/polyaniline composite proton-exchange membrane. J Membr Sci 280:389–396
Tripathi BP, Shahi VK (2011) Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog Polym Sci 36:945–979
Wanga Y, Chen KS, Mishler J, Cho SC, Adroher XC (2011) A review of polymer electrolyte membrane fuel cells: technology, applications, and needs on fundamental research. Appl Ene 88:981–1007
Kerres JA (2001) Development of ionomer membranes for fuel cells. J Membr Sci 185:3–2
Kima DS, Park HB, Rhim JW, Lee YM (2004) Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J Membr Sci 240:37–48
Sforc ML, Yoshida IVP, Nunes SP (1999) Organic–inorganic membranes prepared from polyether diamine and epoxy silane. J Membr Sci 159:197–207
Costamagn P, Srinivasan S (2001) Quantum jumps in the PEMFC science and technology from the 1960s to the year 2000 Part I. Fundamental scientific aspects. J Power Sources 102:242–252
Kumar GG, Nahm KS (2011) Chapter-27: polymer nanocomposites—fuel cell applications. In: Reddy B (ed) Advances in nanocomposites—synthesis, characterization and industrial applications. InTech China, pp 639–660. Available from: http://www.intechopen.com/books/advances-in-nanocompositessynthesis-characterization-and-industrial-applications/polymer-nanocomposites-fuel-cell-applications
Zaidi SMJ (2009) Chapter-2: research trends in polymer electrolyte membranes for PEMFC. In: Zaidi SMJ, Matsuura T (eds) Polymer membranes for fuel cells. pp 7–25
Couture G, Alaaeddine A, Boschet F, Ameduri B (2011) Polymeric materials as anion-exchange membranes for alkaline fuel cells. Prog Polym Sci 36:1521–1557
Siegel C (2008) Review of computational heat and mass transfer modeling in polymer-electrolyte-membrane (PEM) fuel cells. Energy 33:1331–1352
Peighambardoust SJ, Rowshanzamir S, Amjadi M (2010) Review of the proton exchange membranes for fuel cell applications. Int J Hydrogen Energy 35:9349–9384
Wu J, Yuan XZ, Martin JJ, Wang H, Zhang J, Shen J, Wu S, Merida W (2008) A review of PEM fuel cell durability: degradation mechanisms and mitigation strategies. J Power Sources 184:104–119
Yuan XZ, Li H, Zhang S, Martin J, Wang H (2011) A review of polymer electrolyte membrane fuel cell durability test protocols. J Power Sources 196:9107–9116
Millera M, Bazylaka A (2011) A review of polymer electrolyte membrane fuel cell stack testing. J Power Sources 196:601–613
Schmittinger W, Vahidi A (2008) A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J Power Sources 180:1–14
Carmo M, Fritz DL, Mergel J, Stolten D (2013) A comprehensive review on PEM water electrolysis. Int J Hydrogen Energy 38:4901–4934
Ünügür S, Bozkurta A, Hosseini SS (2012) Alternatives toward proton conductive anhydrous membranes for fuel cells: heterocyclic protogenic solvents comprising polymer electrolytes. Prog Polym Sci 37:1265–1291
Wee JH (2007) Applications of proton exchange membrane fuel cell systems. Renew Sustain Energy Rev 11:1720–1738
Li Q, He R, Jensen JO, Bjerrum NJ (2003) Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem Mater 15:4896–4915
Merle G, Wessling M, Nijmeijer K (2011) Anion exchange membranes for alkaline fuel cells: a review. J Membr Sci 377:1–35
Hermanna A, Chaudhuria T, Spagnol P (2005) Bipolar plates for PEM fuel cells: a review. Int J Hydrogen Energy 30:1297–1302
Alizadeh E, Rahgoshay SM, Esbo MR, Khorshidian M, Saadat SHM (2016) A novel cooling flow field design for polymer electrolyte membrane fuel cell stack. Int J Hydrogen Energy 41:8525–8532
Neburchilov V, Martin J, Wang H, Zhang J (2007) A review of polymer electrolyte membranes for direct methanol fuel cells. J Power Sources 169:221–238
Zhang H, Shen PK (2012) Recent development of polymer electrolyte membranes for fuel cells. Chem Rev 112:2780–2832
Zamel N, Li X (2013) Effective transport properties for polymer electrolyte membrane fuel cells with a focus on the gas diffusion layer. Prog Energy Comb Sci 39:111–146
Jiang Z, Jiang ZJ (2014) Plasma techniques for the fabrication of polymer electrolyte membranes for fuel cells. J Membr Sci 456:85–106
Schieda M, Roualdès S, Durand J, Martinent A, Marsacq D (2006) Plasma polymerized thin films as new membranes for iniature solid alkaline fuel cells. Desalination 199:286–288
Noto VD, Zawodzinski TA, Herring AM, Giffin GA (2012) Polymer electrolytes for a hydrogen economy. Int J Hydrogen Energy 37:6120–6131
Ma TY, Dai S, Qiao SZ (2016) Self-supported electrocatalysts for advanced energy conversion processes. Mater Today 19(5):265–27
Sharma S, Pollet BG (2012) Support materials for PEMFC and DMFC electrocatalysts—a review. J Power Sources 208:96–119
Gottesfeld S, Zawodzinski TA (1997). In: Alkire RC, Gerischer H, Kol DM, Tobias CW (eds) Polymer electrolyte fuel cells. Adv Electrochem Sci Eng, 196–301
Othman R, Dicks AL, Zhu Z (2012) Non precious metal catalysts for the PEM fuel cell cathode. Int J Hydrogen Energy 37:357–372
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Activity benchmarks and requirements for Pt, Pt-alloy and non-Pt oxygen reduction catalysts for PEMFCs. App Cataly B: Environ 56:9–35
Ghosh S, Ohashi H, Tabata H, Hashimasa Y, Yamaguchi T (2015) Microstructural pore analysis of the catalyst layer in a polymer electrolyte membrane fuel cell: a combination of resin pore-filling and FIB/SEM. Int J Hydrogen Energy 40:15663–15671
Kim T, Popov BN (2016) Development of highly-active and stable Pt/C catalyst for polymer electrolyte membrane fuel cells under simulated start-up/shut-down cycling. Int J Hydrogen Energy 41:1828–1836
Cho YH, Lim JW, Park HY, Jung N, Ahn M, Choe H, Sung YE (2012) Performance of membrane electrode assemblies using PdPt alloy as anode catalysts in polymer electrolyte membrane fuel cell. Int J Hydrogen Energy 37:5884–5890
Lee KS, Kim DM (2012) Sputtering and heat treatment of pure Ni metal onto a carbon nanotube on carbon paper to fabricate electrocatalysts for the oxygen reduction reaction in PEMFC. Int J Hydrogen Energy 37:6272–6276
Oh SK, Kim MJ, Eom KS, Kyung JS, Kim DH, Cho EA, Kwon HS (2016) Design of MgeNi alloys for fast hydrogen generation from seawater and their application in polymer electrolyte membrane fuel cells. Int J Hydrogen Energy 41:5296–5303
Romanov R, Grigoriev S, Fominski V, Volosova M, Demin M (2015) Preparation and study of thin films of tungsten selenides for electrocatalytic hydrogen evolution. Phys Proced 71:348–353
Xiao P, Chen W, Wang X (2015) A review of phosphide based materials for electrocatalytic hydrogen evolution. Adv Energy Mater 5(24):1–13
Chen GY, Wang C, Lei YJ, Zhang J, Mao ZQ, Guo JW, Wang JL (2016) Catalyst layer doped with phosphotungstic acid for degradation mitigation in polymer electrolyte membrane fuel cells. Int J Hydrogen Energy 1–6 (Accepted) http://dx.doi.org/10.1016/j.ijhydene.2016.04.222
Sanlı LI, Bayram V, Yarar B, Ghobadi S, Gursel SA (2016) Development of graphene supported platinum nanoparticles for polymer electrolyte membrane fuel cells: effect of support type and impregnation-reduction methods. Int J Hydrogen Energy 41:3414–3427
Wang W, Chen S, Li J, Wang W (2015) Fabrication of catalyst coated membrane with screen printing method in a proton exchange membrane fuel cell. Int J Hydrogen Energy 40:4649–4658
Mitzel J, Arena F, Natter H, Walter T, Batzer M, Stefener M, Hempelmann R (2012) Electrodeposition of PEM fuel cell catalysts by the use of a hydrogen depolarized anode. Int J Hydrogen Energy 37:6261–6267
Hickner MA, Ghassemi H, Kim YS, Einsla BR, McGrath JE (2004) Alternative polymer systems for proton exchange membranes (PEMs). Chem Rev 104:4587–4612
Antonacci P, Chevalier S, Lee J, Yip R, Ge N, Bazylak A (2015) Feasibility of combining electrochemical impedance spectroscopy and synchrotron X-ray radiography for determining the influence of liquid water on polymer electrolyte membrane fuel cell performance. Int J Hydrogen Energy 40:16494–16502
Seo DW, Lim YD, Lee SH, Jeong IS, Kim DI, Lee JH, Kim WG (2012) Preparation and characterization of sulfonated poly (tetra phenyl ether ketone sulfone)s for proton exchange membrane fuel cell. Int J Hydrogen Energy 37:6140–6147
Choi JK, Lee DK, Kim YW, Min BR, Kim JH (2008) Composite polymer electrolyte membranes comprising triblock copolymer and heteropolyacid for fuel cell applications. J Polym Sci, Part B: Polym Phys 46:691–701
Souza VC, Quadri MGN (2013) Organic–inorganic hybrid membranes in separation processes: a 10-year review. Braz J Chem Eng 30(04):683–700
Kumar KV, Sundari GS, Jyothi NK (2010) Fabrication and electrical characterization of PEM fuel cell based on (PEO + KHCO3) polymer electrolyte membrane. Int J Inn Res Sci, Eng Technol 2(10):5838–5847
Colicchio I (2008) Silica-based nanocomposite membranes via the sol gel process of polyethoxysiloxane within a sulfonated poly(ether-ether-ketone) matrix: morphology and proton mobility. PhD Theses, Universitäts professor Dr. rer. nat. Martin Möller
Xing P, Robertson GP, Guiver MD, Mikhailenko SD, Kaliaguine S (2004) Sulfonated poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety prepared by direct copolymerization as proton exchange membranes for fuel cell application. Macromol 37:7960–7967
Pei P, Chen H (2014) Main factors affecting the lifetime of proton exchange membrane fuel cells in vehicle applications: a review. Appl Energy 125:60–75
Lian F, Wen Y, Ren Y, Guan HY (2014) A novel PVB based polymer membrane and its application in gel polymer electrolytes for lithium-ion batteries. J Membr Sci 456:42–48
Mishra AK, Bose S, Kuila T, Kim NH, Lee JH (2012) Silicate-based polymer-nanocomposite membranes for polymer electrolyte membrane fuel cells. Prog Polym Sci 37:842–869
Litster S, McLean G (2004) PEM fuel cell electrodes. J Power Sources 130:61–76
Prater KB (1994) Polymer electrolyte fuel cells: a review of recent developments. J Power Sources 51:129–144
Bose S, Kuila T, Hien TX, Nguyen Kimc NH, Laua KT, Lee JH (2011) Polymer membranes for high temperature proton exchange membrane fuel cell: recent advances and challenges. Prog Polym Sci 36:813–843
Yuan W, Tang Y, Yang X, Wan Z (2012) Porous metal materials for polymer electrolyte membrane fuel cells—a review. Appl Ene 94:309–329
Perez LC, Brandaoa L, Sousa JM, Mendes A (2011) Segmented polymer electrolyte membrane fuel cells—a review. Renew Sustain Energy Rev 15:169–185
Kwak SY, Kim SH (2001) Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ Sci Technol 35:2388–2394
Noto VD, Bettiol M, Bassetto F, Boaretto N, Negro E, Lavina S, Bertasi F (2012) Hybrid inorganic-organic nanocomposite polymer electrolytes based on nafion and fluorinated TiO2 for PEMFCs. Int J Hydrogen Energy 37:6169–6181
Devrim Y, Erkan S, Bac N¸ Eroglu I (2012) Improvement of PEMFC performance with Nafion/inorganic nanocomposite membrane electrode assembly prepared by ultrasonic coating technique. Int J Hydrogen Energy 37:16748–16758
Kim DJ, Choi DH, Park CH, Nam SY (2016) Characterization of the sulfonated PEEK/sulfonated nanoparticles composite membrane for the fuel cell application. Int J Hydrogen Energy 41:5793–5802
Lemus J, Eguizabal A, Pina MP (2016) Endurance strategies for the preparation of high temperature polymer electrolyte membranes by UV polymerization of 1-H-3-vinylimidazolium bis(trifluoromethanesulfonyl)imide for fuel cell applications. Int J Hydrogen Energy 41:3981–3993
Noto VD, Boaretto N, Negro E, Giffin GA, Lavina S, Polizzi S (2012) Inorganic-organic membranes based on Nafion, [(ZrO2)$(HfO2)0.25] and [(SiO2)$(HfO2)0.28]. Part I: Synthesis, thermal stability and performance in a single PEMFC. Int J Hydrogen Energy 37:6199–6214
Noto VD, Boaretto N, Negro E, Stallworth PE, Lavina S, Giffin GA, Greenbaum SG (2012) Inorganic-organic membranes based on Nafion, [(ZrO2)-(HfO2)0.25] and [(SiO2)-(HfO2)0.28] nanoparticles. Part II: relaxations and conductivity mechanism. Int J Hydrogen Energy 37:6215–6227
Thampan T, Atwater T, Cook C, Novoa J, Sutorik AC (2016) Hydrogen generation from aluminum hydride for wearable polymer electrolyte membrane fuel cells. Int J Hydrogen Energy 41:9402–9409
Singh S, Modi A, Verma N (2016) Enhanced power generation using a novel polymer-coated nanoparticles dispersed-carbon micro-nanofibers-based air-cathode in a membrane-less single chamber microbial fuel cell. Int J Hydrogen Energy 41:1237–1247
Branchi M, Sgambetterra M, Pettiti I, Panero S, Navarra MA (2015) Functionalized Al2O3 particles as additives in proton-conducting polymer electrolyte membranes for fuel cell applications. Int J Hydrogen Energy 40:14757–14767
Elangovan M, Dharmalingam S (2016) Preparation and performance evaluation of poly (ether-imide) based anion exchange polymer membrane electrolyte for microbial fuel cell. Int J Hydrogen Energy 41:8595–8605
Mack F, Morawietz T, Hiesgen R, Kramer D, Gogel V, Zeis R (2016) Influence of the polytetrafluoroethylene content on the performance of high-temperature polymer electrolyte membrane fuel cell electrodes. Int J Hydrogen Energy 41:7475–7483
Wei Z, Su K, Sui S, He A, Du S (2015) High performance polymer electrolyte membrane fuel cells (PEMFCs) with gradient Pt nanowire cathodes prepared by decal transfer method. Int J Hydrogen Energy 40:3068–3074
Han Z, Yang Z, Han M (2016) Fabrication of metal-supported tubular solid oxide fuel cell by phase-inversion method and in situ reduction. Int J Hydrogen Energy 41:10935–10941
Seol JH, Wona JH, Yoon KS, Hong YT, Lee SY (2012) SiO2 Ceramic nanoporous substrate-reinforced sulfonated poly(arylene ether sulfone) composite membranes for proton exchange membrane fuel cells. Int J Hydrogen Energy 37:6189–6198
Wanga S, Shang Y, Wanga Y, Wanga J (2013) Fabrication and electrochemical performance of Poly (2,5-benzimidazole) (ABPBI)-based MEA by catalyst coated membrane (CCM) method for high-temperature polymer electrolyte fuel cells. Int J Hydrogen Energy 38:11060–11066
Zhong S, Cui X, Gao Y, Liu W, Dou S (2014) Fabrication and properties of poly(vinyl alcohol)-based polymer electrolyte membranes for direct methanol fuel cell applications. Int J Hydrogen Energy 39:17857–17864
Higa M, Hatemura K, Sugita M, Maesowa SI, Nishimura M, Endo N (2012) Performance of passive direct methanol fuel cell with poly(vinyl alcohol)-based polymer electrolyte membranes. Int J Hydrogen Energy 37:6292–6301
Gomes AS, Filho JCD (2012) Hybrid membranes of PVA for direct ethanol fuel cells (DEFCs) applications. Int J Hydrogen Energy 37:6246–6252
Chen Y, Guo R, Lee CH, Lee M, McGrath JE (2012) Partly fluorinated poly(arylene ether ketone sulfone) hydrophilicehydrophobic multiblock copolymers for fuel cell membranes. Int J Hydrogen Energy 37:6132–6139
Lee JR, Wona JH, Yoon KS, Hong YT, Lee SY (2012) Multilayer-structured, SiO2/sulfonated poly(phenylsulfone) composite membranes for proton exchange membrane fuel cells. Int J Hydrogen Energy 37:6182–6188
Filho AAMF, Gomes AS (2012) Sulfonated bisphenol-A-polysulfone based composite PEMs containing tungstophosphoric acid and modified by electron beam irradiation. Int J Hydrogen Energy 37:6228–6235
Fu X, Wang S, Xia Z, Li Y, Jiang L, Sun G (2016) Aligned polyaniline nanorods in situ grown on gas diffusion layer and their application in polymer electrolyte membrane fuel cells. Int J Hydrogen Energy 41:3655–3663
Chen HZ, Chung TS (2012) CO2-selective membranes for hydrogen purification and the effect of carbon monoxide (CO) on its gas separation performance. Int J Hydrogen Energy 37:6001–6011
George D, Suresh PV (2016) Detailed analysis of integrated steam ethanol reformer and high temperature polymer electrolyte membrane fuel cell. Int J Hydrogen Energy 41:1248–1258
Lee KS, Lee BS, Yoo SJ, Kim SK, Hwang SJ, Kim HJ, Cho EA, Henkensmeier D, Yun JW, Nama SW, Lim TH, Jang JH (2012) Development of a galvanostatic analysis technique as an in-situ diagnostic tool for PEMFC single cells and stacks. Int J Hydrogen Energy 37:5891–5900
Cho YH, Yoo SJ, Cho YH, Park HS, Park IS, Lee JK, Sung YE (2008) Enhanced performance and improved interfacial properties of polymer electrolyte membrane fuel cells fabricated using sputter-deposited Pt thin layers. Electrochim Acta 53:6111–6116
Springer TE, Zawodzinski TA, Gottesfel S (1991) Polymer electrolyte fuel cell model. J Electrochem Soc 138(8):2334–2342
Zhang L, Chae SR, Hendren Z, Park JS, Wiesner MR (2012) Recent advances in proton exchange membranes for fuel cell applications. Chem Eng J 204–206:87–97
Caglayan DG, Sezgin B, Devrim Y, Eroglu I (2016) Three-dimensional modeling of a high temperature polymer electrolyte membrane fuel cell at different operation temperatures. Int J Hydrogen Energy 41:10060–10070
Zhang Y, Verma A, Pitchumani R (2016) Optimum design of polymer electrolyte membrane fuel cell with graded porosity gas diffusion layer. Int J Hydrogen Energy 4:8412–8426
Kim DK, Koh JS, Kim MS, Song HH (2015) Experimental and computational study on the dynamic interaction between load variation and back pressure control in a polymer electrolyte membrane fuel cell for automotive application. Int J Hydrogen Energy 40:12370–12381
Torkavannejad A, Sadeghifar H, Pourmahmoud N, Ramin F (2015) Novel architectures of polymer electrolyte membrane fuel cells: efficiency enhancement and cost reduction. Int J Hydrogen Energy 40:12466–12477
Wang Z, Feng K, Li Z, Lu F, Huang J, Wu Y, Chu PK (2016) Self-passivating carbon film as bipolar plate protective coating in polymer electrolyte membrane fuel cell. Int J Hydrogen Energy 41:5783–5792
Feng K, Guo X, Li Z, Yao C, Wu Y (2016) Investigation of multi-coating process treated magnesium alloy as bipolar plate in polymer electrolyte membrane fuel cell. Int J Hydrogen Energy 41:6020–6028
Fecarotti C, Andrews J, Chen R (2016) A petri net approach for performance modelling of polymer electrolyte membrane fuel cell systems. Int J Hydrogen Energy1–19 (Accepted). http://dx.doi.org/10.1016/j.ijhydene.2016.05.138
Chippar P, Kyeongmin O, Kang K, Ju H (2012) A numerical investigation of the effects of GDL compression and intrusion in polymer electrolyte fuel cells (PEFCs). Int J Hydrogen Energy 37:6326–6338
Jereb LK, Sternig C, Fink C, Tatschl R (2016) Membrane degradation model for 3D-CFD analysis of fuel cell performance as a function of time. Int J Hydrogen Energy 1–13 (Accepted). http://dx.doi.org/10.1016/j.ijhydene.2016.05.229
Kozakai M, Date K, Tabe Y, Chikahisa T (2016) Improving gas diffusivity with bi-porous flow-field in polymer electrolyte membrane fuel cells. Int J Hydrogen Energy 1–10 (Accepted). http://dx.doi.org/10.1016/j.ijhydene.2016.05.131
Dhanushkodi SR, Capitanio F, Biggs T, Merida W (2015) Understanding flexural, mechanical and physicochemical properties of gas diffusion layers for polymer membrane fuel cell and electrolyzer systems. Int J Hydrogen Energy 40:16846–16859
Pinar FJ, Rastedt M, Pilinski N, Wagner P (2016) Effect of idling temperature on high temperature polymer electrolyte membrane fuel cell degradation under simulated start/stop cycling conditions. Int J Hydrogen Energy 1–12 (Accepted). http://dx.doi.org/10.1016/j.ijhydene.2016.05.091
Lee HJ, Kim BG, Lee DH, Park SJ, Kim Y, Lee JW, Henkensmeier D, Namb SW, Kim HJ, Kim H, Kim JY (2011) Demonstration of a 20 W class high-temperature polymer electrolyte fuel cell stack with novel fabrication of a membrane electrode assembly. Int J Hydrogen Energy 36:5521–5526
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Chatterjee, A., Hansora, D.P. (2017). Fuel Cells: Construction, Design, and Materials. In: Inamuddin, D., Mohammad, A., Asiri, A. (eds) Organic-Inorganic Composite Polymer Electrolyte Membranes. Springer, Cham. https://doi.org/10.1007/978-3-319-52739-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-52739-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52738-3
Online ISBN: 978-3-319-52739-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)