Abstract
Recent molecular and structural insights have helped to shed light on the embryological origins of the lymphatic vasculature. These discoveries have distinct implications, not only for molecular therapeutics in lymphatic vascular disease but also for the broad field of tumor biology and for the study of vascular malformations.
-
The lymphatic vessels appear substantially later than the blood vascular structures.
-
The lymphatics arise from aggregates of endothelial cells through the combined forces of vasculogenesis and angiogenesis. The earliest identifiable embryonic lymphatic precursors are the jugular lymph sacs, paired structures that are adjacent to the jugular section of the cardinal vein.
-
Both centrifugal and centripetal models for lymphatic vascular development have been proposed, and both likely play a role in mammalian biology.
-
Lymphatic vasculogenesis is thought to occur in four identifiably distinct stages: lymphatic competence, commitment, specification, and vascular coalescence and maturation.
-
Lymphangiogenesis is a critical pathway in embryonic development that has an important, clinically relevant counterpart in wound healing and inflammation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Recent molecular and structural insights have helped to shed light on the embryological origins of the lymphatic vasculature. These discoveries have distinct implications, not only for molecular therapeutics in lymphatic vascular disease but also for the broad field of tumor biology and for the study of vascular malformations.
-
The lymphatic vessels appear substantially later than the blood vascular structures.
-
The lymphatics arise from aggregates of endothelial cells through the combined forces of vasculogenesis and angiogenesis. The earliest identifiable embryonic lymphatic precursors are the jugular lymph sacs, paired structures that are adjacent to the jugular section of the cardinal vein.
-
Both centrifugal and centripetal models for lymphatic vascular development have been proposed, and both likely play a role in mammalian biology.
-
Lymphatic vasculogenesis is thought to occur in four identifiably distinct stages: lymphatic competence, commitment, specification, and vascular coalescence and maturation.
-
Lymphangiogenesis is a critical pathway in embryonic development that has an important, clinically relevant counterpart in wound healing and inflammation.
The lymphatic vasculature was first recognized by Gaspare Aselli more than three centuries ago, and the hypothesized embryonic origin of the lymphatic structures was initially investigated more than one century ago [6]; nevertheless, it only has been recently, during the era of molecular biology, that the mechanisms of mammalian lymphatic development have become increasingly well-understood [1, 2, 7].
Still a subject of some unresolved controversy, the embryological origin of the mammalian lymphatic system has been extensively explored over the last two decades. Recent molecular and structural insights have helped to shed light on this complex and important topic, which also has distinct implications, not only for molecular therapeutics in lymphatic vascular disease but also for the broad field of tumor biology. Furthermore, defects in the growth and development of lymphatic vessels (lymphangiogenesis) underlie numerous disorders, including vascular malformations, lymphedema, and lymphangiectasia [3].
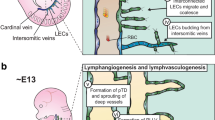
As integral elements of the mammalian circulation, the lymphatic conduits, like all vascular structures, arise from aggregates of endothelial cells, through the concerted forces of vasculogenesis and angiogenesis (◘ Fig. 4.1).
The embryonic development of the vasculature originates from mesodermally derived endothelial cell precursors, termed vasculogenesis. Subsequently, the developing vessels grow and remodel into a mature vascular network by endothelial sprouting and splitting, the process called angiogenesis (Adapted from Oliver [5])
The lymphatic vessels appear substantially later than the blood vascular structures [8]. In the human embryo, this occurs at 6–7 weeks, nearly 1 month after the appearance of the first blood vessels [9]. The earliest identifiable embryonic lymphatic precursors are the jugular lymph sacs, paired structures that are adjacent to the jugular section of the cardinal vein. The origin of these lymph sacs and their relationship to the adjacent cardinal vein has, until quite recently, been central to the theoretical controversy [4]. The «centrifugal» model, originally suggested by Florence Sabin, proposes that the primary lymph sacs arise from the endothelial cell population of the embryonic veins, with subsequent, continued endothelial sprouting from the lymph sacs into the surrounding tissues and organs.
The contrasting «centripetal» model of Huntington relies upon the contribution of mesenchymal precursor cells, termed lymphangioblasts, to give rise to the lymph sacs, a process that occurs independently of the veins.
Although there are lines of evidence to support elements of both of these theories, it seems that the centrifugal model more closely predicts the process in higher mammals.
Support for Sabin’s centrifugal model was initially provided by studies in Prox1-deficient mice [10, 11] and has subsequently been confirmed by others [12, 13]. Nevertheless, mesenchymal cells expressing CD31 and CD45, along with lymphatic endothelial markers (Prox1 and LYVE-1), have been observed in mouse embryos, suggesting that these cells might serve as lymph endothelial precursors [14] . This and other lines of investigation continue to support elements of the centripetal hypothesis [15, 16], particularly in the tissue-specific manner in which brain, cardiac, intestinal, visceral, and dermal lymphatics develop [2].
Prox1 is central to the centrifugal mechanism. This nuclear transcription factor is a homolog of the Drosophila homeobox transcription factor prospero 7 and serves as a master regulator of lymphatic development. The venous origin of the mammalian lymphatic vasculature has been demonstrated by lineage-tracing experiments [12] and supported by studies in zebrafish [13]. It has furthermore become apparent that CCBE1 is crucial for the initial stages of lymphatic vascular development [17, 18].
According to the current prevailing model, lymphatic vasculogenesis is thought to occur in four identifiably distinct stages: lymphatic competence, commitment, specification, and vascular coalescence and maturation (◘ Fig. 4.2).
Lymphatic vasculature development and growth. AM adrenomedullin, Ang angiopoietin, Angptl angiopoietin-like protein, E mouse embryonic day, FGF fibroblast growth factor, GH growth hormone, HGF hepatocyte growth factor, IGF insulin-like growth factor, Nrp2 neuropilin-2, PDGF platelet-derived growth factor, VEGF vascular endothelial growth factor (Reproduced with permission from Cueni and Detmar [4])
Lymphatic competence refers to the cellular capacity to respond to the initial induction signals for lymphatic vascular differentiation [5]. The priming of lymphatic endothelial cells (LECs) to initiate lymphatic development seems to depend on molecular signaling pathways that are distinct from those that direct blood vascular development [17, 18].
LEC competence is recognized through cellular expression of lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) [10, 19] and vascular endothelial growth factor receptor-3 (the flt4 gene encodes for VEGFR3) [4]. Mouse embryos that lack VEGFR3 expression die without lymphatic development. VEGF-C binding to VEGFR3 transduces signals that promote lymphatic endothelial cell survival, proliferation, and migration [20, 21]. Mouse embryos that lack VEGF-C do not develop lymph sacs [22]. VEGF-C/VEGFR3 signaling plays a key role during multiple stages of lymphatic vascular development; while VEGF-C signals can be transduced by means of multiple receptors, VEGFR3 is undoubtedly the most pivotal of these [3]. In addition, more recently, there has been demonstration of the potential for embryonic mechanosensitive activation of VEGFR3 independently of VEGF-C binding [23]. Various defined roles for VEGFR3 signaling in lymphatic vascular development and maturation are summarized in ◘ Table 4.1.
Lymphatic commitment is characterized developmentally and functionally by the expression of Prox1. The expression of this nuclear transcription factor is exclusive to cells of committed lymphatic lineage [10]. Prox1 expression shifts commitment of venous endothelial cells from the default blood vascular fate to a lymphatic lineage [11]. In mammals [2], the initiation of Prox1 expression in venous endothelial cells is dependent on the transcription factors Sox18 [24] and Nr2f2/Coup-TFII [25].
It seems that expression of Prox1 within the embryonic endothelial cell is necessary and sufficient for lymphatic commitment. Prox1-positive lymphatic endothelial.
cells, within both the cardinal and intersomitic veins, subsequently bud and migrate away from the veins in connected streams [2] to form an initial lymphatic plexus and, with further development, lymph sacs [26,27,28]. In addition, the expression of CCBE1 seems to be essential for the egress of the developing LECs.
Lymphatic endothelial cell specification entails the expression of the distinguishing molecular markers that lead to the unique lymphatic endothelial phenotype. As the newly developing LECs leave the veins, they express markers of LEC identity; these include podoplanin and higher levels of VEGFR3 and neuropilin-2 [26,27,28]. Through these developmental steps, the committed lymphatic cell population establishes complete autonomy from the local venous microenvironment. Peripheral migration occurs. Budding and migration precede the formation of primary lymph sacs throughout the embryo. Secondary budding and migration herald the final stages of lymphatic development. The cells thereby form capillaries in a centrifugal fashion, establishing the lymphatic vasculature throughout the bodily tissues and organs [4].
An important event in lymphatic development is the necessary separation between the flow of blood and lymph. A tyrosine kinase, Syk, and an adapter protein, Slp-76, are both critical for lymphaticovenous separation. Deficiency of either Syk or Slp76 has been shown to create abnormal connections between blood vessels and lymphatics, with resultant blood-filled lymphatics and chylous hemorrhage [29]. In the embryo, platelets aggregate at sites of lymphaticovenous connections, triggered by binding of LEC-specific podoplanin to C-type lectin receptor 2 (CLEC-2), which is specifically expressed in platelets; this leads to activation of Syk and Slp-76 [30, 31]. Inadequate megakaryocytes, platelets, podoplanin, CLEC-2, Syk, or Slp-76 in the mouse all can lead to blood-filled lymphatic vessels [2, 29,30,31,32,33,34,35,36].
After the appearance of the embryonic peripheral lymphatic vasculature, these vessels must experience substantial maturation and remodeling. One of the important maturational events is the development of the valve apparatus. A forkhead transcription factor, FOXC2, is highly expressed in adult lymphatic valves. It seems that FOXC2 specifies a collecting lymphatic vessel phenotype [37, 38]. Valve development is also dependent on GATA2 [39]: selective deletion of GATA2 in the murine lymphatic endothelium leads to major defects in valve structure and distended collecting lymphatic vessels. Additional signaling pathways, among them BMP [40], Notch [41], and semaphorin3a-neuropilin-1 [42], play important roles in valvular development.
The ephrins and the angiopoietins also contribute to lymphatic vascular maturation. In mutant mice, faulty expression of EphrinB2 leads to hyperplasia of the collecting lymphatics, absent valve formation, and failure of lymphatic capillary remodeling [43]. There is also a role for EphB4 forward signaling in lymphatic vessel valve development [44]. Angiopoietins 1 and 2 (Ang1 and Ang2) also participate in the maturation of the lymphatic vasculature [45,46,47]. In the lymphatics, Ang2 is a Tie2 receptor agonist, in contradistinction to its role in the blood vasculature [46]. Mice with a deletion of the Tie2 ligand Ang2 exhibit major defects in lymphatic vessel remodeling: their lymphatic vessels prematurely recruit smooth muscle cells and fail to develop valves [45, 46]. The Tie1 receptor also plays a critical role in early stages of lymphatic development [48, 49]. Lymphatic valve development apparently also requires normal expression of integrin-alpha9 and deposition of its ligand, fibronectin-EIIIA, in the extracellular matrix [50]. Reelin signaling apparently participates in vascular maturation, through its regulation of smooth muscle investment of the lymphatic collecting vessels [51].
All of these developmental events are interrelated and complex. New molecular participants in the process continue to be identified. Although lymphangiogenesis is
a critical pathway in embryonic development, it has a counterpart in wound healing
Highlighted References
Nakamura K, Rockson SG. Molecular targets for therapeutic lymphangiogenesis in lymphatic dysfunction and disease. Lymphat Res Biol. 2008;6(3–4):181–9.
Kazenwadel J, Harvey NL. Morphogenesis of the lymphatic vasculature: a focus on new progenitors and cellular mechanisms important for constructing lymphatic vessels. Dev Dyn. 2016;245(3):209–19.
Secker GA, Harvey NL. VEGFR signaling during lymphatic vascular development: from progenitor cells to functional vessels. Dev Dyn. 2015;244(3):323–31.
Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6(3–4):109–22.
Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4(1):35–45.
References
Kanter MA. The lymphatic system: an historical perspective. Plast Reconstr Surg. 1987;79(1):131–9.
Nakamura K, Rockson SG. Biomarkers of lymphatic function and disease: state of the art and future directions. Mol Diagn Ther. 2007;11(4):227–38.
Witte MH, Jones K, Wilting J, Dictor M, Selg M, McHale N, et al. Structure function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006;25(2):159–84.
van der Putte S. The development of the lymphatic system in man. Adv Anat Embryol Cell Biol. 1975;51:3–60.
Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21(3):318–22.
Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21(7):1505–13.
Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21(19):2422–32.
Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12(6):711–6.
Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, et al. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn. 2006;235(6):1554–62.
Pudliszewski M, Pardanaud L. Vasculogenesis and angiogenesis in the mouse embryo studied using quail/mouse chimeras. Int J Dev Biol. 2005;49(2–3):355–61.
Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, et al. Dual origin of avian lymphatics. Dev Biol. 2006;292(1):165–73.
Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41(4):396–8.
Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, et al. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res. 2011;109(5):486–91.
Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60(2):203–12.
Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73.
Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106(10):3423–31.
Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80.
Planas-Paz L, Strilic B, Goedecke A, Breier G, Fassler R, Lammert E. Mechanoinduction of lymph vessel expansion. EMBO J. 2012;31(4):788–804.
Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–7.
Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, et al. The nuclear hormone receptor coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010;24(7):696–707.
Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120(11):2340–8.
Francois M, Short K, Secker GA, Combes A, Schwarz Q, Davidson TL, et al. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol. 2012;364(2):89–98.
Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, et al. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32(5):629–44.
Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299(5604):247–51.
Bertozzi CC, Hess PR, Kahn ML. Platelets: covert regulators of lymphatic development. Arterioscler Thromb Vasc Biol. 2010a;30(12):2368–71.
Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010b;116(4):661–70.
Carramolino L, Fuentes J, Garcia-Andres C, Azcoitia V, Riethmacher D, Torres M. Platelets play an essential role in separating the blood and lymphatic vasculatures during embryonic angiogenesis. Circ Res. 2010;106(7):1197–201.
Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285(32):24494–507.
Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115(19):3997–4005.
Debrincat MA, Josefsson EC, James C, Henley KJ, Ellis S, Lebois M, et al. Mcl-1 and Bcl-x(L) coordinately regulate megakaryocyte survival. Blood. 2012;119(24):5850–8.
Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124(1):273–84.
Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185(3):439–57.
Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10(9):974–81.
Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Secker GA, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest. 2015;125(8):2979–94.
Levet S, Ciais D, Merdzhanova G, Mallet C, Zimmers TA, Lee SJ, et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122(4):598–607.
Murtomaki A, Uh MK, Choi YK, Kitajewski C, Borisenko V, Kitajewski J, et al. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development. 2013;140(11):2365–76.
Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, et al. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res. 2012;111(4):426–36.
Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19(3):397–410.
Zhang G, Brady J, Liang WC, Wu Y, Henkemeyer M, Yan M. EphB4 forward signalling regulates lymphatic valve development. Nat Commun. 2015;6:6625.
Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319(2):309–20.
Gale N, Thurston G, Hackett S, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–23.
Shimoda H, Bernas MJ, Witte MH, Gale NW, Yancopoulos GD, Kato S. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 2007;328(2):329–37.
D'Amico G, Korhonen EA, Waltari M, Saharinen P, Laakkonen P, Alitalo K. Loss of endothelial Tie1 receptor impairs lymphatic vessel development-brief report. Arterioscler Thromb Vasc Biol. 2010;30(2):207–9.
Qu X, Tompkins K, Batts LE, Puri M, Baldwin HS. Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development. 2010;137(8):1285–95.
Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17(2):175–86.
Lutter S, Xie S, Tatin F, Makinen T. Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J Cell Biol. 2012;197(6):837–49.
Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–97.
Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169(3):1080–7.
An A, Rockson SG. The potential for molecular treatment strategies in lymphatic disease. Lymphat Res Biol. 2004;2(4):173–81.
Acknowledgment
The author gratefully acknowledges Shauna Rockson for her artistic contribution to this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Rockson, S.G. (2018). Embryology of the Lymphatic System and Lymphangiogenesis. In: Lee, BB., Rockson, S., Bergan, J. (eds) Lymphedema. Springer, Cham. https://doi.org/10.1007/978-3-319-52423-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-52423-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52421-4
Online ISBN: 978-3-319-52423-8
eBook Packages: MedicineMedicine (R0)