Abstract
Small bowel diverticulitis is a less commonly discussed disease process when compared to colonic diverticulitis; however, the morbidity and mortality are significant. Affected patients tend to have a nonspecific presentation. Complications such as obstruction, hemorrhage and perforation range from 6 to 10%. Axiomatically, high clinical suspicious is critical. This chapter aims to review the common presenting symptoms, diagnostic workup and management of diverticulitis involving the small intestine.
Rare and usually asymptomatic, but be prepared when it rears its ugly head
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Duodenal diverticulitis
- Jejunoileal diverticulitis
- Meckel’s diverticulitis
- ERCP
- Diverticulectomy
- Sphincterotomy
Learning Objectives
-
1.
The learner will be able to formulate a differential diagnosis for small bowel diverticulitis.
-
2.
The learner will be able to describe the evaluation and management of small bowel diverticulitis based on anatomic location and symptomatology.
-
3.
The learner will be able to diagnose and treat symptomatic and incidental Meckel’s diverticula.
Case Scenario
A 67-year-old female presents to the Emergency Department with sudden onset, severe epigastric abdominal pain radiating to her back. Associated symptoms of subjective fevers, nausea and emesis are present. She has a past medical history of HTN, HLD, colonic diverticulosis and denies any surgical history. The patient does note vague intermittent symptoms of crampy abdominal pain, postprandial bloating and nausea for many years. Her vital signs are normal except for a tachycardia of 111. On physical examination, her abdomen is diffusely tender. Laboratory evaluation demonstrated WBC 23,000, BUN 20, and Cr 1.15. A CT scan of her abdomen and pelvis showed thickening of the wall of the duodenum, surrounding fat stranding and a small amount of extraluminal air. The patient was admitted to the medical surgical floor and treated nonoperatively with bowel rest, IV antibiotics and serial abdominal examinations for a contained duodenal diverticular perforation.
Epidemiology/Etiology/Pathophysiology
Diverticular disease of the small intestine is relatively uncommon. Due to its usual asymptomatic presentation, it is often an incidental finding or a diagnosis of exclusion. They are classified based on their location, whether they are congenital or acquired and whether they are true (consisting of all intestinal wall layers) or false diverticula (consisting of mucosa, submucosa and serosa). Forty-five percent of all small bowel diverticula are found in the duodenum, making it the most common location, followed by the ileum and then the jejunum. Less than 4% of small bowel diverticula cause symptoms with the incidence of diverticulitis being 0.3–2.5%.
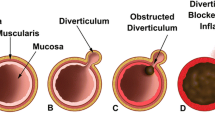
Acquired diverticula develop from dysregulated motility of the intestinal smooth muscle, leading to an increase in intraluminal pressure and herniation at a weakening in the intestinal wall. Bacterial overgrowth and stasis of intestinal transit may lead to a similar pathophysiologic process. Duodenal diverticula are usually solitary in nature and found between the ages 50 and 75. They are most commonly located in the periampullary region, which is within 2 cm of the ampulla in the second portion of the duodenum at the mesenteric border. Jejunoileal diverticula are usually larger in size, multiple and found in the sixth and seventh decade of life (Fig. 20.1).
A Meckel’s diverticulum is the most common true congenital malformation of the gastrointestinal tract. It is a result of the failure of complete obliteration of the omphalomesenteric duct during gestation. Inflammatory changes consistent with diverticulitis are present in 10–20% of those with a Meckel’s diverticulum and can sometimes be clinically indistinguishable from appendicitis.
Differential Diagnosis
Due to the nonspecific presentation associated with small bowel diverticula and their associated complications, the differential includes but is not limited to pancreatitis, cholecystitis, cholangitis, peptic ulcer disease, gastroenteritis and bowel obstruction.
Diagnosis
The diagnosis of small bowel diverticular disease and subsequent development of diverticulitis is often challenging. As with all presentations, a thorough history and physical examination is essential. Signs and symptoms may include nausea, vomiting, abdominal pain, fever and chills. Radiologic studies including plain abdominal films and ultrasound may be helpful in excluding other differential diagnoses. A CT scan of the abdomen is the imaging modality of choice for small bowel diverticulitis and may demonstrate thickening of the bowel wall and surrounding fat inflammation. In the presence of a perforation, extraluminal air or the extravasation of contrast may be visualized. In cases which CT imaging is not able to identify the etiology of a patient’s symptoms, additional studies include esophagogastroduodenoscopy, endoscopic retrograde cholangiopancreatography, fluoroscopic upper gastrointestinal swallow study, or small bowel follow-through may be utilized [1].
Complications
Small bowel diverticula may lead to complications consistent with inflammation, obstruction or hemorrhage. The incidence of these complications ranges from 6 to 10%. Inflammation consistent with diverticulitis may lead to perforation or bleeding, posteriorly into the retroperitoneal space and anteriorly into the peritoneal space or aorta. Mortality associated with complicated small bowel diverticulitis is reported to be as high as 40%.
Management
As with colonic diverticulitis, small bowel diverticulitis may be managed nonoperatively depending on the individual presentation of the patient. An uncomplicated episode of small bowel diverticulitis, including those with a contained perforation, may be treated with bowel rest, antibiotics, serial abdominal examinations and percutaneous drainage if an abscess is present. If the patient presents with hemodynamic instability, generalized peritonitis or failure of medical management, then operative intervention is required. The choice of surgical procedure will depend on the location of the diverticulum and intraoperative findings [2, 3].
Generally, operative intervention for symptomatic duodenal diverticula is reserved for cases not successfully treated with endoscopy (sphincterotomy and stent placement). A simple perforation with limited amount of inflammation may be amenable to a primary closure or diverticulectomy. This is performed after an extensive Kocher mobilization, if located in the duodenum, then primary closure (transversely or obliquely) in a one- or two-layer fashion with permanent suture and drainage of the repair (Fig. 20.2). In situations where a large defect is encountered, a defunctionalized segment of jejunum may be used as a serosal (Thal) patch.
If the diverticulum involves the ampulla of Vater, extensive inflammatory changes are present or it is not anatomically amenable to resection, a diversion procedure is indicated. Diversion may be accomplished with a gastrojejunostomy and pyloric exclusion, Billroth II reconstruction or Roux-en-Y gastrojejunostomy. A segmental resection with primary anastomosis may be utilized for rare cases located in the third or fourth segments of the duodenum or for jejunoileal diverticula. A pancreaticoduodenectomy may also be considered if the inflammation is too severe for safe diversion or if the diverticulum is involving the common bile duct or pancreatic ducts.
Lastly, it is generally agreed that in an adult, incidentally discovered Meckel’s diverticula ought to be left alone, whereas they are resected in a child. Relative indications for adult resections include palpable evidence of ectopic tissue, prior diverticulitis, hemorrhage, intussusception, or the presence of a mesodiverticular band.
Symptomatic Meckel’s diverticulitis (presenting with bleeding, obstruction or inflammation) is treated with surgical management including segmental resection (not simple diverticulectomy as ectopic tissue is often present on the antimesenteric side) and primary ileoileostomy [4]. Appendectomy is generally performed concurrently in order to avoid diagnostic confusion in the future.
References
Akhrass R, Yaffe MB, Fischer C, et al. Small-bowel diverticulosis: perceptions and reality. J Am Coll Surg. 1997;184(4):383–8.
Chiu EJ, Shyr YM, Su CH, et al. Diverticular disease of the small bowel. Hepatogastroenterology. 2000;47(31):181–4.
Kouraklis G, Glinavou A, Mantas D, et al. Clinical implications of small bowel diverticula. Imaj. 2002;4(6):431–3.
Zani A, Eaton S, Rees CM, et al. Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg. 2008;247:276–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Haynes, S. (2017). Small Bowel Diverticulitis. In: Dangleben, D., Madbak, F. (eds) Acute Care General Surgery . Springer, Cham. https://doi.org/10.1007/978-3-319-52255-5_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-52255-5_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52254-8
Online ISBN: 978-3-319-52255-5
eBook Packages: MedicineMedicine (R0)