Abstract
Purpose of Review
Small bowel diverticulosis is a well-known clinical entity whose diagnosis and management has evolved in recent years. This review covers pathophysiology, incidence, and prevalence, and it also provides an update on modern diagnosis and management. Meckel’s diverticula are covered elsewhere in this volume.
Recent Findings
CT scan and MRI have largely supplanted barium follow-through for diagnosis. No intervention is needed in asymptomatic individuals. Endoscopic management is playing an increasing role for both bleeding and resection of intraduodenal diverticula, but surgical intervention remains the only definitive intervention for other complications like diverticulitis and small bowel obstruction.
Summary
Small bowel diverticulosis is an uncommon condition which is associated with numerous possible complications. While endoscopy is playing an increasingly large role in management, surgical resection remains the treatment of choice for most complications. A high index of suspicion is needed in order to diagnose this entity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Small bowel diverticulosis is an uncommon but increasingly recognized cause of various chronic, nonspecific GI tract complaints and also has the potential for causing occasional serious complications. Due to the infrequency of the condition and the challenges associated with diagnosis, much of the existing knowledge about the condition is based on case reports and small case series. Our review will summarize existing knowledge about the condition and provide an overview of diagnostic strategies and management. Meckel’s diverticula are covered elsewhere in this volume and are thus not discussed in this review.
Pathogenesis
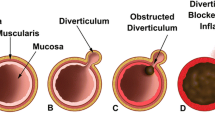
Similar to colonic diverticulosis (but unlike Meckel’s diverticulum), small bowel diverticula contain only mucosal and submucosal layers (and variably, a thin serosal layer) herniating through the muscularis layer and are thus classified as pseudodiverticula [1]. They are thought to arise during periods of increased intraluminal pressure, possibly in the setting of inflammation [2], or intestinal dyskinesia leading to localized areas of high pressure within the small bowel [3, 4]. Evidence of neuromuscular dysfunction of the small bowel was noted in one study that examined histology of small bowel diverticula [5••]. Histologic abnormalities included abnormal myenteric plexus with degeneration of neurons and axons resembling that seen in familial visceral neuropathy, smooth muscle fibrosis resembling that seen in progressive systemic sclerosis, and abnormal smooth muscle cells with degenerated appearance resembling that of visceral myopathy [5••]. Radiographic studies of individuals with small bowel diverticulosis also suggest underlying motor dysfunction as evidenced by small bowel dilation and excess intraluminal fluid and gas [5••].
Duodenal diverticula typically arise along the pancreatic border, distal to the bulb [6]. Periampullary diverticula (PAD), which account for approximately three fourths of all duodenal diverticula [7], are a specific type of duodenal diverticulum occurring within 2–3 cm of the ampulla of Vater or involving the ampulla [7, 8], Fig. 1. In addition to abnormal motility and focal weakness of the duodenal wall, traction of the bile duct on the duodenal wall [8] or sphincter of Oddi dysfunction [7] may also contribute to the formation of PAD.
Jejunoileal diverticula typically arise on the mesenteric border of the small bowel, at locations of weakness of the muscularis layer, generally at sites of entry of vasa recta [3, 9, 10], though location on the anti-mesenteric border has also been described [11••, 12]. Due to their location on the mesenteric border, jejunoileal diverticula can often be located between leaves of the mesentery [11••], which can complicate radiographic visualization and identification. Jejunal diverticula range in size, usually from 1 to 4 cm, but can be as large as 8–9 cm [11••]. Ileal diverticula are generally smaller, ranging from 2 to 15 mm [12].
Prevalence
Small bowel diverticulosis is widely regarded to be an uncommon condition, though there is significant variance in specific estimates of its prevalence. Cited incidence ranges from 0.2 to 4.5% in autopsy studies [13] and 0.5 to 2.3% in small bowel contrast studies [9]. ERCP and EUS studies (which specifically assessed for PAD) have cited incidence ranging from 0.16 to 27% on ERCP [7] and 7.5% on EUS [14••]. This condition can be seen in individuals of all ages, but is more prevalent in adults over age 50 [3, 15]. Diverticulosis is more common in the duodenum compared with the jejunum or ileum; one retrospective review of 208 cases of small bowel diverticulosis noted duodenal location in 79% [4]. An early large case series noted that among diverticula distal to the duodenum, approximately 80% were in the jejunum [11••]. The increased prevalence in the jejunum compared with the ileum is thought to be due to the presence of larger penetrating blood vessels in the proximal jejunum [3]. While small bowel diverticulosis can be solitary [2], most patients have other diverticula elsewhere in the alimentary tract, usually in the colon [15, 16]. An early case series noted approximately 2:1 male predominance of jejunoileal diverticulosis [11••].
Most studies report a decreasing incidence of diverticula in the more distal small bowel [4, 17,18,19], higher prevalence with age [4, 11••, 18], and male prevalence [18]. However, prior studies have also reported no evidence of male predilection [4, 12] and increased rather than decreased incidence in the distal small bowel [12].
Diagnosis
Radiographic imaging of small bowel diverticulosis is currently the test of choice, though it has been suggested that laparotomy is the true gold standard way of diagnosing the condition [9]. Prior to CT and MRI studies, diagnosis was usually made on the basis of barium contrast follow-through studies. Currently, diagnosis is typically made on the basis of abdominal CT scan or MRI. Common CT scan findings include discrete round or ovoid small bowel outpouchings outside the small bowel lumen and without any recognizable small bowel folds, which may contain air, fluid, or contrast [20]. Recent studies have suggested that MR enterography is an effective modality in diagnosis [19], Fig. 2. In the setting of a suspected complication of small bowel diverticulosis, CT scan is considered the preferred diagnostic tool currently, particularly in assessing for diverticulitis [17, 19]. There is no specific CT or MR protocol for detection of small bowel diverticulosis [21]. Both oral and IV contrast are generally preferred, except in cases of acute bleeding, where administration of oral contrast may complicate subsequent endoscopic or angiographic attempts to isolate and control hemorrhage. Small bowel endoscopy (either capsule endoscopy or double balloon endoscopy) has the highest diagnostic yield in diagnosing bleeding secondary to small bowel diverticulosis [22••].
Despite the utility of CT or MRI in the diagnosis of small bowel diverticulosis, diagnosis can still be challenging, as diverticula may be difficult to distinguish from gas or fluid filled loops of small bowel [20]. Diagnosis often requires a degree of clinical suspicion on the part of the radiologist. One study retrospectively examined the records of 28 patients who carried a diagnosis of small bowel diverticulosis on the basis of a barium study (either small bowel follow through or small bowel enema) and underwent a CT scan as well. In this study, the diagnosis of small bowel diverticulosis was made on the initial read of only 7% of CT scans, but on retrospective CT scan review by two radiologists, the diagnosis was made on 75% of these studies [20].
Complications
Most patients with small bowel diverticulosis are asymptomatic [9, 22••] and are often identified on imaging studies aimed at other conditions. Jejunoileal diverticulosis is more likely than duodenal diverticulosis to be associated with chronic symptoms as well as acute complications [4, 23, 24]. Bleeding is thought to be the most common complication of duodenal diverticula, while diverticulitis has been cited as the most common complication of jejunal and ileal diverticulosis [4, 12]. Jejunal diverticula are thought to be more prone to complications than ileal diverticula [12, 17]. Small bowel diverticulosis is also associated with many more serious complications. Cited rates of complications in large case series have ranged from approximately 10–20% [4, 11••]. Common complications of small bowel diverticulosis are summarized in Table 1.
Chronic Symptoms and Bacterial Overgrowth
Small bowel diverticulosis can be associated with nonspecific symptoms like chronic abdominal pain, dyspepsia, nausea/vomiting, borborygmi, and altered bowel habits [3, 9, 19, 25]. These nonspecific symptoms may arise in part due to diverticular distension, which in turn may result from underlying intestinal dyskinesia [4]. The lack of specificity of symptoms and lack of any one pathognomonic clinical feature make diagnosis challenging [26]. There is no correlation between the size of diverticula and the degree of symptoms [9].
Bacterial overgrowth may also be the cause of nonspecific symptoms in small bowel diverticulosis. In addition, bacterial overgrowth can also be associated with vitamin deficiencies. Vitamin B12 deficiency can occur due to disturbances in proximal small bowel pH and resulting inactivation of intrinsic factor by bacterial flora specific to these diverticula [27]. Stasis of intestinal contents can also lead to anaerobic bacterial growth and deconjugation of bile salts, with possible subsequent development of fat soluble vitamin deficiency [9].
Diverticulitis
Diverticulitis is the most common complication of small bowel diverticulosis [18, 25]. While this complication typically presents as acute abdomen, recognition can be challenging, as it frequently presents in a manner similar to more common conditions like biliary colic, renal colic, sigmoid diverticulitis, and pancreatitis [23]. Furthermore, due to the duodenum’s retroperitoneal location distal to the bulb, diverticulitis of this segment of the small bowel may result in less peritoneal irritation, further complicating clinical recognition [6]. With jejunoileal disease, diverticula are usually located on the mesenteric side of the bowel, and associated diverticulitis is often associated with focal inflammation of the adjacent mesenteric fat [19]. While surgical resection is the only definitive treatment, nonoperative management can be considered in cases where only localized peritonitis is noted [1, 3, 9].
Hemorrhage
Bleeding is a recognized complication of duodenal diverticulosis [4] and is being increasingly recognized in more distal small bowel diverticular disease as well. In a retrospective study of 28 individuals [22••], 7.1% mortality was noted [22••]. As previously noted, small bowel endoscopy has shown good diagnostic yield in cases of bleeding. Brisk bleeding may also be detected on traditional angiography or CT angiography, while slower bleeding may be detected on nuclear medicine bleeding scan. Multidetector CT scan can also be a useful test in the setting of suspected small bowel bleeding, as it has high diagnostic yield for small bowel neoplasia, which can give bleeding symptoms similar to that of small bowel diverticulosis [28].
Obstruction
While a less common complication, there are numerous potential causes of obstruction, which include compression from a distended diverticulum, stricture or adhesion formation from prior diverticulitis, intussusception (with a diverticulum serving as lead point), volvulus of a diverticulum bearing segment of small bowel, enterolith formation and impaction, or nonmechanical obstruction due to intestinal dyskinesia [9, 13, 29].
Pancreaticobiliary Disorders
Given their location, periampullary diverticula can cause obstructive jaundice by mechanical compression of the common bile duct (an entity often referred to by the eponym Lemmel’s syndrome) [30, 31], particularly when the papilla is located within a diverticulum [31]. Periampullary diverticula are also a risk factor for bacteriobilia, and colonization with β-glucouronidase producing bacteria can lead to subsequent deconjugation of bilirubin and formation of calcium bilirubinate stones [7]. PAD have also been associated with gallbladder stones [32], CBD stones [32, 33], and idiopathic pancreatitis [7, 33]. Some of these associations are controversial, with one study showing no increase in the risk of acute or chronic pancreatitis with PAD [32]. A recent large retrospective study of 2475 patients using EUS to assess the relationship between PAD and pancreaticobiliary disease noted an association between PAD and cholangitis, dilated CBD, and choledocholithiasis, but no association between PAD and obstructive jaundice, acute or chronic pancreatitis, and recurrent pancreatitis [14••].
Given their proximity to (and frequent involvement of) the ampulla, PAD can complicate successful biliary cannulation during ERCP [8, 32], particularly when the ampulla is located within a diverticulum [7]. They may also lead to an increased risk of postsphincterotomy bleeding [32], though another study showed no such association [8]. Recent studies, however, have shown that techniques like needle knife fistulotomy have led to comparable cannulation success rates even in those with PAD [34]. PAD can cause filling defects on cholangiography that can be confused with periampullary tumor or CBD stone [7].
Management
No treatment is required for asymptomatic small bowel diverticulosis [18, 29]. In cases of diverticulitis, the only definitive intervention is small bowel resection with primary anastomosis [13, 16, 26]. Nonsurgical management can be considered in certain cases [15], generally consisting of broad spectrum antibiotics and close observation for development of perforation or other complication. In those cases responding to supportive management, surgery to prevent recurrence is not recommended [10], as diverticulitis may recur in a different location. Similarly, surgery remains the definitive treatment in cases of severe bleeding. In the setting of overt GI bleeding in which small bowel diverticulosis is the only possible causative lesion noted, it has been recommended to pursue surgical resection [13]. However more recently, successful interventions with argon plasma coagulation and endoclip placement have also been noted [22••]. The increasing availability of double balloon enteroscopy (or other deep enteroscopy) has led to increased utilization of endoscopic control of bleeding rather than surgical management.
Intraluminal Duodenal Diverticulum
Intraluminal duodenal diverticulum (IDD), also known as “Windsock diverticulum” is a specific type of duodenal diverticulum that forms by a different mechanism than traditional small bowel diverticula. This type of diverticulum is thought to form in individuals with a congenital duodenal web or diaphragm (forming due to incomplete foregut recanalization during embryonic development). Over the course of decades, continued peristalsis leads to the formation of a pulsion diverticulum [35••, 36] (Fig. 3), sometimes large enough to lead to clinically significant compression of the true duodenal lumen [35••]. These diverticula almost always form in the second part of the duodenum, just distal to the ampulla of Vater [35••]. Unlike other small bowel diverticula, IDD have epithelial lining on both surfaces [35••, 36] and involve 3 layers of the duodenum [36]. Endoscsopic identification is challenging, as these lesions can commonly be confused with other lesions like choledochocele, periampullary cystic mass, or duodenal duplication cyst [37]. Additionally, a collapsed diverticulum may not be noted [36], or an inverted diverticulum may be mistaken for a large polyp [38]. Thus, a combination of endosopy and radiographic imaging (such as upper GI series or CT scan) is often needed in order to confirm the diagnosis [36,37,38].
Intraluminal duodenal diverticulum, visualized on endoscopy. Arrow indicates false lumen, and circle represents body of diverticulum extending into true lumen. Reproduced with permission from: Anand et al. Two cases of intraluminal “Windsock” diverticula resulting in partial duodenal obstruction. ACG Case Rep J. 2016 Sep 28;3 [4]:e135. [36]. Used with permission from Wolters Kluwer Health
Due to the amount of time taken for Windsock diverticula to form, symptoms (when present) usually do not come on until the third or fourth decade of life. As is the case with traditional small bowel diverticula, symptoms most commonly include nausea, vomiting, postprandial pain, and early satiety [35••, 39], while more serious complications like bleeding [38], partial or complete obstruction [36], and biliary obstruction and possible pancreatitis [37] have been described as well. Gold standard treatment is surgical duodenotomy and diverticulectomy. More recently, however, endoscopic intervention with high success rates has also been described [35••, 39]. While there is significant variation in the specifics of described techniques, most techniques involve either diverticulectomy (with endoscopic removal of the diverticulum, and subsequent incision of the bridge separating diverticular from duodenal lumen) or diverticulotomy (with longitudinal cut made along the diverticulum, often with needle knife papillotome or standard sphinctertome) in order to create a common lumen for both the diverticulum and true duodenum) [35••]. IDD are noted to be very vascular, and delayed bleeding is a noted delayed complication of resection [35••, 39].
Conclusion
Small bowel diverticulosis is a relatively infrequent entity that can cause both acute and chronic gastrointestinal symptoms. While diagnostic and therapeutic modalities have advanced significantly since this disorder was first described (for example, with imaging modalities like enterography now playing a major role in diagnosis, and therapeutic modalities like deep enteroscopy available for treating bleeding complications), a high degree of clinical suspicion is needed for both timely diagnosis and treatment, particularly in elderly individuals in whom this condition is most common.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Kassir R, Boueil-Bourlier A, Baccot S, Abboud K, Dubois J, Petcu CA, et al. Jejuno-ileal diverticulitis: etiopathogenicity, diagnosis and management. Int J Surg Case Rep. 2015;10:151–3.
Ardelt M, Rauchfuss F, Fahrner R, Dondorf F, Hallof G, Ludewig S, et al. Complicated Jejunal diverticulosis: a retrospective single-center evaluation and a possible explanation of pathogenesis. Am Surg. 2017;83(11):e455–8.
Alves Martins BA, Rodrigues Galletti R, Marinho Dos Santos Neto J, Neiva Mendes CA. Case of perforated jejunal diverticulum: an unexpected cause of pneumoperitoneum in a patient presenting with an acute abdomen. Am J Case Rep. 2018;19:549–52.
Akhrass R, Yaffe MB, Fischer C, Ponsky J, Shuck JM. Small-bowel diverticulosis: perceptions and reality. J Am Coll Surg. 1997;184(4):383–8.
•• Krishnamurthy S, Kelly MM, Rohrmann CA, Schuffler MD. Jejunal diverticulosis. A heterogenous disorder caused by a variety of abnormalities of smooth muscle or myenteric plexus. Gastroenterology. 1983;85(3):538–47 Describes common histologic findings in small bowel diverticulosis, with associated insight into underlying pathogenesis.
Miller RE, McCabe RE, Salomon PF, Knox WG. Surgical complications of small bowel diverticula exclusive of Meckel’s. Ann Surg. 1970;171(2):202–10.
Lobo DN, Balfour TW, Iftikhar SY, Rowlands BJ. Periampullary diverticula and pancreaticobiliary disease. Br J Surg. 1999;86(5):588–97.
Jayaraj M, Mohan BP, Dhindsa BS, Mashiana HS, Radhakrishnan G, Dhir V, et al. Periampullary diverticula and ERCP outcomes: a systematic review and meta-analysis. Dig Dis Sci. 2019;64(5):1364–76.
Makris K, Tsiotos GG, Stafyla V, Sakorafas GH. Small intestinal nonmeckelian diverticulosis. J Clin Gastroenterol. 2009;43(3):201–7.
Lebert P, Ernst O, Zins M. Acquired diverticular disease of the jejunum and ileum: imaging features and pitfalls. Abdom Radiol (NY). 2019;44(5):1734–43.
•• Benson RE, Dixon CF, Waugh JM. Nonmeckelian diverticula of the jejunum and ileum. Ann Surg. 1943;118(3):377–93 Classic large case series providing insight into prevalence and associated complications that has been corroborated in numerous subsequent studies.
Parulekar SG. Diverticulosis of the terminal ileum and its complications. Radiology. 1972;103(2):283–7.
Longo WE, Vernava AM. Clinical implications of jejunoileal diverticular disease. Dis Colon Rectum. 1992;35(4):381–8.
•• Bruno M, Ribaldone DG, Fasulo R, Gaia S, Marietti M, Risso A, et al. Is there a link between periampullary diverticula and biliopancreatic disease? An EUS approach to answer the question. Dig Liver Dis. 2018;50(9):925–30 Large study providing detailed data on specific pancreaticobiliary complications noted on EUS, and whether these complications are significantly associated with periampullary diverticulosis.
Lebert P, Millet I, Ernst O, Boulay-Coletta I, Corno L, Taourel P, et al. Acute Jejunoileal diverticulitis: multicenter descriptive study of 33 patients. AJR Am J Roentgenol. 2018;210(6):1245–51.
Yaqub S, Evensen BV, Kjellevold K. Massive rectal bleeding from acquired jejunal diverticula. World J Emerg Surg. 2011;6:17.
Choi JJ, Ogunjemilusi O, Divino CM. Diagnosis and management of diverticula in the jejunum and ileum. Am Surg. 2013;79(1):108–10.
Mantas D, Kykalos S, Patsouras D, Kouraklis G. Small intestine diverticula: is there anything new? World J Gastrointest Surg. 2011;3(4):49–53.
Mansoori B, Delaney CP, Willis JE, Paspulati RM, Ros PR, Schmid-Tannwald C, et al. Magnetic resonance enterography/enteroclysis in acquired small bowel diverticulitis and small bowel diverticulosis. Eur Radiol. 2016;26(9):2881–91.
Fintelmann F, Levine MS, Rubesin SE. Jejunal diverticulosis: findings on CT in 28 patients. AJR Am J Roentgenol. 2008;190(5):1286–90.
Transue DL, Hanna TN, Shekhani H, Rohatgi S, Khosa F, Johnson J-O. Small bowel diverticulitis: an imaging review of an uncommon entity. Emerg Radiol. 2017;24(2):195–205.
•• Yen HH, Chen YY, Yang CW, Soon MS. Diagnosis and management of jejunoileal diverticular hemorrhage: a decade of experience. J Dig Dis. 2012;13(6):316–20 Detailing the evolving role of small bowel endoscopy in evaluation and treatment of small bowel diverticular hemorrhage.
Lacz NL, Zurlo JV. Small bowel diverticulitis: an often overlooked cause of acute abdomen. Emerg Radiol. 2010;17(6):497–501.
Kouraklis G, Glinavou A, Mantas D, Kouskos E, Karatzas G. Clinical implications of small bowel diverticula. Isr Med Assoc J. 2002;4(6):431–3.
Ceuppens A-S, Dhont S, Sneyers B, Schepers C, Ramboer K, Van Hootegem P. Jejuno-ileal diverticulosis : a review of literature. Acta Gastroenterol Belg. 2018;81(4):517–9.
Karas L, Asif M, Chun V, Khan FA. Complicated small bowel diverticular disease: a case series. BMJ Case Rep. 2017;23:2017.
Cooke WT, Cox EV, Fone DJ, Meynell MJ, Gaddie R. The clinical and metabolic significance of jejunal diverticula. Gut. 1963;4:115–31.
Yen H-H, Chen Y-Y, Yang C-W, Liu C-K, Soon M-S. Clinical impact of multidetector computed tomography before double-balloon enteroscopy for obscure gastrointestinal bleeding. World J Gastroenterol. 2012;18(7):692–7.
Tiwari A, Gupta V, Hazrah P, Lal R. A rare case of multiple jejunal diverticulosis presenting as intestinal obstruction. Clin Pract. 2013;3(2):e21.
Tobin R, Barry N, Foley NM, Cooke F. A giant duodenal diverticulum causing Lemmel syndrome. J Surg Case Rep. 2018;2018(10):rjy263.
Rouet J, Gaujoux S, Ronot M, Palazzo M, Cauchy F, Vilgrain V, et al. Lemmel’s syndrome as a rare cause of obstructive jaundice. Clin Res Hepatol Gastroenterol. 2012;36(6):628–31.
Zoepf T, Zoepf DS, Arnold JC, Benz C, Riemann JF. The relationship between juxtapapillary duodenal diverticula and disorders of the biliopancreatic system: analysis of 350 patients. Gastrointest Endosc. 2001;54(1):56–61.
Leivonen MK, Halttunen JA, Kivilaakso EO. Duodenal diverticulum at endoscopic retrograde cholangiopancreatography, analysis of 123 patients. Hepatogastroenterology. 1996;43(10):961–6.
Park CS, Park CH, Koh HR, Jun CH, Ki HS, Park SY, et al. Needle-knife fistulotomy in patients with periampullary diverticula and difficult bile duct cannulation. J Gastroenterol Hepatol. 2012;27(9):1480–3.
•• Law R, Topazian M, Baron TH. Endoscopic treatment of intraluminal duodenal (“windsock”) diverticulum: varying techniques from five cases. Endoscopy. 2012;44(12):1161–4 Detailing common novel endoscopic techniques for managing intraduodenal diverticulum.
Anand V, Provost J, Bakr M, Bach C, Merchant P, Brown C, et al. Two cases of intraluminal “windsock” diverticula resulting in partial duodenal obstruction. ACG Case Rep J. 2016;3(4):e135.
Karagyozov P, Tishkov I, Georgieva Z, Boeva I, Tzankov D. Intraluminal duodenal (“windsock”) diverticulum: a rare cause of biliary obstruction and acute pancreatitis in the adult. Endosc Int Open. 2019;7(1):E87–9.
Eusébio M, Ramos A, Guerreiro H. Intraluminal duodenal (“windsock”) diverticulum: a rare cause of gastrointestinal bleeding. GE Port J Gastroenterol. 2016;23(2):113–5.
Bhalla S, Yu J, Law R. Management of a windsock diverticulum by the use of novel submucosal dissection scissors. VideoGIE. 2019;4(6):247–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Small Intestine
Rights and permissions
About this article
Cite this article
Rangan, V., Lamont, J.T. Small Bowel Diverticulosis: Pathogenesis, Clinical Management, and New Concepts. Curr Gastroenterol Rep 22, 4 (2020). https://doi.org/10.1007/s11894-019-0741-2
Published:
DOI: https://doi.org/10.1007/s11894-019-0741-2