Abstract
Slags play an important role in blast furnace operation, and their compositions are based on the CaO–SiO2–MgO–Al2O3 quaternary system. However, there is not a clear understanding of the effects of gangue on of the formation of FeO-bearing primary-slags process in blast furnace. In this work, the softening and dripping experiments under the blast furnace conditions are designed to explore the influences of CaO-/SiO2-/MgO-/Al2O3 on the softening and melting properties of FeO respectively. The results indicate that additions of CaO or Al2O3 decrease the starting softening temperature and no dripping behaviors are found in comparison with the base case results when only FeO is used. On the contrary, the addition of SiO2 or MgO rises the starting and ending softening temperature, as well as the dripping temperature. The lowest maximum pressure drop is obtained in the case with addition of SiO2. According to XRD analysis results, the initial phase with CaO addition in the primary-slags should be CaO·Fe3O4 and that with SiO2, MgO, Al2O3 additions are fayalite (2FeO·SiO2), magnesioferrite (MgO·FeO), hercynite (FeO·2Al2O3), respectively.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The cohesive zone inside blast furnace, where the ferrous burden softens and melts, significantly affects the productivity of the blast furnace [1]. A Reduction Degree (RD) of a ferrous burden is typically above 50% in the cohesive zone area of a BF [2, 3]. At this point the iron bearing burden consists of metallic iron (Fe), wustite (FeO) and other oxides (CaO, SiO2, MgO, Al2O3 etc.) which form a slag phase. The phase compositions are dependent on the chemistry of the iron bearing burden [4,5,6,7,8]. Many researchers have found that the softening and melting properties of ferrous materials are controlled by reduction degree, basicity, slag viscosity, gangue content and their distribution in the microstructure [9,10,11,12].
However, though various studies are conducted to research the properties of blast furnace slags, no satisfactory knowledge has been acquired about gangue on of the formation of FeO-bearing primary-slags process in blast furnace. Because the mixture between sinter and lump ores in blast furnace was not simply physical mixing, it is of great significance to study the influences of CaO-/SiO2-/MgO-/Al2O3 on the softening and melting properties of FeO respectively [13,14,15]. In the present work, the softening and dripping experiments under the blast furnace conditions are designed to explore the influences of respective CaO-/SiO2-/MgO-/Al2O3 on the softening and melting properties of FeO. The chemical compositions, liquidus temperature of primary-slags were also studied.
Experimental
Experimental Method
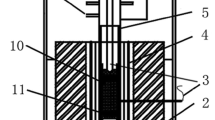
The samples were prepared from CaCO3 (≥99.9%), SiO2(≥99.9%), MgO(≥99.9%), Al2O3(≥99.9%) and FeC2O4·2H2O (≥99.9%) and the powdery CaCO3/SiO2/MgO/Al2O3 were uniformly mixed with FeC2O4·2H2O respectively with a 70:10:10:5:5 (FeO:CaO:SiO2:MgO:Al2O3) mass ratio and then pressed to cylindrical shape samples. The samples were finally roasted to 1173 K (900 °C) with a triple-fired furnace in the inert gas atmosphere (N2, 99.9%). This temperature was held for 1 h, in order to make sure the complete decomposition of FeC2O4·2H2O. Finally, the samples were cooled to the room temperature in the inert gas atmosphere. Both the above mentioned stages were accomplished in the softening-melting equipment with the schematic diagram shown in Fig. 1.
Samples having a layer thickness of 50 mm were charged in a graphite crucible and cokes with a layer thickness of 15 mm were placed over and below the samples. The iron ore samples are 10–15 mm in diameter and the cokes are 6–10 mm in diameter. Subject to the condition of constant layer thickness, the charge of the iron ore samples weighted different due to the difference of density. The inner diameter of the graphite crucible is about 50 mm.
Experimental conditions for the softening and dripping behavior are shown in Fig. 2. The heating up rate is 10 °C/min below 900 °C and 5 °C/min over 900 °C to make sure the iron ore samples are adequately reduced after 900 °C. Gas flow is 5 L/min of N2 below 900 °C and 10 L/min of reducing gas over 900 °C, the composition of reducing gas is CO:N2 in mole fraction = 40:60(%). The load of 1 kg/cm2 is added to the samples in the whole process. The experiment was stopped when pressure drop reached the maximum. The samples are cooled down to room temperature by N2 with the flow rate 5 L/min. In this work, a series of five experiments to determine the impact of CaO-/SiO2-/MgO-/Al2O3 to the softening and melting properties of samples, the chemical compositions of the samples for softening and melting experiment as listed in Table 1.
Results and Discussion
Softening and Melting Properties of Base Case
A typical set of softening and dripping test results is shown in Fig. 3, in which pressure drop, temperature and shrinkage rate are conducted to evaluate the primary slags formation behaviors of iron ore samples. It is difficult to observe the behavior directly as the internal of the softening-melting equipment is not visible. Then some indexes are conducted to evaluate the primary-slags formation behavior of iron ores. T10% is the temperature when the shrinkage rate of the samples reaches 10%, indicating the samples start to soften, softening end temperature (T40%) is the temperature when the shrinkage of the samples reaches 40%. The temperature interval (T40%–T10%) represents the softening zone of samples. Tm is the temperature when the pressure drop of the samples reaches 0.98 kPa, meaning the samples begin to melt. Tp is the temperature when the pressure drop of the samples reaches the highest (ΔPmax), meaning the primary–slags are totally produced. Td is the temperature at which dripping starts and the temperature interval (Td–T10%) reflect the thickness of the cohesive zone.
To study the whole process of the high temperature behaviors of iron ores and gain the primary-slags at Tp, every test contains two steps. Firstly, the whole process of samples from room temperature to dripping finished is conducted. Secondly, stop heating the samples at Tp and cool down the samples to room temperature under the protection of pure N2.
The results of the softening and melting properties of base case were shown in Table 2. The results show that when sample is pure FeO, the softening zone is 111 °C, thickness of the cohesive zone is 652 °C, and the dripping temperature is 1436 °C.
Effects of the Samples Composition on Softening and Melting Properties
In order to study the impact of CaO/SiO2/MgO/Al2O3 on softening and melting properties of iron ores, the samples composition was changed as listed in Table 1.
Table 3 shows the softening and melting properties of samples. For the respective samples, the starting and finishing softening temperature of sample B and C higher than base case and the the softening temperature interval are also wider than base case, of which the sample B temperature is the highest. Figure 4 shows the starting softening temperature of sample D is lower than pure FeO, but the finishing softening temperature is higher than base case, and softening temperature interval is extremely wide. For the sample A, both the starting and finishing softening temperature are lower than base case. While the dripping start temperature of sample B is higher (1525 °C) than base case, indicated that addition of SiO2 increase the softening temperature and dripping start temperature as well as the dripping temperature. Although the maximum pressure drop of the case with the addition of SiO2 decreases to 1.5 kPa, the Tp is the highest among all the cases. The addition of CaO, MgO and Al2O3 have no dripping temperature.
To further study the influence of CaO–SiO2–MgO–Al2O3 on the formation behavior of FeO-bearing primary-slags , the primary-slags samples of A, B, C and D were analyzed through X-Ray diffraction. Figure 5 shows the diffraction patterns of primary-slags of samples. The main primary-slags phases of CaO additions are CaO·Fe3O4 (liquidus temperature 1104 °C) and FeO, and that with SiO2, MgO, Al2O3 additions are fayalite (2FeO·SiO2, 1889 °C), magnesioferrite (MgO·FeO, 1791 °C), hercynite (FeO·2Al2O3, 1780 °C), respectively. The results of the liquidus temperature of the slags were obtained from FactSage6.2. The changes in the softening temperature of the slags are owing to the new compounds generated when compositions changed. Therefore, the decreasing softening temperature of the primary slag is mainly caused by its high CaO. While the slag has a higher SiO2 and higher MgO content, the softening temperature is increased, because it will produce high liquidus temperature substances.
On the other hand, there was no doubt that FeO-bearing primary-slags was circumscribed, because the environment of blast furnace is complexed. The above analysis results inspired to design more experiments to study the impact of slag phase on the softening and melting properties.
Conclusions
The influences of CaO/SiO2/MgO/Al2O3 on the formation behavior of FeO-bearing primary-slags is experimentally demonstrated, and the following conclusions are obtained.
-
1.
Additions of CaO or Al2O3 decrease the starting softening temperature and no dripping behaviors are found in comparison with the base case results when only FeO is used.
-
2.
The addition of SiO2 or MgO rises the starting and ending softening temperature, as well as the dripping temperature. In addition, the addition of SiO2 decreases the maximum pressure drop to as low as 1.5 kPa while increases Tp to as high as 1403 °C.
-
3.
According to the phase diagram and XRD analysis results, the initial phase with CaO addition in the primary-slags should be CaO·Fe3O4 and that with SiO2, MgO, Al2O3 additions are fayalite (2FeO·SiO2), magnesioferrite (MgO·FeO), hercynite (FeO·2Al2O3), respectively.
References
T. Bakker, et al., Softening in the Blast Furnace Process: Local Melt Formation as the Trigger for Softening of Iron Bearing Burden Materials. Delft University of Technology (1999)
P. Kaushik et al., Mixed burden softening and melting phenomena in blast furnace operation Part 2—Mechanism of softening and melting and impact on cohesive zone. Ironmaking Steelmaking 33, 520–528 (2006)
P.F. Nogueira et al., Blast furnace burden softening and melting phenomena: Part I. Pellet bulk interaction observation. Metall. Mater. Trans. B 35B, 829–838 (2004)
P.F. Nogueira et al., Blast furnace burden softening and melting phenomena: Part II. Evolution of the structure of the pellets. Metall. Mater. Trans. B 36B, 583–590 (2005)
P.F. Nogueira et al., Blast furnace burden softening and melting phenomena: Part III. melt onset and initial microstructural transformations in pellets and R. J. Fruehan. Metall. Mater. Trans. B 37B, 551–558 (2006)
J.R. Kim et al., Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 44, 1283–1290 (2004)
M. Hino et al., Simulation of primary-slag melting behavior in the cohesive zone of a blast furnace, considering the effect of Al2O3, FetO, and basicity in the sinter ore. Metall. Mater. Trans. B 30B, 671–683 (1999)
Y.S. Lee et al., Influence of basicity and FeO content on viscosity of blast furnace type slags containing FeO. ISIJ Int. 44, 1283–1290 (2004)
J.R. Kim et al., Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate–based slags containing 10 mass Pct MgO. Metall. Mater. Trans. B 44, 5–12 (2013)
M. Matsunura et al., Improvement of sinter softening property and reducibility by controlling chemical compositions. ISIJ Int. 45, 594–602 (2005)
T. Nishimura et al., Evaluation of softening, shrinking and melting reduction behavior of raw materials for blast furnace. ISIJ Int. 51, 1316–1321 (2011)
S.L. Wu et al., Increasing lump ores proportion in blast furnace based on the high-temperature interactivity of iron bearing materials. ISIJ Int. 50, 686–694 (2010)
A. Shankar et al., Experimental investigation of the viscosities in CaO–SiO2–MgO–Al2O3 and CaO–SiO2–MgO–Al2O3–TiO2 slags. Metall. Trans. B 38B, 911–915 (2007)
S.L. Wu et al., Evaluation of lump ores for use in modern blast furnaces as part of mixed burden practice. Ironmaking Steelmaking 36, 19–23 (2009)
A. Kemppainen et al., Effect of H2 and H2O on the reduction of olivine pellets in CO and CO2 gas. ISIJ Int. 52, 1973–1978 (2012)
Acknowledgements
The authors would like to thank the project supported by the National Natural Science Foundation of China (51304257), and the financial support from the Chongqing Research Program of Basic Research and Frontier Technology (cstc2015jcyjA50014) and National Natural Science Foundation of China (51374263) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Wang, D., Ma, K., Xu, Y., Xu, J., Wen, L. (2017). Influences of CaO/SiO2/MgO/Al2O3 on the Formation Behavior of FeO-Bearing Primary-Slags in Blast Furnace. In: Wang, S., Free, M., Alam, S., Zhang, M., Taylor, P. (eds) Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51091-0_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-51091-0_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51090-3
Online ISBN: 978-3-319-51091-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)