Abstract
Paddy agriculture is one of the major anthropogenic sources of methane (CH4) emission at global level. A decrease in CH4 release in the atmosphere from paddy fields can add significantly to the management of global warming and climate change. Biochar production and application in agriculture prepared from crop straw has been proposed as one of the effective countermeasure to mitigate the greenhouse gas emissions (GHGs) during farming. Biochar, a co-product of a controlled pyrolysis process, can be used as a tool to offset GHGs emissions and as a soil conditioner. Biochar application increased rice productivity, soil pH, soil organic carbon, total N but decreased soil bulk density in the long term. Recent studies have confirmed that the use of biochar in paddy agriculture has the capability to minimise the CH4 production, but its essential mechanism has yet to be clarified. The additions of biochar to the agriculture soil showed higher CH4 consumption because it improves soil aeration and porosity and enhances methanotrophs performance. However, further investigations are needed to evaluate the effect of biochar addition on net CH4 emissions and consumptions, respectively, by methanogens and methanotrophs. Long-term experiments should be conducted to monitor any changes over the years on the influence of biochar amendments on soil–methanotrophs–paddy systems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Methane
(CH4) is one of the most widespread greenhouse gases (GHGs) emitted from paddy
fields and other sources such as wetlands, ruminants, coal mines as well as anthropogenic activities such as leakage from natural gas systems and the raising of livestock. In the early nineteenth century, the atmospheric concentration of CH4 was 700 ppb, but the current concentration is 1750 ppb and has shown a 1 % year−1 increase rate over a century (IPCC 2001; Tiwari et al. 2015). The concentration of CH
4
in the atmosphere is increasing due to discrepancy in CH4 emanation and its removal (Singh 2010). The lifetime of CH4 in the atmosphere is 8–12 years, but it is more efficient in trapping radiation and 23–30 times more potential than CO2 (Tiwari et al. 2015). Global surface temperature has increased by 0.8 °C in the last 100 years and CH4 also contributed to this phenomenon as a potent GHG (Hanson et al. 1996). Recent global estimates of CH4 emission rates from wetland rice fields ranged from 20 to 100 Tg year−1, which corresponds to 6–29 % of the total annual anthropogenic CH4 emission (Neue 1993). According to Demisie and Zhang (2015) the processes of CH4 emission are affected by soil texture, inorganic electron acceptors, soil physico-chemical properties and methanogenic population. CH4 affects the chemistry and oxidation capacity of the atmosphere, e.g. by influencing concentrations of tropospheric ozone, hydroxyl radicals and carbon monoxide. The current burden of CH4 in the atmosphere is approximately 4700 Tg year−1 (Neue 1993). CH4 is produced in flooded paddy
soils by a group of bacteria designated as methanogens (also called marshy soil bacteria). The flooding rice fields restrict the oxygen supply to the soil, which may result in the anaerobic fermentation of soil organic matter and consequently release of sufficient amount of CH4 to the atmosphere. From deeper layer of flooded soil CH4 reaches to the atmosphere by diffusion, ebullition and through aerenchyma conduits of paddy
plant. It is now well accepted that rice cultivation is the substantial source of CH4 emissions therefore, there is need to management of flooded paddy
agriculture to minimise the soil CH4 emissions.
Keppler et al. (2006) demonstrated that significant amounts of CH4 are produced from terrestrial plants and detached leaves. They assumed that living plants and plant litter produce 62–236 Tg year−1 and 1–7 Tg year−1 CH4, respectively. Natural sources are accountable for about 30 % (up to160 Tg year−1) of the CH4 flux; however, the anthropogenic sources are responsible for contributing 70 % (up to 375 Tg year−1) (Mer and Roger 2001). Soil amended with biochar produced less quantities of CH4 than without biochar amendments. Biochar is a co-product of high concentration of carbon and silica which is produced by pyrolysis of biomass/organic material or plant residues under high temperature (400–500 °C) and low oxygen conditions (Lehmann 2007; Lehmann and Joseph 2009). It contains highly condensed aromatic compounds which are resistant to decomposition in soil and thus can effectively sequester the carbon. It is assumed that biochar application in agriculture may improve the soil fertility, crops yields, water holding capacity, degraded land restoration and support CH4-assimilating microorganism, i.e. methanotrophs (Singh and Gupta 2016). Biochar used in agriculture soil as a soil conditioner and plant growth enhancer also increases microbial biomass in paddy ecosystem. Impacts of biochar application on soil physico-chemical properties are widely known, while the research on agriculture productivity and CH4 emission/consumption with reference to biochar application in paddy agriculture is scarce. Therefore, the objectives of this review are (1) to describe the impact of biochar on paddy productivity and CH4 emission/consumption, (2) to assess the role of biochar amendment on soil microbial processes and biomass and (3) to discuss impact of biochar application on soil N dynamics.
7.2 What Is Biochar ?
Biochar is a unique product, which enhances the plant-available nutrients and significantly improves the crop yield. Biochar is produced by pyrolysis of biomass or organic materials and this practice is termed as thermal degradation of biomass such as rice straw, grass, wood, agricultural wastes and manure (Wu et al. 2015). In addition, biochar can significantly improve soil properties by decreasing methane emission, soil bulk density; enhancing soil pH, organic carbon; increasing available nutrients; removing heavy metals and increasing number of methanotrophs , thus ultimately increasing crop yields (Milla et al. 2013). Biochar is fine-grained residue with a high carbon content and works as soil conditioner and carbon sequestrating agent in the soil (Johannes 2007; Gaunt and Johannes 2008; Peter 2007). As stated earlier, biochar enhances the crop yield and it is also indirectly involved in the mitigation of environmental pollution, such as reduction of GHGs. Therefore, most of the studies on biochar concentrated over large-scale production. (Peter 2007; Laird 2008; Johannes 2007; Ghoneim and Ebid 2013). Previously, it was reported that biochar increases the agriculture production and mitigates CH4 emissions. However, biochar also increases the soil methanotrophic community structure and reduces the soil CH4-generating bacteria (methanogens). Therefore, extensive work is required to assess the use of biochar and its impact to restore the methanotrophs niche in the disturbed paddy agriculture and its contribution to stabilise the atmospheric CH4 concentration.
7.3 Biochar Production and Its Properties
Biochar production is a thermal degradation phenomenon of organic material and biomass, using a small-scale reactor and drum method at 400–500 °C with the residence time of up to 1 h. Table 7.1 presents different types of biochar produced from various sources (feedstock) (Gaunt and Johannes 2008; Peter 2007). Ca, Si, Al and K are common elements in biochar but C, N, H and S are also determined by a dry oxidation using an elemental analyser (Hmid et al. 2014). According to Gaunt and Johannes (2008) and Peter (2007) the performance of biochar in their original shapes can be detected by using grinders or sieves, including scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), volatile matter (VM), electrical conductivity (EC), total dissolved solids (TDS) analysis, water holding capacity (WHC) and heavy metal assessment. Peter (2007) and Milla et al. (2013) reported that the sample powder is sprinkled as a thin layer on an adhesive tape placed on the brass sample holder. Excess amounts of the sample are removed with a small manual air blower. The adhered sample is then coated with gold powder using a sputtering device, FTIR spectrometer identified the sample to determine the organic functional groups present for each biomass, especially carbons. Volatile matter in biochar is determined following the ASTM D 3175-07 standard test method. A Beckman Coulter SA 3100 BET analyser containing approximately 0.1000 g to 0.2000 g of each biochar sample is then used at a temperature of 50 °C for 60 min. Electrical conductivity and total dissolved solid analysis are theoretically the best measure to indicate the actual salinity level experienced by the plant root (Peter 2007). Hence, electrical conductivity and total dissolved solids are measured using a portable conductivity meter.
7.4 Biochar Types
Currently, varieties of feedstock are being used as raw material for the preparation of biochar at variable temperatures such as 250, 300, 400, 500, 600 and 700 °C. The composition of various nutrients of biochar varied during its preparation at variable temperatures. A variety of biochar from different feedstock has been presented in Fig. 7.1.
7.4.1 Biochar Produced from Grass
Grass biochar is produced by a variety of grasses such as Switchgrass (Panicum virgatum L.), Sawgrass (Cladium jamaicense), etc. and has been declared as a model bioenergy crop for the production of biochar. These are preferred due to its high yield potential, low input requirements on marginal soils and potentially active in soil carbon sequestration and alleviation of GHGs (McLaughlin and Walsh 1998; Sadaka et al. 2014; Mukherjee and Lal 2013). The switchgrass has a gross calorific value between 18 and 19 MJ kg−1 as compared to hardwoods 20–21 MJ kg−1 (Sadaka et al. 2014). There were several barriers in the way of switchgrass to be used as the sole source of fuel in combustors such as high moisture and ash contents in biomass, which cause ignition and combustion problems. It has been observed that blending of biomass with coal would reduce flame stability problems and will also lead to significant reductions in methane emissions. Consequently, a multitude of studies has investigated about conversion of switchgrass to biochar for the safe and eco-friendly cultivation of agriculture crops (Sadaka et al. 2014).
7.4.2 Woodchips Biochar
Woodchips are a medium-sized solid material made by cutting, or chipping, larger pieces of wood. Today, woodchips are used as a raw material for the production of biochar. It has more carbon concentration as compared to other feedstock biochar including the highest carbon sequestration potential. Woodchips feedstock produce a high quality biochar at 400–500 °C, its good residential time is 2–3 h. Woodchips absorb moisture at 15–20 °C, therefore it requires drying before the pyrolysis (Milla et al. 2013; Lai et al. 2013; Spokas et al. 2009). The Camellia japonica (Japanese Cedar) waste wood chips are used for biochar production by pyrolysis at either 290 °C or 700 °C and called biochar 290 (BC290) and biochar 700 (BC700), respectively (Lai et al. 2013). The percentage amount of C, N, H and available K contents have been found to be about 59.1 %, 0.35 %, 5.73 % and 0.78 g/kg for woodchips biochar 290 (BC290) and 83.0 %, 0.34 %, 2.57 % and 3.90 g/kg for BC700, respectively (Lai et al. 2013).
7.4.3 Rice Husk Biochar
Rice hulls (husk) are the coatings of seeds, or grains, of rice. The husk protects the seed during the growing season, because it is formed from hard materials, including silica, carbon, magnesium and phosphorus. Presently rice husk, used as a raw material for the production of biochar, improves the soil fertility and crop productivity. For making biochar, rice husk is put in a pyrolysis apparatus which consists of a stainless reactor of 500 mm length with a 15 cm inside diameter. The rice husk is then heated externally by an electric furnace (5000 W) to a temperature of 600 °C and it has more concentration of silica and carbon (Zhang and Liu 2012). The use of rice husk biochar in agriculture field in place of synthetic fertilisers is advantageous because the synthetic fertilisers generate many harmful effects such as reduction in microflora, crop yield, nutrient availability, water holding capacity, etc. The studies on cowpea, soybean and maize have also supported the application of biochar as a way to increase crop yields. In Asian region due to elevated production of rice, it is estimated that up to 560 and 112 million tons of rice straw and rice husks are produced, respectively. These residues may be a valuable resource for the production of biochar that may be used in agricultural applications (Masulili and Utomo 2010).
7.4.4 Poultry Litter Biochar
For the production of poultry litter biochar chicken manure (CM), the feedstocks used are wood feedstock, rice husk, plant residue, etc. (Songa and Guo 2012; Demirbas 2001). According to Songa and Guo (2012) CM is a solid waste material, resulting from chicken rearing and is being explored as a feedstock for biofuels and biochar. CM is a mixture of bedding materials of bird feather, hen’s excreta and feed spills. These are pyrolysed by thermochemical conversion technology whereby organic materials are heated in the absence of oxygen. CM can be readily transformed into biochar, biofuel and syngas for the enhancing production of agricultural crop (Songa and Guo 2012; Kim et al. 2009).
7.4.5 Sugarcane Bagasse Biochar
Sugarcane industry produces several pyrolysable residues. These include bagasse (crushed cane stalks), cane trash (leaves and stalk tips removed during harvest) and filter cake, a sludge that is removed via filtration after the juice clarification step and bagasse used for many purposes such as biochar production, biofuels, burning purpose, etc. Currently, sugarcane bagasse is being used on large scale for the production of biochar. The raw material/feedstock should be dry in wet season because moisture content creates difficulties during pyrolysis; dry feedstock has a low residential time (1–2 h) for the production of biochar (Eykelbosh et al. 2014). Sugarcane biochar contains a high concentration of carbon, silica, magnesium, etc. and may play a significant role in agriculture field to enhance the crop production and as a conditioner for saline and degraded soil (Eykelbosh et al. 2014).
7.4.6 Wheat Straw Biochar
Wheat straw containing lignocelluloses biomass is the most abundant organic raw material and is being used widely for biochar production. Wheat straw is collected by a cutting machine and then shipped to the production plant and air-dried. Pyrolysis of wheat straw is performed in a vertical kiln at 350–550 °C, converting 35 % of the biomass to biochar. The biochar mass originally in a particulate form is ground to pass through a 2 mm sieve and mixed thoroughly to obtain a fine granular consistency that would mix more uniformly with the soil mass (Wu et al. 2013).
7.5 Impact of Biochar on Soil and Plant Growth
-
Increases water holding capacity and reduces soil bulk density of the soil
-
Enhances cation exchange capacity
-
Improves fertiliser utilisation by reducing leaching from the root zone
-
Retains minerals in plant available form
-
Supports soil microbial life and biodiversity
-
Plants resistance to diseases and pathogens
-
Reduces soil CH4 emission
-
Increases soil methanotrophs population
-
Improves soil carbon pool
-
Increases nitrogen retention
-
Promotes paddy root growth (Fig. 7.2)
7.6 Impact of Biochar on Crop Yields and Soil Properties
Biochar applications to increasing crop productivity by improving the physico-chemical and biological properties of the soil with variation in crop response. These impacts depend on the chemical and physical properties of the biochar, soil conditions and the crop type (Zwieten et al. 2010; Yamato et al. 2006). Zhanga et al. (2010a, b) found that biochar amendment at 10 t ha−1 and conventional N fertilisation at 300 kg ha−1 enhance the crop yield by 9 %, while only biochar amendment at 40 t ha−1 yields increased by12 %. However, the exact mechanism about the biochar effect on rice yield in presence or absence of fertiliser is still not known. Most of the previously reported field trials have been conducted mostly in tropical regions having relatively poor soils with the rain-fed crops (Zhanga et al. 2010a, b). Zhanga et al. (2010a, b) reported that biochar application increased rice yields by around 10 %. The biochar amendments can increase N availability to crops and that high level of soil organic C accumulation can enhance N efficiency and increase rice productivity in a long-term monitored rice paddy (Pan et al. 2009). This is of particular importance for world’s rice agriculture as the farming has tremendous challenge of N pollution from overuse of N fertilisers (Zhanga et al. 2010a, b).
7.6.1 Paddy Productivity
Biochar amendment significantly impacts the crop yield including the improvement of root length, shoot biomass, panicle length, number of tiller per plan, rice yield, nutrient availability and carbon sequestration (Milla et al. 2013; Abdullah and Wu 2009; Meyer et al. 2011). However, Yang et al. (2015) reported that 2 ton ha−1 biochar application could increase the yield by 5–15 % and biochar of 4 ton ha−1 may increase the yield by about 20 %. The property of cation exchange capacity (CEC), pH and WHC of soils amended with biochar also increases (Yang et al. 2015).
7.6.2 Physico-chemical Properties of Soil
Biochar-amended soil shows the variation in many of its chemical properties, viz. pH, K, Ca, Mg, NH4-N and NO3-N as well as in the ratios of organic C, N and P (Jien and Wang 2013). They demonstrated that pH significantly increased from 7.41 to 9.26 with the application of biochar in the farming land. However, Prommer et al. (2014) reported that, after biochar amendment the soil pH and cation exchange capacity decreased slightly from a preliminary 7.5 to 7.4 (Table 7.2). The biochar-amended soils also showed an enhancement in the mineral content such as K, Ca, Mg, NH4-N and NO3-N, etc. as compared to the control (Agegnehu et al. 2015; Jien and Wang 2013). Biochar significantly increased soil C by 7 % (Mukherjee et al. 2014). In addition, the incubation about 3–4 months after biochar application indicates an increase in the nutrient status of highly weathered soils (Agegnehu et al. 2015; Jien and Wang 2013). The information concerning impact of biochar application on chemical properties of soil is still in an incipient stage; therefore, further research and investigation are required in the area.
Application of biochar in agriculture fields improves soil physical quality for crop production such as electrical conductivity (EC) and WHC. Humus level also increases in the amended soil due to the activity of soil microflora. Therefore, improved soil properties increase the level of nutrients available for the crops. Jien and Wang (2013) reported that addition of biochar in soil decreases the bulk density as compared to control; Mukherjee et al. (2014) also reported that biochar application increased subnanopore surface area of soil by 15 % and reduced soil bulk density by 13 % compared to control. It is reported that biochar-amended soil has an 11 % higher porosity than the unamended soil (Gul et al. 2015). Therefore, biochar plays an effective role to supporting environmental changes with soil microflora and reduction of methane gas emission in soil. The effect of biochar on soil pH and cation exchange capacity may be minimal. Prommer et al. (2014) applied three amendments in silty-loam soil 0.5 % (w/w) in triplicated plots of paddy field: Biochar (oak woodchip), Humic acid (HA) and water treatment residual (WTR) and reported that all amendments significantly augmented soil pH, nevertheless the impact of biochar was the immense. The above results are based on short-term investigation study about the impact of biochar application on soils properties. However, long-term studies with respect to use of biochar on soil physico-chemical properties are yet to be investigated.
7.6.3 Microbial Biomass of Soil
Soil microbial biomass is the key indicator of soil productivity and microbial diversity. The microbial biomass is not only responsible for carrying the nutrient cycles in paddy ecosystems, including carbon (C), nitrogen (N) and phosphorus (P) but also plays a significant role in soil nutrient transformations and acting as a labile nutrient pool offered to plants (Liu et al. 2010). Microbial biomass is responsive to biochar application to the soil of agriculture fields. As the stability period of biochar in soil is assumed to be many years, the changes in microbial biomass size and properties may continue for a long period. Jien and Wang (2013) found some changes in soil microbial activity and microbial biomass after biochar treatment. The highest contents of MBC were found at 21 days for each treated plots, which were 3200 mg kg−1 for 5 % biochar-amended soil, 1145 mg kg−1 for 2.5 % biochar-amended soil and 1759 mg kg−1 for the control, respectively. The pH in the 5 % biochar-amended soil is more suitable for the growth of microbes, particularly for fungal hyphae. Wuddivira et al. (2009) demonstrated that because of higher porosity the biochar-treated soil creates suitable condition for the microbial growth and activity. Biochar has a high concentration of macropores that extends from the surface to the interior and minerals and small organic particles might accumulate in these pores. The increase in microbial biomass as a result of biochar amendment can help detect the presence of a given microbial genera or species via DNA/RNA-based techniques, due to increase in their population size and density in the soil matrix (Gul et al. 2015). This indicated that application of biochar in agriculture could maintain microbial activity in the soils for a longer period. The application of biochar may be considered as a soil conditioner as well as enhancing the microbial activity in benefits of agriculture and environment.
7.6.4 Soil Nitrification
Biochar amendment causes primary changes in soil nutrient cycles, commonly resulting in marked enhancement in crop yields, mostly in saline and unproductive soils having poor soil organic matter contents (Prommer et al. 2014). Prommer et al. (2014) reported that biochar application increased total soil organic carbon but decreased the extractable organic C pool and soil nitrate. Although gross organic N transformation rates were reduced by 50–80 %, the gross N mineralisation process remains unaffected. Biochar application increases the ammonia oxidisers population in soil and consequently more than twofold higher in nitrification rates noted (Ball et al. 2010). Prommer et al. (2014) suggested that addition of any inorganic fertiliser with the combination of biochar may compensate the reduction in organic N mineralisation and as a consequent accelerate the belowground build-up of organic N.
Biochar applications have significant effects on microbial-mediated N transformations (Ball et al. 2010) and ammonia- and methane-oxidising bacterial community composition in paddy soil (Ball et al. 2010). Changes in pH that can start similar responses in soil were not able to explain the observed changes in nitrification. Prommer et al. (2014) after applying biochar, ammonium level increased 0.001 mg kg−1 in the conventionally managed soils (about 88 mg kg−1 dry soils) compared with the organic soils (about 9 mg kg−1 dry soil). After increasing biochar application rate ammonium contents became 66, 30 and 15 mg kg−1, respectively, but does not show significant reductions from the small initial ammonium contents in the organically managed soil. Initial nitrate contents of 5 mg kg−1 increased over the 60 days. Study showed that single or combined application of biochar with any inorganic fertiliser may increase soil organic N in turn enhancing soil carbon sequestration and thereby could play a significant role in future soil and environmental management planning (Prommer et al. 2014).
7.6.5 Soil Mycorrhizal Fungi
Biochar and mycorrhizal applications have been contributing to the sustainable crop production, ecosystem restoration, and soil carbon sequestration and mitigation of methane emission (Warnock et al. 2007). Mycorrhizal fungi are ubiquitous key indicator in nearly all terrestrial vegetation and crop systems, showing a very high degree of specificity and mutualism, enhancing plant growth. Biochar incorporation in soil has a positive impact on mycorrhizal fungi that may influence the nutrient absorption by plant roots (Ishii and Kadoya 1994; Warnock et al. 2007). Biochar can also increase endomycorrhizal plant associations that could enhance P availability in soil (Atkinson et al. 2010). In biochar-amended soil, the favourable soil conditions enhance the ability of MF to resist against plant-fungal pathogen infection through enhanced root colonisation (Atkinson et al. 2010). A number of investigations examined that biochar may influence the mycorrhizal population in terrestrial and paddy ecosystem (Warnock et al. 2007; Ishii and Kadoya 1994) but biochar application in soil and its effect on the diversity of mycorrhizal fungi is still not clear and hence there is need of further detailed study.
7.7 Impact of Biochar on Methanogens and Methanogenesis in Paddy Ecosystem
Biochar amendment affects the methanogenic archaeal community compositions in paddy soils (Dong et al. 2013). No statistically significant differences in methanogenic activities are noted in the rhizosphere of biochar amended and control soil during the rice growing seasons (Dong et al. 2013). But in a field experiment biochar addition at the rate of 9 t ha−1 significantly decreased CH4 emission without affecting the CO2 and N2O emissions (Karhu et al. 2011). But in a laboratory incubation experiment the CH4 emission from paddy soil was completely inhibited compared with the non-amendment control soil (Liu et al. 2011; Bosse and Frenzel 1997). Feng et al. (2012) also reported that amendment of wheat straw biochar significantly reduced CH4 emission from paddy ecosystem. Liu et al. (2011) found that CH4 emission from a rice paddy field was significantly increased (compared with the non-amendment control soil) in the first year after biochar amendment but was not as prominent as in the next year. It has been observed that soil CH4 emission in response to the biochar amendment may vary with biochar types and properties. Most of the studies supported that decreasing methanogenic activity in paddy soil amended with biochar could be due to the increase in porosity of soil in presence of biochar that may inhibit the growth and multiplication of anaerobic methanogens. Although by using rice straw instead of biochar in soil, the rate of methanogenesis can be enhanced because readily degradable carbon in rice straw offered more substrates to methanogenesis to generate CH4 than that in rice straw biochar. In contrast, there was no significant increase in CH4 emissions associated with biochar amendment due to their resistance to decomposition (Liu et al. 2011). However, there is no considerable information about biochar application in paddy fields related to methanogenic activity; methanogens diversity decreases with biochar amendments hence there is need of detailed study on this aspect.
7.7.1 Methane -Producing Bacteria (Methanogens )
Methanogenic archaea (methanogens) are strictly anaerobic microbes that play a vital role in anoxic environments of flooded paddy soil in the generation of CH4 and CO2 (Conrad 1999). Methanogens use acetate (contributes about 80% to CH4 production) as a carbon substrate, but another substrate like H2/CO2 and formats also accelerate 10–30 % CH4 production. According to Methanobacteriales, Methanococcales and Methanomicrobiales orders of methanogens have the ability to fix molecular nitrogen as they have the nif genes (Dannenberg and Conrad 1999). Methane is produced in the anaerobic layers of paddy soil mediated by bacterial decomposition of organic and plant residues (Dubey 2011). The characteristics of methanogens that carried anaerobic degradation of organic matter are described in Table 7.3. Methanogenesis from all substrates requires some unique coenzymes, some of which are exclusively found in methanogens (Ludmila et al. 1998; Yao and Conrad 2001). At least nine methanogen-specific enzymes are involved in the pathway of methane formation from H2 and CO2 (Shima 1998). In paddy soil, acetate and H2 are the two main intermediate precursors for CH4 formation (Yao and Conrad 1999).
7.7.2 Methanogenesis
Biochar affects methanogenesis because numbers of methanogens reduced in anaerobic environments where sulphate and nitrate present in low concentration complete mineralisation of organic matter take place through methanogenic fermentation, which produces CH4 and CO2 according to reaction: C6H12O6 → 3 CO2 + 3 CH4 (Fig. 7.3). Four types of microorganism play important roles in this transformation and convert complex molecules into their simpler forms (Mer and Roger 2001). The transformation takes place by the following steps.
-
Hydrolysis of biological polymers into monomers (glucides, fatty acids, amino acids) by an hydrolytic microflora that can be either aerobic, or facultatively, or strictly anaerobic;
-
Acidogenesis from monomeric compounds and intermediary compounds formed during fermentation (production of volatile fatty acids, organic acids, alcohols, H2 and CO2) by a fermentative microflora that can be either facultatively or strictly anaerobic.
-
Acetogenesis from the previous metabolites by a syntrophic or homoacetogenic microflora; and
-
Methanogenesis from the simple compounds that can be used by methanogens (in particular, H2 + CO2 and acetate) which constitutes the last step of the methanogenic fermentation.
Production, consumption and transfer of CH4 to the atmosphere in paddy fields. Modified from Mer and Roger (2001)
Methanogens have a limited trophic spectra comprised of a small number of simple substrates: H2+CO2, acetate, formate, methylated compounds (methanol, methylamines, dimethyl sulphur) and primary and secondary alcohols.
7.8 Impact of Biochar on Methanotrophs and Methane Oxidation
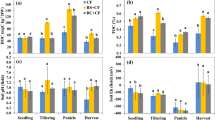
Currently, biochar is used as an environmental and agriculturally supportive agent and hence many parts of world are applying it as a strong soil conditioner for the enrichment of soil nutrient status. The most important aspect related to biochar application in paddy field is the mitigation of methane emission and stimulation of the methane oxidation rate. Reddy et al. (2014) reported that variation in oxidation rates and kinetics of methane in soils depth was variable, therefore samples were taken from different depth of soils and examine that higher oxidation rate was found in upper layer of soil amended with biochar than lower depth of soil. Higher numbers of methanotrophs communities exist in upper layer of soil after amendment of biochar (Feng et al. 2012). Methanotrophs, aerobic bacteria, are present in the upper layer of soil (Reddy et al. 2014; Feng et al. 2012). According to Zhang et al. (2012), biochar plays significant role in the reduction of greenhouse gases mostly methane emissions in paddy soil. The different rates of greenhouse gas emissions in biochar-amended soil are presented in Fig. 7.4.
Biochar plays a significant role in methane mitigation with promoting the methanotrophs population and reducing diversity of methanogens. Paddy is one of the largest anthropogenic sources of CH4 (6–29 % total methane emission) (Neue 1993). Mukherjee and Lal (2013) reported that biochar amendment in soil increases the aeration and porosity therefore, production of CH4 decreases and oxidation of CH4 increases. Furthermore, the aerobic, well-drained soils due to biochar applications can be a sink for CH4 due to the CH4 diffusion and subsequent oxidation by methanotrophs . Hence two mechanisms are involved here: (1) decrease the CH4 production, and (2) increase the CH4 oxidation by methanotrophs may be operational in the biochar-amended soil (Mukherjee and Lal 2013; Zwieten et al. 2009. According to Jien and Wang (2013) increase in soil microbes, nitrogen and phosphorus was observed after 63 and 105 days of biochar application. The highest contents of microbial carbon were found at 21 days for each treated soil, which were 3200 mg kg−1 for 5 % biochar-amended soil (Jien and Wang 2013). This shows that amendment of biochar in soil supports the microbial growth, mostly methanotrophs which play significant role in CH4 uptake. Therefore, an effective process to decrease CH4 emission in paddy soil may be application of biochar (Lehmann 2007). Previous work has shown that CH4-oxidising bacteria are readily enriched within landfill cover soil by exposure to the CH4 generated from the waste (Reddy et al. 2014).
7.8.1 Methanotrophs or Methane -Oxidising Bacteria
Methanotrophs are Gram-negative bacteria that utilise CH4 as their sole source of carbon and energy play a crucial role in reducing global CH4 load due its CH4 consumption characteristics. Studies on CH4 sink measurement from various agro and natural ecosystems showed that the soils of these ecosystems exhibited a significant variation in CH4 sink activity due to methanotrophic bacteria. Paddy soil methanotrophic communities exhibit the highest CH4 sink activity on a global scale (Tiwari et al. 2015). Based on physiology, phylogeny, biochemistry, resting stage, intracellular membrane, genetic characters, ultrastructure and phospholipid ester-linked fatty acid (PLFA) analyses of 14 culturable genera (Han et al. 2009) of aerobic proteobacterial methanotrophs are classified as type I belongs to Gammaproteobacteria group and contain genera Methylobacter, Methylomonas, Methylosphaera, Methylomicrobium, Methylothermus, Methylosarcina, Methylohalobius, and Methylosoma while type II belongs to Alphaproteobacteria group of CH4-oxidising bacteria and include genera Methylocystis, Methylosinus, Methylocapsa, Methylocella. Type I group of methanotrophs is further subdivided into types Ia and Ib (Bodrossy et al. 2003; Krause et al. 2010). Type I subgroup contains several culturable methanotrophs , for example Methylomonas, Methylosarcina, Methylobacter, etc. However, Methylocaldum and Methylococcus come under the subgroup Type Ib or rare type X (Hanson and Hanson 1996; Graef et al. 2011; Giri et al. 2014; Tiwari et al. 2015). Type I methanotrophs also referred as ‘high capacity–low affinity’ methanotrophs are adapted for high CH4 concentrations and assimilate it through RuMP pathway whereas Type II is generally termed as ‘low capacity–high affinity’ methanotrophs capable of using trace quantity of CH4 from the environment and follow the serine pathway for CH4 oxidation (Hanson and Hanson 1996; Tiwari et al. 2015). Verrucomicrobia, a new group of CH4 oxidiser discovered in recent past involved in methane oxidation (Siljanen et al. 2011; Luke et al. 2011; Graef et al. 2011; Tiwari et al. 2015) The methane oxidation pathways by Type I and Type II methanotrophs is presented in Fig. 7.5.
Singh (2010) reported that during last 10 years the extensive study has been done related to population dynamics and diversity of methanotrophic genera bacteria. Currently, 18 genera of cultivated aerobic methanotrophs (Gammaproteobacteria) and five genera of Alphaproteobacteria are represented by approximately 60 different species of the bacteria (Singh 2010). Rising temperature around the earth’s surface is directly associated with the increasing atmospheric level of water vapour, CO2, CH4, N2O, SF6, etc. due to anthropogenic activities (IPCC 2007; EPA 2010; Krause et al. 2010; Li and Wang 2013). Though the atmospheric concentration of CH4 is extremely less than CO2 (IPCC 2007), CH4 is more efficient to trap radiation than CO2 (Solomon et al. 2007; Siljanen et al. 2011; Pandey et al. 2014). It is assumed that methane, 27 times potent GHG than CO2 (Houghton et al. 1995; Phillips et al. 2001; Singh and Gupta 2016), accounting about 15–20 % of the global warming effect (Phillips et al. 2001; Wuebbles and Hayhoe 2002; Jang et al. 2006; IPCC 2007; Dalal and Allen 2008; Tiwari et al. 2015). Being highly reactive in nature, CH4 affects the chemistry and oxidation capacity of the environment by influencing the level of CO, OH−, tropospheric ozone, etc. (Cicerone and Oremland 1988). Global atmospheric concentration of CH4 has almost tripled since pre-industrial times (Krause et al. 2010) increasing rate up to 0.5–1 % year−1 (IPCC 2001, 2007; Tamai et al. 2007; Tiwari et al. 2015). The annual release of CH4 into the atmosphere was 180 Tg year−1 (Khalil and Rasmussen 1994; Mer and Roger 2001; Hill et al. 2016).
In global perspective, most of the atmospheric CH4 is eliminated from the environment through chemical reactions with hydroxyl radicals (OH−) in the troposphere (CH4 + OH− → CH3 − + H2O), and in stratosphere CH4 reacts with the chlorine originated from CFCs (Chlorofluorocarbons) (CH4 + Cl− → HCl + CH3 −.) which involve around 90 % of the total Global CH4 sinks (Schlesinger 1997; IPCC 2001; Hutsch 2001, Mer and Roger 2001; Tiwari et al. 2015). Mer and Roger (2001) state that if equilibrium between by methanogens CH4 emission and methanotrophs CH4 oxidation is positive, the environment may be a CH4 source and if the equilibrium is negative the environment may be a CH4 sink. Aerobic soils are the important biological sink for CH4 due to the presence of unique methanotrophic bacteria (Singh 2010; Tiwari et al. 2015). Methanotrophs utilise CH4 as their carbon and electron source from the surrounding environment. The estimated amount of CH4 consumed by methanotrophic bacteria is between 10 and 40 Tg year−1 and comprises approximately 6–10 % of the total CH4 oxidation of the atmosphere (IPCC 2001; Tiwari et al. 2015). Up to 95 % of the CH4 emitted anoxically may be consumed before destined into the atmosphere (Frenzel et al. 1990; Graef et al. 2011). Therefore, even minute alteration in consumption capacity may have a global significance if key regions such as the Arctic and Antarctica are concerned (Graef et al. 2011). It is assumed that 10–30 % of the CH4 emitted by methanogenic bacteria in submerged conditions of paddy fields is oxidised by methanotrophs linked with the roots of rice crop (King 1997; Schlesinger 1997; IPCC 2001; Mohanty et al. 2007; Tiwari et al. 2015).
7.9 Conclusions and Future Research Directions
Results indicate that biochar and/or compost in a range of combinations added as soil amendments with supplementary fertiliser can improve soil health and boost productivity of paddy crops with the additional environmental benefits of global warming and climate change mitigation. This approach can therefore contribute positively to agricultural and environmental sustainability. Biochar and biochar-compost applications positively impact soil fertility, for example, through their effect on soil physico-chemical properties and plant available nutrients.
Significant increases in various crop yields and plant available soil nutrients were observed due to biochar and compost addition in comparison to the fertiliser only treatment, indicating that application of organic amendments does provide agronomic benefits. The response of paddy crop to biochar and organic amendments could be due to their effects on plant available nutrients, biological N fixation, soil water and nutrient retention, although other mechanisms cannot be discounted. Study indicates that fresh biochar mitigates CH4 emissions immediately after its addition to soil. It has been reported that biochar application to increase CH4 uptake, probably due to better soil aeration and optimum moisture availability.
Application of biochar can significantly improve soil physical quality in terms of bulk density, aeration, porosity and WHC. Biochar has a potentially positive role to play in limiting GHGs emissions but a greater understanding of the mechanisms involved is required. The study showed that biochar addition may reduce the climate change impact of agriculture in both perennial bioenergy crop soils and arable soils. However, further research is required to confirm these results in a variety of agriculture soils using a variety of biochar types. Longer term experiments need to be conducted in order to monitor the effect of biochar on soil CH4 emissions/consumptions following rainfall or N fertilisation events, taking measurements from the day of biochar application onwards. Future studies should investigate whether biochar applications can affect the N use efficiency of paddy agriculture and population dynamics of methanogens/methanotrophs . Additionally, future studies should analyse all of the N-based fertiliser and biochar addition to soil under a range of environmental regimes such as different soil types, N application rates and timings and repeated biochar applications. Future research should make certain that the biochar production and methods of amendments used are sustainable in a social, environmental and economic context.
References
Abdullah H, Wu HW (2009) Biochar as a fuel: properties and grindability of biochars produced from the pyrolysis of Mallee wood under slow-heating conditions. Eng Fuels 23:4174–4181
Agegnehu G, Bass AM, Nelson PN, Muirhead B, Wright G, Michael I (2015) Bird biochar and biochar-compost as soil amendments: effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric Ecosyst Environ 213:72–85
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Bailey VL, Fansler SJ, Smith JL, Bolton JH (2010) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43:296–301
Ball PN, MacKenzie MD, DeLuca TH, Holben WE (2010) Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacteria abundance in dry montane forest soils. J Environ Qual 39:1243–1253
Bodrossy L, Stralis-Pavese N, Murrell JC, Radajewski S, Weilharter A, Sessitsch A (2003) Development and validation of a diagnostic microbial microarray for methanotrophs. Environ Microbiol 5:566–582
Bosse U, Frenzel P (1997) Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryza sativa). Appl Environ Microbiol 63:1199–1207
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46:73–79
Carriera M, Hardieb AG, Urasa U, Gorgensa J, Knoetze JH (2012) Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J Anal Appl Pyrolysis 96:24–32
Cicerone RJ, Oremland RS (1988) Biogeochemical aspects of atmospheric methane. Glob Biochem Cycles 2:299–327
Conrad R (1999) Soil microorganisms oxidizing atmospheric trace gasses (CH4, CO, H2, NO). Ind J Microbiol 39:193–203
Dalal RC, Allen DE (2008) Greenhouse gas fluxes from natural ecosystems. Aus J Bot 56:369–407
Dannenberg S, Conrad R (1999) Effect of rice plant on methane production and rhizospheric metabolism in paddy soil. Biogeochemistry 45:53–71
Demirbas A (2001) Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energ Conver Manag 42:1357–1378
Demisie W, Zhang M (2015) Effect of biochar application on microbial biomass and enzymatic activities in red soil. African J Agric Res 10:755–766
Dong D, Yang M, Wang C, Wang H, Li Y, Luo J, Wu W (2013) Responses of methane emissions and rice yield to applications of biochar and straw in a paddy field. J Soil Sediment 13:1450–1460
Dubey SK (2011) Methane emission and rice agriculture. Curr Sci 81:345–346
EPA (2010) Methane and nitrous oxide emission from natural sources. U.S. Environmental Protection Agency, Washington
Eykelbosh AJ, Johnson MS, Queiroz ESD, Dalmagro HJ, Couto EG (2014) Biochar from sugarcane filtercake reduces soil CO2 emissions relative to raw residue and improves water retention and nutrient availability in a highly-weathered tropical soil. PLoS One 9:98523
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol Biochem 46:80–88
Frenzel P, Therbrath B, Conrad R (1990) Oxidation of methane in the toxic surface layer of deep lake sediment (Lake Constance). FEMS Microbiol Ecol 73:149–158
Gaunt JL, Johannes L (2008) Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ Sci Technol 42:11–4152
Ghoneim AM, Ebid AI (2013) Impact of rice-straw biochar on some selected soil properties and rice (Oryza sativa L.) grain yield. Int J Agron Agric Res 3:14–22
Giri DD, Kumar A, Sahu PK, Mishra PK, Pandey KD (2014) Temperature dependent decline in soil methane oxidizing bacterial population in tropical dry deciduous forest ecosystems. Int J Sci Technol Res 3:2277–8616
Graef C, Hestnes AG, Svenning MM, Frenzel P (2011) The active methanotrophic community in a wetland from the high arctic. Environ Microbiol Rep 3:466–472
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Han B, Chen Y, Abell Y, Jiang H, Bodrossy L, Zhao J, Murrel JC, Xing X (2009) Diversity and activity of methanotrophs in alkaline soil from a Chinese coalmine. FEMS Microbiol Ecol 70:196–207
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
Hmid A, Mondelli D, Fiore S, Fanizzi FP, Ziad Al C, Dumontet S (2014) Production and characterization of biochar from three-phase olive mill waste through slow pyrolysis. Biomass Bioener 71:330–339
Houghton JT, Filho LGM, Bruce J, Lee H, Callander BA, Haites E, Harris N, Maskell K (1995) Radiative forcing of climate change. In: van der Linden PJ, Hanson CE (eds) Climate change. Cambridge University Press, Cambridge
Hutsch BW (2001) Methane oxidation, nitrification, and counts of methanotrophic bacteria in soils from a long-term fertilization experiment (‘Ewiger Roggenbau’ at Halle). J Plant Nutr 164:21–28
Intergovernmental Panel on Climate Change (2001) The science of climate change In: Climate change 2001. Cambridge University Press, Cambridge
Intergovernmental Panel on Climate Change (2007) The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. In: Climate change 2007: climate change and environmental sustainability. Cambridge University Press, New York, pp 433–497
Ishii T, Kadoya K (1994) Effects of charcoal as a soil conditioner on citrus growth and vesicular-arbuscular mycorrhizal development. J Jpn Soc Hortic Sci 63:529–535
Jang I, Lee S, Hong JH, Kang H (2006) Methane oxidation rates in forest soils and their controlling variables: a review and a case study in Korea. Ecol Res 21:849–854
Jien SH, Wang CS (2013) Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 110:225–233
Jindo K, Suto K, Matsumoto K, García C, Sonoki T, Sanchez-Monedero MA (2012) Chemical and biochemical characterisation of biochar-blended composts prepared from poultry manure. Bioresour Technol 110:396–404
Johannes L (2007) Bio-energy in the black. Front Ecol Environ:5–7
Jouiada M, Al-Nofeli N, Khalifa N, Benyettouc F, Yousef LF (2015) Characteristics of slow pyrolysis biochars produced from rhodes grass and fronds of edible date palm. J Anal Appl Pyrolysis 111:183–190
Karhu K, Mattila T, Bergstrom I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity-results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313
Keppler F, Hamilton JTG, Brab M, Rockmann T (2006) Methane emissions from terrestrial plants under aerobic conditions. Nature 439:187–191
Khalil MAK, Rasmussen RA (1994) Global emissions of methane during the last several centuries. Chemosphere 29:833–842
Khan ST, Mubeen U (2012) Wheat straw: a pragmatic overview. Curr Res J Biol Sci 4:673–675
Kim SS, Agblevor FA, Lim J (2009) Fast pyrolysis of chicken litter and turkey litter in a fluidized bed reactor. J Ind Eng Chem 15:247–252
King GM (1997) Responses of atmospheric methane consumption by soils to global climate change. Glob Chang Biol 3:351–362
Krause S, Ke CL, Frenzel P (2010) Succession of methanotrophs in oxygen–methane counter-gradients of flooded rice paddies. Int Soc Microb Ecol J4:1603–1607
Lai W-Y, Lai C-M, Ke G-R, Chung R-S, Chen C-T, Cheng C-H, Pai C-W, Chen S-Y, Chen C-C (2013) The effect of woodchip biochar application on crop yield, carbon sequestration and greenhouse gas emission from soils planted with rice or leaf beet. J Taiwan Inst Chem Eng 44:1039–1044
Laird DA (2008) The Charcoal vision: a win–win–win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. J Agron
Lehmann J (2007) A handful of carbon. Nature 447:143–144
Lehmann J, Joseph S (2009) Biochar for environmental management an introduction. ES-BEM-16:17–23
Li YL, Wang XX (2013) Root-induced changes in radial oxygen loss, rhizosphere oxygen profile, and nitrification of two rice cultivars in Chinese red soil regions. Plant and Soil 36:115–126
Liu S, Li Y, Wu J, Huang D, Su Y, Wei W (2010) Spatial variability of soil microbial biomass carbon, nitrogen and phosphorus in a hilly red soil landscape in subtropical China. Soil Sci Plant Nutr 56:693–704
Liu YX, Yang M, Wu YM, Wang HL, Chen YX, Wu WX (2011) Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J Soil Sediment 11:930–939
Ludmila C, Julia A, Rudoyk RS, Toms M, Lidstrom E (1998) G transfer enzymes and coenzymes were linking methylotrophic bacteria and methanogenic archaea. J Sci 281:99–101
Luke C, Bodrossy L, Lupotto E, Frenzel P (2011) Methanotrophic bacteria associated to rice roots: the cultivar effect assessed by T-RFLP and microarray analysis. Environ Microbiol Rep 3:518–525
Mahinpey N, Murugan P, Mani T, Raina R (2009) Analysis of bio-oil, biogas, and biochar from pressurized pyrolysis of wheat straw using a tubular reactor. Energy Fuel 23:2736–2742
Masulili A, Utomo WH (2010) Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and Its Influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J Agric Sci 2:1
McLaughlin S, Walsh M (1998) Evaluating environmental consequences of producing herbaceous crops for bioenergy. Biomass Bioener 14:317–324
Mer JL, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50
Meyer S, Glaser B, Quicker P (2011) Technical, economical and climate related aspects of biochar production technologies: a literature review. Environ Sci Technol 45:9473–9483
Milla OV, Rivera EB, Huang WJ, Chien CC, Wang YM (2013) Agronomic properties and characterization of rice husk and wood biochars and their effect on the growth of water spinach in a field test. J Soil Sci Plant Nutr 13:251–266
Mohammed IY, Abakr YA, Kazi FK, Yusuf S, Alshareef I, Chi SA (2015) Pyrolysis of Napier grass in a fixed bed reactor: effect of operating conditions on product fields and characteristic. Bio Res 10:6457–6478
Mohanty SR, Bodelier PLE, Conrad R (2007) Effect of temperature on composition of the methanotrophic community in rice field and forest soil. FEMS Microbiol Ecol 62:24–31
Mukherjee A, Lal R (2013) Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 3:313–339
Mukherjee A, Lal R, Zimmerman AR (2014) Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci Total Environ 487:26–36
Neue NU (1993) Methane emission from rice fields. Bioscience 43:466–474
Pan GX, Zhou P, Li ZP, Smith P, Li LQ, Qiu DS, Zhang XH, Xu XB, Shen SY, Chen XM (2009) Combined inorganic/organic fertilization enhances N efficiency and increases rice productivity through organic carbon accumulation in a rice paddy from the Tai Lake region, China. Agric Ecosyst Environ 131:274–280
Pandey VC, Singh JS, Singh DP, Singh RP (2014) Methanotrophs: promising bacteria for environmental remediation. Int J Environ Sci Technol 11:241–250
Peter W (2007) Biochar and bioenergy production for climate change mitigation. New Zealand Sci Rev 64
Phillips R, Whalen SC, Schlesinger WH (2001) Influence of atmospheric CO2 enrichment on methane consumption in a temperate forest soil. Glob Chang Biol 7:557–563
Prommer J, Wanek W, Hofhansl F, Trojan D, Offre P, Urich T, Schleper C, Sassmann S, Kitzler B, Soja G, Hood-Nowotny RC (2014) Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS One 9:86388
Reddy KR, Asce F, Yargicoglu EN, Asce SM, Yue D, Yaghoubi P (2014) Enhanced microbial methane oxidation in landfill cover soil amended with biochar. J Geotech Geoenviron Eng 11:1090–0241
Sadaka S, Sharara MA, Ashworth A, Keyser P, Allen F, Wright A (2014) Characterization of biochar from switchgrass carbonization. Energy 7:548–567
Schlesinger HW (1997) Biogeochemistry: an analysis of global change. Academic Press, New York
Shackley S, Carter S, Knowles T, Middelink E, Haefele S, Sohi S, Cross A, Haszeldine S (2012) Sustainable gasification–biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: context, chemical properties, environmental and health and safety issues. Energy Policy 42:49–58
Shima SL (1998) Mechanism of biological methane formation: structure and function of methyl-coenzyme M reductase. Prote Nucleic Acid Enzyme 43:1461–1467
Siljanen HMP, Saari A, Krause S, Lensu A, Abell GCJ, Bodrossy L, Bodelier PLE, Martikainen PJ (2011) Hydrology is reflected in the functioning and community composition of methanotrophs in the littoral wetland of a boreal lake. FEMS Microbiol Ecol 75:430–445
Singh JS (2010) Methanotrophs: the potential biological sink to mitigate the global methane load. Curr Sci 100:1–10
Singh JS, Gupta VK (2016) Degraded land restoration in reinstating CH4 sink. Front Microbiol 7(923):1–5
Solomon S, Qin D, Manning M, Alley RB, Berntsen T, Bindoff NL, Chen Z, Chidthaisong A, Gregory JM, Hegerl GC, Heimann M, Hewitson B, Hoskins BJ, Joos F, Jouzel J, Kattsov V, Lohmann U, Matsuno T, Molina M, Nicholls N, Overpeck J, Raga G, Ramaswamy V, Ren J, Rusticucci M, Somerville R, Stocker TF, Whetton P, Wood RA, Wratt D (2007) Technical summary. In: Solomon SD, Qin M, Manning Z, Chen M, Marquis KB, Averyt M, Tignor HL, Miller (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Songa W, Guo M (2012) Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J Anal Appl Pyrolysis 94:138–145
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Tamai N, Takenaka C, Ishizuka S (2007) Water soluble Al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil. Soil Biol Biochem 39:1730–1736
Tiwari S, Singh JS, Singh DP (2015) Methanotrophs and CH4 sink: effect of human activity and ecological perturbations. Clim Change Environ Sustainabil 3:35–50
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil concepts and mechanisms. Plant Soil 300:9–20
Wu F, Jia Z, Wang S, Chang SX, Startsev A (2013) Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a chernozemic soil. Biol Fertil Soils 49:555–565
Wu M, Feng Q, Sun X, Wang H, Gielen G, Wu W (2015) Rice (Oryza sativa L) plantation affects the stability of biochar in paddy soil. Scientific Report 5, Article number: 10001
Wuddivira MN, Stone RJ, Ekwue EI (2009) Structure stability of humid tropical soils as influenced by manure incorporation and incubation duration. Soil Sci Soc Am J 73:1353–1360
Wuebbles DJ, Hayhoe K (2002) Atmospheric methane and global change. Earth Sci Rev 57:177–210
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut and soil chemical properties in south Sumatra, Indonesia. Soil Sci Plant Nutr 52:489–495
Yang Y, Ma S, Zhao Y, Jing M, Xu Y, Chen J (2015) A field experiment on enhancement of crop yield by rice straw and corn stalk-derived biochar in Northern China. Sustain 7:13713–13725
Yao H, Conrad R (1999) Thermodynamics of methane production in different rice paddy soils from China, the Philippines, and Italy. Soil Biol Biochem 31:463–473
Yao H, Conrad R (2001) Thermodynamics of propionate degradation in anoxic paddy soil from different rice-growing regions. Soil Biol Biochem 33:359–364
Yargicoglu EN, Sadasivam BY, Reddy KR, Spokas K (2015) Physical and chemical characterization of waste wood derived biochars. Waste Manag 36:256–268
Zhang XC, Liu XH (2012) Effect of biochar on pH of alkaline soils in the loess plateau: results from incubation experiments. Int J Agric Biol 14:745–750
Zhanga A, Biana R, Pana G, Cuia L, Hussaina Q, Li L, Jinwei Z, Zhenga J, Zhanga X, Xiaojun H, Yua X (2010a) Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: a field study of 2 consecutive rice growing cycles. Field Crop Res 127:153–160
Zhanga A, Cuia L, Pana G, Li L, Hussaina Q, Zhanga X, Zhenga J, Crowley D (2010b) Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric Ecosyst Environ 139:469–475
Zwieten V, Singh LB, Joseph S, Kimber S, Cowie A, Yin Chan K (2009) Biochar and emissions of non-CO2 greenhouse gases from soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management. Earthscan, London
Zwieten VL, Kimber S, Morris S, Chan YK, Downie A, Rust J, Joseph S, Cowie A (2010) Effect of biochar from slow pyrolysisi of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Singh, C., Tiwari, S., Boudh, S., Singh, J.S. (2017). Biochar Application in Management of Paddy Crop Production and Methane Mitigation. In: Singh, J., Seneviratne, G. (eds) Agro-Environmental Sustainability. Springer, Cham. https://doi.org/10.1007/978-3-319-49727-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-49727-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49726-6
Online ISBN: 978-3-319-49727-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)