Abstract
Industrial revolution resulted in plenty of contaminants in the environment. Several organic and inorganic pollutants have adversely affected soils and water resources, causing serious health issues in humans. Among inorganic contaminants heavy metals are of prime importance as they are nondegradable in the environment. Arsenic, cadmium, chromium, cobalt, copper, lead, mercury, selenium, zinc, and other metals originating from various point and nonpoint sources are contaminating natural resources. Elevated concentrations of poisonous metals are not only disturbing soil health and microbial ecology but also decreasing crop production and global food security. Entry of metal pollutants into the food chain is dangerous for human health. Serious efforts are needed to mitigate rising threats of metal contamination. Physical, chemical, and biological approaches can be used to remediate such type of pollutants. However, bioremediation is considered as a promising technique, being cost effective and environment friendly with minimum adverse effects, esthetic advantages, and long-term applicability. Phytoremediation is a type of bioremediation to remove toxic metals from soil through hyperaccumulation or phytostabilization in plant cells. Generally, higher contents of toxic metals in soil and water result in more uptake by roots and more translocation toward shoots, causing interference in metabolism and reduced growth. Successful phytoremediation is limited to the plant types, tolerance to the high metal concentrations, accumulation rate, growth rate, adaptability, and biomass production. Metal-tolerant bacteria can help plant to tolerate metal stress via different mechanisms involved including production of different hormones such as auxins, cytokinin, and gibberellic acid or suppressing stress-induced enzymes such as plant ethylene level. This chapter reviews possible interactions between plant and bacteria to make situations more conducive for remediation of metal-contaminated soil. The chapter also covers different strategies/mechanisms adopted by plants and bacteria to mitigate toxic effect of metals on plant growth in metal-contaminated soils.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Modernization has eased human life at the cost of enormous environmental pollution. Human settlements in urban areas and industrial growth have contributed majorly to the environmental concerns. In developing countries where industrial growth has become a prime focus and agricultural economics has been neglected, rural to urban shift has direct implications on soil, air, and water pollutions. Consequently, human health has to face various challenges due to the release of xenobiotics, pollutants, and heavy metals in the environment through industrial effluents. Many of the industries use heavy metals and their effluents containing significant concentrations of heavy metals are dumped without any treatment. The heavy metals released by the industries are deteriorating our soil and water resources. Entry of heavy metals in the food chain can be drastic as many of these heavy metals are carcinogenic to the human. Keeping in view the release of heavy metals to the environment and their associated threats, strategies to rehabilitate our environment must be devised. Microbial-based bioremediation has been considered as promising, cost effective, and environment friendly technique to decontaminate the heavy metals and other toxic compounds from environment (Singh et al. 2011). To understand the gravity of this problem and to cope with the possible consequences of heavy metal pollution, a comprehensive understanding needs to be developed in masses regarding the mechanisms and roles these heavy metals are playing in deterioration of environment and human health.
3.2 Heavy Metals as Soil Pollutants
Heavy metals are the transitional elements with densities higher than 5 g cm−3. Metals such as Iron (Fe), Zinc (Zn), Lead (Pb), Cadmium (Cd), and Mercury (Hg) are some examples of heavy metals. Heavy metal pollution is a major cause of environmental instability, due to their extensive use, distribution, and toxicity to the human. However, low concentrations of some elements such as Iron (Fe) and Zinc (Zn) are required for the proper functioning of human body (Rouphael et al. 2008).
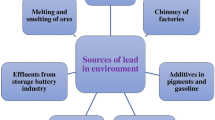
3.3 Sources of Heavy Metals
Metals in soil can accumulate naturally as well as by anthropogenic activities. Weathering of metal rocks, translocation of main land dust particles, and atmospheric secretions from volcanoes are the natural sources of heavy metal release into the environment. Whereas, anthropogenic sources of metal release into soil, water, and environment include exploitation of minerals through mining, agricultural utilization of sewage sludge as organic matter, increased use of electric appliances, metal consumption in industrial process, burning of metal-supplemented fossil fuel in vehicles, and increased reliance on military training to ensure countries defense. The chief man-made sources of heavy metal contamination to the soil are the application of untreated sludge to agricultural lands and industrialization (Shi et al. 2005).
Although industrial effluents are the main cause of heavy metal pollution, yet the domestic waste water also provide significant contribution in this kind of pollution. Agricultural soils in the close proximity of industries are highly polluted with heavy metal; however, the heavy metal pollution has also been found in the suburban to rural areas, where the injudicious use of pesticides, fertilizers, and irrigation with polluted water have contributed to the accumulation of heavy metals in the soil. Industrial wastes are discharged into the rivers, canal, and other water bodies without any sort of treatment. These metals when taken up by the human can be fatal and in acute case death may occur (Sanayei et al. 2009).
3.4 Heavy Metal Concentration
Rising infestation of heavy metals in the environment has hazardous influences on human health and agriculture. In the industrial cities concentrations of Hg, Cd, Pb, As, and Ni already have crossed the permissible limits in soil. In the various geological regions of world, the concentrations of these metals vary from less than 1 to 100,000 ppm. Whereas, permissible limits in the soil are 4960, 120, 480, 810, 460, and 410 ppm for tin, chromium, lead, manganese, nickel, and copper, respectively (Binggan and Yang 2010). The variability in the heavy metal contamination can be due to different agronomic practices. Increased use of phosphate fertilizers and pesticides may also be one reason of contamination of heavy metals in soil (Tumuklu et al. 2007).
3.5 Toxic Effects of Heavy Metals
Plants uptake ions present in the soil solution and utilize them in their metabolism. Simultaneously, nonspecific absorption of soluble heavy metals also occurs. Most of the heavy metals are nonessential for plants and are compartmentalized in the plant tissues (Mohammad et al. 2003). Accumulation of heavy metals is prominent in the crops growing near the industrial areas. Heavy metal exposure to plants at lower concentrations for long duration causes functional syndrome in plants and human. However, metal toxicity accompanied by oxidative stress is caused by high concentrations (John et al. 2009). Production of reactive oxygen species such as superoxide (O2 −), singlet oxygen (O−), hydrogen peroxide (H2O2), and hydroxyl ions (OH−) due to the oxidative stress generated by the heavy metals causes disintegration of cell membranes, imparts cell functioning, and eventually leads to cell death in plants. Bioaccumulation of heavy metals substitutes different enzymes and metals of prime importance by fostering oxidative stress which causes disruption of different functions. It also affects the plant growth by hindering the photosynthetic activity which causes senescence. Heavy metals are more toxic when they are present in their elemental or chemically combined state. Response of plants to these toxic metals depends on their nature and differs from species to species (Talanova et al. 2000). Metal such as cadmium (Cd) reduces the uptake of essential nutrients, decreases the photosynthetic activity, and slows down the plant growth. Reactive oxygen species are produced due to oxidative stress caused by mercury (Hg+2) that disturbs the mitochondrial activity and lead (Pb) at elevated levels dismantle mineral nutrition inhibiting the enzyme activity, causes water imbalance, and alters membrane permeability. In terms of growth, seedling is more susceptible to heavy metal toxicity as compared to seed germination. Moreover, heavy metal toxicity also disturbs many physiological processes such as photosynthesis, transpiration, and enzymatic activity of plants. Various researchers have investigated the harmful effects of heavy metals; Oancea et al. (2005) concluded retardation in growth of tomato and structural damage due to Cr, Hg, Cd, and Zn toxicity; these metals also effected the physiological and biochemical activities of tested plants. Weiqiang et al. (2005) compared the growth of seedlings and seed germination in heavy metals toxicity and found that seedling growth was more susceptible to heavy metal toxicity as compared to seed germination Tuna et al. (2002) evaluated heavy metal toxicity on germination and pollen tubes in tobacco plant (Nicotiana tabacum L.). Outcomes of the experiment showed that with the increasing concentration of heavy metal the pollen length was decreased. Peralta et al. (2004) checked different concentrations of heavy metals including Cd, Cu, Ni on growth of alfalfa (Medicago sativa) plants. Various concentrations of metals, viz., 0, 5, 10, 20, and 40 ppm were used. Results showed that Cd strongly affected the germination and growth of seeds at 10 ppm, while Cu and Ni at 20 ppm and higher concentrations. It has also been learnt that seed germination was not affected by Zn. Gopal and Khurana (2011) tested different heavy metals (Pb, Cr, Cu, Ni, and Cd) on plant growth and stress symptoms were visible at 0.25 mM of metal in soil. It was also observed that these heavy metals decreased leaf mass, plant height, growth, affected enzymatic activity, head size of flowers, delayed flowering, and also caused interveinal chlorosis.
3.6 Techniques Used for Remediation of Metal-Contaminated Soils
Different approaches are used to remediate the metal-contaminated soils. The choice of these approaches relies on the contaminant nature, cost of technologies, characteristics of sites, and time.
3.6.1 Physicochemical Techniques
Physicochemical methods used for remediation of heavy metals involved the following.
3.6.1.1 Isolation
In this technique, heavy metals movement is restricted or metals mobility is prevented. For this technique, physical barriers are used to prevent the vertical and horizontal movement of pollutant (Kabata-Pendias et al. 2010).
3.6.1.2 Separation of Heavy Metals Mechanically
This method involves separation of larger noncontaminated particles from smaller contaminated particles (Wuana and Okieimen 2011).
3.6.1.3 Remediation of Heavy Metals by Chemical Treatment
This technology involves use of chemicals such as hydrogen peroxide and chlorine to reduce the heavy metals movement in situ. This technology is performed in situ and has disadvantage of causing new source of pollution (Kabata-Pendias et al. 2010).
3.6.1.4 Electroremediation
This method involves passing of current having low intensity between anode and cathode in heavy metal polluted soil. In this process, metals can be recovered or removed through precipitation and electroplating (Kabata-Pendias et al. 2010).
3.6.1.5 Binding of Chemicals with Different Chelating Agent
This technique involves use of chemicals that may be organic and inorganic as chelating agent to bind the heavy metals. This process takes place in reactors. The chemicals involved in this process are organic acids and EDTA. The cleaned soil from which metals are removed is then returned to its former location. The efficacy of this process depends on the characteristics of soil (Kabata-Pendias et al. 2010).
3.6.1.6 Removal of Metals by Ion-Exchanging Process
This technique involves use of ion-exchanging materials to remove metal from contaminated soil. Ion-exchanging materials used in this process are chelating resins, zeolites, plant, and microbial biomass. This technique depends on pH and disadvantage of this technique is high cost (Kabata-Pendias et al. 2010).
3.6.2 Remediation of Metals by Biochemical Methods
Biochemical methods of metal remediation are as follows.
3.6.2.1 Bioleaching
This technique involves use of living organisms to extract the heavy metals from their ores. This technique uses several sulfur- and iron-oxidizing bacteria such as Thiobacillus ferrooxidans and Thiobacillus thiooxidans. These species are responsible for the formation of sulfuric acid from the oxidation of inorganic sulfur. This acid acts as metal chelator and used to remove the heavy metals from contaminated soil. Aspergillus niger is also involved in bioleaching process (Mulligan et al. 2004).
3.6.2.2 Biosorption
Biosorption involves concentration and interaction of organic pollutants or toxic metals in the biomass; this is taken as a potential tool for the remediation of metal-contaminated sites and for recovery of costly metals, offering a substitute to old methods such as adsorption and ion exchange on activated carbon. In biosorption, pollutants are bound to bacterial cell wall surface and are used to remove heavy metals from wastewaters, ground waters, and contaminated soils (Chojnacka 2010; Ansari and Malik 2007).
Bioremediation is an in situ remediation technique providing more advantages over the conventional chemical and physical treatments (Radhika et al. 2006).
3.6.2.3 Metal–Microbe Interactions
Metal–microbe interactions are of utmost importance both from plant growth promotion and bioremediation point of view (Ianeva 2009). Although certain heavy metals are needed by the plants for metabolic functioning but their higher concentrations are toxic to the plants (Hynninen et al. 2009). On the contrary, nonessential heavy metals are poisonous to plants and animals. Such metals are arsenic, cadmium, lead, and mercury. They are therefore termed as “toxic metals” (Janssen et al. 2010). These enter into the plant body through the uptake system of essential nutrient elements. At molecular level, these heavy metals get attached with thiol groups and did not allow essential metals to attach. Basically these nontoxic metals displace the essential metals (Ca, K, and Mg) and attach themselves.
Moreover, Pb has the ability to enter into plant body by attaching with the Ca and Zn transport proteins. Resultantly, conformational arrangements of proteins, enzymes, and nucleic acids are disrupted. It also causes disturbance in membrane functions, osmotic balance, and interference with oxidative phosphorylation (Bruins et al. 2000). Bioavailability of metals/metalloids governs their toxicity to the microorganisms. Consequently, with the decrease in pH, the bioavailability of metals to plants increases. It is because of more available concentration of metals in solution form. To overcome this situation, bacteria have adopted mechanisms to resist the higher concentration of these metals. They either pump metals out of their body or hyperaccumulate by converting into less toxic form (Bruins et al. 2000; Ianeva 2009). Additionally, bacterial population exists in the environments with high metal concentration (Bruins et al. 2000). Under heavy metal-stressed environment, microbes have developed numerous mechanisms to help them out. They can mobilize, immobilize, or transform metals. It renders metal ions subjected to plant intake (Shukla et al. 2010). Microorganisms in metal contamination can use either single or combination of mechanisms for existence (Hu et al. 2006).
Isolation of bacterial strains, resilient to the heavy metal toxicity, has been reported in many studies. Since 1970s, aerobic bacteria were mostly found resistant to the heavy metal infestation. Major examples of resistive microorganisms include species of Bacillus and Staphylococcus, in addition to Pseudomonas aeruginosa and Escherichia coli (Bruins et al. 2000). For instance, certain bacteria were identified to survive in Lead (Pb) and Zinc (Zn) mining sites even at massively high 204 μg Pb/g soil (Hu et al. 2006). Resilience to the heavy metal toxicity in bacteria may be due to genetic determinants localized on chromosomes and extra-chromosomal genetic materials like transposons and plasmids (Bruins et al. 2000). Transfer of genetic tendencies to the bacteria has a significant contribution in the heavy metal resistance (Gadd 2010). Among the various mechanisms involved in metal stress tolerance in bacteria (Shukla et al. 2010), the following five are of prime importance:
-
Metal ion efflux
-
Metal exclusion
-
Enzymatic detoxification
-
Intracellular sequestration
-
Extracellular sequestration
Primarily, prokaryotes depict resistance to the toxicity by the active efflux of poisonous metal ions from the cell (Hynninen et al. 2009). Metal resistance system is majorly managed by this active efflux of ions (Bruins et al. 2000). However, intracellular complex formation (mainly in eukaryotes), certain binding factors, and enzyme-mediated reactions such as methylation, demethylation, oxidation, and reduction of metal also contribute significantly in preventing of adverse impact of heavy metals (Hynninen et al. 2009).
3.7 Metal Resistance Mechanisms Used by Microorganisms
Various genera of gram positive and negative bacteria have been identified for metal resilience in polluted soils (Taghavi et al. 2009b). Although in the current literature the mechanism of resistance to metal is still unknown and bacterial approaches of defense against metal toxicity are little known, some important strategies have been identified. In particular, mechanisms of active efflux and precipitation of the heavy metals in insoluble forms are common strategies employed by bacteria against heavy metals tolerance (Mire et al. 2004). Indeed energy-consuming transportation of ions against the gradient by employing ATP-dependent efflux pumps and sequestration of metal ions at the intracellular spaces are effective to remediate highly bioavailable metals accessing the cell membranes.
3.8 Metal Sequestration
Heavy metal-tolerant bacteria have the ability to sequester metals intra as well as extracellular and results in reduced mobilization of the metals. These bacteria actually use intra and extracellular mechanisms to avoid toxicity and it is well reported in the literature. A vast variety of these bacteria can precipitate metals particularly as metal–phosphate and some other forms. Accumulation of sequestered metal depends upon the type of bacterial strain, its growth stage, and environmental conditions containing metals (Mire et al. 2004).
3.8.1 Intracellular Sequestration
Intracellular sequestration is a metal accumulation strategy of bacteria in which metals are accumulated internally especially in cytoplasm to protect the other essential parts of the cell from exposure to toxic metals (Bruins et al. 2000; Mire et al. 2004). These bacteria have the ability to overexpress the Metallothionein (MT) genes after the exposure to heavy metals. MTs are cysteine-containing proteins with low molecular weight. These have high affinity for toxic (Cr and As) and essential (Mn and Fe) metals. Abundant quantity of this protein is also present in fungi, animals, and plants. Proper biological function of MTs is still not illustrated properly but its role for metal sequestration is well documented. In fact the transcription of MT gene is induced by the metal such as Pb, as indicated for a Streptomyces strain’s ability to resist to high concentration of the heavy metals such as Zn, Cu, and Pb (Rifaat et al. 2009). Intracellular sequestrations by binding proteins have also been reported in a Synechococcus sp. by producing MT proteins as a form of resistance (Bruins et al. 2000). Besides, the molecular mechanism remains to be elucidated, resistant strains of Bacillus megaterium, Staphylococcus aureus, Citrobacter freundii, and Vibrio harveyi have been reported to lower the concentration of free lead ions by precipitating lead and accumulating the metal as an intracellular cytoplasmic phosphate salt. In particular, the Vibrio harveyi strain was capable of precipitating Pb in large quantity as phosphate compound (Mire et al. 2004). The product accumulated by the Citrobacter sp. is recognized as PbHPO4 and same precipitate also produced by Staphylococcus aureus strains. Staphylococcus aureus strains, both Pb-resistant and Pb-sensitive strains were able to sequester the lead, but only the Pb-resistant bacteria stored the metal as intracellular lead–phosphate in electron-dense inclusions. Actually metal sequestration by bacteria is a two-step process. In the first step, metals are attached to the negatively charged surface of the microbe. Negative charge on the surface of microbe is due to the negatively charged functional groups on the surface. In the second step, these metals are taken inside the body of microbe. Although Pb-sensitive cells also bind Pb(II) initially, the crystals of Pb–phosphate were not present in different compartments of the cells of sensitive isolates of bacteria as probably lacking the system for precipitating the metal as Pb–phosphate. After examination, negligibly soluble nontoxic phosphate crystals were found and the mechanism was supposed to continue until the Pb(II) concentration overwhelms the binding capacity of the cell (Levinson et al. 1996).
Metal-solubilizing bacteria precipitation has also been reported in a Klebsiella strain cultured in phosphate-limited medium. This bacterium was in fact able to precipitate PbHPO4 granules on the cellular surface as reported for a Citrobacter species grown in the presence of lead, while it accumulates PBS in electron-dense granules in the cells in phosphate-limited cultures (Mire et al. 2004).
3.8.2 Extracellular Sequestration
Metal resistance based on extracellular sequestration results from the binding of toxic metal in a complex, thus it cannot enter the cell membrane. This mechanism has been found in bacteria and even in many species of yeast and fungi. Saccharomyces cerevisiae excretes large amounts of glutathione which may reduce absorption of Ni(II), which binds with great affinity to heavy metals. Other organisms such as yeast form insoluble complexes of phosphate to increase resistance (Bruins et al. 2000).
A lead-resistant Pseudomonas marginalis strain has been reported to avoid lead toxicity by precipitating it as an extracellular polymer. In the absence of Pb, P. marginalis still produced the polymer indicating that it is a metal independent process. Extracellular polymer production is a frequent and unique process by some microbes to overcome metal stress. Detailed and comprehensive investigations of microbial sequestered compounds are scarce. P. fluorescens produced precipitates that contain abundant phosphate and Pb. Furthermore, both phosphate-starved and phosphate-replete P. fluorescens cultures have been reported to generate an insoluble material containing both lead and phosphorus, although phosphate-replete cultures are apparently more efficient at expelling the material.
3.8.3 Plant–Microbe Interactions
Plant root surface and soil area around root called rhizosphere is a very complex medium that contains huge microbial activity. Rhizosphere contains about one to two fold more microbial population as compared to the bulk soil (Maier et al. 2009). It might be due to the high concentration of nutrients in the rhizosphere. As we move from rhizosphere to bulk soil, the nutrient concentration reduces and similar trend is observed in microbial population density. Furthermore, plant roots also produce organic metabolites that act as carbon, energy, and food source for the bacteria. Roots aerate rhizosphere to support the microbial activity (Belimov et al. 2001). Some of the bacteria in reverse have also the ability to support the plant either by enhancing the nutrient availability or by producing the plant growth regulators and protecting plant from the pathogens. This group of bacteria is called plant growth promoting rhizobacteria (PGPR ) . These bacteria consolidate the plant defense mechanisms under stress conditions like heavy metal stress, salinity, and drought (Erturk et al. 2010; Khan et al. 2009; Jing et al. 2007) and ultimately improve the plant growth in heavy metal-contaminated soil (Dary et al. 2010).
3.9 Phytoremediation of Contaminated Soils
It is a process that uses different plants to remediate the metal-contaminated sites either by extracting them out or stabilizing them in the soil. Some of the plants have the capacity to permanently remove the metals from soil by accumulating metal in under and above grounds parts while others produce such rhizospheric compounds that made compounds with metals by reducing their availability to the plants. In this process, metals remain in soil and only their mobility is minimized. The main process involved in the uptake of metals is absorption. This method is among the most economical and eco-friendly approach (Mangkoedihardjo, 2007).
Different processes involved in phytoremediation of heavy metal-contaminated soil are phytoextraction, phytostabilization, phytovolatilization, and microbe-assisted phytoremediation. The process in which contaminants are contained by plant or immobilized in the soil or ground water is known as phytostabilization . It comprises the application of plants to decrease the bioavailability and movement of contaminants in soil. Plants directly stabilize contaminants by adsorption of the contaminants on the root surface, accumulation by the roots, or isolation within the root zone using plants as organic pumps (Pilon-Smits 2005). Phytovolatilization is the movement of a contaminant out of the soil or groundwater and into, through, and out of a plant into the atmosphere. In this process, the contaminant or its metabolite is released into the atmosphere (Pilon-Smits 2005). Phytoextraction (or phytoaccumulation) is the use of plants to remove pollutants from contaminated soil into their above ground parts which can then be harvested and it has been considered as a cost-effective, environment friendly strategy for the cleanup of metal-enriched soils (Manousaki and Nicolas 2009). Actually at the time of plant disposal, which can be composted or incinerated, contaminants are stored in the much smaller plant matter volume than in initially polluted soil or sediments. In fact, plants absorb heavy metals by the root system and concentrate them in the biomass of root and/or transport them into shoots and/or leaves, and plant may continue to uptake these heavy metals until it is harvested. After harvest, a minute concentration of heavy metals will remain in soil, so growth or harvest cycle must be repeated through many crops to get a significant cleanup. After this process, the soil can support other vegetations (Shukla et al. 2010).
However, higher contents of toxic metals in soil and water have resulted in more uptakes by roots and more translocation toward shoots, causing interference in normal metabolism and reduced growth. The success of phytoremediation is limited even with hyperaccumulators due to slow growth and less biomass production because of toxicity and elevated levels of metal ions. Phytoremediation alone is a time-consuming process and its success depends upon the metal tolerance, accumulation, and high biomass production capability of plants (Grčman et al. 2001). So, this situation could be improved and enhanced by assistance of plants with metal-tolerant bacteria having plant growth promotion activities (Ma et al. 2011; Khan et al. 2013).
3.10 Plant Growth-Promoting Rhizobacteria
Heterogeneous group of bacteria that have ability to enhance plant growth in association with plant roots inhabiting around the root is called plant growth-promoting rhizobacteria (PGPR ). Mainly reported PGPR species are Pseudomonas, Azotobacter, Klebsiella, Enterobacter, Arthrobacter, Azospirillum, Burkholderia, Serratia, and Bacillus. In the past few years, comprehensive research work has been carried out to get the better understanding of mechanisms of PGPR (Khan et al. 2009). These bacteria can enhance the plant growth by different direct and indirect mechanisms. Direct mechanisms include nutrient availability (solubilization of mineral phosphates and nitrogen fixation and synthesis of siderophore) and production of plant growth regulators (indole-3-acetic acid (IAA), cytokinins, ethylene, and gibberellic acid). While in indirect mechanism bacteria protect plant from pathogens by producing cyanide and antibiotics. But still exact mechanisms of plant growth promotion are not fully figured out (Erturk et al. 2010; Banerjee et al. 2010).
These bacteria are also helpful for the plant under stressed conditions. Under stressed conditions plants produce more ethylene that has a negative impact on plant growth. PGPR has the ability to reduce the plant ethylene level through enzymatic breakdown of ACC into ammonia and α-ketobutyrate. It is reported that establishment of ACC sink by bacterial population and reduction in the ethylene level consequently cause elongation of root, encourage the formation of longer roots, and decrease hazardous effects of stress that may increase the plant growth and seedling viability. Moreover, rhizobacteria play crucial role in the plant–bacterial interactions through the production of indole-3-acetic acid and phytostimulation efficiency. Under contaminated condition, biosynthesis of auxins and their release into the soil makes important contribution in plant growth promotion (Erturk et al. 2010). It is well documented that under high level of heavy metals condition, even metal-accumulating and tolerant plants are also affected by the heavy metals. So iron deficiency was detected in different plant species in the soil contaminated with heavy metals. Consequently, plant becomes chlorotic due to iron deficiency that causes inhibition of chloroplast development and chlorophyll biosynthesis. However, siderophores–iron complexes can mitigate the iron deficiency and act as a source of iron for plant. Under iron limiting conditions, siderophores produced by bacteria have iron acquisition ability in the form of Fe(III) chelators which is taken up by the plant roots (Kuffner et al. 2008).
3.11 Microbial-Induced Bioremediation
Microbial-induced bioremediation exploits the genetic and biochemical capacities of bacteria for the remediation of organic compounds and heavy metals. Therefore, due to the ability to tolerate metal toxicity, adsorb and accumulate heavy metals ions, or degrade organic pollutants, specific microorganisms can be studied and used in bioremediation of polluted environments. First, it is important to consider that every remediation approach is site specific and has to take into account the peculiar characteristics of the contamination and contaminated area. Moreover, no organisms or groups of organisms are universally applicable to all cases, although some can be metabolically versatile and are capable of degrading a wide spectrum of substrates, thus all procedures will be necessarily site specific. Depending on the detection in the contaminated matrix of metabolic activity functional to the contaminant detoxification, microbe induced-bioremediation relies on two approaches: biostimulation, stimulating native microbial population; and bioaugmentation, which imply an introduction of viable population to the contaminated area (Shukla et al. 2010). Actually if a functional metabolic activity is present, in a biostimulation protocol, soil conditions are modified to enhance catalytic capacities of autochthonous microorganism by supplementing nutrients (nitrogen and phosphorus) and/or electron acceptors (oxygen) until a decontaminated desired threshold is reached. On the other side, in absence of a sufficient metabolic activity, functional to the contaminant remediation, it is possible to introduce a viable population with desired catalytic capabilities adopting a bioaugmentation protocol. In the case, a massive quantity of autochthonous microorganisms previously cultivated or allochthonous microorganisms with desired metabolic characteristics are bioaugmentated to the soil itself (Shukla et al. 2010). Bacteria can adopt bioaccumulation and biosorption mechanism. Bioaccumulation and biosorption involve concentration and interactions of organic pollutants or toxic metals in the biomass, either nonliving (biosorption) or living (bioaccumulation) is taken as a potential tool for the remediation of metal-contaminated sites and for recovery of costly metals, offering a substitute to old methods like adsorption and ion exchange on activated carbon. In biosorption, pollutants are bound to bacterial cell wall surface while in bioaccumulation these become accumulated under the cell. These both techniques are used to remove heavy metals from wastewaters, ground waters, and contaminated soils (Chojnacka 2010).
3.12 Plant Growth-Promoting Rhizobacteria-Assisted Phytoremediation
Phytoremediation can be considered as the most successful methodology for remediation of pollutants from contaminated water and soils. In this method, plant endurance and accumulation ability are very imperative (Paz-Alberto and Sigua 2013). Hyperaccumulators have the capability to extract considerable amounts of pollutants from shallow soil surfaces and water (Garbisu and Alkorta 2003). Different crops such as Indian mustard, sunflower, and alfalfa are efficient hyperaccumulators of Pb from soils but even then these gains are small size. In such a scenario, even by the use of hyperaccumulating plant for the removal of metals it could take years to completely remediate soils.
An alternative strategy to increase the efficiency of the phytoremediation is the inoculation with plant growth-promoting bacteria that facilitate the growth of hyperaccumulator in metal stress. In stress conditions, growth suppressing ethylene in plants can be lowered by inoculation with selected bacteria (Ahmad et al. 2011); such bacteria can also provide the plant with growth regulators and ultimately could improve the efficiency of phytoremediation (Fassler et al. 2010). Numerous findings have been reported, which support application of plant growth-promoting bacteria to facilitate metal phytoextraction (Table 3.1).
In stress conditions, microbial activity is also reduced (Asghar et al. 2012) but plants may help microbes by producing root exudates. Therefore, plant–microbes interaction can improve the phytoremediation efficiency. Phytoremediation assisted by soil rhizobacteria (also called rhizodegradation, rhizoremediation, enhanced rhizosphere biodegradation, microbially assisted phytoremediation) involves the breakdown of contaminants by mutual interaction of plant roots and microbes in the rhizosphere (Shukla et al. 2010). Plant root exudates act as source of carbon, energy, and nutrients for the microflora of soil and promote the activity of microbes (Shukla et al. 2010).
Rhizospheric bacteria have ability to detoxify a variety of toxic metals/compounds efficiently. Different studies on rhizoremediation to explore the symbiotic association reported exhaustion of volatile organic contaminants, naphthalene and polychlorinated biphenyls and trichloroethylene (Shukla et al. 2010). Relationship between plant growth-promoting rhizobacteria and plant to enhance the uptake of toxic metals has been well established and recently phytoremediation associated with microbes has arisen as a successful strategy (Koo and Cho 2009).
PGPR have ability to enhance the growth of the host plant by various mechanisms involving production of specific compounds and increasing nutrient uptake. Further these bacteria can reduce the toxicity of heavy metals and promote the growth of plants under the toxicity of Ni, Pb, or As (Jing et al. 2007). Furthermore, some rhizobacteria can excrete organic acids to enhance the bioavailability of heavy metals and a variety of bacteria (mainly PGPR ) have been reported as phytoextraction assistants, such as Pseudomonas spp., Bacillus spp., Mesorhizobium sp., Microbacterium spp., Rhizobium spp., Sinorhizobium sp., and Achromobacter sp. (Koo and Cho 2009).
Plant roots can also increase metal bioavailability by exuding low molecular weight organic acids and protons that cause decrease in pH of the soil and mobilize the metals. Retardation of heavy metals adsorption and high accumulation is just because of the decrease in the soil pH; moreover, the formation of soluble complex of heavy metals reacting with exuded organic acids also increases bioavailability of heavy metals to plants (Glick et al. 2007).
There are several other mechanisms and bacterial traits that increase the metals phytoremediation along with other previously reported growth promotion activities. For example, increased bioavailability of some metals for phytoremediation is supplemented with metal-binding peptides synthesis by genetically engineered bacteria (Wu et al. 2006). In addition, several scientists have found that efficient phosphate solubilization system present in bacteria can facilitate phytoremediation by its vital role in acquisition of metals. Metals bioavailability is increased when biosurfactant-producing bacteria are used in phytoremediation. The advanced experimentations on PGPR for the remediation of contaminated soils show a novel and innovative prospect for the successive studies. For example, rhizobacteria have proved to increase the acquisition of Cd in Brassica napus (Sheng and Xia 2006), of Ni in Alyssum murale (Abou-Shanab et al. 2007), and significantly improved Cu uptake by B. juncea (Ma et al. 2009). Along with free-living microbial association with plants to mitigate phytoremediation process, symbiotic relationship between metal-resistant rhizobial strains and their respective host has also promising output as metal uptake up to 80 % more in inoculated M. pudica than noninoculated plant has been observed (Chen et al. 2008).
3.13 Conclusions
Bacterial-assisted phytoremediation is considered a promising approach as compared to conventional remediation techniques for metal-contaminated soils. Plant growth-promoting rhizobacteria produce certain plant growth regulators and different enzymes that enhance plant growth under stress conditions. While in response plants provide carbon, energy, and nutrients in the form of root exudates and make conducive environment for the microbes.
However, there are several knowledge barriers that need to be addressed. Prominent among them include the understanding of the ecology and dynamics of PGPR under field conditions. Further, research needs to be focused on understanding the mechanism involved in the remediation process and their genetic characteristics.
References
Abou-Shanab R, Berkum PV, Angle JS (2007) Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 68:360–367
Ahmad M, Zahir ZA, Asghar HN (2011) Inducing salt tolerance in mugbean through co-inoculation with Rhizobium and PGPR containing ACC-deaminase. Can J Microbiol 57:578–589
Ansari MI, Malik A (2007) Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour Technol 98:3149–3153
Asghar HN, Setia R, Marschner P (2012) Community composition and activity of microbes from saline soils and non-saline soils respond similarly to changes in salinity. Soil Biol Biochem 47:175–178
Banerjee S, Rakhi P, Chandan S, Standing D (2010) Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Aust J Crop Sci 4(6):378–383
Belimov A, Safronova V, Sergeyeva T, Egorova T, Matveyeva V, Tsyganov V, Borisov A, Tikhonovich I, Kluge C, Preisfeld A, Dietz K, Stepanok V (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 49(2):151–156
Binggan W, Yang L (2010) A review of heavy metal contaminations in urban soils, urban raod dusts and agricultural soils from China. Microchem J 94:99–107
Bruins MR, Sanjay K, Frederik O (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45(3):198–207
Chen WM, Wu CH, James EK, Chang JS (2008) Metal biosorption capability of Cupriavidus taiwanensis and its effects on heavy metal removal by nodulated Mimosa pudica. J Hazard Mater 151:364–371
Chojnacka K (2010) Biosorption and bioaccumulation-the prospect for practical applications. Environ Int 36:299–307
Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E (2010) “In situ” phytostabilization of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177(1–3):323–330
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107(5):1687–1696
Erturk Y, Ercisli S, Haznedar A, Cakmakci R (2010) Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. J Biol Res 43(1):91–98
Fassler E, Evangelou MW, Robinson BH, Schulin R (2010) Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 80:901–907
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 159:609–643
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56(4):403–407
Garbisu C, Alkorta I (2003) Basic concepts on heavy metal soil bioremediation. Eur J Mineral Process Env Protect 3:229–236
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26(5-6):227–242
Gopal R, Khurana N (2011) Effect of heavy metal pollutants on sunflower. Afr J Plant Sci 5:531–536
Grčman H, Velikonja-Bolta Š, Vodnik D, Kos B, Leštan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation leaching and toxicity. Plant Soil 235:105–114
He LY, Chen ZJ, Ren GD, Zhang YF, Qian M, Sheng XF (2009) Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol Environ Saf 72(5):1343–1348
Hu Q, Hong-yan Q, Jing-hai Z, Hong-xun Z (2006) Bacterial diversity in soils around a lead and zinc mine. J Environ Sci 19(1):74–79
Hynninen A, Thierry T, Leena P, Dominique M, Marko V (2009) An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol Microbiol 74(2):384–394
Ianeva OD (2009) Mechanisms of bacteria resistance to heavy metals. Microbiology 71(6):54–65
Janssen PJ, Rob VH, Hugo M, Pieter M, Nicolas M, Mohammed B, Natalie L, Tatiana V, Alla L, Arlette M, Se'bastien M, Claudine T, Safiyh Sean M, John D (2010) The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS One 5(5):10433
Jing YZ, He Z, Yang X (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci 8:192–207
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: effect on plant growth, biochemical parameters and metal accumulation by Brassica junica L. Int J Plant Prod 3:1735–8043
Kabata-Pendias A (2010) Trace elements in soils and plants. CRC, Boca Raton
Khan MS, Almas Z, Ahmad WP, Mohammad O (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:1–19
Khan MY, Asghar HN, Jamshaid MU, Akhtar MJ, Zahir ZA (2013) Effect of microbial inoculation on wheat growth and phyto-stabilization of chromium contaminated soil. Pak J Bot 45(SI):27–34
Khan AR, Ullah I, Khan AL, Park G, Waqas MS, Hong BK, Jung Y, Kwak Y, Lee I, Shin J (2015) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp RSC-14 inoculation. Environ Sci Pollut Res 22:14032–14042
Koo S, Cho K (2009) Isolation and characterization of a plant growth-promoting Rhizobacterium, Serratia sp SY5. J Microbiol Biotechnol 19(11):1431–1438
Kuffner M, Puschenreiter M, Wieshammer G, Gorfer M, Sessitsch A (2008) Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. Plant Soil 304:35–44
Kumar KV, Singh N, Behl HM, Srivastava S (2008) Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72(4):678–683
Kumar KV, Srivastava S, Singh N, Behl HM (2009) Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. J Hazard Mater 170(1):51–57
Levinson HS, Mahler I, Blackwelder P, Hood T (1996) Lead resistance and sensitivity in Staphylococcus aureus. FEMS Microbiol Lett 145:421–425
Li Y, Wang Q, Wang L, He L, Sheng X (2016) Increased growth and root Cu accumulation of Sorghum sudanense by endophytic Enterobacter sp. K3-2: implications for Sorghum sudanense biomass production and phytostabilization. Ecotoxicol Environ Saf. doi:10.1016/j.ecoenv.2015.10.012
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258
Maier RM, Ian LP, Charles PG (2009) Environmental microbiology. Elsevier Inc, Amsterdam
Mangkoedihardjo S (2007) Phytotechnology integrity in environmental sanitation for sustainable development. J Appl Sci Res 3(10):1037–1044
Manousaki E, Nicolas N (2009) Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environ Sci Pollut Res 16:844–854
Mire CE, Jeanette AT, William FO, Kandalam VR, Gregory BH (2004) Lead precipitation by Vibrio harveyi: evidence for novel Quorum-sensing interactions. Appl Environ Microbiol 70(2):855–864
Mohammad MJ, Malkawi HI, Shibli R (2003) Effects of mycorrhizal fungi and phosphorous fertilization on growth and nutrient uptake of barley grown on soils with different levels of salts. J Plant Nutr 26:125–137
Mulligan CN, Kamali M, Gibbs BF (2004) Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J Hazard Mater 110:77–84
Oancea S, Foca N, Airinei A (2005) Effects of heavy metals on plant growth and photosynthetic activity. Boil Trace Element Res 92:257–273
Paz-Alberto A, Sigua G (2013) Phytoremediation: a green technology to remove environmental pollutants. Am J Clim Chang 2:71–86
Peralta JR, De la Rosa G, Gonzalez JH, Torresdey JLG (2004) Effects of growth stages on the heavy metal tolerance of alfalfa plants. Adv Environ Res 8:679–685
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Radhika V, Subramanian S, Natarajan KA (2006) Bioremediation of zinc using Desulfotomaculum nigrificans: Bioprecipitation and characterization studies. Water Res 40:3628–3636
Reed ML, Glick BR (2005) Growth of canola (Brassica napus) in the presence of plant growth-promoting bacteria and either copper or polycyclic aromatic hydrocarbons. Can J Microbiol 51(12):1061–1069
Rifaat HM, Fathy MK, Wagdy KBK (2009) Effect of heavy metals upon metallothioneins in some Streptomyces species isolated from Egyptian soil. J Appl Sci Environ Sanit 4(3):197–206
Rouphael Y, Cardarelli M, Reab E, Colla G (2008) Grafting of cucumber as a means to minimize copper toxicity. Environ Exp Bot 63:49–58
Sanayei Y, Ismail N, Talebi SM (2009) Determination of heavy metals in Zayandeh Rood River, Isfahan-Iran. World Appl Sci J 6:1209–1214
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WRV, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Shilev S, Fernández A, Benlloch M, Sancho ED (2006) Sunflower growth and tolerance to arsenic is increased by the rhizospheric bacteria Pseudomonas fluorescens. In: Phytoremediation of metal-contaminated soils. Springer, Dordrecht, pp 315–318
Shukla KP, Singh N, Shivesh S (2010) Bioremediation: developments, current practices and perspectives. Genet Eng Biotechnol J GEBJ-3:1–20
Singh JS, Abhilash PC, Singh HB, Singh RP, Singh DP (2011) Genetically engineered bacteria: an emerging tool for environmental remediation and future research perspectives. Gene 480:1–9
Taghavi S, Celine L, Sebastien M, Ruddy W, Max M, Daniel VL (2009) Lead (II) resistance in Cupriavidus metallidurans CH34: interplay between plasmid and chromosomally-located functions. Antonie Van Leeuwenhoek 96(2):171–182
Talanavoa V, Titou AF, Boeva NP (2000) Effect of increasing concentrations of lead and cadmium on cucumber seedlings. Biol Plant 43(3):441–444
Tumuklu A, Yalcin MG, Sonmez M (2007) Detection of heavy metal concentrations in soil caused by Nigde City garbage dump. Polish J Environ Stud 16(4):651–658
Tuna AL, Brum B, Yokas I, Coban E (2002) The effects of heavy metals on pollen germination and pollen tube length in the tobacco plant. Turk J Biol 26:109–113
Weiqiang L, Khan MA, Shinjiro Y, Yujji K (2005) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis Thaliana. Plant Growth Regul 46:45–50
Wu CH, Wood TK, Mulchandani A, Chen W (2006) Engineering plant-microbe symbiosis for rhizoremediation of heavy metals. Appl Environ Microbiol 72(2):1129–1134
Wuana, RA; Okieimen, FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. doi:http://dx.doi.org/10.5402/2011/402647
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Saleem, M. et al. (2017). Prospects of Bacterial-Assisted Remediation of Metal-Contaminated Soils. In: Singh, J., Seneviratne, G. (eds) Agro-Environmental Sustainability. Springer, Cham. https://doi.org/10.1007/978-3-319-49727-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-49727-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49726-6
Online ISBN: 978-3-319-49727-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)