Abstract
In recent years, transoral robotic-assisted surgery (TORS) has revolutionized the surgical management of malignant tumors of the oropharynx that were once only accessible through open procedures such as mandibulotomy and pharyngotomy. TORS has facilitated the de-escalation of chemoradiation therapy in select patients, sparing them the morbidity of these therapies while not compromising oncologic outcome. However, the oncologic efficacy of TORS also relies on the appropriate management of the neck as this greatly influences locoregional and distant recurrence, as well as overall survival. Therefore, neck dissection is an important component of any TORS procedure performed for malignancy. This chapter will discuss the basic elements related to neck dissection for squamous cell carcinoma for oropharyngeal primary tumors resected by TORS and current controversies surrounding neck dissection such as the impact of HPV status on the behavior of nodal metastases. Additionally, relevant complications of neck dissection and preventative measures during neck dissection (i.e., prophylactic transcervical arterial ligation) to reduce the severity of complications of TORS will also be discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
21.1 Introduction
In recent years, transoral robotic-assisted surgery (TORS) has revolutionized the surgical management of malignant tumors of the oropharynx that were once only accessible through open procedures such as mandibulotomy and pharyngotomy. TORS has facilitated the de-escalation of chemoradiation therapy in select patients, sparing them the morbidity of these therapies while not compromising oncologic outcome. However, the oncologic efficacy of TORS also relies on the appropriate management of the neck as this greatly influences locoregional and distant recurrence, as well as overall survival. Therefore, neck dissection is an important component of any TORS procedure performed for malignancy. This chapter will discuss the basic elements related to neck dissection for squamous cell carcinoma for oropharyngeal primary tumors resected by TORS and current controversies surrounding neck dissection such as the impact of HPV status on the behavior of nodal metastases. Additionally, relevant complications of neck dissection and preventative measures during neck dissection (i.e., prophylactic transcervical arterial ligation) to reduce the severity of complications of TORS will also be discussed.
21.2 Oropharyngeal Lymphatic Drainage and Nodal Metastasis
An understanding of oropharyngeal lymphatic drainage patterns is critical to the selection of appropriate cervical nodal dissection levels. Lymphatic drainage patterns of the neck have been extensively studied over several decades and are now well recognized. Based upon the predictive pathways of lymphatic drainage, the neck is typically divided into levels I–VI. Level I is divided into levels Ia (submental) and Ib (submandibular). Level Ia is bounded by the digastric muscles laterally, the mandible superiorly, and the hyoid bone inferiorly. Level Ib is bounded by the anterior and posterior bellies of the digastric muscle and the inferior border of the mandible and represents the submandibular triangle. Levels II–IV contain the internal jugular nodes. Level II (upper jugular) extends from the skull base to the level of the hyoid bone. It is posteriorly bounded by the posterior edge of the sternocleidomastoid muscle and anteriorly by the stylohyoid muscle. Level II is further subdivided into levels IIa and IIb which are separated by the spinal accessory nerve. Level IIa extends anterior to the nerve, while level IIb denotes the area posterior to the nerve. Level III (midjugular) extends from the inferior border of the hyoid to the inferior border of the cricoid cartilage. Its anterior border is the sternohyoid muscle, and its posterior border is the posterior edge of the sternocleidomastoid muscle. Level IV (lower jugular) extends from the cricoid cartilage to the clavicle. Level V (posterior triangle) is the zone bounded by the anterior border of the trapezius muscle and the posterior border of the sternocleidomastoid muscle. It extends inferiorly to the level of the clavicle. Level VI (anterior compartment) is found in the midline and extends from the hyoid to the suprasternal notch inferiorly. Its lateral boundary is the lateral border of the sternohyoid muscle or, as more recently proposed, the medial border of the common carotid artery [1].
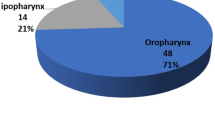
Lymphatic drainage patterns vary significantly from patient to patient, but in general oropharyngeal tumors first drain to the retropharyngeal and internal jugular nodal basins. Lateral pharyngeal tumors tend to spread to the lateral neck nodes, whereas more posteriorly located tumors tend to first drain to the retropharyngeal nodes. A comprehensive analysis of nodal metastasis patterns of oropharyngeal and oral cavity squamous tumors was performed by Shah who reviewed 1,119 elective and therapeutic neck dissections for squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx (Table 21.1) [2]. Of elective neck dissections performed for oropharyngeal primaries, the percentages of metastatic nodes in levels II, III, and IV were 80 %, 60 %, and 27 %, respectively (Fig. 21.1), whereas the percentage of nodal metastasis in levels I and V was both only 7 % each. It should be noted that this pattern of metastasis is different from that of oral cavity squamous cell carcinoma which typically involves levels I–III. When nodal metastases occur outside of levels II–IV, it is typically associated with concurrent level II–IV metastasis. Isolated “skip” lesions are extremely rare; in a study of 333 patients with oropharyngeal/hypopharyngeal primaries, only one patient (0.3 %) was found to have an isolated skip metastasis outside of levels II–IV [3]. Recent studies have also confirmed that nodal metastases in the N0 neck from oropharyngeal primaries mainly occur at levels II–IV [4]. Therefore, in the elective management of the neck for oropharyngeal primaries, dissection of levels II–IV is generally performed as an initial approach for selective neck dissection in these patients. While retropharyngeal nodes are not routinely dissected electively for TORS, surgeons should be aware of the potential for metastasis to these nodes from cancers of the oropharynx. Larger retropharyngeal nodal metastasis may be accessible during TORS, but alternatively most retropharyngeal nodal metastases will need to be included in the fields of radiation therapy if adjuvant treatment is indicated.
21.3 Neck Dissection Classification
Neck dissection can be subclassified into comprehensive neck dissection and selective neck dissection based on the extent of surgical resection of key cervical structures.
21.3.1 Comprehensive Neck Dissection
The most extensive type of neck dissection is radical neck dissection which entails removal of the cervical nodes from levels I to V as well as the sternocleidomastoid muscle, spinal accessory nerve, and internal jugular vein. It is infrequently performed today; radical surgery is usually necessary for therapeutic neck dissection if key structures are involved with extensive nodal disease.
In contrast, modified radical neck dissection involves removal of cervical nodes located at levels I–V but with preservation of one or more of the following structures: the sternocleidomastoid muscle, spinal accessory nerve, and internal jugular vein. Modified radical neck dissection type I involves preservation of only the accessory nerve, while modified radical neck dissection type II involves preservation of the accessory nerve and the internal jugular vein. Type III modified radical neck dissection, also referred to as functional neck dissection, involves preservation of the internal jugular vein, accessory nerve, and sternocleidomastoid muscle. Sacrifice of these structures for TORS oropharyngeal primaries is appropriate only when these structures are clearly involved with disease. A clear surgical plane, not artificially created, should be present to ensure optimal oncologic outcome when preserving these structures but achieving gross total resection of all nodal disease.
21.3.2 Selective Neck Dissection
Selective neck dissection for elective management of the clinically negative neck entails removal of lymph nodes at nodal levels which are at the highest risk for metastatic spread with preservation of the internal jugular vein, sternocleidomastoid, and spinal accessory nerve. Nodal metastasis from oropharyngeal primaries occurs mainly to levels II–IV, and therefore selective neck dissection of the clinically negative neck in patients undergoing TORS for squamous cell carcinoma of the oropharynx should include these levels (also known as lateral neck dissection).
The need for dissection of level IIb, the nodal subdivision defined as the area posterior to the spinal accessory nerve in level II, for squamous cell carcinoma of the oral cavity and oropharynx is debated among surgeons. Recent analyses have shown that dissection of level IIb is beneficial particularly for squamous cell carcinoma of the tonsil and in all patients with oropharyngeal primaries who have clinically N+ disease (within and outside of level II) [5, 6]. In experienced hands, dissection of level IIb adds only minimally increased risk of accessory nerve dysfunction and can be safely performed for appropriate cases.
21.4 Clinicopathological Differences Between HPV Positive and HPV Negative Neck Disease
Recent work has genetically characterized HPV positive and HPV negative tumors as distinct entities in regard to the drivers of their oncogenesis [7]. Therefore, it is not surprising that nodal metastases from these two distinct cancer subtypes have different characteristics and behavior. The percentage of oropharyngeal tumors in the 1990s that were HPV positive is estimated to be approximately 50 %; however, recent analysis has shown that this percentage has dramatically increased to as high as 80 % currently in North America and Europe [8].
As the vast majority of oropharyngeal tumors are HPV positive, an understanding of their distinct characteristics is critical for TORS surgeons. These characteristics can aid surgeons in the preoperative workup of these patients as well as affect intraoperative and postoperative management. It is generally well accepted that HPV positive oropharyngeal squamous cell primaries are characterized by frequent and early nodal spread. This is in part due to the rich lymphatic drainage of the oropharynx. The prognostic impact of early and frequent nodal spread in HPV positive disease is believed to be not as important as nodal metastasis is for HPV negative squamous cell carcinoma. The physical characteristics of HPV positive and HPV negative nodal metastases are also distinct. Cystic cervical nodal metastases from squamous cell carcinoma have been associated with primary tumors which originate from Waldeyer’s ring (which includes the base of the tongue, palatine tonsils, and nasopharynx) in 72–90 % of cases in which the primary tumor is detected [9, 10]. Furthermore, the cystic nature of oropharyngeal nodal metastases has also been linked to HPV positivity [10, 11]. The precise reasons for the occurrence of cystic metastases in oropharyngeal carcinoma are unclear but have been attributed to malignant salivary gland-type cells that metastasize from the oropharynx to cervical nodes and which subsequently express their parental property in these lymph nodes [12]. Alternative explanations involve the transformation of keratinocytes which have an inherent propensity for cyst formation after malignant conversion to a transitional type of squamous cell carcinoma [10]. Regardless of the precise mechanism of formation of cystic nodal metastases in HPV oropharyngeal tumors, surgeons should be aware of their frequent occurrence in the preoperative evaluation of these patients. It should be noted, however, that the presence of cystic cervical node metastases also occurs in other diseases processes as well, such as papillary thyroid carcinoma and hypopharyngeal carcinoma.
21.5 Management of the Neck in HPV Negative and HPV Positive Oropharyngeal Disease
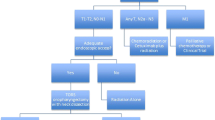
As HPV positive and HPV negative tumors represent biologically distinct tumor entities, we advocate that the elective and therapeutic management strategy of the neck should differ between these two cancer subtypes. Here, we outline our clinical practice in regard to the surgical management of the neck for oropharyngeal squamous cell primaries in the context of HPV status (Fig. 21.2).
21.5.1 The N0 Neck in HPV Positive and HPV Negative Disease
21.5.1.1 Selective Neck Dissection (Levels II–IV)
Occult cervical nodal metastases occur in approximately 30 % of early-stage tumors in both oral cavity and oropharyngeal primaries [13, 14]. As a result, elective neck dissection is usually offered to patients with a clinically and radiographically negative neck. As previously discussed, the nodal basins most commonly involved by both HPV positive and HPV negative oropharyngeal squamous primaries are located at levels II–IV of the ipsilateral neck. Occult metastases outside of these levels are extremely uncommon, and true isolated skip metastases to levels I and V are even rarer. As a result, we advocate elective ipsilateral levels II–IV selective neck dissection for management of the clinically N0 neck in well-lateralized HPV positive and HPV negative oropharyngeal tumors. Bilateral elective neck dissection of levels II–IV needs to be considered for base of tongue lesions that are centrally located or approaching the midline.
A recent publication reported an overall survival advantage for early-stage oral cavity cancer after elective neck dissection [15]. However, these results cannot be extrapolated meaningfully to the oropharynx because of the distinct biological behavior of HPV-related oropharynx cancer. On the other hand, the obvious utility of elective neck dissection in any head and neck cancer including oropharyngeal primaries is its ability to provide definitive histopathologic staging information that is otherwise not available from any other existing investigative modality including modern radiographic imaging. This information can then be used by the multidisciplinary team for designing an individualized therapeutic plan for the patient based on risk versus benefit rather than an empiric estimation of the possibility of nodal metastatic disease.
Elective radiation to the neck can be performed in select patients who have contraindications to or refuse elective neck dissection or whose primary tumor is amenable to treatment with radiation therapy alone. While generally outside of our treatment paradigm, close observation followed by surgical salvage if necessary may be an alternative option in these patients.
21.5.1.2 Role of Sentinel Node Biopsy
As the majority of patients with early-stage oropharyngeal cancer will not harbor occult nodal metastases when staged clinically and radiographically N0, some have advocated sentinel node biopsy in an effort to avoid the morbidity of elective neck dissection. Sentinel node biopsy entails lymphatic mapping in order to selectively identify nodes that are most likely to be involved via metastatic lymphatic spread. Current techniques employ the use of preoperative lymphoscintigraphy with a radiolabeled colloid solution which is injected around the primary tumor. Specialized gamma cameras and handheld gamma probes are used to identify the flow of radiolabeled colloid solution to the sentinel nodes. Once identified intraoperatively, these nodes are biopsied, and the need for subsequent treatment is determined based on the histological analysis of the biopsied sentinel node(s) as cancer metastases usually spread in a serial fashion and the first encountered nodes (sentinel nodes) will harbor cancer cells before progressive spread to subsequent nodal basins.
There is a paucity of data surrounding the accuracy of sentinel node biopsy for oropharyngeal cancer. Furthermore, logistic and technical difficulties exist with the injection radioactive tracer material preoperatively for hard to access areas within the oropharynx. A large multi-institutional trial specifically examining oral cavity squamous cancers demonstrated accurate prediction of the pathologically negative neck based on negative sentinel nodes as high as 96 % [16]. A recent trial evaluating the efficacy of sentinel node biopsy in oral cavity cancer (including oropharyngeal-bordering tumors) demonstrated a negative predictive value of 95 % [17]. It is unclear how well these results will translate to oropharyngeal primaries and sentinel node biopsy for oropharyngeal primaries is not currently recommended as standard-of-care outside of clinical trials.
21.5.2 Management of the N+ Neck in HPV Negative Disease
The management of the clinically N+ neck differs from that of the N0 neck. In a series of comprehensive therapeutic neck dissections done for oropharyngeal primaries, Shah demonstrated the presence of a significant number of level I and V nodal metastases as compared to those of patients who underwent comprehensive neck dissection for clinically N0 disease [2] (Table 21.1). We therefore advocate comprehensive dissection of levels I–V in patients with HPV negative oropharyngeal primaries with evidence of clinically N+ disease. Additionally, any grossly invaded cervical structures such as the sternocleidomastoid muscle, internal jugular vein, or spinal accessory nerve should be resected for optimal oncologic outcome.
21.5.3 Management of the N+ Neck in HPV Positive Disease
Previous studies examining the impact of nodal metastases on patient outcome did not take into account the effect of HPV status on tumor behavior and prognosis. As previously discussed, we now understand that HPV positive and HPV negative tumors are very different biological cancer subtypes that also have distinct clinical behavior. Given these inherent differences, questions have arisen regarding the ideal management of the clinically N+ neck in HPV positive oropharyngeal cancer and whether treatment paradigms should be the same as N+ disease in HPV negative cancers. A recent large retrospective analysis of 201 patients with surgically resected oropharyngeal cancer from our institution has provided significant insight regarding the differences in prognostic factors between HPV positive and HPV negative tumors [18]. Interestingly, pathologic nodal status had no impact on survival for HPV positive patients but showed a trend toward significance in HPV negative patients (Fig. 21.3). This suggests that nodal metastases in HPV positive patients are more indolent and generally do not portend worse clinical outcome as compared to HPV negative nodal metastases. As a result, our clinical practice for clinically N+ oropharyngeal HPV positive squamous cell primaries is to perform an ipsilateral selective neck dissection of levels II–IV (including any clinically involved neck levels). Some of these patients will go on to receive adjuvant postoperative radiation therapy based on their pathologic characteristics. Radiation therapy appears to be sufficient to address the rare occult nodal metastases in levels I and V that are not addressed surgically. The addition of postoperative radiation therapy in N1 disease remains at the discretion of the surgeon and multidisciplinary treatment team. N1 nodal disease that has been satisfactorily resected without adverse features such as extensive extracapsular nodal spread can be observed without the addition of postoperative radiation therapy. In contrast, N1 nodal disease that possesses adverse features such as extensive extracapsular spread may receive postoperative radiation at the discretion of the multidisciplinary treatment team. Further discussion of postoperative radiation therapy following neck dissection is detailed below.
Kaplan-Meier plots demonstrating the impact of pathologic cervical nodal status in HPV positive and HPV negative tumors of the oropharynx on patient survival (Reproduced with permission from Iyer et al. Annals of Surgical Oncology, 2015 [18])
21.6 Pharyngeal Defects Following Primary Tumor TORS and Neck Dissection
When neck dissection is carried out concurrently with TORS resection of the primary tumor, there is always the possibility that a full thickness defect can be created through the pharyngeal musculature into the neck. Because of this risk, some surgeons prefer to delay the neck dissection until 2–4 weeks after TORS of the primary. However, the majority of surgeons now carry out neck dissection in conjunction with TORS in order to facilitate adjuvant radiation treatment in a timely fashion. Surgeons must therefore be aware of the potential for pharyngeal defects to result from such combined surgeries and be prepared to repair such defects. TORS of a large oropharyngeal primary can have significant implications for neck dissection. Through-and-through defects can create an open communication between the neck and pharynx that must be addressed intraoperatively. The presence of a pharyngocervical salivary fistula in close proximity to an exposed carotid artery increases the risk of carotid rupture postoperatively. Thus large primary pharyngeal resection beds may require primary closure or coverage with local flaps or free tissue transfer if a salivary communication exists or is likely to develop. Recently, the Classification of Oropharyngeal Robotic Defects (CORD) has been proposed to help guide reconstruction defects following TORS [19, 20]. This classification characterizes the surgical defect in terms of size, location, extent of oropharyngeal resection, presence of pharyngocervical fistula, and exposure of the carotid artery. Reconstruction can proceed, primarily, with local flaps or free tissue transfer through combined transoral and open approaches through the neck. Clearly, prophylactic transcervical arterial ligation (discussed below) should be avoided when reconstruction with microvascular free tissue transfer is anticipated. As discussed elsewhere in this book, a number of free flap reconstructive options have been used to repair pharyngeal/hypopharyngeal defects following TORS including radial forearm, anterolateral thigh, and jejunal flaps. Pedicled flaps have also been used including pectoralis major and supraclavicular artery flaps. Primary closure techniques with musculomucosal advancement flap pharyngoplasty have been described in order to decrease fistula rates and improve functional outcome following surgery [21]. In many of these techniques, including free flap reconstruction, the surgical robot has been utilized in performing parts of the reconstruction, including the microvascular anastomosis [22]. If the defect is small, most surgeons will allow the resection bed to heal by secondary intention, and neck dissection can thus proceed without any additional considerations for reconstruction of the primary site. Large resection beds are subject to salivary secretions and continuous movement of the oropharynx during deglutition, making the resection bed vulnerable to wound breakdown. This may increase the risk for postoperative pharyngocervical fistula, cervical infection, and/or vascular breakdown resulting in oropharyngeal hemorrhage. For these reasons, proper selection of cases for TORS is crucial, and we recommend avoidance of leaving large areas of the oropharynx to heal by secondary intention. Local, pedicled, or free flaps can aid in providing healthy tissue to cover the resection bed and can be inset during the time of neck dissection through combined open and transoral techniques.
21.7 Extracapsular Nodal Extension in HPV+ Oropharyngeal HSNCC
Traditionally, extracapsular nodal extension of head and neck cancers portended worse outcomes and survival [23,24,25]. This has led to the recommendation for adjuvant chemoradiation therapy for patients with evidence of extracapsular spread (ECS) following surgery. Locoregional control and survival have indeed been shown to be improved after chemoradiation in these patients in several studies [26,27,28]. However, these studies group all head and neck squamous cancers together, including HPV positive and HPV negative oropharynx cancer, in their analyses. It is now clear that HPV positive and HPV negative tumors represent distinct oncologic entities, and a significant survival advantage is seen in HPV positive tumors as compared to HPV negative tumors [29]. This has led to recent speculation regarding the prognostic effects of ECS in head and neck squamous cell nodal metastases. In a recent study by Sinha et al., disease-free survival was no better in p16-positive (which serves a surrogate measure for HPV positivity) patients with ECS treated with adjuvant chemoradiation therapy as opposed to patients who were not treated with adjuvant chemoradiation therapy despite ECS [30]. A subsequent study from the University of Pittsburgh confirmed that ECS was not an independent predictor of worse survival in HPV positive tumors suggesting that ECS alone may be insufficient criteria to merit adjuvant chemoradiation [31]. More recently, Iyer et al. demonstrated by retrospective analysis that ECS was prognostic in HPV negative tumors but had no statistically significant effect on survival in HPV positive tumors (Fig. 21.4). These early studies suggest that HPV positive patients with ECS may possibly be able to be spared adjuvant chemotherapy; however, randomized prospective trials will be needed first before definitive recommendations can be made regarding the sparing of chemotherapy in these patients, and these trials are indeed underway [32].
Kaplan-Meier plots demonstrating the impact of extracapsular spread (ECS) of cervical nodal metastases in HPV positive and HPV negative tumors of the oropharynx on patient survival (Reproduced with permission from Iyer et al. Annals of Surgical Oncology, 2015 [18])
These studies on the impact of nodal ECS are particularly important in patient selection for TORS surgery. Traditionally, if chemoradiation appeared inevitable despite surgical resection, then the additional morbidity of surgery was considered as an argument to favor chemoradiation therapy as primary treatment for these patients. However, if ECS proves not to be a worse prognostic factor for patient survival and locoregional recurrence, then perhaps select patients may be better served with TORS surgery or radiation alone in order to avoid the morbidity of upfront primary chemoradiation. In a study from the University of Pittsburgh, TORS was able to obviate or reduce the need for additional therapy in 76 % of stage I/II and 46 % of stage III/IV patients [33].
21.8 Adjuvant Radiation Therapy Following TORS/Neck Dissection
Clearly, avoidance and de-escalation of adjuvant therapy is a potential benefit of primary surgical treatment. As previously discussed, HPV positive HNSCC represents a biologically less aggressive subtype compared to HPV negative disease. Therefore, the need for conventional full-dose radiation therapy in patients with HPV positive tumors has fallen into question. It may be possible to de-escalate the total dose of radiation therapy in lower-risk HPV positive patients if surgically derived histopathologic information is used in rational decision-making. In an effort to more definitely answer this question, the Eastern Cooperative Group (ECOG) 3311 trial (NCT01898494) was designed to study the effect of radiation dose de-escalation in intermediate-risk HPV positive patients undergoing transoral surgery (Fig. 21.5). Low-risk patients are observed without any adjuvant therapy, whereas high-risk patients are treated with standard chemoradiation therapy. Here, low-risk patients are defined as those with T1–T2, N0–N1 disease with clear margins and no evidence of ECS, perineural invasion, or lymphovascular invasion. High-risk patients are defined as those with positive margins, extensive ECS, or greater than five positive metastatic lymph nodes. These treatment paradigms for low- and high-risk patients are in line with previous and current practice. Intermediate-risk patients are of particular interest in this study. Intermediate-risk patients are defined in this study as patients with either at least one close (<3 mm) margin, minimal (<1 mm) nodal ECS, two to four metastatic lymph nodes, perineural invasion, or lymphovascular invasion. Patients in the intermediate subgroup are randomized to receive either standard-dose radiation therapy at 60 Gy or de-escalated to receive 50 Gy. The trial is ongoing, and it will be important to see if de-escalation can offer equivalent survival and recurrence outcomes as full-dose radiation therapy. If oncologic outcomes are equivalent and functional results are superior, this may support the use of TORS in select “intermediate”-risk patients to decrease the toxicity of adjuvant treatment.
Eastern Cooperative Oncology Group (ECOG) 3311 protocol for the evaluation of de-escalation of intermediate-risk patients following TORS. Low-risk patients receive observation postoperatively, and high-risk patients receive chemoradiotherapy (Reproduced with permission from: http://ecog-acrin.org/clinical-trials/e3311-educational-materials)
A recent retrospective analysis was performed on 175 patients with p16+ oropharyngeal SCC with ECS and/or close positive margins treated with either 66 Gy or 60 Gy postoperatively [34]. The authors found there was no difference in locoregional recurrence-free survival between the two groups. These data further suggest that HPV+ oropharyngeal nodal metastases represent a biologically less aggressive disease entity and may not require an aggressive adjuvant therapy as compared to HPV negative disease.
21.9 Transcervical Arterial Ligation During Neck Dissection for the Prevention of TORS-Related Hemorrhage
A particularly relevant concern for TORS is the risk of severe postoperative hemorrhage. This risk, albeit small, can lead to life-threatening complications secondary to the aspiration of large-volume blood loss. The reported frequency of postoperative hemorrhage varies widely in the literature and ranges from 1.5 % to 11.5 % with severe or life-threatening bleeds occurring only rarely [35,36,37,38,39,40,41,42]. The average time to postoperative hemorrhage is roughly 1 week, when most patients are no longer in the inpatient setting [39]. Severe complications in patients who suffer from life-threatening hemorrhage tend to occur in patients who are unable to protect their airway at the time of hemorrhage which underscores the need for appropriate patient selection for TORS [39].
Prophylactic transcervical arterial ligation performed at the time of neck dissection has been proposed as a means to mitigate the severity of postoperative hemorrhage following TORS in patients who experience such events. Evidence from high-volume TORS centers supports the use of prophylactic transcervical arterial ligation as a means to potentially decrease the overall severity of post-TORS hemorrhage [39, 40]. Consequently, many institutions, including our own, routinely perform prophylactic transcervical arterial ligation at the time of neck dissection to reduce the risk of postoperative hemorrhage in high-risk patients. We advocate distal vessel ligation of the superior thyroid, ascending pharyngeal, facial, and lingual arteries on the ipsilateral side of tumor resection. Bilateral vessel ligation should not be performed as this may significantly compromise end-organ blood flow.
It has been suggested that external carotid artery ligation may contribute to the development of first bite syndrome postoperatively through selective denervation of the cervical sympathetic plexus that accompanies the external carotid and ultimately innervates the parotid gland [43]. However, these events are exceedingly rare, and a clear association has yet to be established. The potential benefits of transcervical arterial ligation in preventing potentially life-threatening oropharyngeal hemorrhage greatly outweigh the small risk of developing first bite syndrome from ligation. Nevertheless, we advocate distal selective vessel ligation of the branches of external carotid supplying the oropharynx (e.g., ascending pharyngeal, lingual, facial arteries) rather than the main external carotid to minimize disruption of the sympathetic plexus. Additionally, preliminary functional cadaveric studies from our institution suggest that distal vessel ligation of external carotid artery branches may be more efficacious than external carotid artery ligation alone in the prevention of severe post-TORS hemorrhage given the extensive collateral flow from the contralateral carotid system as well as the ipsilateral internal carotid arterial system.
Prophylactic ligation is one method to help potentially reduce the adverse impact of postoperative hemorrhage following TORS. Other means include placing a temporary tracheostomy (<2 weeks) in high-risk patients at the time of neck dissection (or primary tumor resection) to assist airway protection in the event of a postoperative bleed. In the future more sophisticated technologies may allow for the closure of the tumor resection bed either primarily or with local flaps transorally. These interventions may help to eliminate the occurrence of severe postoperative hemorrhage in these patients.
21.10 Chylous Fistula
Chylous fistula following neck dissection can be distressing to the patient and prolong hospital stay following surgery. Its reported incidence is low and is approximately 1–2 % following neck dissection. During neck dissection, the thoracic duct is found in the left neck and can have a highly variable branching pattern. Two or more branches are seen in up to 40 % of patients. The duct typically terminates in the internal jugular vein and is vulnerable to injury at this location (particularly along the medial wall of the vein) as this region is highly accessible during surgery. Intraoperative measures taken during neck dissection can facilitate prevention. Particular attention to the extravasation of chyle during dissection of level IV is critical in preventing postoperative fistula. Prophylactic clamping and tying of adipose tissue in level IV as the nodal packet is dissected close to the jugular vein can reduce the occurrence of thoracic duct fistula. Surgeons should be aware of the location of the phrenic and vagus nerves during this process to avoid inadvertent injury to these structures. The thoracic duct or its branches are often not visualized during neck dissection, and meticulous ligation in level IV is therefore crucial. Intraoperative Valsalva maneuver can be used to aid in the visualization of extravasated chyle from an injured lymphatic duct. Any visualized extravasation should be immediately addressed intraoperatively with suture ligation.
Chylous fistulas that develop postoperatively may be initially managed conservatively if patients are asymptomatic and chylous drain output is less than 600 mL in a 24 h period. Chylous drain output greater than 300 mL per day for 3 days is unlikely to resolve with conservative measures alone [44]. Conservative management of chylous fistula involves dietary measures including a nonfat or low-fat diet which decreases the flow/production of lymphatic fluid/chyle. In more severe cases, oral intake can be entirely restricted, and nutritional support is provided parenterally until chyle leakage is controlled. If patients are receiving nutritional tube feedings, formulations with medium-chain triglycerides are recommended as medium-chain triglycerides bypass the lymphatic system via the portal vein and are thus transferred directly to the liver. Somatostatin analogs such as octreotide are also useful in the conservative management of chylous fistula. These agents decrease chyle production and are often used in the management of low-output chyle leaks. In fact, some evidence suggests that octreotide may be useful in the treatment of high-flow chyle leaks as well [45]. Compressive dressings may also be useful in the conservative management of chylous fistula, particularly if accumulation of fluid is observed under intact skin flaps.
If chyle output exceeds the abovementioned thresholds or remains persistent despite conservative management, surgical intervention should be considered. Exploration and suture ligation in the operative room can be technically challenging but is an effective means to address persistent or high-volume chylous fistula. Thoracic duct embolization and transthoracic endoscopic thoracic duct ligation have also been described as minimally invasive alternatives to surgical exploration. While highly successful in some cases, the overall reported success rates are highly variable, and the procedure is not without its potential complications.
21.11 Contraindications and Patient Selection
The advent of TORS with neck dissection has significantly expanded the treatment options for cancers of the oropharynx. It has provided surgical access to the oropharynx that was once only accessible via more invasive open procedures. However, as other treatment modalities such as radiation and chemoradiation therapy are also effective, appropriate selection of patients is critical to provide the most efficacious and safe treatment for the patient. While an exhaustive discussion of relative and absolute contradictions for TORS is not presented here, key contraindications as they relate specifically to neck dissection are presented.
Frank involvement of carotid artery by tumor or tumor nodal metastases can present a challenge to successful neck dissection. Patients who have tumor encasing the carotid artery are best treated nonsurgically because complete surgical resection is not feasible or safe even with carotid artery resection and grafting, especially in those who have extensive disease at the skull base. Other contraindications include invasion of the prevertebral fascia, paraspinal muscles, and brachial plexus.
21.12 Summary
Management of the regional lymphatics is a critical component of treatment selection and surgical planning in management of patients with squamous cell carcinoma of the oropharynx. The patterns of nodal metastases from the oropharynx have been well recognized for several decades, but we now know that nodal metastases have different characteristics and behavior depending on HPV status of the tumor. Improved understanding of these differences has led to an evolution of management strategies of not only the primary tumor but also of cervical nodal metastases. Individualized patient selection balancing risk versus benefit based on multidisciplinary interaction is crucial for successful outcome after surgical treatment of oropharyngeal squamous cell carcinoma.
References
Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134(5):536–8.
Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–9.
Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990;12(3):197–203.
Lim YC, Koo BS, Lee JS, Lim JY, Choi EC. Distributions of cervical lymph node metastases in oropharyngeal carcinoma: therapeutic implications for the N0 neck. Laryngoscope. 2006;116(7):1148–52.
Corlette TH, Cole IE, Albsoul N, Ayyash M. Neck dissection of level IIb: is it really necessary? Laryngoscope. 2005;115(9):1624–6.
Seethala RR. Current state of neck dissection in the United States. Head Neck Pathol. 2009;3(3):238–45.
Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82.
O’Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440–51.
Gourin CG, Johnson JT. Incidence of unsuspected metastases in lateral cervical cysts. Laryngoscope. 2000;110(10 Pt 1):1637–41.
Thompson LD, Heffner DK. The clinical importance of cystic squamous cell carcinomas in the neck: a study of 136 cases. Cancer. 1998;82(5):944–56.
Stelow EB, Jo VY, Stoler MH, Mills SE. Human papillomavirus-associated squamous cell carcinoma of the upper aerodigestive tract. Am J Surg Pathol. 2010;34(7):e15–24.
Regauer S, Beham A, Mannweiler S. CK7 expression in carcinomas of the Waldeyer’s ring area. Hum Pathol. 2000;31(9):1096–101.
van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177(2):379–84.
Ross GL, Soutar DS, MacDonald DG, Shoaib T, Camilleri IG, Robertson AG. Improved staging of cervical metastases in clinically node-negative patients with head and neck squamous cell carcinoma. Ann Surg Oncol. 2004;11(2):213–8.
D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373(6):521–9.
Civantos FJ, Zitsch RP, Schuller DE, Agrawal A, Smith RB, Nason R, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(8):1395–400.
Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA, et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur J Cancer. 2015;51(18):2777–84.
Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-positive and negative oropharyngeal cancer. Ann Surg Oncol. 2015;22(13):4411–21.
de Almeida JR, Genden EM. Robotic assisted reconstruction of the oropharynx. Curr Opin Otolaryngol Head Neck Surg. 2012;20(4):237–45.
de Almeida JR, Park RC, Genden EM. Reconstruction of transoral robotic surgery defects: principles and techniques. J Reconstr Microsurg. 2012;28(7):465–72.
Genden EM, Park R, Smith C, Kotz T. The role of reconstruction for transoral robotic pharyngectomy and concomitant neck dissection. Arch Otolaryngol Head Neck Surg. 2011;137(2):151–6.
Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg. 2010;126(6):1978–87.
Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA. Prognostic factors of neck node metastasis. Clin Otolaryngol Allied Sci. 1982;7(3):185–92.
Johnson JT, Myers EN, Bedetti CD, Barnes EL, Schramm Jr VL, Thearle PB. Cervical lymph node metastases. Incidence and implications of extracapsular carcinoma. Arch Otolaryngol. 1985;111(8):534–7.
Ferlito A, Robbins KT, Shaha AR, Pellitteri PK, Kowalski LP, Gavilan J, et al. Current considerations in neck dissection. Acta Otolaryngol. 2002;122(3):323–9.
Johnson JT, Wagner RL, Myers EN. A long-term assessment of adjuvant chemotherapy on outcome of patients with extracapsular spread of cervical metastases from squamous carcinoma of the head and neck. Cancer. 1996;77(1):181–5.
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–44.
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–52.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
Sinha P, Lewis Jr JS, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118(14):3519–30.
Maxwell JH, Mehta V, Wang H, Cunningham D, Duvvuri U, Kim S, et al. Quality of life in head and neck cancer patients: impact of HPV and primary treatment modality. Laryngoscope. 2014;124(7):1592–7.
Washington University School of Medicine. Post operative adjuvant therapy de-intensification trial for human papillomavirus-related, p16+ oropharynx cancer (ADEPT). ClinicalTrialsgov [Internet] Bethesda: National Library of Medicine (US) 2000- [6/2016] Available from: https://clinicaltrials.gov/ct2/show/study/NCT01687413 NLM Identifier: NCT01687413. 2016.
Gildener-Leapman N, Kim J, Abberbock S, Choby GW, Mandal R, Duvvuri U, et al. Utility of up-front transoral robotic surgery in tailoring adjuvant therapy. Head Neck. 2016;38:1201–7.
Chin RI, Spencer CR, DeWees T, Hwang MY, Patel P, Sinha P, et al. Reevaluation of postoperative radiation dose in the management of human papillomavirus-positive oropharyngeal cancer. Head Neck. 2016;38:1643–9.
Asher SA, White HN, Kejner AE, Rosenthal EL, Carroll WR, Magnuson JS. Hemorrhage after transoral robotic-assisted surgery. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2013;149(1):112–7.
Chia SH, Gross ND, Richmon JD. Surgeon experience and complications with Transoral Robotic Surgery (TORS). Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2013;149(6):885–92.
Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2011;145(2):248–53.
Lorincz BB, Mockelmann N, Busch CJ, Knecht R. Functional outcomes, feasibility, and safety of resection of transoral robotic surgery: single-institution series of 35 consecutive cases of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Head Neck. 2015;37(11):1618–24.
Mandal R, Duvvuri U, Ferris RL, Kaffenberger TM, Choby GW, Kim S. Analysis of post-transoral robotic-assisted surgery hemorrhage: frequency, outcomes, and prevention. Head Neck. 2016;38(1):E776–82. doi: 10.1002/hed.24101. Epub 2015 Jul 15.
Pollei TR, Hinni ML, Moore EJ, Hayden RE, Olsen KD, Casler JD, et al. Analysis of postoperative bleeding and risk factors in transoral surgery of the oropharynx. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1212–8.
Richmon JD, Feng AL, Yang W, Starmer H, Quon H, Gourin CG. Feasibility of rapid discharge after transoral robotic surgery of the oropharynx. Laryngoscope. 2014;124(11):2518–25.
Vergez S, Lallemant B, Ceruse P, Moriniere S, Aubry K, De Mones E, et al. Initial multi-institutional experience with transoral robotic surgery. Otolaryngol Head Neck Surg: Off J Am Acad Otolaryngol Head Neck Surg. 2012;147(3):475–81.
Chiu AG, Cohen JI, Burningham AR, Andersen PE, Davidson BJ. First bite syndrome: a complication of surgery involving the parapharyngeal space. Head Neck. 2002;24(11):996–9.
Scorza LB, Goldstein BJ, Mahraj RP. Modern management of chylous leak following head and neck surgery: a discussion of percutaneous lymphangiography-guided cannulation and embolization of the thoracic duct. Otolaryngol Clin N Am. 2008;41(6):1231–40. xi
Barili F, Polvani G, Topkara VK, Dainese L, Roberto M, Aljaber E, et al. Administration of octreotide for management of postoperative high-flow chylothorax. Ann Vasc Surg. 2007;21(1):90–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Mandal, R., Ganly, I., Patel, S.G. (2017). Management of the Neck for Oropharyngeal Squamous Cell Carcinoma in the Era of Transoral Robotic-Assisted Surgery (TORS). In: Gil, Z., Amit, M., Kupferman, M. (eds) Atlas of Head and Neck Robotic Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-49578-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-49578-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49576-7
Online ISBN: 978-3-319-49578-1
eBook Packages: MedicineMedicine (R0)