Summary
Nitric oxide (NO) is emerging as a signaling molecule in plants. Its metabolism, site and mode of action in chloroplasts are still not clear. Chloroplasts are emerging as an alternative site for NO synthesis in plants. However, exogenous NO donors show direct evidence on the action of this molecule on chloroplasts under stress as well non-stress conditions. Nitric oxide is also implicated in the development and senescence of the organelle. The effects of NO on chloroplasts, particularly on photosynthetic and antioxidative processes are described. The target sites and probable sites of action are enumerated.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Nitric Oxide
- Chloroplast
- Electron transport
- Photosynthesis
- Photosystems
- Photophosphorylation
- Greening
- Senescence
- Development
- Abiotic stress
14.1 Introduction
Nitric oxide (NO) is a gaseous molecule with a signaling role in plant growth , development and responses to environmental changes (Neill et al. 2008; Palavan-Unsal and Arisan 2009; Misra et al. 2010a, b, 2011, 2012). The effects of NO in plants can be direct or through intermediate effector molecules regulating cellular metabolism (Krasylenko et al. 2010). Nitric oxide action is achieved also by modifying the redox state of the cell and can modulate the activity of proteins, through reversible reactions with functional groups such as thiols and heme. It is well known that iron is a necessary element for synthesis and development of chloroplast and NO plays an important role in the distribution of iron in the chloroplasts in plant leaves (Sun et al. 2007). Nitric oxide, a highly unstable free radical, has been described both as a cytotoxin and a cytoprotectant in plants, as well (Beligni and Lamattina 1999a, 2001a). This signal molecule appears to take part in the regulation of cellular redox homeostasis, acting either as an oxidant or as an antioxidant (Stamler et al. 1992). However, at lower concentrations, NO promotes normal plant growth and development (Beligni and Lamattina 2001b). Nitric oxide stimulates leaf expansion, prevents etiolation, retards leaf senescence and induces stomatal closure (Leshem et al. 1998; Beligni and Lamattina 2000; García-Mata and Lamattina 2001). When applied at relatively high doses to plants, NO clearly perturbs normal metabolism and reduces the net photosynthesis in leaves of oats and alfalfa (Hill and Bennett 1970). Nitric oxide in concentrations above optimal (above 10−6 M) inhibits the expansion of leaf lamina, increases the viscosity of simulated thylakoid lipid monolayers and potentially impairs photosynthetic electron transport (Leshem et al. 1998; Leterrier et al. 2012).
Chloroplasts are highly specialized semiautonomous photosynthesizing organelles found in green plants. There is wide diversity in chloroplast structure , function and adaptation. The chloroplasts encode a large number of their own RNAs and proteins, in addition to that synthesized by the nuclear genes, economizing the cellular energy demand for its structural organization. Chloroplast develops from a progenitor known as proplastid accompanied with the coordinated regulation of plastid and nuclear-encoded genes (Baumgartner et al. 1989; Dilnawaz et al. 2001; Joshi et al. 2013). The chloroplasts are the only organelle that supports autotrophy in plants through its role in photosynthesis and also sustains life on earth. The process involves coordination between the primary photochemical processes in the thylakoid membrane and reduction of CO2 in the stroma of chloroplasts . The photochemical process includes solar energy trapping, photolysis of water, electron transfer and generation of reductants for the reduction of CO2 to carbohydrates in the stroma – the soluble fraction of chloroplasts . The thylakoid membrane has four main multi-subunit protein complexes: photosystem II (PSII ), photosystem I (PSI ), cytochrome b 6 f and ATPase (Nelson and Yocum 2006).
Studies on the effect of NO on chloroplasts are crucial for understanding its role in green plants. In addition, chloroplasts are reported to be one of the several cellular sites for the synthesis of endogenous nitric oxide (Guo and Crawford 2005; Jasid et al. 2006; Galatro et al. 2013; Tewari et al. 2013). However, till date there is a lack of a precise report on the effect of the NO on the regulation of different physiological, biochemical and molecular processes in chloroplasts . In this review, we consolidate the up-to-date studies on the effect of NO on chloroplasts . An emphasis will be given in this chapter for the effect of NO on the photochemical efficiency in chloroplasts under physiological conditions and abiotic stress .
14.2 Sources of Nitric Oxide in Plants
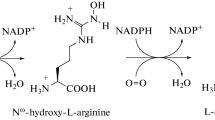
The nitric oxide production in plant cells is compartmentalized and is mediated through several different pathways (Gupta et al. 2011). It has been shown to produce NO from nitrite (Desikan et al. 2002), from L – arginine by NOS like activity (Guo et al. 2003) and from S-nitrosoglutathione decomposition (Jasid et al. 2006) in chloroplasts . Non-enzymatic production of NO from nitrite involving plastid pigments such as carotenoids has also been reported (Cooney et al. 1994). Interestingly, NO synthesis in response to iron, elicitors, high temperatures, salinity or osmotic stress is first detected in chloroplasts using NO-sensitive diaminofluorescein probes (Foissner et al. 2000; Gould et al. 2003; Arnaud et al. 2006). In spite of several ifs and buts, these results corroborate the hypothesis that plastids are key players in the control of NO levels in plant cells. Nitric oxide originates in chloroplasts through the reduction of nitrite to NO and/or through nitric-oxide synthases (NOS) like activities (NOA) mediated NO biosynthetic pathway using arginine as a precursor molecule.
14.3 Effect of Nitric Oxide on Photosynthetic Pigment Dynamics
Nitric oxide improves the accumulation of chlorophylls (Chls) and even imitates red light responses in greening leaves (Beligni and Lamattina 2000). It is well known that the photosynthetic pigments, Chls in particular, are visible markers for chloroplast development and senescence in leaves (Misra and Biswal 1980, 1982; Misra and Misra 1986, 1987; Biswal et al. 2001; Dilnawaz et al. 2001). The synthesis of Chls and plastid proteins is intricately connected and is essential for the stability of Chl-protein complexes in vivo (Dilnawaz et al. 2001; Neill et al. 2003; Joshi et al. 2013). Seedlings grown in darkness develop etioplasts from proplastids, which ultimately transform into well organized chloroplasts in light (Misra and Misra 1987; Joshi et al. 2013). But, genes for nuclear-encoded Chl a/b – binding antennae and plastid-encoded Chl a – binding polypeptides are obligatorily dependent on incidence of light only. These photo-regulatory processes have several light receptors such as phytochrome and cryptochrome (Pogson and Albrecht 2011; Lepistö and Rintamäki 2012).
Nitric oxide donor sodium nitropruside (SNP) enhanced Chl synthesis and accumulation of light-harvesting chlorophyll a/b complex of PSII (LHCII) and PSIA/B, primary photochemistry of PSII and effective quantum yield of PSII of the developing chloroplasts in greening of barley leaves (Zhang et al. 2006). Nitric oxide scavenger PTIO (2-phenyl-4,4,5,5-tetramentylimidazoline-1-oxyl-3-oxide) or NOS inhibitor L-NNA (nitro-nitro-L-arginine) retarded the greening process. Moreover, sodium ferrocyanide, an analog of SNP, nitrite and nitrate etc. do not have any effect on the greening process, suggesting a positive role of NO in the greening process (Zhang et al. 2006). The endogenous NO content of greening leaves also increased in parallel with the greening (measured by Chl accumulation) of leaves indicating a direct role of plastid NO in leaf greening (Zhang et al. 2006). Leaf senescence is associated with the symptoms of Chl degradation through various enzymatic and oxidative processes in the green cells (Biswal et al. 2001; Misra et al. 2006). Endogenous and exogenous NO at lower concentrations delayed leaf senescence , but at higher concentrations accelerated leaf senescence (Leshem et al. 1997; Guo and Crawford 2005; Mishina et al. 2007; Selcukcam and Cevahir 2008; Prochazkova and Wilhelmova 2011).
14.4 Interaction of Nitric Oxide with Oxygen Evolving Complex
Photosystem II is one of the sites of action for NO in chloroplasts (Wodala et al. 2008). At the electron donor side of PSII it acts at the oxygen-evolving complex (OEC ). This complex is a part of the PSII , which is a multi-subunit chlorophyll -protein complex that uses light energy to oxidize water and form molecular oxygen, with a concomitant reduction of plastoquinone to plastoquinol (Debus 1992; Britt 1996). The functional conformation of the Mn cluster is expected to be maintained by a 33 kDa hydrophilic protein subunit of OEC attached to the luminal side of the D1/D2 heterodimer. During the oxidation of two water molecules to one oxygen molecule and protons, the OEC cycles through five intermediate redox states termed S0–S4 (Fig. 14.1). Dark adapted photosynthetic apparatus contains S0 and S1 states. The most reduced state is S0, while S1, S2 and S3 represent higher oxidation states and molecular oxygen being evolved during the transition from S4 to S0 states (Haumann et al. 2005; Penner-Hahn and Yocum 2005).
Schematic presentation of the Kok’s model of oxygen evolution , consisting of four stabile states (S0–S3) and one transient state (S4). The possible sites of action of NO on the cycle are shown by asterisks. The back reduction of S-states by NO and possible over-reduction to S-n states are shown by dashed arrows
It is suggested that NO interacts with Mn cluster of PSII and leads to rapid destabilization of the excited states of OEC (Schansker and Petrouleas 1998). Studies with PSII and the di-manganese catalase have shown a similar mode of interaction of NO with the different oxidation states of the Mn clusters (Ioannidis et al. 2000). It is also suggested by Ioannidis et al. (2000) that one-electron reduction of the cluster occurs followed by release of NO2 ─ as described below:
Schansker et al. (2002) studied the oxygen oscillation patterns of PSII -enriched membranes and observed shift of the maximum flash-induced oxygen yield from flash 3 to flash 6/7 in the NO-treated samples. Considering these observations, the authors suggested the reduction of Mn cluster to the S−2 state by NO, which is assigned to the formation of Mn(II)-Mn(III) dimer. During catalysis the enzyme appears to cycle between the states Mn(II)-Mn(II) and Mn(III)-Mn(III) (Khangulov et al. 1990; Waldo and Penner-Hahn 1995).
Ioannidis et al. (2000) proposed a rapid interaction of NO with S3 state of the OEC . This is explained by a metallo-radical characteristic of the S3 state. A probable role of Tyr YD in oxidizing of Mn complex to the lower oxidizing state S0 than the S1 state was proposed by Styring and Rutherford (1987). Sanakis et al. (1997) proposed the formation of a Tyr-NO species which can act as an electron donor to PSII . An iminoxyl radical is formed upon light-induced oxidation of this species, which is the first example of a chemical modification of one of the tyrosines of PSII to produce a photochemically active species. Our recent in vitro study demonstrated that the exogenous NO donor SNP (above 5 μM) has a clearly pronounced damaging effect on the primary oxygen-evolving reactions at the electron donor side of photosynthetic apparatus (Vladkova et al. 2011). In addition, our investigation for influence of exogenous NO donor SNP on isolated thylakoid membranes also revealed a dramatic increase of PSII population in the most reduced S0 state and an increase of the turnover time of the oxygen-evolving centers, i.e. delayed the process of the electron donor capturing or Si states turnover (Vladkova et al. 2011).
14.5 Effect of Nitric Oxide on Photosynthetic Electron Transport
One of the important reactions of NO in biology is interaction with metal complexes (Wink and Mitchell 1998). Because NO possesses an unpaired electron, it has high affinity to transition metals to form metal-nitrosyl complexes (Wink and Mitchell 1998). Due to this reason, proteins containing transition metal ions in either heme or non-heme complexes can be the potential targets for NO (Wink and Mitchell 1998). Nitric oxide is able to influence the photosynthetic electron transport chain directly by binding to such non-heme iron in the core complex of the PSII (Wodala et al. 2008). The important binding sites of NO in PSII are the non-heme iron between QA and QB binding sites (Diner and Petrouleas 1990; Petrouleas and Diner 1990) and YD, the Tyr residue of D2 protein (Sanakis et al. 1997). Electron paramagnetic resonance (EPR) studies confirmed the NO binding to non-heme iron and that NO competes with bicarbonate for its binding (Diner and Petrouleas 1990). Formate , an anion which also competes with bicarbonate , binds simultaneously with NO (Diner and Petrouleas 1990) besides the other anions like fluoride (Sanakis et al. 1999).
Experiments with isolated thylakoids indicated that NO binding slows down the rate of electron transfer between QA and QB (Diner and Petrouleas 1990). Binding of NO to the QAFe2+QB complex is facilitated in the presence of reduced QA acceptor, as this reduction weakens the bond between bicarbonate and iron (Goussias et al. 2002). Nitric oxide binding to PSII can also decrease the rate of electron transport on the donor side as well, since in vitro experiments have proven that NO interacts with the YD• tyrosine residue and the OEC . The latter is reduced to the S−2 state by NO, as shown by oxygen electrode, fluorescence and EPR measurements (Schansker et al. 2002). Measurements in the presence of DCMU demonstrated that NO induces inhibition of QA − recombination with the S2 state of the OEC . This donor side inhibition of electron transport may sufficiently be accounted by the reduction of either the OEC , or the YD• residue by NO. To the contrary, our recent results showed that the NO donor SNP is probably the only NO donor which stimulates the electron transport through PSII at sub-μmolar concentrations (Vladkova et al. 2011). Nitric oxide interacts with the tyrosine residue of the D2 protein (Sanakis et al. 1997) and the resulting YD•–NO couple has a decreased redox potential low enough to become a more efficient electron donor in isolated thylakoid membranes than the immediate redox-active tyrosine residue (YZ) located on the D1 protein. The probable binding sites and sites of action in thylakoid membranes are summarized in Fig. 14.2.
Summary and schematic presentation of the probable binding of NO to different components of PSII , cytochrome b 6 f, PSI , ATPase complexes. The nitrosylation of membrane lipids and of both thylakoid and stromal polypeptides are shown as red colored circular asterisks. These binding and nitrosylation affect photosynthetic processes
Chlorophyll fluorescence studies have provided contradictory effects of NO on chloroplasts in vivo. However, these results depend on the used NO donor. In the leaves, NO derived from S-nitroso-N-acetylpenicillinamine (SNAP) showed no effect on the maximum quantum efficiency of photosynthesis , but that from SNP and S-nitrosoglutathione (GSNO) decreased this parameter (Takahashi and Yamasaki 2002; Yang et al. 2004; Wodala 2006; Wodala et al. 2008). All the NO donors induced a decrease in effective quantum efficiency, which is related to photochemical quenching (qP). These studies indicated that NO increases the proportion of closed PSII reaction centers in intact leaves (Wodala 2006; Wodala et al. 2008). However, all these results are not uniform and unequivocal. Wodala et al. (2008) suggested that the different chemical properties of NO donors and the different experimental conditions generate conflicting experimental results in vivo. Fast Chl fluorescence induction kinetics of GSNO-treated leaf disks confirmed significant donor and acceptor side inhibition of electron transport (Wodala et al. 2008).
It has been found that NO influences the non-photochemical quenching (NPQ ). Besides reducing steady-state NPQ values, NO changes the amplitude and kinetics of an NPQ transient, which resembles reaction-center NPQ described by Finazzi et al. (2004). Reaction-center NPQ arises upon the onset of illumination of dark-adapted leaves and, at low light intensities, it is relaxed rapidly after a few min of illumination. On the basis of its fast relaxation and ΔpH-dependency, Finazzi et al. (2004) showed that reaction-center NPQ is caused by the rapid and transient over acidification of the thylakoid lumen, which is created by the immediate onset of the photochemistry. In addition, they suggested that the ΔpH may be further increased by cyclic and pseudo-cyclic electron transport (Mehler-reaction) and explain the relaxation of this transient form of NPQ by the activation of the carbon fixation apparatus, which decreases ΔpH and redox pressure. Although a potential effect of NO on Calvin cycle activation would account for changes in this NPQ transient, steady-state NPQ values below control values indicate that NO does not decrease the maximum rate of the Calvin cycle (Finazzi et al. 2004).
Photosynthetic studies on stomata of peeled epidermal strips respond to exogenous NO by instantly decreasing photochemical fluorescence quenching coefficients (qP and qL), the operating quantum efficiency of PSII , and NPQ to close to zero. However, NO effect in vivo is reversible. The reversible inhibition by NO of the electron transport rate could be restored by bicarbonate , a compound known to compete with NO for one of the two coordination sites of the non-heme iron (II) in the QAFe2+QB complex (Ordog et al. 2013).
14.6 Effect of Nitric Oxide on the Photophosphorylation in Chloroplasts
Previous studies revealed that NO donor SNAP inhibits the linear electron transport rate and light-induced pH formation (ΔpH) across thylakoid membrane, and decreased the rate of ATP synthesis (Takahashi and Yamasaki 2002). The inhibitory effect of NO on the photophosphorylation can be prevented by a supplemental high concentration of bicarbonate . It has been reported that high concentrations of bicarbonate enable the bound NO to liberate from the reaction center and recover electron transport activity (Diner and Petrouleas 1990). Thus, sensitivity of photosynthesis against NO would depend on a local concentration of bicarbonate within thylakoid membranes. It is plausible that the decreased rate of electron transport, due to the NO-induced dissociation of bicarbonate from the specific sites of thylakoids, is involved in the mechanism of the inhibitory effect of NO on the photophosphorylation. The overall effect of NO on the electron transport and the photophosphorylation as well its resultant effect on the stromal enzymes is summarized and shown in Fig. 14.2.
14.7 Ameliorating Effect of Nitric Oxide on Photosynthetic Stress Responses
Plants produce substantial amounts of NO in their natural environments (Wilson et al. 2008). In recent years, there has been increasing evidence that NO is involved in regulating, if not all, many key physiological processes in plants under normal and stress conditions. NO is reported to ameliorate several stress responses in plants (García-Mata and Lamattina 2001, 2002; Arasimowicz and Floryszak 2007; Krasylenko et al. 2010; Misra et al. 2011). Abiotic stresses, such as drought , high and low temperature, salinity, heavy metals, UV-B and oxidative stress is reported to induce NO production in plants (Shi et al. 2005; Arasimowicz and Floryszak 2007; Qiao and Fan 2008; Misra et al. 2011). Stressors form free radicals and other oxidants, resulting in increased level of reactive oxygen species (ROS) in plant cells (Mittler 2002; Qiao and Fan 2008). NO eliminates the superoxide radicals and as a signal molecule interacts with plant hormones and ROS (Laxalt et al. 1997; Zhao et al. 2004; Qiao and Fan 2008). In addition to its signal roles, NO may also function as a regulator of gene expression (Kopyra and Gwozdz 2004; Qiao and Fan 2008). A large amount of NO may combine with O2− to form peroxynitrite (ONOO¯), which has been reported to damage lipids , proteins and nucleic acids (Yamasaki et al. 1999).
14.7.1 Nitric Oxide Under Osmotic Stress
Osmotic stress is one of the major abiotic factors limiting crop productivity and natural status of the environment, affecting functions of the plants (Misra et al. 2001). Drought , high salinity and freezing impose osmotic stress on plants. Plants respond to this stress in part by modulating gene expression, which eventually leads to the restoration of cellular homeostasis, detoxification of ROS and recovery of growth (Xiong and Zhu 2002). One of the most important responses of the plants under osmotic stress is increased synthesis of abscisic acid (ABA) (Neill et al. 2008). Nitric oxide maintains leaf water content by regulating ABA-induced stomatal closure during osmotic stress. But the physiological role of NO-induced ABA accumulation remains unknown. Reactive oxygen species are one of the main damaging compounds that are produced during stress and osmotic stress in particular (Beligni and Lamattina 1999a, b). The protective action of NO against oxidative damage can be explained by two mechanisms . Firstly, NO operates as a signaling molecule, which activates cellular antioxidant enzymes (Huang et al. 2002; Shi et al. 2005; Zhao et al. 2008). Secondly, NO might detoxify ROS directly. Mutant analysis of genetically modified Arabidopsis showed evidences that NO is an endogenous regulator of the tolerance of the plants to salt stress (Martinez et al. 2000; Zhao et al. 2004, 2007). These authors showed that Atnos 1 mutant with decreased NO production due to a T-DNA insertion in AtNOS gene was hypersensitive to oxidative stress induced by NaCl (Martinez et al. 2000; Zhao et al. 2004, 2007).
14.7.2 Nitric Oxide Under Temperature Stress
Extreme temperatures either high or low (chilling) are stressful to plants affecting photosynthesis in plants (Misra and Misra 1986; Misra et al. 1997). Heat stress can also cause an overproduction of ROS, which could be involved in triggering defence responses against potentially damaging temperatures (Suzuki and Mittler 2006; Volkov et al. 2006; Kotak et al. 2007; Locato et al. 2008). A significant rise in NO production under heat stress in alfalfa sprouts (Leshem et al. 1998) and in tobacco leaf cells (Gould et al. 2003) are reported. Conversely, pea plants exposed at 38 °C for 4 h reduced the NO content of leaves, but it was found that the S-nitrosothiol (SNOs) content increases threefold and that the protein nitrosylation is enhanced (Corpas et al. 2008a). Protein nitrosylation can cause an inhibition of the activities of photosynthetic enzymes such as carbonic anhydrase and of ferredoxin-NADP reductase (Chaki et al. 2011). In Arabidopsis, several mutants have been identified to have impairment in the GSNOR1 gene, showing the involvement of this gene in the mechanism of response against heat stress. Thus, the mutant HOT5 (sensitive to hot temperatures) showed that GSNOR modulates the intracellular level of SNOs, enabling thermo-tolerance, as well as the regulation of plant growth and development (Lee et al. 2008). In calluses of reed, the exogenous application of SNP or ABA elevated thermo-tolerance by alleviating ion leakage, lipid peroxidation and growth suppression induced by heat stress (45 °C for 2 h). On the other hand, exogenous ABA notably activated NOS activity and increased NO release, maintaining the heat tolerance (Song et al. 2008). Studies so far unequivocally report an increase in NO content and increase in thermo-tolerance of plants.
Chilling (below 4 °C) or cold (below 10 °C) stress is also detrimental for plant systems (Misra et al. 1997). A proteomic analysis showed that the chilling stress induced S-nitrosylation of Rubisco, which correlated with the inhibition of photosynthesis (Abat and Deswal 2009). Pea plants exposed to low temperature (8 °C for 48 h) showed an enhanced activity of L-arginine NOS and GSNOR, and an increase in the content of SNOs (Sharma et al. 2005). Low temperature caused an imbalance of the ROS and reactive nitrogen species metabolism in leaves, triggering a rise in the lipid oxidation and the protein tyrosine nitration, which indicates an induction of oxidative and nitrosative stress (Corpas et al. 2008b; Airaki et al. 2012). Similar responses and an increase in NO content have been reported in Arabidopsis thaliana exposed to chilling (4 °C for 1–4 h) stress or during cold acclimation (Zhao et al. 2009; Cantrel et al. 2011). It is most probable that temperature stress induced increase in NO and stress ameliorating effect of NO could be due to the antioxidative action of NO (Desikan et al. 2002).
14.7.3 Nitric Oxide Under High Light Stress and UV Radiation
High light causes photoinhibition of photosynthesis and generates ROS in green plants (Misra et al. 1997). Protective role of NO during photoinhibition has been reported in higher plants. Pronounced increases of NO production are found in tall fescue leaves after exposure to high-light stress (Xu et al. 2010). Nitric oxide might act as a signaling molecule to enhance antioxidant enzyme activities, further protecting against injuries caused by high light stress (Xu et al. 2010). However, Chlamydomonas reinhardtii cells under very high light conditions induces 1O2 (singlet oxygen) accumulation due to a decrease in the 1O2 scavenging capacity caused by NO-mediated inhibition of carotenoid synthesis and PSII electron transport , which in turn leads to oxidative damage and cell death (Chang et al. 2013).
The UV-B radiation (280–320 nm) clearly affects plant growth and usually also induces oxidative stress. Moreover, UV-B triggered a rise in ROS widely distributed in chloroplasts and mesophyll cells, causing cell damage. It has been observed that apocynin reduces UV-B-induced oxidative damage because it reduces the Chl breakdown caused by H2O2, and this is correlated with NO production mediated by an enhanced NOS activity (Tossi et al. 2009). In the case of maize leaves, the UV-B irradiation provoked a simultaneous rise in the concentration of ABA, H2O2 and NO in leaves. These authors also reported that the accumulation of endogenous NO is ABA-dependent and is responsible for tolerance to high doses of UV-B radiation (Tossi et al. 2012). Exogenous NO partially alleviated the UV-B effect characterized by a decrease in Chl contents and oxidative damage to the thylakoid membrane in bean seedlings (Shi et al. 2005). Zhang et al. (2009) suggested that under UV-B stress, NO production is mediated by H2O2 through the enhancement of NOS activity. It is well established that H2O2 induces NO synthesis and accumulation and vice versa (cf. Mazid et al. 2011).
The other possible explanation of the protective action of NO on the photosynthesis under UV and temperature stress can be either due to the modification of the OEC after interaction with NO (for SNP treated thylakoid membranes), which leads to a strong increase of the most reduced S0 state in the dark or an increase of PSII open centers (Vladkova et al. 2011). It is well established that the most sensitive component to UV and temperature stress in photosynthetic membranes is LHCII-PSII supercomplex (Ivanova et al. 2008; Dankov et al. 2009; Apostolova and Dobrikova 2010; Dobrikova et al. 2013). Apostolova et al. (2006) showed that there is a relationship between the organization of PSII complex and oxidation state of the Mn clusters of the OEC . On the other hand, it has been demonstrated that UV radiation causes increase of PSII centers in the S0 state, as one of the reasons for the UV-induced inhibition of the oxygen evolution is the direct absorption of UV light by Mn ions in Mn(III) and Mn(IV) oxidation states (see in Ivanova et al. 2008; Dobrikova et al. 2013). Therefore, it could be assumed that modification of the oxido-reduction states (i.e. more reduced states) of the Mn cluster in OEC after NO interaction is also a possible reason for protection of photosynthetic apparatus under abiotic stress .
14.7.4 Nitric Oxide Under Heavy Metals
The sensitivity of the photosynthetic processes to heavy metals is studied extensively (Arellano et al. 1995; Barón et al. 1995; Boucher and Carpentier 1999; Prasad 2004; Rouillon et al. 2006). One of the primary sources of metal toxicity in chloroplasts is through the generation of ROS, which affects chloroplast structure and function , as in other abiotic stresses. Recently, Saxena and Shekhawat (2013) reported the role of NO in heavy metal tolerance in plants. As reported for other abiotic stresses, NO has both beneficial and harmful effects during heavy metal stress, depending on the concentration and location of NO in the plant cells. Nitric oxide decreases the harmful effects of the ROS generated by heavy metal stress, interacts with other target molecules and regulates the expression of stress responsive genes (Saxena and Shekhawat 2013).
Recently it has been shown that the metalloid arsenic (As) also triggers the NO and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis (Leterrier et al. 2012). Arsenic-treated seedlings showed a significant decrease in growth and an increase in lipid oxidation due to an alteration in antioxidant enzymes with a significant increase in NO content, protein tyrosine nitration and also the S-nitrosoglutathione reductase (GSNOR) activity, which reduced the glutathione and S-nitrosoglutathione content. Talukdar (2013) reported that the exogenous addition of NO significantly reversed the As-induced oxidative stress in Phaseolus vulgaris seedlings, maintaining H2O2 in a certain level through balanced alterations of antioxidant enzyme activities. The role of SNP donated NO in the process of amelioration has ultimately been manifested by significant decrease of the membrane damage and improvement of growth performance in plants grown on As + SNP medium. Nitric oxide synthesis inhibitor PTIO accelerates As-induced oxidative damage, which clearly demonstrates the role of NO in ameliorating metal stress in plants. Yu et al. (2013) studied the effect of Cd-induced stress in cucumber seedlings. Both leaf pigments and net photosynthesis , and antioxidant activity decreased after Cd treatment, which could be reversed by the application of exogenous NO (100 μM SNP). Similarly, Srivastava and Dubey (2012) showed ameliorating effect of NO on the ROS scavenging machinery in Mn-induced oxidative stress in rice seedlings.
14.7.5 Nitric Oxide Under Herbicides
The widespread use of herbicides in agriculture has resulted in increasing pollution of soil and water with these toxic compounds. Herbicides are one of the major abiotic stressors as that of salinity, drought , temperature extremes, flooding, toxic metals, high light intensity and UV-radiation. All of these are the major causes of yield loss in cultivated crops worldwide and pose major threats to agriculture and food security (Rodríguez et al. 2005). The primary site of action of many herbicides is chloroplast , besides the mitochondria. As many other abiotic stressors (Qiao and Fan 2008; Misra et al. 2010a, b, 2011), the herbicides also elicit the production of NO (Klepper 1979; Mallick et al. 2000; Sakihama et al. 2002). Exogenous NO has been shown to reduce herbicide toxicity by its protective effects on chloroplast membrane and by retarding herbicide induced loss of Chl (Beligni and Lamattina 1999a, b; Hung et al. 2002).
One of the most widely used herbicides is atrazine, which belongs to the triazine group of chemicals. The primary site of atrazine action is blocking the PSII electron transport via binding to the QB-binding site on the D1 polypeptide of PSII reaction center and inhibition of light-driven electron transport from QA to QB in PSII (Trebst 1987; Draber et al. 1991). A high sensitivity of the photosynthetic apparatus to atrazine is well documented (Qian et al. 2009; Vladkova et al. 2009; Apostolova et al. 2011; Rashkov et al. 2012). Qian et al. (2009) have shown that in unicellular green algae Chlorella vulgaris, atrazine (100 μg/L) or glufosinate (10 mg/L) with low concentrations of NO donor SNP (10–20 μM) significantly decreased herbicide induced ROS generation and membrane peroxidation and increased the chlorophyll content of leaves.
Other widely used herbicide in agriculture is paraquat (also known as methyl viologen), which belongs to the bipyridinium herbicides . This herbicide exerts its toxic effects by catalyzing the electron transfer from PSI to molecular oxygen, producing oxygen radicals that cause lipid peroxidation and membrane damage (Cha et al. 1982). Hung et al. (2002) have evaluated the protective effect of NO against paraquat toxicity of rice leaves. They showed that NO-donors (PBN, N-tert-butyl-α-phenylnitrone, SNP, sodium nitropruside and SIN-1, 3-morpholinosydnonimine), as well as the ascorbic acid and NaNO2 are effective in reducing paraquat toxicity in rice leaves, most likely mediated through an increase in antioxidant enzyme activities and decrease in lipid peroxidation.
Recently, Sood et al. (2012) revealed the effects of the exogenous NO (donor SNP) on the paraquat treated Azolla microphylla. The authors results suggested that SNP released NO can work both as cytoprotective and cytotoxic in concentration dependent manner and involvement of NO in protecting Azolla against paraquat toxicity. Paraquat (8 μM) alone increased the activities of antioxidant enzymes SOD, CAT, GPX, APX and the amount of H2O2. The supplementation of SNP (8–100 μM) suppressed the activities of antioxidant enzymes and the amount of H2O2 compared to paraquat alone. The addition of NO scavengers along with NO donor in paraquat treated fronds neutralized the effect of exogenously supplied NO, indicating that NO can effectively protect Azolla against paraquat toxicity by quenching ROS. Higher SNP concentration (200 μM) is reported to reverse the effect of NO.
Diquat, like as the paraquat is a bipyridinium herbicide and serves as an artificial electron acceptor of PSI (Beligni and Lamattina 1999a, b), influencing also the level of NO in the plants. Beligni and Lamattina (2002) reported that diquat triggered lipid peroxidation, ribulose-1,5-biphosphate carboxylase/oxygenase (Rubisco) and D1 protein loss. Supplementation of NO-donors SNP and S-nitroso-N-acetylpenicillamine greatly reduced lipid peroxidation, rapid protein turn-over and mRNA breakdown caused by the application of a high dose of diquat to potato leaf pieces or isolated chloroplasts . Moreover, diquat caused an increase in the rate of photosynthetic electron transport in isolated chloroplasts and NO restored it back to the control levels (Beligni and Lamattina 2002).
Lactofen is a diphenylether herbicide , which inhibits Chl biosynthesis by blocking the enzyme protoporphyrinogen-IX oxidase activity, which catalyses the oxidation of protoporphyrinogen-IX to protoporphyrin-IX (proto-IX) (Matringe et al. 1989). Accumulation of protoporphyrins leads to ROS generation causing oxidative stress in the plants. SNP donated NO was able to scavenge ROS generated by the lactofen action in soybean plants, avoiding the photosynthetic pigment breakdown, but the lipid peroxidation was not completely prevented (Ferreira et al. 2010). Later, Ferreira et al. (2011) demonstrated that the lactofen-induced morphological and physiological alterations in soybean leaves are reduced with NO.
14.8 Conclusion
This review clearly shows NO effects on the chloroplast structure and function under physiological and stress conditions. Many investigations revealed the role of the nitric oxide as a key signal molecule in plants. It has also been shown that NO participates under abiotic stress . Nitric oxide production increases in the plants as a response to abiotic stress (Qiao and Fan 2008). Under stress, NO enhances activities of the antioxidant enzymes (Shi et al. 2005; Neill et al. 2008) and as a signal molecule interacts with plant hormones , and affects physiological processes (Laxalt et al. 1997; Zhao et al. 2004; Misra et al. 2006; Qiao and Fan 2008; Misra et al. 2011). The other possibility of the protection of NO on the photosynthesis under abiotic stress can be due to the direct action on the thylakoid membranes, which leads to an increase of the open PSII centers and a modification of the OEC (Vladkova et al. 2011). These changes influence the structure of the LHCII-PSII supercomplex in response to stress factors (Ivanova et al. 2008; Dankov et al. 2009; Apostolova and Dobrikova 2010). On the other hand, it has been shown that there is relationship between the organization of the PSII complex and the oxidation state of the Mn cluster in the OEC (Apostolova et al. 2006). It has been proposed that the modification of the oxido-reduction state of the Mn cluster after NO interaction is a possible reason for the protection of the photosynthetic apparatus under abiotic stress .
Abbreviations
- PSI:
-
photosystem I
- PSII:
-
photosystem II
- LHCII:
-
light-harvesting chlorophyll a/b complex of PSII
- GSNO:
-
S-nitrosoglutathione
- GSSG:
-
glutathione disulphide
- NO:
-
nitric oxide
- qL:
-
coefficient of photochemical fluorescence quenching assuming interconnected PSII antennae
- qP:
-
coefficient of photochemical fluorescence quenching assuming non-interconnected PSII antennae
- NPQ:
-
non-photochemical quenching
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase
- PTIO:
-
2-phenyl-4,4,5,5-tetramentyl-imidazoline-1-oxyl-3-oxide
- NOS:
-
nitric oxide synthase
- L-NNA:
-
Nω-nitro-L-arginine
- SNP:
-
sodium nitropruside
- OEC:
-
oxygen-evolving complex
- CPTIO:
-
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-l-oxyl-3-oxide
References
Abat JK, Deswal R (2009) Differential modulation of S-nitrosoproteome of Brassica juncea by low temperature: change in S-nitrosylation of Rubisco is responsible for the inactivation of its carboxylase activity. Proteomics 9:4368–4380
Airaki M, Leterrier M, Mateos RM, Valderrama R, Chaki M, Barroso JB, del Río LA, Palma JM, Corpas FJ (2012) Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ 35:281–295
Apostolova EL, Dobrikova AG (2010) Effect of high temperature and UV-A radiation on the photosystem II. In: Pessarakli M (ed) Handbook of Plant and Crop Stress, 3rd ed. CRC Press, BocaRaton, pp 577–591
Apostolova EL, Dobrikova AG, Ivanova PI, Petkanchin IB, Taneva SG (2006) Relationship between the organization of the PSII supercomplex and the functions of the photosynthetic apparatus. J. Photochem Photobiol B 83:114–122
Apostolova EL, Dobrikova AG, Rashkov GD, Dankov KG, Vladkova RS, Misra AN (2011) Prolonged sensitivity of immobilized thylakoid membranes in cross-linked matrix to atrazine. Sens Acuat B 156:140–146
Arasimowicz M, Floryszak J (2007) Nitric oxide as a bioactive signaling molecule in plant stress response. Plant Science 172:876–887
Arellano JB, Lázaro JJ, López-Gorgé J, Barón M (1995) The donor side of Photosystem II as the copper-inhibitory binding site. Photosynth Res 45:127–134
Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F (2006) An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J Biol Chem 281:23579–23588
Barón M, Arellano JB, López-Gorgé J (1995) Copper and photosystem II: A controversial relationship. Physiol Plant 94:174–180
Baumgartner BJ, Rapp JC, Mullet JE (1989) Plastid transcription activity and DNA copy number increase early in Barley chloroplast development. Plant Physiol 89:1011–1018
Beligni MV, Lamattina L (1999a) Is nitric oxide toxic or protective? Trends in Plant Science 4:299–300
Beligni MV, Lamattina L (1999b) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344
Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and deetiolation, and inhibits hypocotyls elongation, three light-inducible responses in plants. Planta 210:215–221
Beligni MV, Lamattina L (2001a) Nitric oxide in plants: the history is just beginning. Plant Cell Environ 24:267–278
Beligni MV, Lamattina L (2001b) Nitric oxide: A non-traditional regulator of plant growth. Trends in Plant Sci 6:508–509
Beligni MV, Lamattina L (2002) Nitric oxide interferes with plant photooxidative stress by detoxifying reactive oxygen species. Plant Cell Environ 25:737–748
Biswal AK, Dilnawaz F, David KAV, Ramaswamy NK, Misra AN (2001) Increase in the intensity of thermoluminescence Q-band during leaf ageing is due to a block in the electron transfer from QA to QB. Luminescence 16:309–313
Boucher N, Carpentier R (1999) Heat-stress stimulation of oxygen uptake by Photosystem I involves the reduction of superoxide radicals by specific electron donors. Photosynth Res 59:167–174
Britt RD (1996) Oxygen evolution, In Ort DR, Yocum CF (eds) Advances in Photosynthesis: Oxygenic Photosynthesis, The Light Reactions. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 137–164
Cantrel C, Vazquez T, Puyaubert J, Rezé N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E (2011) Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol 189: 415–427
Cha LS, McRae DG, Thompson JE (1982) Light-dependence of paraquat-initiated membrane deterioration in bean plants. Evidence for the involvement of superoxide. Physiol Plant 56:492–499
Chaki M, Valderrama R, Fernández-Ocãna A, Carreras M, Gómez-Rodríguez V, Pedrajas JR, Begara FJ, Morales JC, Sánchez-Calvo B, Luque F, Leterrier M, Corpas FJ, Barroso JB (2011) Mechanical wounding induces anitrosative stress by down-regulation of GSNO reductase and an increase in S-nitrosothiols in sunflower (Helianthus annuus) seedlings. J Exp Bot 62:1803–1813
Chang HL, Hsu YT, Kang CY, Lee TM (2013) Nitric Oxide down-regulation of carotenoid synthesis and photosystem II activity in relation to very high light-induced singlet oxygen production and oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol 58:1296–1315
Cooney RV, Harwood PJ, Custer LJ, Franke AA (1994) Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspect 102:460–462
Corpas FJ, Chaki M, Fernández-Ocãna A, Valderrama R, Palma JM, Carreras A, Begara FJ, Morales JC, Airaki M, del Río LA, Barroso JB (2008a) Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol 49:1711–1722
Corpas FJ, del Río LA, Barroso JB (2008b) Post-translational modifications mediated by reactive nitrogen species: Nitrosative stress responses or components of signal transduction pathways? Plant Signaling Behaviour 3:301–303
Dankov K, Taneva S, Apostolova EL (2009) Freeze-thaw damage of photosynthetic apparatus. Effect of the organization of LHCII-PSII supercomplex. Comp Rend Acad Bulg Sci 62:1103–1110
Debus RJ (1992) The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta 1102:269–352
Desikan R, Griffiths R, Hancock J, Neill S (2002) New role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Nat Acad Sci, USA 99:16314–16318
Dilnawaz F, Mahapatra P, Misra M, Ramaswamy NK, Misra AN (2001) The distinctive pattern of photosystem II activity, photosynthetic pigment accumulation and ribulose-1, 5-bisphosphate carboxylase/oxygenase content of chloroplasts along the axis of primary wheat leaf lamina. Photosynthetica 39:557–563
Diner BA, Petrouleas V (1990) Formation by NO of nitrosyl adducts of redox components of the Photosystem II reaction center. II: Evidence that HCO3−/CO2 binds to the acceptor-side non-heme iron. Biochim Biophys Acta 1015:141–149
Dobrikova AG, Krasteva V, Apostolova EL (2013) Damage and protection of the photosynthetic apparatus from UV-B radiation I. Effect of ascorbate. J Plant Physiol 170:251–257
Draber W, Tietjen K, Kluth JF, Trebst A (1991) Herbicides in photosynthesis research. Angew Chem 30:1621–1633
Ferreira LC, Cataneo AC, Remaeh LMR, Corniani N, Fumis TDF, Souza YAD, Scavroni J, Soares BJA (2010) Nitric oxide reduces oxidative stress generated by lactofen in soybean plants. Pest Biochem Physiol 97:47–54
Ferreira LC, Cataneo AC, Remaeh LMR, Búfalo J, Scavroni J, Andréo-Souza Y, Cechin I, Soares BJA (2011) Morphological and physiological alterations induced by lactofen in soybean leaves are reduced with nitric oxide. Planta Daninha 29:837–847
Finazzi G, Johnson GN, Dall.osto L, Joliot P, Wollman FA, Bassi RA (2004) Zeaxanthin-independent non photochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci, USA 101:12375–12380
Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Galatro A, Puntarulo S, Guiamet JJ, Simontacchi M (2013) Chloroplast functionality has a positive effect on nitric oxide level in soybean cotyledons. Plant Physiol Biochem 66:26–33
García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204
García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128:790–792
Gould KS, Lamotte O, Klinger A, Pugin A, Wendehenne D (2003) Nitric oxide production in tobacco leaf cells: A generalized stress response? Plant Cell Environ 26:1851–1862
Goussias C, Deligiannakis Y, Sanakisk Y, Ioannidis N, Petrouleas V (2002) Probing subtle coordination changes in the iron-quinone complex of photosystem II during charges separation by the use of NO. Biochemistry 41:15212–15223
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase 1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302:100–103
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends in Plant Science 16:160–168
Haumann M, Liebisch P, Müller C (2005) Photosynthetic O2 formation tracked by time-resolved X-ray experiments. Science 310:1019–1021
Hill AC, Bennett JH (1970) Inhibition of apparent photosynthesis by nitrogen oxides. Atmospheric Environ 4:341–348
Huang X, Rad U, Durner J (2002) Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215:914–923
Hung KT, Chang CJ, Kao CH (2002) Paraquat toxicity is reduced by nitric oxide in rice leaves. J Plant Physiol 159:159–166
Ioannidis N, Schansker G, Barynin VV, Petrouleas V (2000) Interaction of nitric oxide with the oxygen evolving complex of photosystem II and manganese catalase: A comparative study. J Biol Inorg Chem 5:354–363
Ivanova PI, Dobrikova AG, Taneva SG, Apostolova EL (2008) Sensitivity of the photosynthetic apparatus to UV-A radiation: a role of light-harvesting complex II – photosystem II supercomplex organization, Radiat Environ Biophys 47:169–177
Jasid S, Simontacchi M, Bartoli CG, Puntarulo S (2006) Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol 142:1246–1255
Joshi P, Misra AN, Nayak L, Biswal B (2013) Response of mature, developing and senescing chloroplast to environmental stress. In: Advances in Photosynthesis and Respiration, Volume 36. Springer, Dordrecht, pp 641–668
Khangulov SV, Barynin VV, Antonyuk-Barynina SV (1990) Manganese containing catalase from Thermus thermophilus peroxide-induced redox transformation of manganese ions in the presence of specific inhibitor of catalase activity. Biochim Biophys Acta 1020:25–33
Klepper LA (1979) Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmosph Environ 13:537–542
Kopyra M, Gwozdz EA (2004) The role of nitric oxide in plant growth regulation and responses to abiotic stress. Acta Physiol Plant 26:459–472
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10:310–316
Krasylenko YA, Yemets AI, Blume YB (2010) Functional role of nitric oxide in plants. Russian J Plant Physiol 57:451–461
Laxalt AM, Beligni MV, Lamattina L (1997) Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. European J Plant Pathol 103:643–651
Lee U, Wie C, Fernández M, Feelisch BO, Vierling E (2008) Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis, Plant Cell 80:786–802
Lepistö A, Rintamäki E (2012) Coordination of plastid and light signaling pathways upon development of Arabidopsis leaves under various photoperiods. Mol Plant 5:799–816
Leshem YY, Haramaty E, Iluz D, Malik Z, Sofer Y, Roitman L, Leshem Y (1997) Effect of stress nitric oxide (NO): Interaction between chlorophyll fluorescence, galactolipid fluidity and lipoxygenase activity. Plant Physiol Biochem 35:573–579
Leshem YY, Wills RBH, Ku VVV (1998) Evidence for the function of the free radical gas – nitric oxide (NO•) – as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36:825–833
Leterrier M, Airaki M, Palma J, Chaki M, Corpas FJ (2012) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pol 166:136–143
Locato V, Gadaleta C, De Gara L, De Pinto MC (2008) Production of reactive species and modulation of antioxidant network in response to heat shock: A critical balance for cell fate. Plant Cell Environ 31:1606–1619
Mallick N, Mohn FH, Rai L, Soeder CJ (2000) Impact of physiological stresses on nitric oxide formation by green alga, Scenedesmus obliquus. J Microbiol Biotechnol 10:300–306
Martinez GR, Mascio PD, Bonini MG, Augusto O, Briviba K, Sies H (2000) Peroxynitrite does not decompose to singlet oxygen (1gO2) and nitroxyl (NO-). Proc Nat Acad Sci, USA 97:10307–10312
Matringe M, Camadro JM, Labbe P, Scalla R (1989) Protoporphyrinogen oxidase as a molecule target for diphenyl ether herbicides. Biochem J 260:231–235
Mazid M, Khan TA, Mohammad F (2011) Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: A synergistic signalling approach. J Stress Physiol Biochem 7:34–74
Mishina TE, Lamb C, Zeier J (2007) Expression of a nitric oxide degrading enzyme induces a senescence programme in Arabidopsis. Plant Cell Environ 30:39–52
Misra AN, Biswal UC (1980) Effect of phytohormones on the chlorophyll degradation during aging of chloroplasts in vivo and in vitro. Protoplasma 105:1–8
Misra AN, Biswal UC (1982) Differential changes in the electron transport properties of chloroplasts during aging of attached and detached leaves, and of isolated chloroplasts. Plant Cell Environ 5:27–30
Misra AN, Misra M (1986) Effect of temperature on senescing rice leaves. I. Photoelectron transport activity of chloroplasts. Plant Science 46:1–4
Misra AN, Misra M (1987) Effect of age and rehydration on greening of wheat leaves. Plant Cell Physiol 28:47–51
Misra AN, Ramaswamy NK, Desai TS (1997) Thermoluminescence studies on photoinhibition of pothos leaf discs at chilling, room and high temperature. J Photochem. Photobiol B: Biology 38:164–168
Misra AN, Srivastava A,; Strasser RJ (2001) Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol 158:1173–1181
Misra AN, Latowski D, Strzalka K (2006) The xanthophylls cycle activity in kidney bean and cabbage leaves under salinity stress. Russian J Plant Physiol 53:102–109
Misra AN, Misra M, Singh R (2010a) Nitric oxide biochemistry, mode of action and signalling in plants. J Med Plants Res 4:2729–2749
Misra AN, Misra M, Singh R (2010b) Nitric oxide: An ubiquitous signaling molecule with diverse role in plants. African J Plant Sci 5:57–74
Misra AN, Misra M, Singh R (2011) Nitric oxide ameliorates stress responses in plants. Plant Soil Env 57:95–100
Misra AN, Misra M, Singh R (2012) Nitric oxide signaling during senescence and programmed cell death in leaves. In: Ekinci D (ed) Chemical Biology, Intech Open. pp 159–186
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci 7:405–410
Neill S, Desikan R, Hancock J (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, strimal closure, and abiotic stress. J Exp Bot 59:165–176
Nelson N, Yocum CF (2006) Structure and function of Photosystems I and II. Annu Rev Plant Biol 57:521–565
Ordog A, Wodala B, Rozsavolgyi T, Tari I, Horvath F (2013) Regulation of guard cell photosynthetic electron transport by nitric oxide. J Exp Bot 64:1357–1366.
Palavan-Unsal N, Arisan D (2009) Nitric oxide signaling in plants. Bot Res 75:203–229
Penner-Hahn JE, Yocum CF (2005) The photosynthesis “oxygen clock” gets a new number. Science 310:982–983
Petrouleas V, Diner BA (1990) Formation by NO of nitrosyl adducts of redox components of the Photosystem II reaction center. I. NO binds to the acceptor-side non-heme iron. Biochim Biophys Acta 1015:131–140
Pogson BJ, Albrecht V (2011) Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol 155:1545–1551
Prasad MNV (ed.) Heavy metal stress in plants: From biomolecules to ecosystems,. 2004. Springer-Verlag, Berlin-Heidelberg, pp 1–391
Prochazkova D, Wilhelmova N (2011) Nitric oxide, reactive nitrogen species and associated enzymes during plant senescence. Nitric Oxide 24:61–65
Qian H, Chen W, Li J, Wang J, Zhou Z, Liu W, Fu W (2009) The effect of exogenous nitric oxide on alleviating herbicide damage in Chlorella vulgaris. Aqua Toxicol 92:250–257
Qiao W, Fan LM (2008) Nitric oxide signaling in plant responses to abiotic streses. J Integrative Plant Biol 50:1238–1246
Rashkov GD, Dobrikova AG, Pouneva ID, Misra AN, Apostolova EL (2012) Sensitivity of Chlorella vulgaris to herbicides. Possibility of using it as a biological receptor in biosensors. Sens Actuat: B. Chemical 161:151–155
Rodríguez M, Canales E, Borrás-Hidalgo O (2005) Molecular aspects of abiotic stress in plants. Biotecnol Aplic 22:1–10
Rouillon R, Piletsky SA, Breton F, Piletska EV, Carpentier R (2006) Photosystem II biosensors for Heavy Metals Monitoring. In: Giardi MT, Piletska E (eds) Biotechnological Applications of Photosynthetic Proteins: Biochips, Biosensors and Biodevices. Springer US, pp 166–174
Sakihama Y, Nakamura S, Yamasaki H (2002) Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: an alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol 43:290–297
Sanakis Y, Goussias C, Mason RP, Petrouleas V (1997) NO interacts with the tyrosine radical YD• of photosystem II to form an iminoxyl radical, Biochemistry 36:1411–1417
Sanakis Y, Petasis D, Petrouleas V, Hendrich M (1999) Simultaneous binding of fluoride and NO to the nonheme iron of Photosystem II: Quantitative EPR evidence for a weak exchange interaction between the semiquinone QA – and the iron-nitrosyl complex. J Am Chem Soc 121:9155–9164
Saxena I, Shekhawat GS (2013) Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 32:13–20
Schansker G, Goussias C, Petrouleas V, Rutherford AW (2002) Reduction of the Mn cluster of the water-oxidizing enzyme by nitric oxide: Formation of an S−2 state. Biochemistry 41:3057–3064
Schansker G, Petrouleas V (1998) In: Garab G (ed) Photosynthesis: mechanisms and effects. Kluwer, Dordrecht., Vol. 2, pp 1319–1322
Selcukcam EC, Cevahir OG (2008) Investigation on the relationship between senescence and nitric oxide in sunflower (Helianthus annuus L.) seedlings. Pak J Bot 40:1993–2004
Sharma P, Sharma N, Deswal R (2005) The molecular biology of the low-temperature response in plants. Bioessays 27:1048–1059
Shi SY, Wang G, Wang YD, Zhang LG, Zhang LX (2005) Protective effect of nitric oxide against oxidative stress under ultraviolet-B radiation. Nitric Oxide 13:1–9
Song L, Ding W, Shen J, Zhang Z, Bi Y, Zhang L (2008) Nitric oxide mediates abscisic acid induced thermotolerance in the calluses from two ecotypes of reed under heat stress. Plant Sci 175:826–832
Sood A, Kalra C, Pabbi S, Uniyal PL (2012) Differential responses of hydrogen peroxide, lipid peroxidation and antioxidant enzymes in Azolla microphylla exposed to paraquat and nitric oxide. Biologia 67:1119–1128
Srivastava S, Dubey RS (2012) Nitric oxide alleviates manganese toxicity by preventing oxidative stress in excised rice leaves, Acta Physiol Plant 34:819–825
Stamler JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898–1902
Styring S, Rutherford AW (1987) In the oxygen-evolving complex of photosystem II the S0-state is oxidized to the S1-state by D+ (Signal II slow). Biochemistry 26:2401–2405
Sun J, Jiang H, Xu Y, Li H, Wu X, Xie Q, Li C (2007) The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis, Plant Cell Physiol 48:1148–1158
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Takahashi S, Yamasaki H (2002) Reversible inhibition of photophosphorylation in chloroplasts by nitric oxide. FEBS Lett 512:145–148
Talukdar D (2013) Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19:69–79.
Tewari RK, Prommer J, Watanabe M (2013) Endogenous nitric oxide generation in protoplast chloroplasts, Plant Cell Rep 32:31–44
Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181:871–879
Tossi V, Cassia R, Bruzzone S, Zocchi E, Lamattina L (2012) ABA says NO to UV-B: a universal response? Trends in Plant Sci 17:510–517
Trebst A (1987) The three-dimensional structure of the herbicide binding niche on the reaction center polypeptides of photosystem II. Z Naturforsch 42c:742–750
Vladkova R, Ivanova P, Krasteva V, Misra AN, Apostolova E (2009) Assessment of chlorophyll fluorescence and photosynthetic oxygen evolution parameters in development of biosensors for detection of QB binding herbicides. Compt rend Acad bulg Sci 62:355–360
Vladkova R, Dobrikova AG, Singh R, Misra AN, Apostolova E (2011) Photoelectron transport ability of chloroplast thylakoid membranes treated with NO donor SNP: Changes in flash oxygen evolution and chlorophyll fluorescence. Nitric Oxide 24:84–90
Volkov RA, Panchuk II, Mullineaux PM, Schöfl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61:733–746
Waldo GS, Penner-Hahn JE (1995) Mechanism of Manganese Catalase Peroxide Disproportionation – Determination of Manganese Oxidation-States During Turnover. Biochemistry 34:1507–1512
Wilson ID, Neill SJ, Hancock JT (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31:622–631
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Rad Biol Med 25:434–456
Wodala B (2006) Combined effects of nitric oxide and cyanide on the photosynthetic electron transport of intact leaves. Acta Physiol Szeg 50:185–188
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F (2008) In vivo target sites of NO in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol 146:1920–1927
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Envron 25:131–139
Xu YF, Sun XL, Jin J-W, Zhou H (2010) Protective roles of nitric oxide on antioxidant systems in tall fescue leaves under high-light stress. Afr J Biotechnol 9:300–306
Yamasaki H, Sakihama Y, Takahashi S (1999) An altenative pathway for nitric oxide production in plants: new features of an old enzyme. Trends in Plant Sci 4:128–139
Yang JD, Zhao HL, Zhang TH, Yun JF (2004) Effects of exogenous nitric oxide on photochemical activity of photosystem II in potato leaf tissue under non-stress condition. Acta Sin 46:1009–1014
Yu L, Gao R, Shi Q, Wang X, Wei M, Yang F (2013) Exogeneous application of sodium nitroprusside alleviated cadmium induced chlorosis, photosynthesis inhibition and oxidative stress in cucumber. Pak J Bot 45:813–819
Zhang L, Wang Y, Zhao L, Shi S, Zhang L (2006) Involvement of nitric oxide in light-mediated greening of barley seedlings. J Plant Physiol 163:818–826
Zhang L, Zhou S, Xuan Y, Sun M, Zhao L (2009) Protective effect of nitric oxide against oxidative damage in Arabidopsis leaves under ultraviolet-B irradiation. J Plant Biol 52:135–140
Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134:849–857
Zhao M, Zhao X, Wu Y, Zhang L (2007) Enhanced sensitivity to oxidative stress in an Arabidopsis nitric oxide synthase mutant. J Plant Physiol 164:737–745
Zhao L, He J, Wang X, Zhang L (2008) Nitric oxide protects against polyethylene glycol-induced oxidative damage in two ecotypes of reed suspension cultures. J Plant Physiol 165:182–191
Zhao MG, Chen L, Zhang LL, Zhang WH (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767
Acknowledgements
This is a part of the UGC MRP No. F. 36-302/2008 and DBT BUILDER project No. BT/PR9028/INF/22/193/2013 to ANM and is the result of International cooperation grants BIn-01/07 of the NSF of Bulgaria and project Grant No. INT/BULGARIA/B70/06 DST, India. MM acknowledges the UGC, India grant [No. F.15-14/11 (SA-II)] of PDF for Women.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Misra, A.N., Singh, R., Misra, M., Vladkova, R., Dobrikova, A.G., Apostolova, E.L. (2017). Nitric Oxide Mediated Effects on Chloroplasts. In: Hou, H., Najafpour, M., Moore, G., Allakhverdiev, S. (eds) Photosynthesis: Structures, Mechanisms, and Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-48873-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-48873-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48871-4
Online ISBN: 978-3-319-48873-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)