Abstract
Animal communication is based on signals that provide information to receivers regarding specific aspects of the environment and individual traits of the signaler. Many animals produce acoustically different call types depending on the different behaviors or general contexts they experience. The acoustic structure within a call type typically varies and conveys socially relevant information specific to individual identity, sex, age, social rank, relatedness, or group membership. Both specific referents to the context and referents to individual and group signatures enable receivers to extract diverse information and to incorporate it into their decisions at different levels of complexity in social interactions. From the production side, it is difficult to prove what cognitive mechanisms underlie the emission of specific call types, but recent empirical studies support the fact that it cannot be based on simple emotional expressions. More likely, multiple information processes are involved that integrate the individual traits and an animal’s perceptions of different referents, the overall context, or other external stimuli, to produce the final acoustic outcome. Research on the cognitive mechanisms that underlie the perception of different types of referents reveals that on the receiver side, information use likely has both innate and learned components. As such, in all cases, a cognitive representation of the eliciting stimuli expressed by the specific call structure is likely learned by receivers based on simple association of the signal’s acoustic structure and the context or the individual traits of the signaler. In the case of functionally referential signals, referents to external stimuli seem to play an influential role in affecting the response of receivers, allowing less flexibility to integrate additional information, compared to other, less context-specific calls, due to the urgency of responding. The different referents in a call should generally reflect the social and ecological constraints a species experiences.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
Animals coordinate their activities with conspecifics by communicating with each other and thereby produce a variety of signals relevant to their survival and reproduction, and hence their evolutionary fitness . Communication plays a particularly important role within group-living species in allowing individuals to coordinate their daily activities and form social relationships. Vocal communication is common in many birds (Chaps. 2 and 3) and mammals (Chaps. 9, 10 and 11), although olfactory, visual, and tactile signals are also frequently used (Bradbury and Vehrencamp 2011). While it is obvious that any signal produced has some level of reference (Marler et al. 1992), the debate over which animal vocalizations can be regarded as referential signals remains contentious, and a similarly controversial issue persists regarding the semantics of vocalizations, that is, the meaning of the signal to the receiver (e.g., Stegman 2013; Scarantino and Clay 2015; Wheeler and Fischer 2012, 2015). Referents and semantics in animal vocalizations are the topics of this chapter.
Both of these issues—referents and semantics—were initially introduced into the study of animal communication through research on alarm calling by vervet monkeys , Chlorocebus pygerythrus (formerly Cercopithecus aethiops). This work described and quantified the production of, and responses to, alarm calls that were specific to different types of predators (Seyfarth et al. 1980; Marler et al. 1992). By confirming Struhsaker’s (1967) observations on alarm call production, and by using playback experiments to isolate the context-independent information provided by signals, Seyfarth et al. (1980) provided the first evidence of referential communication in animals (Manser 2013). They not only confirmed that vervet monkeys produce distinct alarm calls for leopards, eagles, snakes, and baboons . They also quantified the distinctive behavioral responses elicited by each of these predator types. For example, vervets ran up into a tree when a leopard appeared. When an eagle appeared, individuals on the ground ran into a bush or tree, whereas individuals already in a tree moved down from its top to the center of the tree out of harm’s way. When a snake was encountered, the animals all stood up bipedally and looked around on the ground. In field playback tests, hearing the distinctive alarm calls evoked by each type of predator, broadcast in the absence of the predator itself, elicited the same behavioral responses that were appropriate for the type of predator that had originally elicited the call used as a stimulus. That is, when the animals heard an alarm call originally evoked by a leopard, for example, they responded as if a leopard were actually present. Within the different call types, some variation in call structure (e.g., call length, call interval, or amplitude) appeared to be arousal-related or to express individual traits. Although, for example, an increase in alarm call length increased responsiveness in some cases, these acoustic properties did not affect the qualitative distinctions among responses to the predator-specific call types.
Based on this seminal work on vervet monkeys , “functionally referential” signals have been defined as signals that refer to external objects and events and convey a specific semantic meaning to receivers (Macedonia and Evans 1993). In this chapter, I seek to develop a somewhat broader framework for considering referential signals that unifies work on several related topics in the field of animal communication. In addition to including the typical definition of referential signals as referring to external objects and events, this framework, outlined in Sect. 8.2, also recognizes two additional types of referents (Table 8.1) . First, it recognizes that vocal signals commonly carry information that refers to the phenotypic traits or social status of individuals, as well as to their membership in various social categories and their social relationships. In Sect. 8.2.1, I discuss studies of recognition and discrimination of individuals and other social categories as well as recognition of third-party social relationships . Second, this framework recognizes that signals can also contain references to the behavioral context and ongoing expressions of specific behaviors. I discuss these issues in Sect. 8.2.2. In Sect. 8.2.3 I return to a discussion of alarm signaling as an example of signals for which external objects and events are the referents. The development of this framework is then followed in Sect. 8.3 by a general discussion of semanticity in animal communication. Section 8.4 briefly considers the psychological mechanisms potentially involved in producing and receiving the three different types of referential signals. Throughout the chapter, I will distinguish between the production and the perception side of signaling behavior and address the question of whether similar cognitive mechanisms underlie communication involving the three broad types of referents in animal vocalizations (Table 8.1). To facilitate the discussion, I will focus on the work we have been doing over the last two decades on meerkats (Suricata suricatta) (Fig. 8.1a) and, to a lesser extent, the banded mongoose (Mungos mungo) (Fig. 8.1b). In parallel, I integrate results from this work, where possible, with research on other non-primate mammal and bird species and compare it with primates . Before turning to the types of references in communication signals in Sect. 8.2, I wish to provide a brief background on some of the important issues at hand and on the main study animal to be discussed.
Two mammalian study systems for investigating referents and semantics in animal vocalizations. (a) A study group of meerkats. The dominant male wears a radio transmitter that allows researchers to locate the group at any time (photo courtesy Tim Clutton-Brock). (b) A study group of banded mongooses (photo courtesy Feargus Cooney)
8.1.1 Overview of the Issues
Signals evolve if they bring an advantage to the signaler as well as to the receiver. The signaler influences the behavior or physiology of the receiver to its own advantage (Maynard-Smith and Harper 2003), and the receiver typically responds in a way that is of advantage to itself. In cooperative situations, the interests of signalers and receivers overlap, while in conflict situations, signalers and receivers have different motivations in the production of the signal and how to respond, and benefits to the communicative partners may differ substantially. In general, the mechanisms underlying the production of signals by the signaler differ from the mechanisms that are involved in the perception of the signal and subsequent generation of a response (Seyfarth and Cheney 2003). Vocal signals had long been regarded as motivational or emotional expressions of animals (Darwin 1872; Morton 1977). Following the study describing alarm calls specific to different predator types in vervet monkeys (Struhsaker 1967), however, researchers realized that specific external stimuli can elicit highly context-specific calls. Subsequently, the discussion emerged as to whether these calls refer to external events or objects (Seyfarth et al. 1980; Marler et al. 1992; Macedonia and Evans 1993) and whether receivers have a cognitive representation of the eliciting stimuli that can be evoked by only hearing the vocalizations (Zuberbühler et al. 1999). However, recent discussions about the cognitive mechanisms underlying vocal perception question whether these highly context-specific calls induced by an external event or object should be regarded as different than calls associated with a specific behavior or the individual traits of the signaler (Wheeler and Fischer 2012).
Vocal signals for most mammal species have been described as innate and hardwired, leaving little room for adjustment and flexibility in different social and ecological contexts. This is particularly true for the production side with regard to the signal’s overall acoustic structure, but is less true for the usage and comprehension of calls (Smith 1965, 1981; Chaps. 9 and 10). So far only a few species have been documented to adjust their calls as adults and, for example, to conform as a social group to a vocal signature shared in common (humpback whales , Megaptera novaeangliae , Payne and Payne 1985; chimpanzees, Pan troglodytes, Crockford et al. 2004) or to imitate conspecifics (bottlenose dolphins , Tursiops truncatus , King and Janik 2013) or heterospecifics (harbor seals , Phoca vitulina , Ralls et al. 1985; Asian elephants, Elephas maximus, Stoeger et al. 2012). More frequently, mammal and bird species have been reported to exhibit high flexibility in tailoring signal usage to their social environment (Seyfarth and Cheney 2010). Similarly, from the perception side, we observe considerable variability in the likelihood that receivers respond to the same call types and also in the strength of their responses.
Regarding underlying cognitive mechanisms, these patterns suggest differences between call production , call usage, and call comprehension. In many species, call production seems primarily genetically determined and triggered by specific external or internal factors, without much control and flexibility on the part of the signaler. Whereas in call usage and call comprehension, the signaler and receiver show much more flexibility and capacity to adjust their signals to their social and ecological environment (see Chap. 10). Therefore, the key questions we are interested in addressing are what causes variation in the acoustic structure of a signaler’s calls, and what aspects of this variation do receivers perceive as meaningful in terms of changing their behavior? The follow-up questions are then, what are the underlying cognitive mechanisms on the production side, in terms of call usage, and on the perception side, in terms of generating a response, and in what ways do they differ? We are, in particular, interested in asking these questions separately as they pertain to the three different types of references in animal vocalizations mentioned above (Table 8.1) and outlined in more detail in Sect. 8.2.
8.1.2 Meerkats
Meerkats, a cooperatively living mongoose species of the family Herpestidae , live in despotic societies with the dominant pair monopolizing reproduction (Fig. 8.1a; Clutton-Brock et al. 1998). They occupy the open, dry habitat of a semidesert, with scarce food availability and high predation pressure (Clutton-Brock et al. 2001). The group typically consists of between 3 and 50 individuals, with several subordinate adult and subadult individuals, juveniles, and pups (Clutton-Brock et al. 2006). Each group defends a territory of about 2–5 km2 (Manser and Bell 2004) by marking along their territory boundaries, and other important locations, using feces and anal gland secretion (Jordan et al. 2007). The dominant female can produce up to 4 litters a year with 1–7 pups per litter (Clutton-Brock et al. 2001). Dominant females contribute up to 80 % of the pups in the population, and the dominant male sires about 80 % of the dominant female’s pups (Griffin et al. 2003; Spong et al. 2008; Nielsen et al. 2012). Subordinate individuals typically forego their own reproduction and help raise the dominant pair’s offspring (Clutton-Brock et al. 1998), although under some circumstances, they are able to reproduce and even raise their own offspring. A group can be highly stable over many years and have the same dominant pair, but in other groups, dominant pairs, or at least one of the dominant individuals, may change frequently, bringing instability to the group, often with the result of decreased reproduction (unpublished data, long-term Kalahari Meerkat Project).
The dominant pair exhibits distinctive behaviors to assert their dominance position, such as by frequent anal markings at obvious locations within their home range, as well as by regularly marking other group members. Subordinates periodically show clear submissive behaviors by initiating grooming and emitting specific vocalizations (Kutsukake and Clutton-Brock 2006). A recent analysis focusing on social interactions among subordinate females has shown that there is also subtle competition and that a social hierarchy exists, whereby age and condition seem to be the determining factors in what relative ranking a specific individual assumes (Thavarajah et al. 2014).
Meerkats have evolved a rich repertoire of signals in several different modalities that are used to coordinate their cooperative behavior and cohesive group movement, as well as in forming social relationships. This includes a sophisticated vocal system (Fig. 8.2; Manser et al. 2014), in addition to olfactory (Jordan et al. 2007) and visual signals. They produce at least 30 different call types, and, particularly in the context of predator avoidance, calls are associated with high variation in their acoustic structures (Manser et al. 2014). Due to their cohesive foraging , and the fact that a single individual must trade off being vigilant for predators against digging for prey in the sand, they also coordinate their movement while foraging using several different call types. Finally, calls also play an important role in both affiliative and agonistic social interactions. Pup vocalizations differ from the adult vocal repertoire (White 2001). In particular, several different types of begging calls are produced to elicit provisioning by the older group members (Manser and Avey 2000; Kunc et al. 2007). After individuals reach approximately 6 months of age, the full range of adult vocalizations is in place (Hollén et al. 2008).
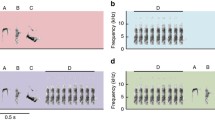
Spectrograms of the different alarm calls of meerkats (Suricata suricatta). Top row shows the highest-urgency calls; bottom row, the lowest-urgency calls, which are not predator-type specific. Middle rows (framed) show the predator-specific calls (aerial, terrestrial, recruitment) in relation to the urgency (low, high) of the situation (Reproduced from Manser 2009)
Olfactory, visual, and tactile cues and signals also play critical roles in meerkat communication, though to date they have been less thoroughly investigated. Olfactory cues or signals include the deposition of feces, urine, and scent marks from different glands, but mainly anal glands (Jordan et al. 2007). These signals are used to maintain the dominance hierarchy within a group, signal territory boundaries, and potentially advertise the reproductive state of females within the group . Visual signals include different body postures, but also glares that engage others visually, or changes in tail positions (M. B. Manser, personal observations) that, in particular, occur during social interactions.
8.2 Types of Referential Vocal Signals
Animal communication has been defined as functionally referential if a signaler produces a signal that is associated with a specific object or event in the external environment and allows the receiver to extract that information and use it in generating an accordingly specific response (Marler et al. 1992; Macedonia and Evans 1993). Calls related to the behavioral state of the signaler were regarded as the expression of the motivation or emotion of the caller (Darwin 1872; Morton 1977; for review Manser 2009). Recent discussions have questioned whether a distinction of functionally referential calls in comparison to other less context-specific call types or calls referring to the signaler’s behavioral state is justified, based on the cognitive mechanisms involved in perceiving and responding to signals (Wheeler and Fischer 2012). The basic argument is that any call type, whether highly context specific to the external environment of the signaler or referring to the signaler’s behavioral state, conveys some referential information to the receiver. In this section, I describe an expanded framework for referential signals that considers how acoustic variation in calls relates to different types of references in vocal communication in terms of conveying specific information to receivers (Table 8.1 ).
8.2.1 Reference to Individual Traits and Social Categories
Phenotypic variation of traits related to the individuality or social category of the signaler allows social recognition at different levels (Wiley 2013; Chap. 7). This variation, thus, conveys a type of reference to the receiver by differentiating animals according to their group membership, kin, rank, sex, age, or quality, with the most fine-grained categorization occurring at the individual level, or even within an individual (e.g., due to hormonal changes related to reproductive stage or health condition). Testing whether individual differences in vocalizations are meaningful to the receiver has challenged researchers. One particular recurring hurdle is whether receivers just discriminate among individuals or categories of individuals or whether they recognize the individual and have some cognitive representation of it (Proops et al. 2009).
Categorizing conspecifics is important for any animal but in particular for animal species that live in groups with repeated encounters and individualized interactions. In such social settings, it becomes advantageous to distinguish among individuals based on differences in relationships (Wittig et al. 2007) or contribution to cooperative tasks (Krams et al. 2008). In almost all mammal species, animals produce individually distinctive vocalizations and olfactory signals due to differences in their morphology or physiology, irrespective of whether there has been past selection for individuality (Sheehan et al. 2014). In this section, I will evaluate the different types of social recognition in general, and in meerkats in particular, to identify the underlying cognitive mechanisms.
8.2.1.1 Individual Discrimination and Recognition
Despite the fact that individuality in most vocalizations has been described early on, such as Beecher (1982, 1991) emphasizing when individuality is likely to be evolutionarily advantageous, it has taken a long time to collect evidence that individually specific signals are used by receivers. In the past, researchers typically recorded calls of several individuals and quantified the differences in vocalizations. Most of the time, this was done from one sample of recordings, and we often do not know how stable individual traits in calls are in relation to the ontogeny of an animal. Some call structures vary also within an individual, for example, due to hormonal influences related to dominance rank , condition, or health state, such as cortisol or testosterone having a direct influence on pitch or call rate and length.
The strength of selection for individuality depends on interactions among group members, the group composition, and in particular also on the group size. In species where group members aggregate but do not show individualized interactions, recognition is less important than in groups where such individualized interactions have evolved. In such species, being individually recognized is more difficult in larger groups (Pollard and Blumstein 2011). Therefore, in social animals, for which recognizing different individuals brings benefits, increased group size may select for increased individuality. Evidence in support of this hypothesis exists in analyses of contact calls of bats (Wilkinson 2003) and alarm calls in sciurid rodents (Pollard and Blumstein 2011). It remains to be investigated whether similar relationships exist in the different social mongoose species, which vary in maximal group size, with dwarf mongoose groups having up to 30 members, those of meerkats ranging up to 50 individuals, and those of the banded mongoose extending up to 70 individuals (Manser et al. 2014). Also, the question arises as to whether these effects of group size may also apply within a species, with the calls in larger groups becoming more individually distinct with smaller within-individual variation and larger among-individual variation. Furthermore, some types of vocalizations, either due to their acoustic structure or their function and the context they are related to, may be more individually distinct than others (Rendall et al. 2009).
Methods to identify how animals categorize their social environment differ depending on the level of recognition required. Individual discrimination is based on a simple mechanism, where receivers detect acoustic differences between the calls of different individuals. In contrast, individual recognition requires receivers to have a cognitive representation about which specific individual is associated with different call structures (Seyfarth and Cheney 2015). Individual or categorical discrimination can be tested by simple habituation-discrimination experiments (Johnston and Bullock 2001; Chap. 7), whereas the question of individual or categorical recognition is better investigated by violations of expectation experiments (Proops et al. 2009). In habituation-discrimination experiments , a subject is presented with a set of different stimuli of the same category until it no longer shows a continued response (Johnston and Bullock 2001). Once habituation has occurred, a stimulus of a different category is presented. If this new stimulus causes a response similar to that at the very beginning, we have evidence that the animals distinguish between the two stimulus types. If the subject’s response does not recover, it becomes difficult to interpret the result (see discussions of this issue in Chaps. 6 and 7). For example, did the subject fail to respond because the difference between stimuli was smaller than the corresponding just-noticeable difference (JND) ? Did the difference exceed the subject’s JND but fall below its just-meaningful difference (JMD; Nelson and Marler 1990) ; that is, were the differences perceptually discriminable but behaviorally irrelevant? Or was the experimental setup somehow flawed or simply not adequately realistic or representative? To exclude failures of discrimination due to failures of experimental design, it is imperative to simultaneously run appropriate control conditions (Hare and Atkins 2001; Schibler and Manser 2007; Karp et al. 2014).
One of the most convincing studies on individual recognition of vocalizations was conducted with domestic horses ( Equus caballus ) by employing a cross-modal expectancy violation experiment (Proops et al. 2009). In expectancy violation experiments, a cue or signal is presented that goes together with previous information about an event or detected object (congruent situation). Then a test is performed to determine whether the animal responds in a surprised manner if the presented cue or signal does not correspond to the original information (incongruent situation). In the study by Proops et al. (2009), subjects were first presented with a specific horse from their group who was then led behind a visual barrier. This generated a visual expectancy regarding which horse was blocked from view. Using playbacks of horse contact calls from the direction of the barrier, which were either congruent or incongruent with the horse hidden behind the barrier, this expectancy was either confirmed or violated. If the vocalization and the horse the test subject was exposed to were congruent, the response was minimal. In the situation where the horse vocalization being broadcast was from a different individual, the test subject showed surprised behavior. These results have since been confirmed in other animals using similar setups testing cross-modality between vocal and visual cues (large-billed crows, Corvus macrorhynchos , Kondo et al. 2012; rhesus monkeys, Macaca mulatta , Adachi and Hampton 2011) and also between vocal and olfactory cues (ring-tailed lemurs, Lemur catta , Kulahci et al. 2015).
The maintenance of the social structure displayed by meerkats seems to require individual recognition and not just individual discrimination. This recognition could occur within the visual, olfactory, or vocal modalities. With experiments we have shown that they vocally distinguish among individuals and that this has to be at the recognition level rather than just discriminating among calls of different individuals (Townsend et al. 2012a; Reber et al. 2013). With an expectancy violation experiment in the spatial arrangement of foraging meerkats, we first showed that meerkats discriminate between calls of different individuals (Townsend et al. 2012a). In a follow-up experiment, we showed that subordinate meerkats also distinguish between any other subordinate female in the group and the dominant female (Reber et al. 2013). Here, we controlled that it was not just a dominance expression in the acoustic structure of the calls but rather the recognition of the dominant females. Based on these two experiments, we concluded that meerkats recognize individuals from their calls, at least their “close calls,” by recognizing the acoustic variation and associating it with specific group members.
Individual discrimination or recognition is not always used by receivers even if individual differences in signals exist. In meerkats we have shown in several different contexts that calls vary individually, including alarm calls (Schibler and Manser 2007), sentinel calls (Manser 1998), and close calls (Townsend et al. 2010). However, the receivers seem not to use this information in all circumstances. When testing individual discrimination of alarm calls with a habituation-discrimination experiment , meerkats showed the same response, whether it was an individual that had been made unreliable or another reliable group member (Schibler and Manser 2007). This indicated that the identity of the animal producing the alarm call was either not perceived or was not important in a receiver’s decision to respond. Such a lack of discrimination may be understandable in a high-risk situation such as alarm calling. Although in other species, such as marmots (Marmota marmota) , receivers take the identity of the signaler into account in similar high risk situations (Blumstein and Daniel 2004). In meerkats, unreliable signalers may be less frequent due to the high predation risk, such that they cannot afford to cheat (Schibler and Manser 2007).
Another situation showing the flexibility of receivers is the context dependence of meerkat responses to close calls. Close calls are the most frequently emitted calls while foraging , with a large variation in call rate, but also individual variation in call structure, as well as more extensive group differences (Townsend et al. 2010). The response of receivers to the dominant female’s close calls described in the above experiment only becomes obvious when there is a conflict between the listener and the dominant female, but not during stable, relaxed periods (Reber et al. 2013). Similar results have been reported in chacma baboons ( Papio ursinus ) where a female responded to another female’s threat grunt only if she had recently been threatened by that female. If she had recently groomed with that same female, she ignored the call (Engh et al. 2006). These experiments clearly show that receivers discriminate individual differences in vocalizations and that they are also able to represent the signaler in ways that go beyond the memory of their individually distinct signals. However, if there is no motivation to show a response, we are not able to distinguish the causes of no response. It then becomes difficult to judge whether individual differences are not important or the experimental setup was not appropriate to the species’ natural behavior, as may have been the case when we tested the same setup in the banded mongoose, and no variation in response was detected (Jansen et al. 2013).
8.2.1.2 Social Category Discrimination and Recognition
Social recognition is not only about individual recognition but also occurs at other levels, such as group, kin, rank, sex, age, or the advertisement of quality. Several species have evolved group signatures in their vocalizations and scents. In theory, such group signatures facilitate the immediate identification of the group a signaler belongs to and, as such, can be used to help advertise or defend home ranges or group resources. Group-specific calls may be due to genetic differences, as shown for Gunnison’s prairie dogs ( Cynomys gunnisoni , Travis et al. 1997) and squirrel monkeys ( Saimiri sciureus , Lieblich et al. 1980), or due to vocal learning , as shown in humpback whales (M. novaeangliae, Payne and Payne 1985), yellow-naped amazons (Amazona auropalliata, Wright and Wilkinson 2001), and chimpanzees (P. troglodytes, Crockford et al. 2004).
In meerkats we have shown that individuals in different groups vary in vocal and olfactory signals individually, but also appear to have a group signature (Townsend et al. 2010; Wadewitz 2010). However, while we found a strong response to olfactory cues of foreign meerkats (Wadewitz 2010), the animals did not show much interest in the calls of foreign individuals (Townsend et al. 2010; Reber et al. 2013). The same result was found for the banded mongoose , in which subjects respond particularly strongly to the olfactory cues of neighbors and less strongly to those of strangers (Müller and Manser 2007), but when playing contact calls of neighbors, they do not respond at all (Müller 2007).
The group signature in meerkat vocalizations is unlikely to be under strong selection given that it is not used by receivers to distinguish between their own group and foreign groups (Townsend et al. 2010). Olfactory signals , however, may be much more important in these contexts. This is not surprising if we consider that vocalizations are used mainly during within-group communication and not in between-group interactions or in advertisements to potential mates. The one context where it may be beneficial to adjust vocalizations to other group members is for male immigrants, so as not to be too different from others in the newly joined group and, thus, to facilitate being recognized as a group member. However, as long as having a distinct individual signature is not disadvantageous, there may be no selection pressure to change it, and conformity in meerkats, due to social or ecological factors, may not be expected. Ongoing work is currently investigating how immigrant adult males adjust their individual signature in calls given in their new social group. This may differ in regard to olfactory signals , as they are used to communicate within and between groups, and this is true for meerkats (Jordan et al. 2007; Wadewitz 2010) and banded mongooses (Jordan et al. 2010).
Kin also represent an important social category for which advertisement and recognition can be beneficial. This is particularly true in mate choice contexts, in which inbreeding avoidance is paramount, or in cooperative breeding contexts, in which investing help in closely related individuals is key to increasing indirect fitness benefits. For bell miners ( Manorina melanophrys ), a colonial honeyeater from Australia, the calls of more closely related individuals are more similar in comparison with unrelated individuals, though helping effort correlated not with genetic relatedness but with acoustic similarity (McDonald and Wright 2011). While evidence for kin recognition of vocalizations in mate choice is missing, olfactory signals play an important role in mate preference (Le Claire et al. 2013). Several different mechanisms may facilitate kin recognition, including familiarity (i.e., growing up in close spatial proximity with ample opportunities for social interactions) or phenotype matching, in which animals assess their own phenotype or that of the phenotype of their familiar kin and compare it to the encountered unfamiliar individuals (Lacy and Sherman 1983; Le Vin et al. 2010).
Meerkats live in social groups with several overlapping generations and in which mainly the females are philopatric and the males disperse typically at the age of 1–3 years (Clutton-Brock et al. 1998; Young and Clutton-Brock 2006). Females disperse after they have been forced out of their natal group by the dominant female and then sometimes meet up with males to found a new group. Within groups, meerkat shows clear inbreeding avoidance, in which the dominant female does not breed with her sons, the dominant male does not breed with his daughters, and full-siblings or half-siblings do not breed with each other (Nielsen et al. 2012). The question was how dispersing females and males avoid inbreeding, as they might encounter full-siblings or half-siblings they had never encountered before and, thus, could not rely on familiarity as a mechanism for inbreeding avoidance. The most obvious signals to use are olfactory , as vocal signals are not frequently given in such encounters, and visual cues, although there seem to be some similarities among group members, may not be as reliable. In a study testing kin recognition in meerkats via olfactory signals , in particular anal gland secretions, females invested more time inspecting the scent of related than unrelated unfamiliar individuals, suggesting that they use a phenotype-matching mechanism to discriminate kin from non-kin (Le Claire et al. 2013).
The recognition of social rank may be based on simple mechanisms where each individual in the group divides its social companions into two groups: those ranked above itself and those ranked below. Slightly more complicated mechanisms may involve an individual understanding of the rank relations among every other individual in the group, which has been shown in chacma baboons ( Papio ursinus , Bergman et al. 2003) and pinyon jays ( Gymnorhinus cyanocephalus , Paz-y-Miño et al. 2004). Here, rank order recognition may involve transitive inference , meaning that subjects recognize if A dominates B and B dominates C, then A dominates C. In meerkats we have the clear distinct role of the dominant pair , where within the same sex, they are dominant to everybody else. Among the subordinates, a rank order also exists and appears to be related to age given that, within litter/age cohorts, same-sex siblings establish their relative rankings by showing dominance assertion to each other (Thavarajah et al. 2014). Existing data suggest meerkats accept older individuals as higher ranked, but compete for ranks within their cohort. If the dominant position were freed up, the most dominant individual in the next cohort has an advantage because it can quickly assume dominance without long, costly competition for the position against other same-sex individuals in the group.
Recognition of other social categories, such as sex or quality, seems not to play an important role in the vocal behaviors of meerkats. This contrasts with some other mammal species, in which, for example, we find clear differences between females and males in the structure of alarm calls (e.g., vervet monkeys, Seyfarth and Cheney 1990; green monkeys , Chlorocebus sabaeus , Price and Fischer 2013), suggesting that the function of alarm calls may be different for the two sexes . That we do not find a sex difference in meerkats might be related to the fact that we have little evidence that quality advertisement plays a role in recruiting mates. However, quality advertisement could be important in the direct competition within the different sexes, when competition is most obvious. This may be the case during dominance changes within each sex, when individuals compete very obviously with each other (Clutton-Brock et al. 2006; Kutsukake and Clutton-Brock 2006), but also among subordinates during relaxed periods, when there seems to be a continuous assertion of dominance, in particular among litter mates (Thavarajah et al. 2014). We are currently addressing these questions to see whether the most frequently emitted close calls or any other vocalization types change in individuals during the transition from subordinate to dominant. Such a change would not be surprising, as females experience a secondary growth period during this stage (Russell et al. 2004). The question would still remain as to whether such changes are at all meaningful to receivers.
8.2.1.3 Recognition of Third-Party Relationships
Recognition of relationships among other group members, that is, third-party relationships, is considered to be a particularly complex cognitive challenge (Seyfarth and Cheney 2015). Recognizing third-party relationships requires more complex cognitive mechanisms than the ability to individually recognize conspecifics. It requires observing and appropriately interpreting third-party interactions and integrating this information into one’s future behavioral decisions . Even in small groups, and in particular in larger groups, where a large number of dyadic or even triadic interactions are possible, a substantial memory of individualized interactions is required. Several studies of chacma baboons ( Cheney and Seyfarth 1999) and also of spotted hyenas (Crocuta crocuta, Engh et al. 2005) have provided evidence for the recognition of third-party relationships. In meerkats, it is currently unclear whether convincing evidence for the recognition of relationships among other group members exists, except for the relationship between dominant female and male as the dominant pair. The problem may be partly that we are not able to detect such relationships, as there are no obvious contexts in which specific individuals seem to support each other more or have a stronger relationship than with others.
8.2.2 References to Behavioral Contexts
Many animals vocally express their current behavior with specific vocalizations emitted on a regular or ongoing basis while they are engaged in the behavior. Such calls can be related to broad contexts, such as foraging as a cohesive group. In some species, variation in call structure maps directly onto specific events such as searching and feeding events (Jansen et al. 2012) or travelling between food patches (Boinski 1991). Some acoustically very similar calls, which we may categorize as the same call type, are used in several different behavioral contexts to which a signaler is exposed or according to the behavior it performs. For example, in chacma baboons , acoustically similar calls are used as contact barks while foraging and as alarm barks to warn others of predators (Fischer et al. 2001). Many other behaviors are much more confined to a specific context but are also accompanied by vocalizations, such as aggression in competitive interactions over resources, as well as various affiliative behaviors, including grooming.
Such calls have been suggested to express the motivation and also the emotional state of the signaler (Darwin 1872; Morton 1977). For example, an imminent attack might be signaled with harsh aggressive calls, or submission might be signaled when social partners express fear in order to avoid likely aggression. Based on these observations, Morton (1977) put forward some empirical evidence that signal design follows so-called “motivational-structural rules” in that a signaler’s emotions or motivations are clearly represented by specific acoustic features. Several studies in birds and mammals provided additional empirical support for this, but other studies did not find this pattern (Manser 2009). On the perception side, this means that receivers need additional information on context to distinguish different situations when callers emit acoustically similar calls purely on an emotional basis.
In meerkats, the majority of behavioral contexts are accompanied by a specific vocalization, which may explain the large repertoire of distinct call types they have evolved (Manser 1998; Manser et al. 2014). The main contexts in which vocalizations are emitted involve coordinating group cohesion (e.g., while foraging and moving in their home range), avoiding predation (e.g., predator warnings, mobbing, recruiting others to inspect predator cues, or coordinating sentinel activities), and interacting socially with conspecifics (e.g., affiliative or aggressive behaviors). Some of the same call types are used in several different contexts. For example, the same call types are given during sentinel activities and when individuals are standing in the sun to warm themselves, as well as during grooming. Other distinct call types are limited to one context only. Lead calls, for example, are only given when an individual wants to move and the group to follow; a submission call is only emitted when the animal submits to a more dominant individual . Currently we do not fully understand why some call types occur across contexts, in particular when they are associated with broad categories of potentially very different emotions . One explanation could be that meerkats categorize the calls differently than we do based on our available quantitative analytical methods.
8.2.3 References to External Objects and Events
Following the early studies of alarm calling in vervet monkeys, several studies have now demonstrated that other mammal and bird species produce highly object-specific and context-specific vocalizations that refer to features of the external environment, primarily in the context of predator alarm calls and food calls (reviewed in Townsend and Manser 2013; Gill and Bierema 2013). In meerkats, many of the identified call types are associated with a specific behavior of the signaler (Manser 1998; Manser et al. 2014). In addition, like vervet monkeys , meerkats emit predator-type specific calls that are restricted specifically to the approach of predators and that induce stereotyped escape behaviors with some flexibility along an urgency continuum (Manser 2001; Manser et al. 2001, 2002). They also have a call that is elicited by the detection of other animals in their environment, but only when moving, independent of whether the detected animals are dangerous or harmless birds or mammals, foreign conspecifics, or predators (Manser 2009). Besides their predator-type specific terrestrial and aerial alarm calls, meerkats produce other alarm calls that have a more general alert function, and receivers need additional contextual information to perceive the details of the situation (Manser 2001, 2009) (Fig. 8.2).
The acoustic structures of predator-type specific calls in meerkats vary depending on how close the threat is and what risk it poses (Manser 2001; Manser et al. 2001, 2002). This means the calls convey information to the receiver in regard to multiple aspects of the situation. Firstly, the call refers to the predator type, although it is not clear whether this information is about the predator identity or the spatial area and direction from which it approaches (e.g., aerial raptors approaching from the sky versus terrestrial predators approaching on the ground). Secondly, the acoustic structure changes along the same dimension within the predator-specific calls conveying information on the distance and the risk the approaching predator poses (Manser 2001). These multidimensional aspects of variation related to different external factors make it difficult to distinguish whether calls refer to the external event or are the expression of the emotional state of the signaler, as the whole discussion on predator-type specific calls versus urgency-level-based alarm calls has shown (e.g., Furrer and Manser 2009).
To be considered a functionally referential call, a high degree of perception specificity must exist on the part of receivers, as demonstrated by their appropriate response to the call in the absence of the stimulus that elicits the call from signalers (Marler et al. 1992). In meerkats, playbacks of different predator-type specific calls (see Fig. 8.2) caused them to respond in qualitatively different ways (Manser et al. 2001). In response to an aerial alarm call , they would run to the next shelter, typically a bolthole. In response to a terrestrial alarm call , they would look around and gather together to then either move to a sleeping burrow system for shelter or, if no predator was to be seen, resume foraging . In response to a recruitment call, receivers would move towards the caller or loudspeaker. When testing the different predator-type specific calls that differed in their urgency level, which is clearly expressed in changes in acoustic structure (Fig. 8.2), receivers showed quantitative changes in the intensity of their responses, but responses remained qualitatively the same within a predator type.
Emitting an alarm call is not only affected by the external stimuli eliciting the call but also by the social environment of the caller in meerkats (Townsend et al. 2012b) and also in other species (Papworth et al. 2008). Such findings suggest that, even though the emission of alarm calls may generally be a rather stereotyped, genetically determined behavior, there is some flexibility on the production side , and different information processes are involved in the decision of whether to give a predator-type specific call. Likewise, on the perception side, not all receivers respond in the same way. Instead, we find variation among individuals in terms of whether they respond, how fast and strong they respond, and how fast they resume foraging after hearing an alarm call . In particular, it appears that individuals that have invested time into digging for a prey item in the sand are more reluctant to run immediately for shelter, and if they do run, they are the first to resume foraging again and return to their digging spot (Amsler 2008). These observations support the idea that behavioral responses to a call with a specific acoustic structure are not simple reflexes. Instead, receivers appear to incorporate additional information into their decisions about responding.
8.3 Semanticity in Animal Communication
The debate over the potential referential and semantic nature of signals must be considered from both the production and perception sides (Macedonia and Evans 1993; Seyfarth and Cheney 2003). The highly context-specific alarm calls of vervet monkeys were initially termed functionally referential (Marler et al. 1992) to emphasize that the underlying cognitive mechanisms driving production and perception may well be different from those underlying the referential use of words in human language . For many years, people have questioned whether animal communication involves information transfer or instead involves manipulation of receivers by signalers (Dawkins and Krebs 1978; Stegmann 2013). Owings and Morton (1998) built up their “assessment-management ” approach to vocal communication based on Morton’s (1977) motivational structural rules . However, there is increasing evidence that the production of call types differing in usage and structure cannot simply be explained as merely the expression of the signaler’s motivational and emotional state, but rather involves multiple information processes (Manser 2009; Crockford et al. 2012, Watson et al. 2015).
The perception side of functionally referential vocalizations has also been reconsidered in recent years. Owren and Rendall (1997) put forward the “affect conditioning ” or “affect induction” model, where animals do not respond in a specific way because of the information being transferred in a signal, but due to the effect of the signal’s specific acoustic structure on the receiver’s low-level perceptual, attentional , and motivational processes. However, there is also plenty of evidence that the affect model has its limitations, for example, when it comes to explaining the flexible responses of receivers to the same acoustic structure of vocalizations (Seyfarth et al. 2010), and that semantics and referential information likely play important roles in generating receiver responses.
Arguments over the semantic properties of functionally referential signals are based on their purported effects in evoking cognitive representations of the eliciting objects or events in the mind of the receiver. The clearly varied responses to the various alarm call types in vervet monkeys raises questions about whether a simple association between a specific call type and an external stimulus might allow receivers to respond appropriately (perceptual semanticity) or whether an evoked cognitive representation of the signal-eliciting stimulus induces the response in the receiver (conceptual semanticity) (Zuberbühler et al. 1999). There is evidence from prime-probe experiments on Diana monkeys (Cercopithecus diana) that habituation was transferable across semantically similar calls but not across acoustically similar ones (Zuberbühler et al. 1999). Such findings suggest the animals were not responding to the acoustic features alone; instead, their responses were mediated by the similarity of the meaning of the presented stimuli. Evans and Evans (2007) supported this idea with experiments on chickens with regard to their food calls being representational signals. Nevertheless, the common associations in both of these examples could be formed via associative learning and may not necessarily involve more complex cognitive representations (Adams and Beighley 2013).
If we accept that animals are able to recognize individuals from their vocalizations in a cross-modal, representational way (Proops et al. 2009), it could be argued that functionally referential food and predator vocalizations are processed in a similar way. Specifically, responses to such vocalizations may be mediated by a receiver’s cognitive representation of the object eliciting the call type (Zuberbühler et al. 1999; Hurford 2007). In all cases of different referent types (Table 8.1), animals appear capable of learning to make these types of associations . This view is supported by experiments with golden-mantled ground squirrels (Spermophilus lateralis, Shriner 1999) and, more recently, by experiments on superb fairy wrens (Malurus cyaneus), in which predators were associated with artificial sounds (Magrath et al. 2015). The receivers showed clear escape behavior after few exposures, whereas they did not show a response to a new call brought in without association to a predator.
Another recent suggestion discourages distinguishing in a categorical way between functionally referential calls and behavior-related calls in favor of considering all animal vocalizations as existing along a continuum from low to high context specificity (Wheeler and Fischer 2012). These arguments are based on the cognitive processes involved from the perception side, with one of the main assumptions being that, to the receiver of the calls, there is no inherent difference between those associated with external referents or internal features of the signaler. This may be true for the perception side, but a critical question remains on the production side: what underlying cognitive processes cause the emission of the specific call types in the first place? Currently, we seem unable to convincingly identify the relevant processes involved at the level of what specific aspects of external stimuli drive the production of specific call types, much less at a neurobiological level . Neither do we fully understand the cognitive processes involved in the production of calls relating to specific behaviors of a caller. Clearly much work remains before we will fully understand the semanticity of signals from the perspectives of both signalers and receivers.
8.4 Mechanisms for Producing and Receiving Referential Signals
In this section, I briefly address issues related to the underlying mechanisms involved in producing and receiving referential signals as they pertain to the three types of referential signals discussed in Sect. 8.2 and summarized in Table 8.1.
From the production side, considering the sources and patterns of variation in signals is key to gaining insight into their production mechanisms . For example, the variation in signals that is correlated with differences in individual traits may often be due to anatomical and physiological differences related to sound production mechanisms (see Chap. 7). That is, the vocal expression of individual traits may be more or less fixed because production mechanisms limit the signaler’s influence on the signal’s individually distinctive acoustic structure, perhaps depending on the signal type (e.g., noisy versus tonal) or also its function (Rendall et al. 2009). Consequently, many of the signals animals produce will necessarily include some reference to individual traits or membership in various social categories . This form of referential signaling, therefore, likely occurs independently of whether the signals are also used to convey information about specific behavioral contexts or external objects and events.
It is more difficult to identify mechanisms driving signalers to produce calls that reference specific behavioral contexts or external objects and events. In terms of signaling-specific behavioral contexts, variation in signals may arise due to variation in the signaler’s own behavioral or motivational state, which may show large variability depending on the signaler’s immediate environment. For example, to emphasize a situation as becoming threatening in fights over resources, signalers may first start with low levels of aggression and then change into high levels of aggression. In turn, these state-level changes may be reflected in an increase in the modulation, harshness, and amplitude of signals (Manser et al. 2014). In many instances, however, adjustments in signal production appear to reflect the signaler’s ability to attend to differences in its social environments, often in ways that depend on its own situation and the situation of one or more intended receivers. Indeed, there is increasing empirical evidence that, instead of being a simple expression of emotion or motivation , the production and usage of signals is more flexible than previously described. Signalers tailor the production of their calls to the social environment, including both whether and what categories of conspecifics are around (Townsend et al. 2012b; Gyger et al. 1987), whether or not receivers attend to the signal (Wich and de Vries 2006), and whether or not conspecifics are in danger (Papworth et al. 2008). For example, there is evidence from predator-type specific calls (Townsend et al. 2012b) and food-type specific calls (Gros-Louis 2004) that signalers do, in fact, adjust their signaling behavior in relation to their social environment. This necessarily means that signalers may often possess cognitive mechanisms for processing information about their social environment and making decisions that give them flexibility to change their vocal production based not only on the behavior and responses of receivers but also, at least in some species, on the knowledge they perceive the receiver to possess (Crockford et al. 2012; see also Chap. 9), implying a qualitatively different level of social awareness (but see Seyfarth and Cheney 2015). A signaler that tries to influence the receiver to produce a specific response may be at a significant advantage if it is able to anticipate the action of the receiver and change its own vocal behavior accordingly.
On the perception side, receiver mechanisms for processing information in referential signals and generating appropriate behavioral responses appear to depend on the type of referent (Table 8.1) to which receivers benefit most from attending. As noted previously, referents to behavioral contexts and also to external objects and events all seem to also include referents to individual traits. Yet, the attention of receivers to calls referring to external objects and events seems primarily biased to this dimension and thus less to individual traits. This bias, in turn, accordingly guides the behavior of the receiver to be more stereotyped and less dependent on the signaler’s identity. This bias and its behavioral effects are likely due to signals referring to external objects and events that are commonly associated with high arousal (Meise et al. 2011) and a high urgency to generate the most appropriate response. Predator-type specific calls related to danger, for example, induce fear. Food-type specific calls related to high rewards induce positive excitement or the desire to move to the location of a food source. Regarding signals referring to the signaler’s behavioral context, there is seldom the need to respond as immediately as to functionally referential calls, and the cognitive integration of the reference on individual traits, as well as other context-related information, can easily become more important. This would support the suggestion by Wheeler and Fischer (2012) that in less context-specific signals, animals need to acquire additional information to decide how best to respond, and this may be cognitively more demanding. However, it may be overestimated that in highly context-specific calls, other information sources may be fully filtered out, as variation in the responses of receivers to functionally referential calls suggests flexibility in several species (Seyfarth et al. 1980; Manser et al. 2001; Price and Fischer 2013).
8.5 Summary and Future Directions
Producing and receiving referential vocal signals, at least in social mammals and birds , should be considered in a broader framework that recognizes three different types of referents (Table 8.1): those related to individual traits and social categories , those related to behavioral context, and those related to external objects and events. In this chapter, I have illustrated the benefits of adopting such a framework using examples largely drawn from our work on vocal communication and social behavior in meerkats. The meerkat vocal system seems to be characterized by an exceptionally broad range of predator-specific (external referent) and behavior-specific (pertaining to the signaler) vocalizations. This may be explained by the fact that meerkats use their vocalizations mainly to coordinate group cohesion and antipredator behaviors, both of which are keys to survival in the open habitat they live in with scarce food availability and high predation pressure. Although meerkat social interactions are also accompanied by specific vocalizations, dyadic interactions in meerkats seem to be more important in organizing social relationships than attending to third-party relationships, as shown for more socially structured groups in primates or hyenas. Olfactory and visual signals and cues also play important roles in meerkat social interactions by providing additional, multimodal information about the characteristics of an individual, thereby freeing them from sole reliance on mainly vocal signals (see Chap. 5). The predator-type specific calls produced by signalers elicit distinct and obvious escape responses from receivers that are qualitatively consistent and appropriate to the type of predator. Nevertheless, variation in the strength of a response depends on the behavior of the receiver at the moment the signal is perceived.
To understand the function and meaning of vocalizations, future efforts will need to identify both the causes of the acoustic variation observed in the production of the signals and the consequences of that variation in terms of the responses of receivers. Being aware that we have different references in vocalizations, with each based on different underlying cognitive mechanisms at least on the production side, though potentially less so on the perception side, may help to make clear predictions for what variation we can expect in specific situations. For example, the demands of social recognition will differ depending on the social system or social structure of an organism and will select more or less for the expression of individual traits or the coordination of different behavioral tasks. Ecological constraints, such as those related to predation risk and foraging , may differentially favor the evolution of signals referring to external object or events.
While over the recent years a lot of progress has been made toward identifying in detail acoustic variation as it relates to specific referents, we still do not know much about the specific underlying information processes related to the production or the perception of acoustic variation. For example, we have only limited knowledge about the conditions that enhance selection for individual variation or uniformity and what acoustic parameters are typically expressing them under what conditions. We still do not fully understand what acoustic structures elicit specific behaviors, or why in some species the same signal type is used in several behavioral contexts, while in other species, several different signal types seem to elicit the same behaviors. In terms of the so-called functionally referential signals that refer to external objects and events, we generally have not adequately identified whether the calls really refer to spatial area versus predator type, whether the signals are given as a command, or whether they merely express the caller’s emotional state. Likewise, on the perception side, we still lack a complete understanding about what information processes underlie responses to specific variation in the acoustic features of signals. Although, evidence is slowly emerging that some animals do seem to have cognitive representations for individuality and predator types, empirical studies are very rare, and it is difficult to draw firm or broad conclusions about what cognitive mechanisms for information processing and integration are involved.
The biological world is very seldom divided into distinct black and white categories , even though it may be much easier for us to comprehend and quantify discrete rather than continuous structures and patterns. It is, therefore, not surprising that we as observers tend to focus on the most obvious, or on the very novel and exciting aspects of a system, and suppress the additional “noisy” effects that are difficult to place in the conceptual frameworks we develop to make sense of the world. For example, the first papers on the functionally referential alarm calls of vervet monkey focused on the predator specificity of signals and the qualitatively different responses of receivers, but largely disregarded the variation in signals and responses in relation to other factors (Seyfarth et al. 1980). Recent studies on the same species highlight this variation and suggest the calls are not as predator-type specific as originally described (Price et al. 2015).
While we gain from advancing single concepts as the main explanation, it becomes increasingly clear that animals are exposed to a vast variety of inputs that subsequently function to stimulate signal production and, on the receiver side, function to make the best decision in their situation to respond to specific vocalizations. One aspect I have learned from our detailed work on communication and cognition in meerkats is that we are still far from understanding how these animals categorize their world and in what way this influences their communication system. To understand animals’ decisions and the underlying cognitive mechanisms, it is critical that we first identify in detail what input from the social and ecological environment is relevant and influences the production of signals and responses to them.
References
Adachi I, Hampton R (2011) Rhesus monkeys see who they hear: spontaneous cross-modal memory for familiar conspecifics. PLoS One 6(8):e23345
Adams F, Beighley SM (2013) Information, meaning and animal communication. In: Stegmann UE (ed) Animal communication theory: information and influence. Cambridge University Press, Cambridge, pp. 399–420
Amsler V (2008) How urgency levels in alarm calls influence the foragers response in meerkats (Suricata suricatta). MSc thesis, University of Zurich, Zurich
Beecher MD (1982) Signature systems and kin recognition. Am Zool 22(3):477–490
Beecher MD (1991) Successes and failures of parent-offspring recognition systems in animals. In: Hepper PG (ed) Kin recognition. Cambridge University Press, Cambridge, pp. 94–124
Bergman T, Beehner JC, Cheney DL, Seyfarth RM (2003) Hierarchical classification by rank and kinship in baboons. Science 302:1234–1236
Blumstein DT, Daniel JC (2004) Yellow-bellied marmots discriminate between the alarm calls of individuals and are more responsive to calls from juveniles. Anim Behav 68(6):1257–1265
Boinski S (1991) The coordination of spatial position: a field study of the vocal behaviour of adult female squirrel monkeys. Anim Behav 41(1):89–102
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, MA
Cheney DL, Seyfarth RM (1999) Recognition of other individuals’ social relationships by female baboons. Anim Behav 58:67–75
Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O’Rian MJ, Skinner JD (1998) Infanticide and expulsion of females in a cooperative mammal. Proc R Soc Lond B 265(1412):2291–2295
Clutton-Brock TH, Brotherton PNM, Russell AF, O’Riain MJ, Gaynor D, Kansky R, Griffin A, Manser M, Sharpe L, McIlrath GM, Small T, Moss A, Monfort S (2001) Cooperation, control, and concession in meerkat groups. Science 291(5503):478–481
Clutton-Brock T, Hodge S, Spong G, Russell A, Jordan N, Bennett N, Sharpe L, Manser M (2006) Intrasexual competition and sexual selection in cooperative mammals. Nature 444(7122):1065–1068
Crockford C, Herbinger I, Vigilant L, Boesch C (2004) Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110(3):221–243
Crockford C, Wittig RM, Mundry R, Zuberbühler K (2012) Wild chimpanzees inform ignorant group members of danger. Curr Biol 22:142–146
Darwin CH (1872) The expression of the emotions in man and animals. Murray J, Publ, London
Dawkins R, Krebs JR (1978) Animal signals: information or manipulation. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific Press, Oxford, pp. 282–309
Engh AL, Siebert ER, Greenberg DA, Holekamp K (2005) Patterns of alliance formation and postconflict aggression indicate spotted hyenas recognize third-party relationships. Anim Behav 69:209–217
Engh AL, Hoffmeier RR, Cheney DL, Seyfarth RM (2006) Who, me? Can baboons infer the target of a vocalization? Anim Behav 71:381–387
Evans CS, Evans L (2007) Representational signalling in birds. Biol Lett 3(1):8–11
Fischer J, Metz M, Cheney DL, Seyfarth RM (2001) Baboon responses to graded bark variants. Anim Behav 61(5):925–931
Furrer R, Manser MB (2009) The evolution of urgency-based and functionally referential alarm calls in ground-dwelling species. Am Nat 173:400–410
Gill SA, Bierema AMK (2013) On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119:449–461
Griffin AS, Pemberton JM, Brotherton PNM, McIlrath G, Gaynor D, Kansky R, O’Riain J, Clutton-Brock TH (2003) A genetic analysis of breeding success in the cooperative meerkat (Suricata suricatta). Behav Ecol 14(4):472–480
Gros-Louis J (2004) The function of food-associated calls in white-faced capuchin monkeys, Cebus capucinus, from the perspective of the signaller. Anim Behav 67:431–440
Gyger M, Marler P, Pickert R (1987) Semantics of an avian alarm call system: the male domestic fowl (Gallus domesticus). Behaviour 102:15–40
Hare JF, Atkins BA (2001) The squirrel that cried wolf: reliability detection by juvenile Richardson’s ground squirrels (Spermophilus richardsonii). Behav Ecol Sociobiol 51:108–112
Hollén L, Clutton-Brock TH, Manser MB (2008) Factors affecting the development of alarm-call production, usage and responses in meerkats (Suricata suricatta). Behav Ecol Sociobiol 62:821–829
Hurford JR (2007) The origins of meaning: language in the light of evolution. Oxford University Press, Oxford
Jansen DA, Cant MA, Manser MB (2012) Segmental concatenation of individual signatures and context cues in banded mongoose (Mungos mungo) close calls. BMC Biol 10(1):97
Jansen DAWAM, Cant MA, Manser MB (2013) Testing for vocal individual discrimination in adult banded mongooses. J Zool 291:171–177
Johnston RE, Bullock TA (2001) Individual recognition by use of odours in golden hamsters: the nature of individual representation. Anim Behav 61:545–557
Jordan NR, Cherry MI, Manser MB (2007) Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Anim Behav 73(4):613–622
Jordan NR, Mwanguhya F, Kyabulima S, Rüedi P, Cant MA (2010) Scent marking within and between groups of wild banded mongooses. J Zool 280(1):72–83
Karp D, Manser MB, Wiley EM, Townsend SW (2014) Nonlinearities in meerkat alarm calls prevent receivers from habituating. Ethology 120(2):189–196
King SL, Janik VM (2013) Bottlenose dolphins can use learned vocal labels to address each other. Proc Natl Acad Sci 110(32):13216–13221
Kondo N, Izawa E, Watanabe S (2012) Crows cross-modally recognize group members but not non-group members. Proc R Soc Lond B 279:1937–1942
Krams I, Krama T, Igaune K, Mänd R (2008) Experimental evidence for reciprocal altruism in the pied flycatcher. Behav Ecol 62:599–605
Kulahci IG, Drea CM, Rubenstein DI, Ghazanfar AA (2015) Individual recognition through olfactory–auditory matching in lemurs. Proc R Soc Lond B 281(1784):20140071
Kunc HP, Madden JR, Manser MB (2007) Begging signals in a mobile feeding system: the evolution of different call types. Am Nat 170(4):617–624
Kutsukake N, Clutton-Brock TH (2006) Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats, Suricata suricatta. Behav Ecol Sociobiol 59(4):541–548
Lacy R, Sherman PW (1983) Kin recognition by phenotype matching. Am Nat 121:489–512
Le Claire S, Nielsen JF, Thavarajah NK, Manser M, Clutton-Brock TH (2013) Odour-based kin discrimination in the cooperatively breeding meerkat. Biol Lett 9(1):20121054
Le Vin AL, Mable BK, Arnold KE (2010) Kin recognition via phenotype matching in a cooperatively breeding cichlid, Neolamprologus pulcher. Anim Behav 79:1109–1114
Lieblich AK, Symmes D, Newman JD, Shapiro M (1980) Development of the isolation peep in laboratory-bred squirrel monkeys. Anim Behav 28(1):1–9
Macedonia JM, Evans CS (1993) Essay on contemporary issues in ethology: variation among mammalian alarm call systems and the problem of meaning in animal signals. Ethology 93:177–197
Magrath RD, Haff TM, McLachlan JR, Igic B (2015) Wild birds learn to eavesdrop on heterospecific alarm calls. Curr Biol 25:2047–2050
Manser, M.B. (1998) The evolution of auditory communication in suricates Suricata suricatta. PhD thesis, Cambridge University, Cambridge
Manser MB (2001) The acoustic structure of suricate alarm calls varies with predator type and the level of urgency. Proc R Soc Lond B 268:2315–2324
Manser MB (2009) What do functionally alarm calls refer to? In: Dukas R, Ratcliffe J (eds) Cognitive ecology II. The University of Chicago Press, Chicago, IL and London, pp. 229–248
Manser MB (2013) Semantic communication in vervet monkeys and other animals. Anim Behav 86:491–496
Manser MB, Avey G (2000) The effect of pup vocalisations on food allocation in a cooperative mammal, the meerkat (Suricata suricatta). Behav Ecol Sociobiol 48(6):429–437
Manser MB, Bell MB (2004) Spatial representation of shelter locations in meerkats, Suricata suricatta. Anim Behav 68(1):151–157
Manser MB, Bell MB, Fletcher LB (2001) The information that receivers extract from alarm calls in suricates. Proc R Soc Lond B 268:2485–2491
Manser MB, Seyfarth RM, Cheney DL (2002) Suricate alarm calls signal predator class and urgency. Trends Cogn Sci 6:55–57
Manser MB, Graw B, Hollén LI, Bousquet CA, Furrer RD, le Roux A (2014) Vocal complexity in meerkats and other mongoose species. Adv Stud Behav 46:281–310
Marler P, Evans CS, Hauser MD (1992) Animal signals: reference, motivation, or both? In: Papousek H, Jürgens U, Papousek M (eds) Nonverbal vocal communication: comparative and developmental approaches. Cambridge University Press, Cambridge, pp. 66–86
Maynard-Smith JM, Harper D (2003) Animal signals. University Press, Oxford, New York, NY
McDonald PG, Wright J (2011) Bell miner provisioning calls are more similar among relatives and are used by helpers at the nest to bias their effort towards kin. Proc R Soc Lond B 278(1723):3403–3411
Meise K, Keller C, Cowlishaw G, Fischer J (2011) Sources of acoustic variation: implications for production specificity and call categorization in chacma baboon (Papio ursinus) grunts. J Acoust Soc Am 129:1631–1641
Morton ES (1977) On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am Nat 111:855–869
Müller, C.A. (2007) Environmental knowledge in the banded mongoose (Mungos mungo). PhD thesis, University of Zurich, Zurich
Müller CA, Manser MB (2007) ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proc R Soc Lond B 274(1612):959–965
Nelson DA, Marler P (1990) The perception of birdsong and an ecological concept of signal space. In: Berkley MA, Stebbins WC (eds) Comparative perception: volume II. Wiley, New York, NY, pp. 443–478
Nielsen JF, English S, Goodall-Copestake WP, Wang J, Walling CA, Bateman AW, Flower TP, Sutcliffe RL, Samson J, Thavarajah NK, Kruuk LE, Clutton-Brock TH, Pemberton JM (2012) Inbreeding and inbreeding depression of early life traits in a cooperative mammal. Mol Ecol 21(11):2788–2804
Owings DH, Morton ES (1998) Animal vocal communication: a new approach. Cambridge University Press, Cambridge
Owren MJ, Rendall D (1997) An affect-conditioning model of nonhuman primate vocal signaling. Perspect Ethol 12:299–346
Papworth S, Böse AS, Barker J, Schel AM, Zuberbühler K (2008) Male blue monkeys alarm call in response to danger experienced by others. Biol Lett 4:472–475
Payne K, Payne R (1985) Large-scale changes over 19 years in song of humpback whales in Bermuda. Z Tierpsychol 68:89–114
Paz-y-Miño CG, Bond AB, Kamil AC, Balda RP (2004) Pinyon jays use transitive inference to predict social dominance. Nature 430:778e782
Pollard KA, Blumstein DT (2011) Social group size predicts the evolution of individuality. Curr Biol 21:413–417
Price T, Fischer J (2013) Meaning attribution in the West African green monkey: influence of call type and context. Anim Cogn 17:277–286
Price T, Hammerschmidt K, Wadewitz P, Cheney DL, Seyfarth RM, Fischer J (2015) Vervets revisited: a quantitative analysis of alarm call structure and context specificity. Sci Rep 5:13220
Proops L, McComb K, Reby D (2009) Cross-modal individual recognition in domestic horses (Equus caballus). Proc Natl Acad Sci USA 106(3):947–951
Ralls K, Fiorelli P, Gish S (1985) Vocalizations and vocal mimicry in captive harbor seals: Phoca vitulina. Can J Zool 63:1050–1056
Reber SA, Townsend SW, Manser MB (2013) Social monitoring via close calls in meerkats. Proc R Soc Lond B 280:1–7
Rendall D, Notman H, Owren MJ (2009) Asymmetries in the individual distinctiveness and maternal recognition of infant contact calls and distress screams in baboons. J Acoust Soc Am 125(3):1792–1805
Russell AF, Carlson AA, McIlrath GM, Jordan NR, Clutton-Brock T (2004) Adaptive size modification by dominant female meerkats. Evolution 58(7):1600–1607
Scarantino A, Clay Z (2015) Contextually variable signals can be functionally referential. Anim Behav 100:e1–e8
Schibler F, Manser MB (2007) The irrelevance of individual discrimination in meerkat alarm calls. Anim Behav 74:1259–1268
Seyfarth R, Cheney D (1990) The assessment by vervet monkeys of their own and another species’ alarm calls. Anim Behav 40(4):754–764
Seyfarth RM, Cheney DL (2003) Signalers and receivers in animal communication. Annu Rev Psychol 54:145–173
Seyfarth RM, Cheney DL (2010) Production, usage, and comprehension in animal vocalizations. Brain Lang 115(1):92–100
Seyfarth RM, Cheney DL (2015) Social cognition. Anim Behav 103:191–202
Seyfarth RM, Cheney DL, Marler P (1980) Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav 28:1070–1094
Seyfarth RM, Cheney DL, Bergman T, Fischer J, Zuberbühler K, Hammerschmidt K (2010) The central importance of information in studies of animal communication. Anim Behav 80:3–8
Sheehan MJ, Straub MA, Tibbetts EA (2014) How does individual recognition evolve? Comparing responses to identity information in Polistes species with and without individual recognition. Ethology 120(2):169–179
Shriner WM (1999) Antipredator responses to a previously neutral sound by free-living adult golden-mantled ground squirrels, Spermophilus lateralis (Sciuridae). Ethology 105:747–757
Smith WJ (1965) Message, meaning and context in ethology. Am Nat 99:405–409
Smith WJ (1981) Referents of animal communication. Anim Behav 29:1273–1274
Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH (2008) Factors affecting the reproductive success of dominant male meerkats. Mol Ecol 17(9):2287–2299
Stegmann U (2013) Animal communication theory: information and influence. Cambridge University Press, Cambridge
Stoeger AS, Mietchen D, Oh S, de Silva S, Herbst CT, Kwon S, Fitch WT (2012) An Asian elephant imitates human speech. Curr Biol 22(22):2144–2148
Struhsaker TT (1967) Auditory communication among vervet monkeys (Cercopithecus aethiops). In: Altmann SA (ed) Social communication among primates. University of Chicago Press, Chicago, IL, pp. 281–324
Thavarajah NK, Fenkes M, Clutton-Brock TH (2014) The determinants of dominance relationships among subordinate females in the cooperatively meerkat. Behaviour 151:89–102
Townsend SW, Manser MB (2013) Functionally referential communication in mammals: the past, present and the future. Ethology 119:1–11
Townsend SW, Hollén LI, Manser MB (2010) Meerkat close calls encode group significant signatures, but fail to discriminate. Anim Behav 80:133–138
Townsend SW, Allen C, Manser MB (2012a) A simple test of vocal individual recognition in wild meerkats. Biol Lett 8:179–182
Townsend SW, Rasmussen M, Clutton-Brock TH, Manser MB (2012b) Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behav Ecol 23:1360–1364
Travis SE, Slobodchikoff C, Keim P (1997) DNA fingerprinting reveals low genetic diversity in Gunnison’s prairie dog (Cynomys gunnisoni). J Mammal:725–732
Wadewitz P (2010) Olfactory discrimination of predators and conspecifics in meerkats (Suricata suricatta). MSc thesis, University of Zurich, Zurich
Watson SK, Townsend SW, Schel AM, Wilke C, Wallace EK, Cheng L, West V, Slocombe KE (2015) Vocal learning in the functionally referential food grunts of chimpanzees. Curr Biol 25(4):495–499
Wheeler BC, Fischer J (2012) Functionally referential signals: a promising paradigm whose time has passed. Evol Anthropol Issues News Rev 21:195–205
Wheeler BC, Fischer J (2015) The blurred boundaries of functional reference: a response to Scarantino & Clay. Anim Behav 100:e9–e13
White SM (2001) Juvenile development and conflicts of interest in meerkats. PhD thesis, University of Cambridge, Cambridge
Wich SA, de Vries H (2006) Male monkeys remember which group members have given alarm calls. Proc R Soc Lond B 273:735–740
Wiley RH (2013) Specificity and multiplicity in the recognition of individuals: implications for the evolution of social behaviour. Biol Rev 88(1):179–195
Wilkinson GS (2003) Social and vocal complexity in bats. In: de Waal FBM, Tyack PL (eds) Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press, Cambridge, MA, pp. 322–341
Wittig RM, Crockford C, Seyfarth RM, Cheney DL (2007) Vocal alliances in chacma baboons, Papio hamadryas ursinus. Behav Ecol Sociobiol 61:899–909
Wright TF, Wilkinson GS (2001) Population genetic structure and vocal dialects in an amazon parrot. Proc R Soc Lond B 268:609–616
Young AJ, Clutton-Brock T (2006) Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol Lett 2(3):385–387
Zuberbühler K, Cheney DL, Seyfarth RM (1999) Conceptual semantics in a nonhuman primate. J Comp Psychol 113:33–42
Acknowledgments
I thank the editors for the invitation to write this chapter and in particular Mark Bee for his editing efforts. Also many thanks to Simon Townsend and two referees on their helpful comments on earlier drafts of the chapter. The University of Zurich has funded this project and also the long-term research on the different mongoose species in addition to the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Manser, M.B. (2016). Referents and Semantics in Animal Vocalizations. In: Bee, M., Miller, C. (eds) Psychological Mechanisms in Animal Communication. Animal Signals and Communication, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-319-48690-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-48690-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48688-8
Online ISBN: 978-3-319-48690-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)