Abstract
The similarities between self-experienced and vicarious pain have led research to suggest that both experiences may be facilitated by shared neural representations. Indeed, neuroimaging evidence demonstrates an overlap in neural patterns during self- and other-pain. Such comparable brain activity may facilitate an empathic understanding of the current state of the individual in pain by stimulating relevant pain associations in the own sensory, affective and cognitive systems. However, research further shows the distinct contributions of neural activity during vicarious pain processing, in particular in brain regions related to perspective-taking, attention and top-down response regulation. Likewise, such activity may underpin response formation to the observed pain, such as empathic or withdrawal behaviors. This chapter reviews 31 fMRI, six EEG/MEG and four TMS studies exploring the neural correlates of vicarious pain in healthy individuals. Both shared and distinct neural contributions to stimulus and response processing during vicarious pain are discussed. Notably, an integrative model of vicarious pain is introduced which brings such contributions together in a comprehensive manner. Moreover, the chapter highlights inconsistencies and research gaps in current literature with the aim of stimulating further scientific investigation. This is pertinent to the detection of neurobiological markers and intervention targets for empathic deficits which characterize a wide variety of clinical health issues.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Vicarious pain is characterized by the observation of individuals who are experiencing acute pain [48, 110]. The empathic ability to relate to the affective state of these individuals has social and physical benefits as it enables observes to adjust their behavior according to the context [156]. Not only can this enhance social relationships through the display of compassion, but it also promotes adequate assessment of situational cues that require prompt withdrawal responses. Thus, stimuli that are potentially threatening can be removed before causing further harm to either the individual in pain or the observer [14, 61, 62, 75]. In line with those essential survival functions, evidence from imaging studies using electroencephalography (EEG), magnetoencephalography (MEG), transcranial magnetic stimulation (TMS), and functional magnetic resonance imaging (fMRI) suggests that the human brain is wired to facilitate empathic understanding through shared and distinct neural representations of self-experienced and observed pain [4, 67, 78, 81, 105, 110, 111, 178, 205]. Nonetheless, inconsistencies in current literature highlight that further exploration is critical to acquiring a complete understanding of the neural mechanisms underpinning vicarious pain and empathy. Research in this area is pertinent to the detection of neurobiological markers and intervention targets for empathic deficits which characterize a wide variety of clinical health issues, such as autism, schizophrenia and motor neuron disease [12, 18, 35, 172].
2 Vicarious Pain, Empathy and the Perception-Action Model
Acute self-pain is experienced in the own body directly as an “unpleasant sensory and emotional experience” of sharp quality “associated with (…) tissue damage” [94, p. 5]. In contrast, although vicarious pain is defined as the observation of pain in others, the affective, cognitive, and sensory aspects that accompany this experience are challenging to pinpoint. Particularly, empathic responding to such pain observation has not yet been clearly defined [155]. It is generally described as an understanding of affective states in others. However, more narrow definitions of empathy have specified that the platform for such understanding arises when observation or imagination of a person in a particular emotional state elicits a similar emotion in the self that remains conceptually separate from personal distress [74, 98]. Furthermore, empathy has been associated not only with emotional relatedness, but also a cognitive and somatosensory understanding of the observed pain. In line with this, research suggests that empathizing with vicarious pain can have affective, cognitive and sensory effects for an individual that are similar to self-pain [67, 156]. Reflecting this similarity, the Perception-Action Model (PAM) of empathy advances that vicarious processing is subserved by shared neural representations underlying self- and observed pain [81, 110, 111, 155, 156, 178]. It relies on the mirror neuron system which is characterized by neurons that respond both when an action is actively performed and passively observed [158]. More explicitly, during pain observation motor mirror neurons are activated that correspond to muscle groups involved in acute self-pain . This activation promotes understanding of vicarious pain through the mirroring of such pain within the own motor system [160]. In consequence, a neural network is automatically stimulated, containing learned sensory and affective information for self-pain which can be used to predict and evaluate the suffering of others. This may facilitate other-oriented empathy as well as self-oriented distress and withdrawal responses [81]. Moreover, the PAM advocates indirect sensory, affective and cognitive mirroring in pain processing regions which are not directly implicated in the mirror neuron system, such as the cingulate and prefrontal cortices. Among those, similar neural patterns during self- and vicarious pain may reflect comparable stimulus processing, resulting in shared neural pain representations. Such representations also trigger an associative pain network and facilitate a swift, concurrent holistic appraisal of the observed pain [110, 155].
Research provides evidence for both motor mirror neurons and shared neural representations in vicarious pain processing. First, studies have shown neural activity in the inferior frontal gyrus (IFG) and inferior parietal lobule (IPL) during pain observation, which are considered core regions of the human mirror neuron system [14, 65, 77, 110, 158, 171, 178, 193]. Second, brain activation in the well-established pain matrix has been reported to overlap for self- and vicarious pain forming shared neural representations of these pain experiences [48, 110]. As shown in Fig. 1, the pain matrix includes the primary (SI) and secondary somatosensory cortices (SII), thalamus, prefrontal cortex (PFC), insula (INS), and cingulate cortex (CC).
It is suggested that SI, SII and the thalamus encode the sensory aspects of noxious stimuli, including location and intensity. In contrast, INS, CC and PFC play a role in cognitive–affective evaluation and top-down control [42, 43, 151, 186]. These regions are comparably implicated in vicarious pain. However, current methodological issues, such as coarse spatial neuroimaging resolution, prevent firm conclusions to which extent the neural patterns of self- and other-pain are identical [86, 111]. On the contrary, Zaki et al. [205] had indicated that self- and vicarious pain have differed functional connectivities across brain regions and thus, unique inter-regional communication may reflect the qualitative differences between these pain experiences. Likewise, activity in the same brain regions as self-pain may nonetheless make distinct contributions to other-pain processing [110]. Research suggests that mirroring may be neither necessary nor sufficient for empathy induction (for a critical review, see [111]. For example, individuals reported empathic distress upon learning that their partner was in pain without activation in the IFG or IPL, deeming these regions unnecessary for affective empathy [179]. Furthermore, individuals tend to exhibit appropriate empathic responses in situations where individuals are in pain, even though mirroring alone would imply that they are not [57, 112]. Accordingly, additional neural activity is required for adequate appraisal of vicarious pain [111, 149]. Thus, the involvement of aforementioned brain regions in vicarious pain is likely to go beyond mirroring [77].
Moreover, aside from appraising observed pain by means of shared and distinct neural correlates, neural patterns in observers may underpin distinct responses to the pain. The PAM predicts other-oriented empathic understanding to automatically arise from shared pain representations. Indeed, ample papers supporting the PAM report an association between empathic abilities and neural mirroring activity, proposing that the evaluation of observed pain through bottom-up mirroring induces empathy (e.g. [158, 160]). Empathy has been shown to elicit altruistic behaviors that have the aim of helping the individual in pain. These promote social relationships and their protective benefits [155, 156]. However, vicarious pain perception has also been found to induce self-oriented distress and withdrawal behaviors to the observed pain threat [65, 110, 111]. In line with this, brain regions active during pain observation have also been associated with emotional contagion, which is measured by affective distress ratings. Such contagion may elicit avoidance when observing negative affect in others and, thus, stands in opposition to the altruistic acts induced by empathy [60, 111]. While Preston and Hofelich [157] recently speculated that these concepts may also be evoked by neural mirroring, they did not extensively elaborate on the mechanisms behind this. Further contrasting the PAM , recent evidence suggests that neural activity during vicarious pain reflects top-down sensorimotor pain predictions based on higher cortical analysis rather than bottom-up mirroring. These predictions are dynamically compared with the pain observation and may evoke self-oriented motor withdrawal preparation [54, 111, 200]. Accordingly, brain responses to vicarious pain may reflect other-oriented empathy or self-oriented withdrawal. Notably, neural responses to empathy and distress have not yet been reliably teased apart. Given these issues, current findings of shared and distinct neural activation between self- and vicarious pain should be interpreted with caution [111]. At this time, the roles of each brain region involved in pain observation are not clearly established. However, this chapter will present noteworthy speculations that have been made and lend themselves to extensive future research.
The following sections will provide an overview of brain regions associated with vicarious pain processing. The chapter will first discuss evidence for shared and distinct neural substrates of self- and other-pain before exploring correlates underpinning empathic understanding and self-oriented behaviors. An integrative model will be presented that extends the well-established PAM with the aim of incorporating recent findings that offer a more rounded understanding of the complexity of vicarious pain processing. A summary of studies and reported brain activity appears in Table 1. This table presents only research directly exploring the neural correlates of vicarious pain in the healthy population and in absence of modulating factors, such as group membership [7, 91]. As can be inferred, the majority of research in this field utilizes fMRI for brain investigations (for details on fMRI methods, see [84]. To date, there are only four TMS studies [4,5,6, 128]; for details on TMS methods, see Rossi et al. [126, 162] and two EEG studies [27, 194]; for details on EEG methods, see [183] that investigate the neural activity during pain observation.
3 Neural Responses to Vicarious Pain
Across neuroimaging research, the most consistent brain activations during vicarious pain experience lie in the INS, CC and PFC. These regions have been implicated in the affective–cognitive processing of self- and observed pain (e.g. [49, 89, 147, 163, 179, 192]). In contrast, findings for the motor regions have been less consistent. While several studies report IFG, IPL and motor cortical activity in response to pain observation [113, 137, 193], many find none [48, 89, 179]. Likewise, activation in the somatosensory cortices remains variable [28]. Nonetheless, when contrasting brain responses to videos of limbs and objects subjected to equivalent noxious stimulation, the motor and somatosensory cortices responded exclusively to painful limbs, suggesting that sensorimotor mirroring may play an essential role in the identification of human pain [49]. Contradictory neuroimaging findings may result from the sensitivity of vicarious pain correlates to attentional focus. In an image- and coordinate-based meta-analysis, Lamm et al. [110] propose that the distinct recruitment of sensorimotor compared to affective brain areas depends on which pain components the observer highlights. Accordingly, attending to sensory factors, such as pain intensity, should be subserved by neural correlates of sensorimotor processing. On the other hand, an affective focus, such as rating pain unpleasantness, should activate affective pain substrates [110, 113]. In both cases, increased stimulus complexity should be associated with cognitive regions [88, 112, 149].
The common stimuli used to induce empathy for vicarious pain allow for such differentiation (Fig. 2). Most typically, participants are presented with images of hands or feet in painful or non-painful scenarios that are likely to evoke a sensory focus as the emphasis is on the body part (e.g., [6, 89, 98, 137]). In contrast, requiring participants to infer pain from facial expressions, abstract cues or imagination taps into affective processing [22, 98]. Furthermore, complex pain scenarios entail greater cognitive analysis of the presented context [149]. The corresponding effects of focus have been reflected in neuroimaging findings with sensory focus activating areas IFG, IPL, SI, SII and motor cortices, affective focus correlating with anterior cingulate cortex (ACC) and anterior INS (aINS) activity [4, 136, 179], and cognitive involvement eliciting anterior midcingulate cortex (aMCC) and PFC activity [88, 110]. Notably, most studies with sensory-focused stimuli reveal activity in both sensory and affective brain areas, suggesting that affective processing of observed pain is more readily activated than sensory processing [105, 110, 111].
Typical stimuli in vicarious pain research. facial pain and neutral expressions and hands and feet in non-painful and painful scenarios [193]
4 Shared Neural Representations
As proposed by the PAM , shared neural representations of self- and vicarious pain arise from neural mirroring and facilitate an accelerated understanding of the sensory, affective and cognitive experience of the individual in pain [155, 156]. While motor and somatosensory neural activity may underpin direct pain mirroring, affective–cognitive activity reflects indirect mirroring evoked through the similar processing requirements of self- and other-pain features. Due to the current lack of evidence for cognitive mirroring, the PFC is not included in this section and its distinct contributions to vicarious pain processing will be discussed at a later point.
4.1 The Sensorimotor Regions
4.1.1 The Mirror Neuron System: Inferior Parietal Lobule and Inferior Frontal Gyrus
During vicarious pain, the IPL and IFG have been respectively implicated in sensory [131, 158] and affective mirroring [171]. Identical neurons in the IPL and IFG respond to self-performed and observed actions, thus reflecting motor mirroring of observed pain [26, 39, 45, 163, 171, 193]. Supporting this, both regions react to physical rather than abstract pain cues, indicating that they require visual perception of relevant motor information [110, 179]. Vachon-Presseau et al. [193] report greater IPL responsiveness to body parts in pain and consistent bilateral IFG activation to both facial and bodily pain cues (Fig. 3).
a Observing body limbs is associated with increased activation in sensorimotor regions compared to facial expressions. b Observing facial expressions is associated with increased activation in medial PFC (mPFC) and Superior temporal sulcus (STS) (associated with perspective-taking) compared to limbs. c Vicarious pain is associated with increased activation in IFG, IPL and EBA compared to neutral images. d Increased activation of IPL during vicarious pain observed in limbs compared to vicarious facial pain expressions. For all images p < 0.001, uncorrected; error bars represent standard error of mean; **p < 0.01; ***p < 0.001 [193]
Furthermore, the IPL is associated with motor movement, spatial processing and increased intensity ratings of observed pain [26, 163]. As such functions require the analysis of sensory pain components, this suggests that the IPL mirrors sensorimotor aspects of vicarious pain [26, 113, 137, 146, 193]. In contrast, the IFG is involved in the extraction of affective meaning from faces, including anger and happiness expressions [141]. Correspondingly, studies indicated greater IFG activation during pain observation when participants were required to attend to the emotional meaning of pain [26, 196]. Such activity has been associated with higher self-rated affective empathy but shows no correlation with sensory pain intensity ratings, substantiating the role of the IFG in affective pain processing during vicarious pain [22, 163, 192, 193, 196]. Notably, IFG responses to pain observation can modulate higher cortical emotion centers, such as the aINS [31], suggesting that the IFG identifies motor activity in observed pain and communicates associated affective meanings to higher regions for further analysis [158]. Accordingly, the PAM advocates that motor mirroring in the IPL stimulates sensory pain associations while IFG mirroring activates affective pain associations. Thus, both provide a neural base for translating observed facial and bodily pain cues into self-correlates, creating shared representations of self- and other-pain observation [31, 147, 155, 156, 158]. This may facilitate rapid appraisal of the observed pain and corresponding emotional contagion or empathic understanding of the suffering individual [82, 171, 192]. Nonetheless, at present no intracellular recordings of human IPL or IFG neurons during pain observation exist. Reliance on vague spatial resolution of noninvasive neuroimaging techniques make it challenging to confirm that IPL and IFG activity occurs in the same neurons involved in self-pain . Thus, motor mirroring of pain in these regions is derived from previous research that is not specific to vicarious pain. Further pain-related research is needed to verify such a notion.
4.1.2 The Motor Cortices
The premotor cortex and Supplementary Motor Area (SMA) have been associated with action understanding of vicarious pain [100, 134, 163, 186]. In line with the PAM , the premotor cortex has been pinpointed as a neural correlate of motor imitation and is suggested to encode observed actions via motor mirroring (for a meta-analysis on the mirror neuron system in imitation, see [130]). Notably, somatotopical organizations within the premotor cortex facilitate the localization of perceived body parts [25]. Furthermore, the SMA has been implicated in event sequencing for the analysis and understanding of witnessed behaviors [112, 113, 136, 163]. Accordingly, these regions may analyze the motor cues of facial and bodily pain expressions, creating shared neural representations of self- and other-pain and activating relevant associations for pain evaluation [130, 155, 159]. The role of the motor cortex in motor mirroring is substantiated by TMS research. All three available TMS studies recorded an inhibition of motor-evoked potentials in participants watching videos of hands or feet being deeply penetrated by needles. This inhibition was specific to the muscle subjected to noxious stimulation. In contrast, gentle touch of humans or needle penetration of nonhuman objects had no such effect (Fig. 4) [4, 6, 128].
Suppression of MEP amplitude in response to vicarious pain [4]. Abbreviations: MEP motor-evoked potentials; FDI first dorsal interosseous; ADM abductor digiti minimis. a MEPs recorded from FDI muscle that was penetrated by needle or touched by Q-tip. Significant amplitude decrease occurred for specific FDI when penetrated by needle compared to Q-tip (p = 0.01) or compared to non-corporeal object (p = 0.01). b MEPs collected from ADM muscle which was not stimulated. No significant effect was found for ADM muscle, indicating that motor suppression was specific to the muscle targeted by the observed noxious stimulation. (*) identify significant post hoc comparisons (p < 0.02) [4]
As equivalent motor inhibition has been noted in response to self-pain [79], these findings have been interpreted as evidence for motor mirroring that reflects a direct resonance of the witnessed pain in the motor system of the observer [5]. Furthermore, the revealed neural inhibition was associated with increased pain intensity ratings, but not with affective measures of pain or empathy measures, implicating it exclusively in sensory processing of vicarious pain [3, 4]. Such findings support the PAM , substantiating the formation of shared neural representations within the motor cortices during vicarious pain.
4.1.3 The Somatosensory Cortices
Although the involvement of the somatosensory cortices is well-established for self-pain , findings are less robust for vicarious pain. The majority of vicarious pain studies do not include somatosensory areas in regions of interest analyses, making it challenging to determine how frequently such activation takes place. Of those that do, some studies find no activation in the somatosensory areas during pain observation [22, 48, 89, 134, 135, 179], while others report neural activity in at least one of the two regions [49, 98, 137, 147, 148]. Contradicting the PAM , both Singer et al. [179] and Morrison et al. [135] reported that the somatosensory cortices were only active when participants received painful electric shocks on their own hand, but not when abstract or visual cues informed them that another individual received equivalent stimulation.
However, both a systematic review [105] and a comprehensive meta-analysis [110] highlight the need to focus on sensory aspects of vicarious pain in order to engage somatosensory processing. Evidence is provided that presenting participants with limbs in pain is more likely to activate SI and SII than using faces or abstract pain cues [110, 113]. Indeed, Bufalari et al. [27] recorded increased SI activity while witnessing body parts in pain which concurred with higher pain intensity but not unpleasantness ratings, confirming that the SI specifically encodes sensory pain components. As demonstrated in Fig. 5, such activity dissociated painful from non-painful vicarious tactile stimulation. Specifically, gentle touch decreased amplitudes in the SI, while painful touch increased them [27]. Nonetheless, SI and SII have also been implicated in the undifferentiated mirroring of sensory cues, independent from whether stimulation is noxious or not, contesting that their activity is specific to pain (for a review, see [28, 110, 165]). As fine-grained MEG analysis can differentiate painful and non-painful self-touching, such methods may aid in clarifying the pain-specificity of vicarious somatosensory responses [153].
Change in P45 Amplitude during vicarious pain compared to baseline according to condition. a P45 amplitude during vicarious pain (red), vicarious gentle touch (blue) and static hand (green). Significant increase of P45 amplitude during vicarious pain compared to hand or touch (p = 0.0001). b Topographic distribution of P45 during each condition. c Percentage change in P45 amplitude compared to baseline according to condition. *p < 0.05; **p < 0.001 [27]
As similar activity has been noted during self-pain , the PAM interprets SI and SII activation during vicarious pain as bottom-up sensory mirroring [158]. In line with this, the SII has been implicated in the mirror neuron system due to its connections to the IPL [194]. Furthermore, the SI and SII contain somatosensory maps that may enhance the identification of observed body parts [16, 122]. Likewise, infrequent reports of thalamus activity suggest that this region may contribute to vicarious pain processing in its role in the transmission of mirrored sensory input to the cortex [98]. These findings support the notion of shared neural pain representations which provide a reference point from which individuals interpret the pain they observe in others [16, 44]. Correspondingly, Valeriani et al. [194] found that participants rated their own heat pain higher when simultaneously witnessing other individuals receiving pain stimulation. It was suggested that pain observers map the viewed pain onto the own body, intensifying self-experienced pain. Likewise, Osborn and Derbyshire [148] found that somatosensory activity during vicarious pain was associated with subjective reports of feeling pain sensations within the own body, substantiating the concept of shared sensory pain experiences. Notably, this neural activity was absent in participants who did not report similar sensations. Activation in affective pain areas, such as aINS, was similar across groups, suggesting that sensory and affective pain mirroring are dissociable [148].
Research has yet to reveal which factors mediate the group differences in somatosensory responsiveness to observed pain. Such factors may be of significant clinical relevance as they may underpin sources of empathic deficits in clinical disorders as well as dysfunctional pain behaviors. However, at present such research is in its infancy [7, 8]. Moreover, although evidence for sensory mirroring in the somatosensory cortices, and tentatively the thalamus, during vicarious pain is provided, this neural activity is neither confirmed to unambiguously overlap with self-pain activations nor to be pain-specific. Likewise, the functions subserved by SI and SII have not been differentiated, although these regions are dissimilarly implicated in self-pain processing [184]. Thus, more research is needed to establish the manner in which shared sensory representations arise from such activation during observed pain.
4.2 The Affective Regions
4.2.1 The Insula
INS activation has been proposed to subserve interoception and affective vicarious pain processing [22, 51, 72, 98, 166, 175, 179, 192, 203]. Interoception is defined as a process by which several sources of information are integrated to form an internal representation of the current bodily state and corresponding emotional responses [50]. This is pertinent for self-pain as it allows individuals to assess their physical state. Given the comparable affective processing requirements of self- and other-pain, it is likely that the INS may play a similar role in pain observation [89, 113]. Indeed, Gu et al. [87] highlight that the INS is essential for empathy induction, having found INS lesions to inhibit empathic responding. In line with the PAM , there is evidence for an overlap in increased aINS activation during self- and vicarious pain when comparing brain activity within the same individuals [48, 146]. Likewise, between-subject paradigms have revealed consistent aINS activity during pain observation within established INS correlates of the pain matrix [88, 89, 98, 99, 112, 113, 186]. These findings provide support for indirect affective pain mirroring from which shared neural pain representations arise [22, 146, 179, 205].
Furthermore, comparable aINS activation has been demonstrated during both direct evaluative pain judgments as well as identification of limb laterality for images of limbs in pain, substantiating the notion of automaticity in such mirroring [89]. Such automatic mirroring is likely to achieve an immediate representation of the current state of the individual in pain [155, 156]. Moreover, Cheng et al. [36] instructed participants to imagine observed pain from the perspective of either a stranger or a loved one. The latter condition elicited increased aINS activation as well as greater neural overlap for self- and other pain, indicating that increased emotional attachment evokes greater affective mirroring of the observed pain within one’s own system. Notably, aINS activity distinguishes between painful and non-painful vicarious stimulation [112] and responds to general negative encounters, such as self-experienced and vicarious disgust [93, 199]. Accordingly, shared neural representations in the INS may not be pain-specific, but instead encode adversity [13, 48]. aINS involvement in the anticipation of aversive stimuli and its connections to well-established emotion centers, such as the amygdala, substantiate its likely contribution to the affective processing of vicarious pain [110, 164, 166, 174]. Given the findings, it is plausible that affective mirroring gives rise to shared affective pain representations during vicarious pain, in particular when high levels of emotions are involved. However, further fine-grained analysis is required to assess to what extent neural patterns during self- and other-pain are identical.
4.2.2 The Cingulate Cortex
During self- and vicarious pain, the ACC has been has been associated with affective pain processing, [70, 76] while the midcingulate cortex (MCC) is associated with both affective and sensory pain components [43]; for review on CC see, [83, 110, 198]; for systematic review on CC in vicarious pain, see [202]. The PAM suggests that these similar roles give rise to mirroring and shared neural pain representations. However, while intracellular recordings have confirmed nociceptive neurons in the CC for self-pain , such an investigation has not yet been performed for vicarious pain [92]. In line with the role of the ACC in affective processing, neural activity in the subgenual and rostral ACC (sgACC; rACC) as well as aMCC has been found via pain observation in limbs, facial pain expressions and abstract pain cues [22, 26, 134, 163]. Supporting the PAM , the activations are reported to be overlapping and partially anterior to those commonly found during self-pain [26, 134]. Notably, Singer et al. [179] revealed ACC activation when participants received abstract information about their significant other receiving painful electric shocks, showing that emotional attachment was sufficient to induce a neural representation of pain unpleasantness. A direct comparison of neural activity confirmed that the ACC regions activated during self- and other-pain overlapped. Furthermore, similar to self-pain , greater rACC activity during other-pain was associated with increased other-pain evaluations [179]. However, to date, no correlations between affective pain measurements and ACC activity have been analyzed for facial and abstract pain cues. Thus, the extent to which the ACC subserves affective processing of vicarious pain is speculative. In contrast, studies presenting limbs in pain have investigated such correlations and confirmed that higher neural responses are indeed associated with higher unpleasantness ratings [113]. Furthermore, Jackson et al. [100] reported that ACC activity is specific to imagining the observed body part from an emotional first-person perspective and correlates with ratings of observed pain levels (Fig. 6). These findings support a role of the ACC in affective processing for vicarious pain which is comparable to affective self-pain processing and thus may contribute to shared affective pain representations [22, 48, 193]. Notably, the revealed ACC activity may not be pain-specific, but instead reflect the mirroring of a general negative affect [13].
ACC and aINS activation during vicarious pain. Abbreviations: AIC anterior insula. a ACC and aINS activation while observing another individual in pain. b Increased ACC activity was associated with increased subjective pain ratings (MNI Coordinates) [99]
In contrast, vicarious pain research has failed to find aMCC activity in abstract or facial cues of pain, suggesting that it may not subserve affective pain representations as it does for self-pain . Instead, the aMCC has been found explicitly responsive to images of limbs in pain, regardless of whether individuals focused on the sensory or affective aspects of the presented body part [113] or imagined the pain from a first- or third-person perspective [98]. In line with its responsiveness to body parts, research proposes that the aMCC may reflect sensory rather than affective mirroring. Furthermore, it may underpin distinct roles in pain observation [147]. Indeed, lesion studies indicate that an intact aMCC is not required for affective empathy [87]. Taken together, findings for CC activation suggest that neural patterns may overlap for self- and vicarious pain, reflecting neural mirroring and shared pain representations [48, 110, 134, 170, 195]. Nonetheless, the exact functions of the CC in mirroring and distinct contributions to vicarious pain processing need further clarification.
5 Distinct Neural Contributions
Several brain regions are implicated in making distinct contributions to the cognitive processing of vicarious pain. These include self-other distinction and attentional control, which are associated with IPL, SI, SII, INS, CC and PFC, as well as contextual pain appraisal and top-down regulation, which draw on the PFC. Notably, although it is proposed that in their cognitive roles these regions reflect processing that is independent from shared neural representations, the revealed activation has not been firmly excluded from underpinning mirror processing.
5.1 Self-other Distinction
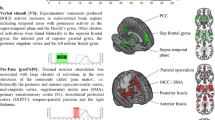
During vicarious pain, the IPL, temporoparietal junction (TPJ), somatosensory regions, aINS and PFC have been implicated in distinguishing one’s own sensory and affective experiences from those of the individual in pain. Uddin et al. [188] demonstrated that TMS stimulation to the right IPL disrupted the ability to discriminate self- and other-faces, which suggests that this brain region plays a key role in maintaining a distinct sense of self. Likewise, the somatosensory cortices may contribute to such a self-other distinction. Jackson et al. [98] reported somatosensory activation exclusive to imagining noxious stimulation from a first-person but not a third-person perspective. The lack of activation in the latter condition may be a mechanism by which SI and SII separate sensory experiences observed in others from their own sensory state while a first-person perspective may contribute to shared sensory experiences [98]. Moreover, both the INS and PFC have been associated with interoceptive self-awareness during vicarious pain that establishes an understanding of the self-state as a reference point against which to compare external pain cues [169]. Brooks et al. [23] have provided evidence of a somatotopic organization in the aINS that facilitates such interoception [74, 100]. Lending tentative support, Ebisch et al. [73] showed that for vicarious touch the pINS is involved in differentiating self- and other-states; however, studies have not yet explored this specifically for vicarious pain. Furthermore, when pain observation elicits discomfort in the observer, however not in the observed individual, the PFC has been proposed to promote self-other distinction for adequate context assessment [111, 149]. Likewise, the TPJ, which has been associated with perspective-taking, in particular, is responsive in such circumstances. Cheng et al. [37] report that the TPJ subserves self-other distinction and aids the understanding of individuals when the observer does not rely on neural pain mirroring. Substantiating this, imaging the observed pain from the perspective of a stranger has been associated with decreased activation of the pain matrix and increased TPJ involvement. This indicates that greater perspective-taking is applied when individuals have less emotional attachment to the individual suffering pain (Figs. 7 and 8). Such findings are reflected in negative functional connectivity between TPJ and aINS and positive connectivity between TPJ and superior frontal gyrus during pain observation from a stranger perspective. These suggest that the aINS is less involved in the encoding of stranger pain than of loved-one pain [36, 37].
Double dissociation between pain matrix and TPJ responses during vicarious pain imagined from different perspectives. Imaging pain from the perspective of a loved-on was associated with increased ACC, INS, SMA and PAG activity during pain observation, resembling self-perspective responses. Stranger perspective was associated with increased TPJ activity compared to the other two perspectives [36]
Negative association between TPJ and closeness in relationships. Abbreviations: IOS Scale Inclusion of others in self Scale; rTPJ right TPJ; SFG superior frontal gyrus. rTPJ, SFG and mPFC showed increased activation when imagining observed pain from a stranger perspective compared to self- or loved-one perspective. Increased self-reported overlap of self and others in close relationships was associated with decreased rTPJ activity during pain observation [36]
Self-other distinctions are an essential component of empathy [75, 98]. Although observing another individual in pain can have similar internal effects as self-pain for the observer, understanding that his or her pain is distinct from the self is crucial for adequate empathic responses that promote social survival. In contrast, a strong transference of other-pain to the self may trigger emotional contagion, thus evoking self-preserving withdrawal responses. Forthcoming systematic exploration may aim to further pinpoint the neural substrates of self-other distinction in the context of vicarious pain and empathy. Deficits in these correlates may be of significance for dysfunctional empathic responding [116, 197].
5.2 Salience Detection and Attention
The INS and aMCC have been associated with salience detection and attention in self- and vicarious pain processing [53, 104, 138]. Contrasting shared neural representations of the PAM , mINS and pINS activity has been reported more frequently for self- than other-pain [23, 80, 96]. Upon comparing brain activity in the same individuals, Ochsner et al. [146] found that the mINS only showed activation during application of heat pain to one’s own skin, but not when the same stimulus was observed on another individual. It was highlighted that the aINS has connections to affective processing areas while the mINS is linked to cognitive processing regions and is implicated in attention. As self-pain may require increased attentional resources due to greater stimulus salience compared to observed pain, it may specifically draw on the mINS for attention regulation [53, 104, 125, 146]. Nevertheless, fine-grained Multi-Pattern Variate Analysis (MPVA) has revealed overlapping mINS patterns for self- and vicarious pain, indicating that further research is required to clarify the role of the INS in salience detection and attention during pain observation [48]. Moreover, aMCC activation in established attention regions has been consistently reported for vicarious pain [26, 98, 99, 135,136,137, 147, 163, 205]. Similar to self-pain , observed pain signals threat and thus draws attention to the need for prompt responses [61, 75]. In line with the PAM , it is possible that comparable processing requirements of self- and other-pain stimuli may induce cognitive mirroring. However, it is equally likely that the aMCC directly process salient environmental hazards and regulate attention, independent from such mirroring [155, 156]. At present only three neuroimaging studies have explored attention in vicarious pain and thus no comprehensive conclusions can be drawn [78, 88, 89]. Fan and Han [78] demonstrated that participants identified and empathized with observed pain faster when pain was specifically attended to. In contrast, neural activity associated with vicarious pain processing has been found no longer significant when individuals focus away from pain cues while counting the number of presented limbs in pain. Accordingly, top-down attentional control may modulate vicarious pain processing and accordingly limit its automaticity as proposed by the PAM [88]. Moreover, when cognitive load was held constant and required similar attention levels during neutral and vicarious pain tasks, aMCC responses were equivalent for both painful and neutral images. This suggests that aMCC activation is not pain-specific but instead underpins attentional control [89]. Taken together, current research advocates that the aMCC, and tentatively the INS, facilitate attention during pain observation [9, 88, 89] and that vicarious pain processing, and thus potentially empathy, is vulnerable to resource competition from cognitive tasks [78, 88, 132]. Both premises have been established for self-pain . However, whether such processing reflects shared or distinct neural attention correlates for self- and other-pain needs to be disentangled in further research in order to gain a full understanding of the effects of salience and attention during vicarious pain [9, 59].
5.3 Context Appraisal and Top-Down Regulation
Vicarious pain studies speculate that the PFC subserves the assessment and cognitive integration of multiple inputs for adequate pain appraisal [77, 117, 185, 195]. Furthermore, it may play a role in top-down regulation of responses to the observed pain [76, 85, 117, 185, 201]; for review on PFC, see [127]. While these functions resemble those during self-pain , it has not yet been established whether PFC activity reflects shared or distinct neural representations during other-pain [26, 112, 145, 193]. Although it differentiates between painful and non-painful observations [26, 149], the majority of vicarious pain research fails to find PFC involvement. Such inconsistencies may be an effect of task differences across studies, which require varying levels of cognitive resources. For example, Lamm et al. [112] presented participants with images of individuals who had their hands penetrated by needles or touched by Q-tips and informed them that these individuals showed either normal or abnormal responses corresponding to a neurological condition. PFC activity was only found for abnormal conditions, such as when participants were told that needles elicited no pain and Q-tips elicited pain. Likewise, Perry et al. [149] reported greater EEG suppression in the frontal areas both during the observation of painful needles as well as when individuals responded with abnormal pain to non-painful Q-tip stimulations. Both studies proposed that PFC processing reflects the detection and integration of conflicts, such as pain, and thus promotes accurate appraisal of the observed context. This is particularly required when witnessed responses fail to correspond to behaviors associated with noxious stimulation [112, 149]. In contrast, passive processing of straightforward pain reactions may occur at an automatic level without significant PFC activation [112]. Corresponding to PFC involvement in more complex encoding of pain observations, the medial PFC has been specifically associated with the decoding of facial pain expressions [26, 127, 193]. As facial expressions convey less noxious information than limbs in pain, greater cognitive evaluation is required for pain assessments. Accordingly, the PFC is proposed to subserve the integration of affective pain information with stored associations about social consequences of the observed pain [26, 156, 169]. In line with this, the PFC has been implicated in the analysis of the internal states and predicted intentions of other individuals [97, 102, 169]. More specifically, the mPFC has been associated with human perspective-taking when imagining observed pain from the first- or third-person perspective, but not as an artificial object, as shown in Fig. 9 [98]. Therefore, the PFC may have the role to complement neural pain mirroring with contextual information to enable accurate response formation. Notably, the precuneus, superior temporal sulcus (STS) and cerebellum, which are similarly associated with the ability to accurately attribute internal states to others, have been infrequently shown to respond during vicarious pain (Fig. 3) [147, 161, 193]. Although this tentatively suggests that these regions also contribute to active understanding of the suffering individual during pain observation, more consistent findings are required to evaluate their role in vicarious pain. Furthermore, higher functional connectivity has been registered between dorsomedial PFC and IFG during the complex vicarious pain conditions. Given the role of the IFG in perspective-taking and the retrieval of pain-related memories [103], it is plausible that the two regions work conjunctly to infer an understanding of the current state of the individual in pain [112].
mPFC activation associated with perspective-taking during vicarious pain. a mPFC activation when imaging presented pain from self-perspective compared to object perspective. b mPFC activation when imaging presented pain from other-perspective compared to object perspective. c mPFC responses in the three different conditions: self-pain as blue, other-pain as yellow and artificial pain as red [98]
Moreover, functional connectivity analyses have revealed increased neural connectivity for the PFC with the somatosensory cortices, CC and INS, which may reflect top-down regulation of responses to vicarious pain [88, 205]. More specifically, Cheng et al. [37] revealed that during pain observation, participants who are accustomed to seeing pain show greater PFC responses and decreased activity in SI, SII, INS and CC regions than controls (Fig. 10). Comparable to self-pain literature, increased activation in the PFC was associated with decreased emotional reactivity and lower pain intensity ratings [9, 24, 29, 33, 75, 154, 195]. These results indicate that the PFC exerts downregulatory control over regions involved in the processing of sensory and affective vicarious pain components.
Neural activity for pain experts and controls during vicarious pain through needles. a Non-experienced controls showed INS, PAG, ACC and SMA activation while experts showed IPL and mPFC activation. b Controls showed higher pain intensity and unpleasantness ratings compared to experts. c Compared to experts, controls showed higher aINS activation only for needles, but not for Q-Tips. Compared to controls, experts showed higher mPFC activation during needles, but not for Q-Tips [37]
Indeed, studies show that when cognitive analysis of the context implies that individuals in pain are to blame for the pain they are enduring [66] or are unfair to others [180], INS and CC activity is decreased and pain observers report less empathy. Such top-down regulation may occur at early pain processing stages [68]. Crucially, the revealed functional connectivity patterns between cognitive and sensory-affective regions were specific to vicarious pain and virtually absent during self-pain in the same individuals. While the role of such connectivities has not been confirmed, this substantiates that although brain regions are shared with self-pain processing, distinct neural communication may make unique contributions to vicarious pain [76, 110, 185, 205]. Furthermore, such top-down regulation may be a source of dysfunctional pain and empathy expressions. Ochsner et al. [146] advanced that the PFC encodes observed knowledge related to pain and pain-appropriate responses. In particular, increased activity in the rostrolateral PFC during pain observation has been associated with higher trait anxiety scores (Fig. 11). Such increased neural response is proposed to reflect increased encoding and learning of environmental threat cues in anxious populations, which may perpetuate dysfunctional self-pain anxiety [32, 146, 152]. This lends itself to future exploration of PFC substrates in maladjusted pain and empathy behaviors. Taken together, findings for PFC activation reinforce the role of the PFC in contextual analysis and top-down regulation during vicarious pain [2, 169]. However, as different PFC regions may contribute distinct processes, research specific to pain observation may reveal in which manner the subsections of the PFC are involved [76, 117, 201]. Likewise, it has not yet been established which neural patterns overlap with those during self-pain , as suggested by the PAM , and which activity is independent from neural mirroring [155]; for a meta-analysis on mPFC contribution to empathy, see [169].
Correlations between vicarious pain activity and anxiety scores. Abbreviations: FPQ fear of pain questionnaire; STAI state-trait anxiety inventory; rlPFC rostrolateral PFC. Higher rlPFC activity correlated with higher STAI scores during vicarious pain. No correlation with FPQ during other-pain. Higher ACC activity correlated with higher FPQ scores during self-pain. No effect for STAI during self-pain [146]
6 Other-Oriented Empathy and Self-oriented Withdrawal
The PAM proposes that shared neural representations of self- and other-pain evoke other-oriented empathic understanding that initiates altruistic behavior. Such behavior enhances the protective benefits of social cooperation and thus contributes to social survival. Nevertheless, the inherent threat cues of observed pain may instead induce emotional contagion and trigger self-oriented withdrawal responses. These facilitate physical survival. Nonetheless, research has not only failed to tease apart the neural patterns of these two distinct responses, but the factors mediating which response is chosen also remain unclear.
6.1 Motor Empathy and Motor Preparation
In line with the PAM , motor mirroring during vicarious pain may promote empathic response [155, 156]. However, meta-analytic evidence proposes that, similar to self-pain , the IPL, IFG, motor cortices and aMCC motor zones are actively involved in self-oriented pain predictions as well as subsequent motor preparation and coordination [40, 46, 47, 64, 77, 139, 173, 182]. Research advances that as IPL motor mirroring typically occurs to active movement, motionless pain presentations require the IPL to derive implied movement from the observed scene [34, 46]. For example when images of hands penetrated by needles are shown, the IPL may predict the anticipated removal of the hand rather than mirroring muscle cues [101]. Likewise, in line with its involvement in serial motor prediction, the SMA may predict the motor consequences of observed pain [113, 163]. Csibra [54] proposes that such predictions result from top-down analysis and are dynamically compared against the concurrent pain context within the sensorimotor regions in order to prepare motor responses such as withdrawal. This explains findings indicating that goal-directedness is necessary to elicit premotor activity during vicarious pain as preparatory actions are goal-oriented [99]. Thus contrasting the PAM , instead of constituting the first step in stimulus analysis through bottom-up mirroring, sensorimotor activation during vicarious pain may reflect top-down predictions subsequent to higher cortical processing of the observed pain [54, 111, 155]. Accordingly, sensorimotor activity during vicarious pain may contribute to prediction and preparation of withdrawal from presented threats instead of facilitating empathy [111].
In favor of such a notion, the IPL and aMCC have been implicated in the priming of motor reactions. For example, Morrison et al. [136] displayed animations and required participants to indicate via button press whether items struck or missed hands. When noxious items struck hands, participants showed increased reaction times and increased aMCC activity compared to non-noxious items and noxious miss conditions. It was concluded that voluntary movements were facilitated when corresponding to withdrawal movements that have been triggered through vicarious pain perception (Fig. 12) [21, 136, 193]. Such interference has been reported in particular for body parts compared to faces, indicating that greater sensorimotor relevance of presented pain images elicits greater motor prediction [191]. Notably, this contrasts studies that have found the motor regions of the aMCC are only responsive to self-pain [179].
Interaction between noxious items, hit or hiss conditions and motor response. a 1. MCC and PCC areas of interaction associated with increased reaction times during noxious-hit conditions. 2. Main effect of noxiousness (yellow), main effect of motor response (green), and interaction between noxiousness, motor response and hit (red). b. Decreased reaction times in milliseconds during noxious-hit conditions [136]
Furthermore, increased IFG activation has been reported in chronic pain patients who are more expressive about their own pain and also show increased vicarious pain responses. Although this neural activity may underpin greater receptiveness to the suffering of others mediated through self-pain sensitivity [192], alternative views propose that the overlap represents increased withdrawal urges in response to the comparable threats of both self- and other-pain. In line with this, IFG activity has been associated with self-oriented distress reactions to pain observation [111]. Such findings advocate that the sensorimotor regions do not facilitate empathy induction, but instead promote self-oriented avoidance responses [62, 64]. Substantiating this, IFG, IPL and motor activity have been seen in response to aversive stimuli, such as fear-inducing weapon images, which do not present motor cues yet trigger motor withdrawal [60]. Furthermore, imagined motor movement activates the premotor cortex and SMA in absence of motor cues, indicating that this system has functions beyond motor mirroring [118]. In line with this, SMA activity during vicarious pain has been associated with self-directed emotional distress, which may reflect greater motor preparation in response to resulting self-oriented withdrawal urges [108]. Nonetheless, Preston [155] counters that motor imagination requires an internal representation of the anticipated movement and thus utilizes similar mirroring structures as direct observation. Moreover, Avenanti et al. [5] highlight that not all motor inhibition in response to vicarious pain subserves self-oriented withdrawal responses [114, 190]. Research has shown that motor retraction reflexes to self-pain result in nonspecific suppression of all muscles in the concerned limb [79, 126]. In contrast, vicarious pain motor inhibition is specific to the exact muscle underlying the observed stimulation, corroborating its direct mirroring function. Such motor inhibition was associated with increased self-rated empathy and, in contrast, negatively correlated with personal distress. This indicates that motor inhibition reflecting bottom-up mirroring takes place during pain observation and may provide a platform for empathic understanding [5, 6]. Nonetheless, it does not exclude the possibility of concurrent top-down motor predictions within IPL, IFG and motor cortices, which facilitate self-oriented withdrawal.
Notably, sensorimotor bottom-up and top-down processing may work in conjunction. Functional connectivity analyses have revealed that sensory pain ratings were associated with increased neural synchronization between the thalamus, SI and the motor cortices during pain observation [15, 143]. Moreover, Carr et al. [31] report that the IFG receives somatosensory information from the IPL upon which it encodes the goal of the observed act. Research advances that such circuits may present reciprocal feedback loops by which sensory and motor cues mutually contribute to context-appropriate pain responses. In line with this, increased sensory signaling in self-pain has been attenuated by motor cortical stimulation resulting in a decreased pain report [19, 20, 115, 150, 187]. Likewise, similar to self-pain, increased connectivity between the aMCC motor zones and motor cortical regions during vicarious pain implicates aMCC motor zones in preparing withdrawal responses through projections to motor cortices [93, 138]. The aMCC regions for pain and motor processing have been found to lie adjacent and interact, potentially providing a direct feedback loop for motor and pain analysis [136]. Therefore, recorded sensorimotor activation during vicarious pain may reflect both initial motor mirroring and subsequent preparatory responses based on sensory and motor feedback [41, 105]. However, no confident conclusions can be drawn without further systematic investigations that pinpoint the neural pathways of these distinct functions [17, 99, 110, 111, 118, 163]. While initial motor activation may contribute to action understanding via direct mirroring of perceived motor cues within the motor regions [158, 160], feedback from higher cortical structures are likely to contribute to subsequent top-down action predictions and motor preparation [54, 119, 200].
6.2 Sensory Empathy and Sensory Preparation
Contrasting the sensory mirroring of the PAM , Morrison et al. [137] suggest that SI and SII are involved in integrating sensory information with action during pain observation in order to predict the sensory consequences of observed pain. Thus, rather than inducing empathy, SI and SII activity may be a product of higher cortical output that evokes self-oriented avoidance behaviors [54]. Such notion is supported by self-pain research that shows high SI susceptibility to top-down regulation; however, it has not been explored for vicarious pain [30, 184]. Notably, SI and SII activations have been linked to the bias of falsely reporting self-experienced tactile stimulation subsequent to observing pain in others [137]. Likewise, Valeriani et al. [194] found that participants that were concurrently subjected to self-pain while observing pain in others demonstrated increased self-pain ratings. The PAM suggests that such hypersensitivity to tactile threat is triggered when individuals evaluate noxious stimuli via their own sensory neurons. However, alternative views suggest that it results from top-down somatosensory threat predictions and facilitates both increased vigilance and withdrawal from external hazards [14, 74, 111, 137, 155]. Interestingly, somatosensory activity during pain observation also increased when limbs in pain were presented in the context of happy and pain faces compared to neutral faces. Such affective faces may increase arousal, which impacts top-down pain analysis and can lead to increased projections of threatening pain predictions to SI and SII [90]. Crucially, increased tactile hypersensitivity during vicarious pain is associated with withdrawal responses rather than empathic , altruistic behaviors [63]. Nonetheless, somatosensory activity in response to observing limbs in painful compared to non-painful scenarios has been associated with increased self-rated empathic abilities [38]. Accordingly, while SI and SII may underpin threat predictions and evoke self-oriented avoidance responses during vicarious pain, both regions also show involvement in other-oriented empathy. Future investigation may disentangle the activations corresponding to distinct bottom-up or top-down processing, instigating empathy or withdrawal.
6.3 Empathic Distress and Personal Distress
aINS, ACC and amygdala involvement in affective processing is well-established, and it is particularly challenging to tease apart which neural patterns underpin other-oriented empathy as opposed to self-oriented affective distress during vicarious pain. More specifically, aINS activity has been associated with both higher self-reported empathy and increased intensity ratings of observed pain [95, 163, 179, 193]. Research advances that the interoceptive functions of the aINS may be the mechanism by which the state of the person suffering pain is mapped onto the own state, intrinsically triggering empathic understanding [31, 89, 177]. In line with this, Hein et al. [91] found increased aINS activity during pain observation to correlate with empathic concern and, more importantly, predict helpful behavior. No association was found for aINS activity and self-oriented distress, supporting its role in other-oriented empathy. This suggests that greater responsiveness of this brain region may be a predictor of greater empathic response. Clinical findings for patients with congenital insensitivity to pain further support such a notion. This disorder is characterized by the inability to experience pain, and, thus patients cannot use sensory and affective pain mirroring to evaluate the pain experienced by another individual [57]. Alternatively, during vicarious pain the revealed aMCC and aINS activation in those individuals is more likely to reflect an empathic understanding of emotionally aversive events. However, as the stage at which deficits in congenital pain sensitivity originate is unclear, a form of pain mirroring may remain effective. Further research is required to gain a comprehensive understanding of these mechanisms [140]. Moreover, these findings do not exclude that the aINS also encodes self-oriented distress responses to observed pain [111]. The INS has been associated with increased emotional responses to self-pain , and, similarly activity during vicarious pain may reflect increased emotional distress [69, 107, 168]. Indeed, research has reported correlations between aINS activity and self-rated negative affect during pain observation, in particular when emotional attachment to the individual in pain is high [36, 179]. Likewise, Akitsuki and Decety [1] demonstrated a positive correlation between activity in the left aINS and emotional contagion. This was associated with motor preparation, implicating the aINS in a self-oriented distress response that instigates withdrawal. Furthermore, compassion training, which involves the regulation of self-experienced affect during empathic response failed to elicit aINS activation, supporting a role of this region in coding personal distress responses [106]. Thus, inconsistent findings prevent firm conclusions on whether aINS activity during vicarious pain reflects other-oriented empathy or self-oriented distress. Notably, Lamm et al. [108] suggest that the role of such activity is modulated by the perspective from which the observed pain is imagined. First-person perspectives may thus elicit personal distress while third-person perspectives elicit empathic understanding, both of which are mediated by the aINS. Furthermore, the aINS may moderate the separation of self- and other-affect during pain observation rather than underpinning one or the other [77].
Moreover, research suggests that the amygdala can differentiate other-oriented empathy from self-oriented distress during pain observation in its function in fear processing [176]. Akitsuki and Decety [1] revealed higher amygdala activation when individuals viewed images of limbs on which pain was inflicted intentionally, in particular when imagining the pain from a first-person perspective [1, 108]. As the threat value of such stimuli is high, this supports a role of the amygdala in threat detection. Substantiating this, comparisons between self- and vicarious pain have found amygdala activation specific to other-pain, independent of the perspective used to imagine the observed pain. These results indicate that observing the administration of noxious stimuli to other individuals arouses the threat detection system even when individuals do not fear the pain itself (Fig. 13) [146]. Accordingly, the danger cues of observed pain may activate a self-oriented fear response that evokes withdrawal behaviors [1, 142].
Increased amygdala activity during vicarious pain compared to self-pain. Small-volume-corrected clusters of amygdala activity and their mean beta values with standard deviations as indicated by bars [146]
Interestingly, the amygdala shows such fear processing specifically to male but not to female pain expressions. This indicates that while male pain may be interpreted as self-directed risk, female pain may activate other-oriented empathic responses [176]. Furthermore, functional connectivity analyses indicate that the amygdala may project fear information to both the somatosensory cortices and the aINS, potentially contributing to somatosensory predictions and affective appraisal of the observed pain [176]. In turn, the aINS projects information to the ACC for further processing and response selection [89]. Notably, the ACC has been associated with increased personal distress responses during vicarious pain. In line with this, activation in this brain region decreases when pain stimulation is presented in the context of several affective facial expressions. Increasing levels of affective information should increase ACC activity that underpins empathy as this information contributes to a more comprehensive emotional appraisal of the pain observation. Thus, the decrease may instead correspond to the decrease in self-oriented affective processing of vicarious pain due to the distraction value of the presented faces [90]. Taken together, there is evidence for an involvement of the aINS, amygdala and CC in other-oriented empathy and self-oriented distress processing during observed pain. In particular, the INS may underpin empathy [31, 91, 95], while the amygdala and CC may subserve personal distress [1, 176]. Nonetheless, future research needs to pinpoint exact neural patterns for firm conclusions to be drawn. Exploring amygdala activity during vicarious pain may be especially fruitful due to its well-known connection to fear. While fear can be other-oriented, its strong evolutionary connection to self-oriented survival makes it likely to underpin personal affective distress during pain observation.
7 An Integrative Model of Vicarious Pain and Research Gaps
Available literature has demonstrated the involvement of sensorimotor, affective and cognitive processing regions in pain observation. Notably, the PAM offers essential theoretical insight into the mechanisms of vicarious pain and empathy. Nonetheless, the inclusion of distinct neural activations, empathy and withdrawal responses in such a theoretical framework may provide a more comprehensive account of vicarious pain processing. Such an integrative model of vicarious pain, as displayed in Fig. 14, can also visualize current research gaps that provide a podium for future scientific investigation.
Integrative model of vicarious pain. Vicarious pain is processed at stimulus level, including shared and distinct neural activations underpinning bottom-up and top-down processing of the observed pain. Two response options may result, including empathy and distress. All four concepts may interact, but further research is needed to elucidate such notion
Brain activation during vicarious pain may underpin stimulus and response processing. At the stimulus processing level, the PAM suggests that overlapping brain activation during self- and other-pain reflects sensorimotor and affective bottom-up pain mirroring that stimulates associations relevant for stimulus appraisal [155,156,157]. Extending this, such activation may also underpin top-down predictions about the observed pain, which are compared against the concurrent context and guide behavioral responses [54, 200]. Notably, the revealed bottom-up and top-down pain processing may form a feedback loop. Accordingly, two distinct, yet interactive, mechanisms may be subserved by shared neural pain representations. More specifically, initial bottom-up pain processing may direct which top-down analysis is conducted. Subsequently, top-down predictions and corresponding motor preparation may be adjusted according to feedback from context comparisons [111]. Likewise, distinct brain activations during vicarious pain are indicated to contribute to both bottom-up processing as well as cognitive analysis and top-down regulation of responses to pain observation [77, 112, 188]. In particular, the qualitative differences between self-experienced and observed pain may be reflected in unique bottom-up neural pathways for each pain experience. Furthermore, complex pain contexts may be decoded in distinct neural patterns to enable individuals to adjust the representation conveyed in bottom-up neural mirroring according to supplementary cognitive information. Thus, shared and distinct neural representations of vicarious pain may have similar interactions within bottom-up and top-down processing as well as with one another. Such dynamic feedback systems are reflected in increased functional connectivities between corresponding brain regions; however, they have not yet been systematically tested [15, 143, 205]. While such interactions are a novel theoretical extension of the PAM for vicarious pain, these have been established in other fields, such as visual perception. In particular, bottom-up processing has been found to impact higher cortical activity, and top-down processing can shape the bottom-up perception of stimuli [10, 124]. Accordingly, it is likely to take effect in vicarious pain experiences and contribute to the evaluation of observed pain. Nonetheless, while there is evidence of an overlap in activation in the mirror neuron system and regions of the self-pain matrix during pain observation, the exact neural correlates have not yet been identified. Therefore, the extent to which neural correlates are shared or distinct is subject to further verification. Similarly, neural substrates of bottom-up and top-down pain processing have not yet been teased apart, thus making it challenging to investigate their interactions [77, 105, 110, 111].
At the response processing level, brain activation during vicarious pain may subserve other-oriented empathic understanding and altruistic behaviors [157] or self-oriented distress and withdrawal behaviors [36, 136, 176, 191]. However, the neural correlates of these two distinct response options have not been directly compared, and, thus no firm conclusions can be drawn. Moreover, research has shown that at early processing stages, individuals show greater neural responses and empathic responding to individuals with whom they can identify and feel positively about [66, 180]. In line with this, habituated empathic or distress responses may shape early neural processing of the observed pain, reinforcing neural pathways that may be dysfunctional [7]. Nonetheless, the factors mediating whether empathy or withdrawal is induced remain largely under-researched. Likewise, it is unclear whether these responses are evoked subsequent to the stimulus processing of pain observation or occur concurrently [111]. Concurrent stimulus and response processing of vicarious pain may contribute to remarkably complex feedback interactions. Elucidating the manner in which these responses are formed is the first step in understanding and tackling habitual dysfunctional empathy or avoidance behaviors [71]. Moreover, uncovering the interactions within and between stimulus and response processing levels is likely to contribute to a comprehensive concept of vicarious pain.
In order to shed light on the neural correlates of pain observation and their functions, behavioral and neuroimaging methods may be combined. Notably, as the roles of the different brain regions involved are not reliably confirmed for self-pain , revealing identical neural patterns for self- and other-pain may not enable research to derive their corresponding functions [93, 185, 186]. Accordingly, systematic and well-controlled paradigms may engender more robust findings. Furthermore, regions of interest analyses may be extended beyond the mirror and pain system. For example, the amygdala responds to aversive events such as facial pain expressions [31]. However, this region remains largely untouched by vicarious pain research. Pinpointing neural patterns that underlie pain observation is challenging as present neuroimaging tools are subject to coarse spatial resolution and individual brain differences can further decrease accuracy [105, 112, 113]. Likewise, these techniques may fail to detect weak levels of activation, and therefore activity in other regions cannot be confidently excluded. Ideally, single-cell recording could confirm both identical and distinct neural activation, but this is subject to immense practical and ethical restrictions [92]. Nonetheless, improvements in analytical methodology may minimize the impact of equipment issues. For example, MPVA has proven useful for detailed neural explorations and is likely to contribute to teasing apart brain responses to stimulus and response processing of vicarious pain [48]. Moreover, functional connectivity analysis as well as EEG, MEG and TMS may collectively contribute to an understanding of neural communication during pain observation. In particular, these methods can establish temporal processing sequences and elucidate the directions of such communication. Such methods may shed light on the extent and effects of shared and distinct neural activity and interaction as well as elucidate the neural correlates of empathic and withdrawal responses. Notably, although systematic paradigms are robust in identifying correlational and causal relationships, they struggle with ecological validity. Thus, findings from artificial laboratory settings may not be directly transferred to the complexity of vicarious pain in the natural environment. Nevertheless, a deeper understanding of the various aspects of vicarious pain will provide a solid basis in which more complex paradigms can be confidently rooted [204].
8 Clinical Relevance
Elucidating the neural underpinnings of vicarious pain and empathic or withdrawal responses is of significant relevance for the detection and management of clinical issues that are characterized by dysfunctional pain or empathy behaviors. Structural and functional brain abnormalities have been associated with empathic deficits in clinical disorders [35, 57, 58, 129, 133, 181]. For example, Cummins et al. [55] provided behavioral evidence for a link between motor coordination difficulties and decreased emotion recognition in others. As abnormal motor processing may be associated with defective mirror neuron systems, blunted empathy may result from inadequate motor mirroring that fails to engage evaluative associations. Indeed, motor disorders have been associated with decreased gray matter volume in the IFG [35]. Likewise, individuals with developmental and psychotic disorders, such as autism and schizophrenia, have consistently shown abnormal motor inhibition during action observation as well as decreased empathic abilities. This substantiates that dysfunctional mirroring contributes to empathic deficits [123, 129, 144]. Furthermore, abnormal aINS, aMCC and PFC functioning has been associated with decreased empathic understanding concurrent with deficits in attentional control in ADHD [189], negativity biases in depression [56, 167] and antisocial conduct [181]. Accordingly, processing deficits in affective–cognitive substrates of vicarious pain, empathy and withdrawal may underpin dysfunctional behaviors. While clinical research has focused on empathy toward facial emotional expressions, investigating potential interactions between pain and empathic understanding may provide novel intervention targets for dysfunctions in either domain. For example, based on shared neural representations between self- and other-pain, self-pain treatments may fine-tune the neural pain mirroring system and thus have spillover effects on increasing empathic understanding [52]. Notably, social and physical pain have been shown to share neural correlates during vicarious processing. Given the strong association between empathic deficits and social rejection, research may further uncover factors that perpetuate such cycles [95]. Identifying neural and behavioral sources of dysfunctional empathic behaviors may enable better management of such deficits which can increase the quality of life for many individuals suffering from clinical disorders. Moreover, such identification contributes to improved health care. As accurate empathic awareness has been found to increase diagnosis accuracy, enhanced empathic skills may facilitate both enriched treatment and patient-clinician relationships [11]. In line with this, training programs teaching self-regulation of affect during empathic understanding have been successful. Individuals reported lower self-oriented distress during pain observation, but displayed greater other-oriented altruistic behaviors than individuals without this training. As such, emotion regulation and compassion may be useful for enhancing context-appropriate responses [106]. Crucially, not only can the clinical realm benefit from establishing neuroimaging correlates of vicarious pain and empathy, but vice versa, clinical research can contribute to this by revealing dysfunctional activity associated with deficits. Such studies can confirm functional speculations derived from healthy populations.
9 Conclusion
Current vicarious pain research provides tentative evidence for shared and distinct neural representations of self- and other-pain. Nevertheless, the neural substrates of vicarious pain experiences are subject to confirmation in further systematic paradigms. These should combine neuroimaging and behavioral methodology to investigate brain responses and their corresponding functions during pain observation. Extending the PAM , an integrative model of vicarious pain is recommended as a platform for future comprehensive scientific inquiry.
Abbreviations
- IFG:
-
Inferior Frontal Gyrus
- IPL:
-
Inferior Parietal Lobule
- SI, SII:
-
Primary Somatosensory Cortex, Secondary Somatosensory Cortex
- PFC:
-
Prefrontal Cortex
- INS:
-
Insula
- aINS, mINS, pINS:
-
Anterior Insula, Mid-Insula, Posterior Insula
- CC, ACC, PCC:
-
Cingulate Cortex, Anterior Cingulate Cortex, Posterior Cingulate Cortex
- sgACC, rACC:
-
subgenual ACC, rostral ACC
- MCC, aMCC:
-
Midcingulate Cortex, Anterior MCC
- dlPFC, dmPFC, mPFC, rlPFC:
-
dorsolateral PFC, dorsomedial PFC, medial PFC, rostrolateral PFC
- SMA:
-
Supplementary Motor Area
- fMRI:
-
functional Magnetic Resonance Imaging
- EEG:
-
Electroencephalography
- MEG:
-
Magnetoencephalography
- TMS:
-
Transcranial Magnetic Stimulation
- PAM:
-
Perception Action Model
References
Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47(2):722–34.
Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–77.
Avenanti A, Aglioti SM. The sensorimotor side of empathy for pain. In: Psychoanalysis and neuroscience. Berlin: Springer; 2006. p. 235–56.
Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8(7):955–60.
Avenanti A, Minio-Paluello I, Bufalari I, Aglioti SM. The pain of a model in the personality of an onlooker: influence of state-reactivity and personality traits on embodied empathy for pain. Neuroimage. 2009;44(1):275–83.
Avenanti A, Paluello IM, Bufalari I, Aglioti SM. Stimulus-driven modulation of motor-evoked potentials during observation of others’ pain. Neuroimage. 2006;32(1):316–24.
Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr Biol. 2010;20(11):1018–22.
Azevedo RT, Macaluso E, Avenanti A, Santangelo V, Cazzato V, Aglioti SM. Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Hum Brain Mapp. 2013;34(12):3168–81.
Bantick Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(2):310–9.
Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15(4):600–9.
Bellet PS, Maloney MJ. The importance of empathy as an interviewing skill in medicine. JAMA. 1991;266(13):1831–2.
Benedetti F, Bernasconi A, Bosia M, Cavallaro R, Dallaspezia S, Falini A, Poletti S, Radaelli D, Riccaboni R, Scotti G. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114(1):154–60.
Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. J Neurosci. 2008;28(4):923–31.
Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1–23.
Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F. Synchronous with your feelings: sensorimotor γ band and empathy for pain. J Neurosci. 2009;29(40):12384–92.
Bingel U, Lorenz J, Glauche V, Knab R, Gläscher J, Weiller C, Büchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage. 2004;23(1):224–32.
Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99(1):313–21.
Blair RJR. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14(4):698–718.
Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S, Smith AR, Madan A, Patterson D, George MS. A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin J pain. 2011;27(6):486.
Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, Shelley N, Branham RK, Thomas KJ, George MS. Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag (The Journal of the Canadian Pain Society). 2007;12(4):287.
Botvinick M, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8(12):539–46.
Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25(1):312–9.
Brooks J, Zambreanu L, Godinez A, Craig A, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27(1):201–9.
Brown CA, Jones AKP. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin Neurophysiol. 2008;119(10):2370–9. doi:10.1016/j.clinph.2008.06.014.
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13(2):400–4.
Budell L, Jackson P, Rainville P. Brain responses to facial expressions of pain: emotional or motor mirroring? Neuroimage. 2010;53(1):355–63.
Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17(11):2553–61.
Bufalari I, Ionta S. The social and personality neuroscience of empathy for pain and touch. Front Human Neurosci. 2013;7.
Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain. 2005;113(1):172–84.
Bushnell M, Duncan G, Hofbauer R, Ha B, Chen J-I, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci. 1999;96(14):7705–9.
Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci. 2003;100(9):5497–502.
Carter AE, Carter G, Boschen M, Al Shwaimi E, George R. Pathways of fear and anxiety in dentistry: a review. World J Clin Cases WJCC. 2014;2(11):642.
Carter CS, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–79.
Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50(3):1148–67.
Cerami C, Dodich A, Canessa N, Crespi C, Iannaccone S, Corbo M, Lunetta C, Consonni M, Scola E, Falini A. Emotional empathy in amyotrophic lateral sclerosis: a behavioural and voxel-based morphometry study. Amyotrophic Lateral Scler Frontotemporal Degeneration. 2014;15(1–2):21–9.
Cheng Y, Chen C, Lin C-P, Chou K-H, Decety J. Love hurts: an fMRI study. Neuroimage. 2010;51(2):923–9.
Cheng Y, Lin C-P, Liu H-L, Hsu Y-Y, Lim K-E, Hung D, Decety J. Expertise modulates the perception of pain in others. Curr Biol. 2007;17(19):1708–13.
Cheng Y, Yang C-Y, Lin C-P, Lee P-L, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage. 2008;40(4):1833–40.
Chong TT-J, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol. 2008;18(20):1576–80.
Churchland MM, Byron MY, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006;26(14):3697–712.
Cioni B, Meglio M. Motor cortex stimulation for chronic non-malignant pain: current state and future prospects. In: Operative neuromodulation. Berlin: Springer; 2007. p. 45–9.
Coghill R. Individual differences in the subjective experience of pain: new insights into mechanisms and models. Headache: J Head Face Pain 2010;50(9):1531–5. doi:10.1111/j.1526-4610.2010.01763.x.
Coghill R, McHaffie JG, Yen Y-F. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci. 2003;100(14):8538–42.
Coghill R, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–43.
Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behav Brain Sci. 2014;37(02):177–92.
Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15.
Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J Neurosci. 2011;31(49):17996–8006.
Costantini M, Galati G, Romani GL, Aglioti SM. Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. Eur J Neurosci. 2008;28(6):1222–30.
Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66.
Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95.
Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci. 2010;107(40):17433–8.
Crombez G, Eccleston C, Baeyens F, Eelen P. Attentional disruption is enhanced by the threat of pain. Behav Res Ther. 1998;36(2):195–204. doi:10.1016/S0005-7967(97)10008-0.
Csibra G. Action mirroring and action understanding: an alternative account. Sensorymotor Found Higher Cogn Attention Perform. 2008;XXII:435–59.
Cummins A, Piek JP, Dyck MJ. Motor coordination, empathy, and social behaviour in school-aged children. Dev Med Child Neurol. 2005;47(7):437–42.
Cusi AM, Nazarov A, Holshausen K, MacQueen GM, McKinnon MC. Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci JPN. 2012;37(3):154.
Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61(2):203–12.
Danziger N, Prkachin KM, Willer J-C. Is pain the price of empathy? The perception of others’ pain in patients with congenital insensitivity to pain. Brain. 2006;129(9):2494–507.
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain-and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77(6):3370–80.
De Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci U S A. 2004;101(47):16701–6.
De Vignemont F, Jacob P. What is it like to feel another’s pain?*. Philos Sci. 2012;79(2):295–316.
De Vignemont F, Singer T. The empathic brain: how, when and why? Trends in cognitive sciences. 2006;10(10):435–41.
De Waal FB. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol. 2008;59:279–300.
Decety J. To what extent is the experience of empathy mediated by shared neural circuits? Emot Rev. 2010;2(3):204–7.
Decety J. Dissecting the neural mechanisms mediating empathy. Emot Rev. 2011;3(1):92–108.
Decety J, Echols S, Correll J. The blame game: the effect of responsibility and social stigma on empathy for pain. J Cogn Neurosci. 2010;22(5):985–97.
Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3(2):71–100.
Decety J, Yang C-Y, Cheng Y. Physicians down-regulate their pain empathy response: an event-related brain potential study. Neuroimage. 2010;50(4):1676–82.
Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73(3):431–45.
Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306.
Drwecki BB, Moore CF, Ward SE, Prkachin KM. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain. 2011;152(5):1001–6.
Duerden EG, Arsalidou M, Lee M, Taylor MJ. Lateralization of affective processing in the insula. Neuroimage. 2013;78:159–75.
Ebisch SJ, Ferri F, Salone A, Perrucci MG, D’Amico L, Ferro FM, Romani GL, Gallese V. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci. 2011;23(7):1808–22.
Eisenberg N, Fabes RA. Empathy: conceptualization, measurement, and relation to prosocial behavior. Motiv Emot. 1990;14(2):131–49.
Eisenberg N, Morris AS. The origins and social significance of empathy-related responding. A review of empathy and moral development: implications for caring and justice by ML Hoffman. Social Justice Res. 2001;14(1):95–120.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93.
Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35(3):903–11.
Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46(1):160–73.
Farina S, Tinazzi M, Le Pera D, Valeriani M. Pain-related modulation of the human motor cortex. Neurol Res. 2003;25(2):130–42.
Farrell MJ, Laird AR, Egan GF. Brain activity associated with painfully hot stimuli applied to the upper limb: A meta-analysis. Hum Brain Mapp. 2005;25(1):129–39.
Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36(4):171–80.
Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends cogn Sci. 2004;8(9):396–403.
Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 2013;37(3):340–8.
Gracely RH, Sundgren PC. Neuroimaging of pain. In: Functional neuroradiology. Berlin: Springer; 2012. p. 273–90.
Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Nuñez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cogn Brain Res. 2005;25(1):153–60.
Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst). 2001;107(1):293–321.
Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(9):2726–35.
Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36(1):256–67.
Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30(10):3739–44.
Han S, Fan Y, Xu X, Qin J, Wu B, Wang X, Aglioti SM, Mao L. Empathic neural responses to others’ pain are modulated by emotional contexts. Hum Brain Mapp. 2009;30(10):3227–37.
Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–60.
Hutchison WD, Davis K, Lozano A, Tasker R, Dostrovsky J. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2(5):403–5.
Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back). Exp Brain Res. 2010;205(1):1–12.
IASP IAftSoP. Classification of chronic pain. 2 ed. Seattle: IASP Press; 1994.
Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proc Natl Acad Sci. 2009;106(19):8021–6.
Isnard J, Magnin M, Jung J, Mauguière F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152(4):946–51.
Isoda M, Noritake A. What makes the dorsomedial frontal cortex active during reading the mental states of others? Front Neurosci. 2013;7.
Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–61.
Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–9.
Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125(1):5–9.
Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J Neurophysiol. 2010;104(1):128–40.
Kang P, Lee J, Sul S, Kim H. Dorsomedial prefrontal cortex activity predicts the accuracy in estimating others’ preferences. Front Human Neurosci. 2013;7.
Kelly S, Lloyd D, Nurmikko T, Roberts N. Retrieving autobiographical memories of painful events activates the anterior cingulate cortex and inferior frontal gyrus. J Pain. 2007;8(4):307–14.
Keogh E, Moore DJ, Duggan GB, Payne SJ, Eccleston C. The disruptive effects of pain on complex cognitive performance and executive control. PLoS ONE. 2013;8(12):e83272.
Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11(6):417–28.
Klimecki OM, Leiberg S, Ricard M, Singer T. Differential pattern of functional brain plasticity after compassion and empathy training. Social Cogn Affect Neurosci. 2013;nst060.
Kulkarni B, Bentley D, Elliott R, Youell P, Watson A, Derbyshire S, Frackowiak R, Friston K, Jones A. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21(11):3133–42.
Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Cogn Neurosci J. 2007;19(1):42–58.
Lamm C, Decety J. Is the extrastriate body area (EBA) sensitive to the perception of pain in others? Cereb Cortex. 2008;18(10):2369–73.
Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–502.
Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality in empathy—a critical comment. Neurosci Res. 2015;90:15–24.
Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2010;22(2):362–76.
Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007;2(12):e1292.
Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Di Lazzaro V, Tonali PA, Arendt-Nielsen L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol. 2001;112(9):1633–41.
Lefaucheur J, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen J. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J Neurol Neurosurg Psychiatry. 2008;79(9):1044–9.
Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, Suckling J, Baron-Cohen S, Consortium MA. Atypical neural self-representation in autism. Brain. 2010;133(2):611–24.
Lorenz J, Minoshima S, Casey K. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(5):1079–91.
Lotze M, Montoya P, Erb M, Hülsmann E, Flor H, Klose U, Birbaumer N, Grodd W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11(5):491–501.
Mattiassi AD, Mele S, Ticini LF, Urgesi C. Conscious and unconscious representations of observed actions in the human motor system. J Cogn Neurosci. 2014;26(9):2028–41.
May A. Neuroimaging: visualising the brain in pain. Neurol Sci. 2007;28(2):S101–7.
May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol. 2009;5(4):199–209.
Mazzola L, Isnard J, Mauguiere F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex. 2006;16(7):960–8.
McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res: Neuroimaging. 2012;201(3):233–9.
Mechelli A, Price CJ, Friston KJ, Ishai A. Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex. 2004;14(11):1256–65.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67.
Mercier C, Leonard G. Interactions between pain and the motor cortex: insights from research on phantom limb pain and complex regional pain syndrome. Physiother Can. 2011;63(3):305–14.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202.
Minio-Paluello I, Avenanti A, Aglioti SM. Left hemisphere dominance in reading the sensory qualities of others’ pain? Soc Neurosci. 2006;1(3–4):320–33.
Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry. 2009;65(1):55–62.
Molenberghs P, Cunnington R, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci Biobehav Rev. 2009;33(7):975–80.
Molnar-Szakacs I, Kaplan J, Greenfield PM, Iacoboni M. Observing complex action sequences: the role of the fronto-parietal mirror neuron system. Neuroimage. 2006;33(3):923–35.
Morelli SA, Lieberman MD. The role of automaticity and attention in neural processes underlying empathy for happiness, sadness, and anxiety. Front Human Neurosci. 2013;7.
Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G. Empathy and judging other’s pain: an fMRI study of alexithymia. Cereb Cortex. 2007;17(9):2223–34.
Morrison I, Downing PE. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage. 2007;37(2):642–51.
Morrison I, Lloyd D, Di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4(2):270–8.
Morrison I, Peelen MV, Downing PE. The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17(9):2214–22.
Morrison I, Tipper SP, Fenton-Adams WL, Bach P. “Feeling” Others’ painful actions: the sensorimotor integration of pain and action information. Hum Brain Mapp. 2013;34(8):1982–98.
Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101(6):3258–69.
Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–69.
Nagasako EM, Oaklander AL, Dworkin RH. Congenital insensitivity to pain: an update. Pain. 2003;101(3):213–9.
Nakamura K, Kawashima R, Ito K, Sugiura M, Kato T, Nakamura A, Hatano K, Nagumo S, Kubota K, Fukuda H. Activation of the right inferior frontal cortex during assessment of facial emotion. J Neurophysiol. 1999;82(3):1610–4.
Norris CJ, Chen EE, Zhu DC, Small SL, Cacioppo JT. The interaction of social and emotional processes in the brain. J Cogn Neurosci. 2004;16(10):1818–29.
Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage. 2008;43(3):571–80.
Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn Brain Res. 2005;24(2):190–8.
Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1):69–77.
Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Social Cogn Affect Neurosci. 2008;3(2):144–60.
Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2007;17(5):1139–46.
Osborn J, Derbyshire SW. Pain sensation evoked by observing injury in others. Pain. 2010;148(2):268–74.
Perry A, Bentin S, Bartal IB-A, Lamm C, Decety J. “Feeling” the pain of those who are different from us: modulation of EEG in the mu/alpha range. Cogn Affect Behav Neurosci. 2010;10(4):493–504.
Peyron R, Garcia-Larrea L, Deiber M, Cinotti L, Convers P, Sindou M, Mauguiere F, Laurent B. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62(3):275–86.
Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Clin Neurophysiol. 2000;30(5):263–88.
Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JNP. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–81.
Ploner M, Schmitz F, Freund H-J, Schnitzler A. Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol. 2000;83(3):1770–6.
Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206–14.
Preston SD. A perception-action model for empathy. Empathy Mental Illness 2007;428–47.
Preston SD, De Waal F. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25(01):1–20.
Preston SD, Hofelich AJ. The many faces of empathy: parsing empathic phenomena through a proximate, dynamic-systems view of representing the other in the self. Emot Rev. 2012;4(1):24–33.
Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92.
Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn Brain Res. 1996;3(2):131–41.
Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2(9):661–70.
Roldan-Gerschcovich E, Cerquetti D, Tenca E, Leiguarda R. The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase. 2011;17(3):270–5.
Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Group SoTC. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39.
Saarela MV, Hlushchuk Y, Williams ACdC, Schürmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cereb Cortex. 2007;17(1):230–7.
Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 2000;20(19):7438–45.
Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Hum Brain Mapp. 2009;30(9):2722–30.
Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, Sammer G, Vaitl D. The insula is not specifically involved in disgust processing: an fMRI study. NeuroReport. 2002;13(16):2023–6.
Schneider D, Regenbogen C, Kellermann T, Finkelmeyer A, Kohn N, Derntl B, Schneider F, Habel U. Empathic behavioral and physiological responses to dynamic stimuli in depression. Psychiatry Res. 2012;200(2):294–305.
Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz H-G, Rolke R, Treede R-D, Bartenstein P, Birklein F. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology. 2005;64(7):1175–83.
Seitz RJ, Nickel J, Azari NP. Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology. 2006;20(6):743–51. doi:10.1037/0894-4105.20.6.743.
Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain. J Neurophysiol. 2007;97:3651–9.
Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17(1):18–24.
Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology. 2007;21(4):431.
Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80(6):3247–60.
Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. NeuroReport. 2004;15(14):2261–5.
Simmons W, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34(11):2944–58.
Simon D, Craig KD, Miltner WH, Rainville P. Brain responses to dynamic facial expressions of pain. Pain. 2006;126(1):309–18.
Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in cognitive sciences. 2009;13(8):334–40.
Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156(1):81–96.
Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62.
Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–9.
Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–42.
Swick D, Ashley V, Turken U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9(1):102.
Teplan M. Fundamentals of EEG measurement. Measure Sci Rev. 2002;2(2):1–11.
Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86(3):1499–503.
Tracey I. Imaging pain. Br J Anaesth. 2008;101(1):32–9.
Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–91.
Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. In: Advances in stereotactic and functional neurosurgery 9. Berlin: Springer; 1991. p. 137–9.
Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self–other discrimination. Social Cogn Affect Neurosci. 2006;1(1):65–71.
Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann B, Hebebrand J, Daum I, Wiltfang J, Kis B. Social cognition in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 2010;34(5):734–43.
Urban P, Solinski M, Best C, Rolke R, Hopf H, Dieterich M. Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle Nerve. 2004;29(5):663–9.
Vachon-Presseau E, Martel MO, Roy M, Caron E, Jackson PL, Rainville P. The multilevel organization of vicarious pain responses: effects of pain cues and empathy traits on spinal nociception and acute pain. Pain. 2011;152(7):1525–31.
Vachon-Presseau E, Roy M, Martel M-O, Albouy G, Sullivan MJ, Jackson PL, Rainville P. The two sides of pain communication: effects of pain expressiveness on vicarious brain responses revealed in chronic back pain patients. J Pain. 2013;14(11):1407–15.
Vachon-Presseau E, Roy M, Martel M, Albouy G, Chen J, Budell L, Sullivan M, Jackson P, Rainville P. Neural processing of sensory and emotional-communicative information associated with the perception of vicarious pain. Neuroimage. 2012;63(1):54–62.
Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM. Seeing the pain of others while being in pain: a laser-evoked potentials study. Neuroimage. 2008;40(3):1419–28.
Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain. 2004;109(3):399–408.
Van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc Neurosci. 2007;2(3–4):179–222.
van Veluw SJ, Chance SA. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014;8(1):24–38.
Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388–97.
Wicker B, Keysers C, Plailly J, Royet J-P, Gallese V, Rizzolatti G. Both of us disgusted in < i > My </i > insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64.
Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychol Bull. 2005;131(3):460.
Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19(5):1019–27. doi:10.1093/cercor/bhn147.
Yesudas EH, Lee T. The role of cingulate cortex in vicarious pain. BioMed Res Int. 2015.
Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–9.
Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. 2012;15(5):675–80.
Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2(3–4):276–91.
Acknowledgements
This work was supported by the May Endowed Professorship of The University of Hong Kong, the Research Grants Council Humanities and Social Sciences Prestigious Fellowship (Ref: HKU703-HSS-13). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Terrighena, E.L., Lee, T.M.C. (2017). The Neuroimaging of Vicarious Pain. In: Saba, L. (eds) Neuroimaging of Pain. Springer, Cham. https://doi.org/10.1007/978-3-319-48046-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-48046-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48044-2
Online ISBN: 978-3-319-48046-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)