Abstract
The mechanical properties of a pig’s skin which is used as the human skin substitute in the studies carried out in vitro are important for a number of applications, including surgery and biomechanics. In this study, uniaxial tensile experiments were performed on porcine skin for the two directions of the samples taken (parallel and perpendicular to the spine) to investigate the tensile stress-strain response. The experimental results show that pig’s skin exhibits anisotropic and non-linear behavior. The Ogden model was adopted to describe tensile behavior of the pig’s skin. The Ogden model provides a good tensile curve fit for the animal skin tissue in the low range of deformation (first and second stage of elongation curve).
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Intoduction
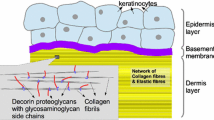
The human skin is a complex tissue which consists of several heterogeneous layers: the epidermis, the dermis, and the hypodermis. Each layer has a unique structure and function [5, 13]. Due to the legal regulations and the ethical issues, obtaining human skin for experimental tests is difficult. A literature analysis shows that substitute of human skin like animals’ skin is used in the majority of in vitro skin examinations. The similarity of anatomical features and mechanical properties makes the pig’s skin most commonly used human skin substitute [10, 16]. The mechanical examinations carried on towards recognizing skin tissue properties show that it is an anisotropic, viscoelastic and non-linear material [13]. The exact determination of skin’s mechanical properties is still an open question, and there is no one and only standard that has been established in determining skin’s behavior. Additionally, tests results are influenced by the conditions of samples taken (e.g. a direction of sample taken, a type of animal, parameters of samples storage: medium, temperature, time, humidity). There are several methods of samples storage, e.g. cooling, freezing [5].
The determination of mechanical properties of skin is essential both in engineering and medicine, in such fields like surgery and dermatology to predicting the effect of surgical treatment. The knowledge acquired in experimental tests in vivo and in vitro is used for designing surgical tools, medical robots and in criminology to estimation the history of damage formation and the nature of skin lesions due to mechanical injuries or traffic accidents [13].
Constitutive modeling of biological tissues is important in many fields such as biomechanics of collisions [4, 7, 12], rehabilitation, tissue engineering, surgical simulations [3, 9], drug delivery systems. For skin, existing hyperelastic models such as Neo-Hookean [1], Mooney-Rivlin [15], Ogden [2, 15] have been employed.
In this study, in vitro, tensile uniaxial stress versus strain response for pig’s skin tissue and the approximation of experimental results with the use of the Ogden model were presented.
2 Material and Methodology
The pieces of skin for examinations were taken from the back of a 9-month domestic pig (the weigh of animal was 135 kg). Hairs and the fat were removed from the skin using a sharp scalpel blade and then skin samples were cut in two directions: parallel and perpendicular in the reference to the spine. All samples had the same dimensions: the length 100.0 ± 0.2 mm, the measurement base for all samples was 50 ± 0.2 mm, the width 10.0 ± 0.2 mm and the average thickness was 2.2 ± 0.2 mm. The samples were stored at the temperature of \(-18\,^{\circ } \)C wrapped in polypropylene foil. The tests were carried out after 1 hour from defrosting in the temperature of 22 \(\pm \, 2\,^{\circ }\)C. The uniaxial tensile tests were carried out with the use of the MTS Insight 50 testing machine, the range of measuring head was 1 kN. The samples were mounted using flat clamps and they were elongated at the constant rate of 5 mm/min (the strain rate of 0.0017 1/s), which is commonly used for soft tissues [17]. Each set of samples for tension testing (the samples divided according to the direction of their taking) contained minimum 5 samples. In the results the average stress-strain curves were presented. Next, the Ogden model with two material constants was used to describe the tensile behavior of the pig’s skin.
3 Results of Tensile Test
The characteristic tensile curves for the parallel and the perpendicular directions of sample taking were presented in Figs. 1, 2 and 3. The elongation curves for the skin present non-linear characteristic with two quasi-linear segments. The shape of the curves allows to distinguish three phases, what is specific for soft tissues. The first phase (I) showed in Figs. 1 and 2, is known as “toe region”, in which the elastin fibres are mainly responsible for stretching mechanism, while the contribution of the collagen fibres is negligible. In the second phase (II) of stretching, the collagen fibres align themselves in the direction of applied load and become straighter, the force-extension relation becomes approximately linear. In the third phase (III), the ultimate tensile strength is reached [5, 6]. In dependence of sample direction taking, the curves had different slope and the range of maximal extension. The tensile test of skin samples was carried out to characterize the mechanical parameters of skin material, the tensile strength for the perpendicular samples was 11.87 ± 1.69 MPa, 10.65 ± 1.75 MPa for parallel samples and the strain was respectively 0.66 ± 0.05 and 0.34 ± 0.02.
4 Constitutive Model
To describe the measured constitutive response of skin tissue, the Ogden model is commonly employed [15]. The Ogden model is used to describe non-linear behavior of hyperelastic material, such as polymers, rubber and soft tissue. The model assumes that materials behavior may be characterized by a strain energy density function. On the base on this function stress-strain relationship is obtained. A strain energy density function W is given as (1):
where \( \alpha \) and \( \mu \) are material constants - shear modulus and strain hardening exponent. For incompressible material, the principal stretch ratios are described by the formula (2):
A strain energy function can be given as the function of two independent stretches with the use of formulas (1) and (2):
Under plane stress condition it is assumed \( \sigma _{3} =0\), nominal stress \( \sigma _{i} \) is described by dependence [14]:
For uniaxial elongation carried out by the load in the direction of the long axis, the nominal stress can be given as (5):
The materials constants for the Ogden model (\( \alpha \) i \( \mu \)) can be obtained experimentally. During the calculation of the Ogden model, the values of shear modulus and strain hardening exponent were set up as variables larger than zero. The procedure of calculation was carried out until the value of \( R^{2} \) (coefficient of determination) was higher than 0.99.
4.1 Results and Discussion
In Fig. 4, the tensile stress-strain curves fitted with the Ogden model at 0.0017/s strain rate were shown. The Ogden model provided a good approximation of the experimental data obtained in the tensile test for pig’s skin but only for low stretching ratio value, first and second stage of stress-stain curves (Fig. 5). This corresponds to a range of elastic deformation of elastin and collagen fibers, where initially the non-linear shape of the stress-strain curve changes the character on the similar to a straight line. For the third stage of elongation, the stress-stain curves didn’t fit with the Ogden model (for stretching ratio above 1,15 for perpendicular samples and 1,3 for parallel samples). The Ogden constants determined experimentally for two directions of samples taken were summarized in Table 1.
In our research, one value of the strain rate and variable values of shear modulus and strain hardening exponent were used. However in the literature, the noticeable influence of the strain rate on changing the value of the Ogden coefficient \( \mu \) and its negligible influence on the Ogden exponent \( \alpha \) was observed [14]. The data used by Manan et al. [11] for numerical investigation of influence of the Ogden coefficients (\( \mu \) and \( \alpha \)) on stress-stretch ratio curves showed its quasi-linear dispersion. According to [11] the low value of shear modulus will influence the stress-stretch ratio curve linearly, where by increasing the values will cause the curve to behave highly non-linear.
In the literature, elongation curves fitting by the Ogden model are shown for the elastic behavior of the material. In the research carried out by Shergold et al., the Ogden material model was used to detail the mechanical characteristics of pig’s skin under uniaxial tension [15]. The results showed that the Ogden model provides a good curve fit for the pig’s skin. A good elongation curve fit was obtained by Lim et al. for the stretch ratio of about 2,3 for specimen perpendicular to spine and 1,4 for the specimen parallel to spine [8]. The value of shear modulus for perpendicular and parallel pig’s skin samples agrees with the shear modulus reported by Shergold et al. (0,4–7,5 MPa in dependence on strain rate and constant \( \alpha =12\)). In our research, the parallel specimens have higher values of strain hardening exponent (about 40 %) and shear modulus (about 17 %) than perpendicular samples. The low strain rate shear modulus of human skin \( \mu =0.11\) MPa and \( \alpha =9\) are close to our values. The study conducted by Ni Annaidh et al., shows the value of mechanical properties for human skin: ultimate tensile stress 21,6 ± 8,4 MPa, but in the literature 1–32 MPa, failure strain 0,54 ± 0,17, but in the literature 0,17–2,07 [13]. Wide range of values depends on age, orientation/location of specimens, conditions of in vivo or in vitro tests.
5 Conclusions
An analysis of the stress versus strain response for pig’s skin tissue under quasi-static uniaxial tensile test of samples taken in two directions, parallel and perpendicular with respect to the long axis of the animal (identified by the axis of the spine) was performed. The experimental results indicate the non-linear nature of the behavior and the effect of anisotropy of skin tissue on the stress-strain characteristics. Presented results demonstrate that the Ogden model can provide a good approximation to experimental data, particularly in the range of low stretch ratio. For the good approximation of constitutive model, it is essential to obtain the values of material constants for different strain rates, particularly shear modulus which is dependent on strain rate [8, 15]. The understanding of skin behaviour under applied load is important for the production of skin substitutes, temporary and permanent. The anisotropic tensile properties are significant for surgery simulation and skin graft. Mechanical properties of pig’s skin should be investigated and compared with human data.
References
Delalleau, A., Josse, G., Lagarde, J.M., Zahouani, H., Bergheau, J.M.: A nonlinear elastic behavior to identify the mechanical parameters of human skin in vivo. Skin Res. Technol. 14(2), 152–164 (2008)
Evans, S.L.: On the implementation of a wrinkling, hyperelastic membrane model for the skin and other materials. Comput. Methods Biomech. Biomed. Eng. 12(3), 319–332 (2009)
Famaey, N., Vander Sloten, J.: Soft tissue modelling for applications in virtual surgery and surgical robotics. Comput. Methods Biomech. Biomed. Eng. 11(4), 351–366 (2008)
Forbes, P.D., Cronin, D.S., Deng, Y.C., Boismenu, M.: Numerical human model to predict side impact thoracic trauma. In: IUTAM Symposium on Impact Biomechanics: From Fundamental Insights to Applications, Dublin (2005)
Geerligs, M.: Skin layer mechanics. Ph.D. thesis, Technische Universiteit Eidhoven (2010)
Hendriks, F.M.: Mechanical behavior of human skin in vivo: a literature review. Nat. Lab. Unclassified report 820, Philips Research Laboratories (2001)
Ivancic, P.C., Pearson, A.M., Tominaga, Y., Simpson, A.K., Yue, J.J., Panjabi, M.M.: Mechanism of cervical spinal cord injury during bilateral facet dislocation. Spine 32(22), 2467–2473 (2007)
Lim, J., Hong, J., Chen, W.W., Weerasooriya, T.: Mechanical response of pig skin under dynamic tensile loading. Int. J. Impact Eng. 38, 130–135 (2011)
Lim, Y., Kim, K., Park, K.: ECG recording on a Bed Turing Steep without direct skin-contact. IEEE Trans. Biomed. Eng. 54(4), 718–725 (2007)
Liu, Z., Yeung, K.: The preconditioning and stress relaxation of skin tissue. J. Biomed. Pharmaceut. Eng. 2(1), 22–28 (2008)
Manan, N.F.A., Noor, S.N.A.M., Azmi, N.N., Mahmud, J.: Numerical investigation of Ogden and Mooney-Rivlin material parameters. ARPN J. Eng. Appl. Sci. 10(15), 6329–6335 (2015)
Muggenthaler, H., Merten, K., Peldschus, S., Holley, S., Adamec, J., Praxl, N., Graw, M.: Experimental tests for the validation of active numerical human models. Forensic Sci. Int. 177(2–3), 184–191 (2008)
Annaidh, A.N., Bruyere, K., Destrade, M., Gilchrist, M.D., Ottenio, M.: Characterizing the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 5(1), 139–148 (2012)
Ogden, R.W., Saccomandi, G., Sgura, I.: Fitting hyperelastic models to experimental data. Comput. Mech. 4(6), 11 (2004)
Shergold, O.A., Fleck, N.A., Radford, D.: The uniaxial stress versus strain response of pig skin and silicone rubber at low and high strain rates. Int. J. Impact Eng. 32, 1384–1402 (2006)
Swindle, M.M., Makin, A., Herron, A.J., Clubb, F.J., Frazier, K.S.: Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49(21), 344–356 (2012)
Żak, M., Kuropka, P., Kobielarz, M., Dudek, A., Kaleta-Kuratewicz, K., Szotek, S.: Determination of the mechanical properties of the skin of pig fetuses with respect to its structure. Acta Bioeng. Biomech. 13(2), 37–43 (2011)
Acknowledgements
The work was realized due to statutory activities M-1/6/2015/DS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Łagan, S., Liber-Kneć, A. (2017). Application of the Ogden Model to the Tensile Stress-Strain Behavior of the Pig’s Skin. In: Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E. (eds) Innovations in Biomedical Engineering. Advances in Intelligent Systems and Computing, vol 526. Springer, Cham. https://doi.org/10.1007/978-3-319-47154-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-47154-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47153-2
Online ISBN: 978-3-319-47154-9
eBook Packages: EngineeringEngineering (R0)