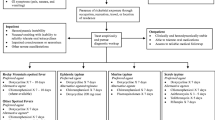
Abstract
When a rickettsiosis is suspected, prompt empiric antibiotic therapy should be initiated. Clinical recognition is key to timely treatment. Early administration of effective antibiotics can quickly abate illness and prevent severe complications. Tetracyclines are the drug class of choice for all these infections, with doxycycline being the preferred agent. Where available, chloramphenicol can be used for spotted fever group rickettsioses, typhus group rickettsioses, and scrub typhus, but the drug is not effective for infections caused by Ehrlichia spp. and Anaplasma phagocytophilum. Other alternative agents are available, but their efficacy against the different genera of this order is not as generalizable as that of tetracyclines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spotted fever group rickettsioses
- Typhus group rickettsioses
- Scrub typhus
- Anaplasmosis
- Ehrlichiosis
- Doxycycline
- Chloramphenicol
- Fluoroquinolones
- Azithromycin
- Rifampin
1 Introduction
Syndromes associated with organisms in the order Rickettsiales range from mild illness, which may fail to prompt the seeking of medical attention, to severe life-threatening infections. Infections with these agents are not limited to those with severe medical comorbidities or immunocompromised conditions; rather, anyone exposed to potential vectors (even the young, active, and healthy) is at risk for acquiring an infection. Fortunately, if the possibility of a rickettsiosis is promptly recognized, the prompt initiation of an effective antibiotic can prevent morbidity and mortality (Hamburg et al. 2008), especially in the setting of a severe disease such as Rocky Mountain spotted fever (RMSF) . Effective therapy is readily available and generally inexpensive, but many empiric antibiotic choices such as beta-lactams or sulfonamides, targeting more frequently occurring syndromes (e.g., pharyngitis, upper respiratory tract infections, urinary tract infections), have no activity against these rickettsial pathogens (Rolain et al. 1998; Branger et al. 2004). This chapter is intended to guide the reader on appropriate pharmacologic therapy for those infected with organisms in the order Rickettsiales. The following sections will outline the general principles when approaching a patient with a suspected rickettsiosis; discuss important pharmacologic properties of active antimicrobial agents; discuss specifics regarding important clinical syndromes; and finally, touch upon some important aspects regarding the treatment of children and pregnant women.
2 General Principles
As largely undifferentiated febrile illnesses with signs and symptoms mimicking many other infectious diseases, it is imperative for clinicians to be aware of the specific rickettsial agents endemic to their region of practice and to recognize the potential for acquiring these infections while traveling (Parola et al. 2013). The patient’s history in regard to occupation, recreational activities, and exposure to potential arthropod vectors and mammalian reservoirs is paramount in formulating the differential diagnosis in a patient with a fever without an obvious source. Although rash is often the sign that clues a physician to include a rickettsial illness as a consideration, the presence of a typical rash varies depending on the infecting agent. For example, rash occurs in 90 % with RMSF (R. rickettsii) (Helmick et al. 1984) versus 54 % with murine typhus (R. typhi) (Dumler et al. 1991). Rash may occur late in the course of illness (50 % after day 3 in those with RMSF) (Helmick et al. 1984). Furthermore, rash is a less frequent occurrence in human monocytotropic ehrlichiosis (HME) (Fishbein et al. 1994; Olano et al. 2003) and human granulocytotropic anaplasmosis (HGA) (Aguero-Rosenfeld et al. 1996; Bakken and Dumler 2008). Since delay of treatment can have severe consequences and portend a higher case fatality rate (Kirkland et al. 1995), clinicians should not rely on the presence of rash when considering empiric treatment. Subtle physical exam findings, such as an eschar, may be hidden and require close examination of normally clothed areas of the skin (genitalia, buttock, or axilla), but their presence may be a strong indicator of the site of bacterial inoculation. In those with darkly pigmented skin, a subtle rash may not be easily noticed, as it may be in a patient with lightly pigmented skin .
When infection with these agents is suspected, prompt empiric antimicrobial therapy should be started. Confirmatory laboratory diagnosis with serology, the mainstay of diagnostic techniques, is retrospective in nature, requiring seroconversion or fourfold increase in antibody titers from acute- and convalescent-phase serum samples. Therefore, antimicrobials should not be withheld while awaiting confirmation of diagnosis (Chapman et al. 2006).
The antibiotics used to treat these infections are relatively bioavailable by the oral route, facilitating outpatient management—provided the patient is able to tolerate oral medications and has access to appropriate follow-up. Since nausea and vomiting often accompany severe disease, admission to the hospital for initiation of parenteral antibiotics may be necessary. Other indications for hospitalization, and perhaps admission into an intensive care unit, may include the need for close clinical monitoring, management of hemodynamic instability with intravenous fluids and/or vasopressors, and other supportive measures (e.g., ventilatory management and hemodialysis). The increased vascular permeability associated with severe infections may complicate the balance of maintaining end organ perfusion through maintenance of the intravascular fluid compartment versus extravasation of fluid into the interstitial third space. For the sake of optimal ventilatory support, this may necessitate the need for Swan Ganz catheterization to monitor pulmonary capillary wedge pressure .
3 Antimicrobial Agents
Many antimicrobials frequently used for the empiric treatment of a presumed bacterial infection are ineffective for the treatment of rickettsioses (e.g., penicillins, cephalosporins, sulfonamides, and aminoglycosides) (Rolain et al. 1998; Branger et al. 2004). Sulfonamide formulations, such as trimethoprim-sulfamethoxazole, have even been associated with increased severity and poorer outcomes (Ruiz Beltran and Herrero Herrero 1992a). For the most part, treatment for these illnesses is fairly similar and lean on the tetracycline antibiotic class as the treatment of choice. For the exception of prospective studies performed in those with Mediterranean spotted fever (MSF) , most treatment recommendations are based on retrospective data supporting the efficacy of tetracyclines. Susceptibilities for antibiotics against rickettsial agents are not standardized or validated in the same manner as they are for more typical bacterial agents. The following section will highlight important aspects of antimicrobial agents with in vitro activity to clinically relevant rickettsioses. Although not meant to be as extensive in detail, nor substitute for a pharmacologic text, information will include usual dosages, potential side effects, and contraindications. When available, the MICs for drugs against these pathogens are mentioned. Following sections will touch upon caveats of therapy regarding specific disease syndromes and in special circumstances .
3.1 Tetracyclines
The tetracyclines are the treatment of choice for all agents in the order Rickettsiales. This class includes tetracycline hydrochloride, doxycycline , and minocycline . Tetracycline hydrochloride is a short half-life drug, which is given four times daily. The presence of food inhibits its absorption. As a consequence of having to take on an empty stomach, gastrointestinal side effects are fairly common. Although inexpensive and readily available throughout the world, the drug is not widely available in the United States. Doxycycline and minocycline have good bioavailability with food. In fact, the presence of food in the stomach can alleviate the potential side effects of nausea and dyspepsia. The longer half-lives of doxycycline and minocycline allow for twice daily dosing, another factor that improves adherence. These medications should be taken with ample water to ensure passage through the gastroesophageal junction and to avoid pill esophagitis (Moffa and Brook 2015).
The in vitro susceptibility of tetracyclines to spotted fever group rickettsiae (SFGR) , typhus group rickettsiae (TGR) , Orientia tsutsugamushi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum are excellent and appear better than that of antibiotics in other classes (McDade 1969). The MICs of the SFGR and TGR to tetracyclines are 0.06–0.25 μg/mL (Rolain et al. 1998); those for Orientia tsutsugamushi are 0.15–0.31 μg/mL (Raoult and Drancourt 1991); those for Ehrlichia chaffeensis are <0.5 μg/mL (Brouqui and Raoult 1992; Branger et al. 2004); and those for Anaplasma phagocytophilum are 0.03–0.25 μg/mL (Klein et al. 1997; Horowitz et al. 2001; Maurin et al. 2003; Branger et al. 2004). The standard dose of doxycycline and minocycline in adults is 100 mg oral or intravenous every 12 h. In those with severe illness, the first administration should be given as a 200 mg loading dose. Subsequent doses should then resume at the standard 100 mg every 12 h. In children weighing less than 45 kg, 1.1 mg/kg every 12 h should be given. The duration of therapy is discussed in sections pertaining to particular syndromes below.
3.2 Chloramphenicol
Chloramphenicol has long been considered the primary alternative to tetracyclines for SFGR, TGR, and scrub typhus . For the SFGR and TGR, the MICs are 0.25–2.0 μg/mL (Raoult and Drancourt 1991; Rolain et al. 1998). The MIC of chloramphenicol against O. tsutsugamushi is 1.25–2.5 μg/mL (Raoult and Drancourt 1991). Choramphenicol has poor in vitro activity against E. chaffenesis and A. phagocytophilum (Brouqui and Raoult 1992; Klein et al. 1997; Horowitz et al. 2001; Maurin et al. 2003; Branger et al. 2004). Although inexpensive and readily available in much of the world, severe adverse reactions such as aplastic anemia should temper its use when the risk to benefit ratio is not favorable. Chloramphenicol is no longer manufactured or available in its oral form in the U.S. In addition, there have been shortages of the intravenous formulation. Therefore, in the U.S., the drug is very difficult to obtain and may not be a viable treatment option when clinicians are faced with those with a severe allergery to drugs in the tetracycline class. Where available, and when drugs in the tetracycline class are absolutely contraindicated, chloramphenicol is given to adults at a dose of 500 mg every 6 h oral or intravenously. In children, the dose is 12.5 mg/kg every 6 h intravenously.
3.3 Fluoroquinolones
The fluoroquinolones are a class of relatively broad-spectrum antibiotics with in vitro activity to organisms in the genus Rickettsia. The MIC of readily available fluoroquinolones (i.e., ciprofloxacin, ofloxacin, and levofloxacin) range from 0.25 to 1.0 μg/mL to the SFGR and TGR tested (Jabarit-Aldighieri et al. 1992; Maurin and Raoult 1997; Rolain et al. 1998). Although ciprofloxacin has appeared effective against O. tsutsugamushi in vitro (Kelly et al. 1995) and in a mouse model (McClain et al. 1988), there have been clinical failures in humans treated with the antibiotics. Subsequent studies have detected mutations in the gyrA gene (target site for fluoroquinolones) of O. tsutsugamushi (Tantibhedhyangkul et al. 2010; Jang et al. 2013). The presence of this mutation in the organism’s genome confers resistance and should preclude the use of fluoroquinolones in those with scrub typhus . Although E. chaffeensis is not susceptible to fluoroquinolones, these agents have some activity against A. phagocytophilum (MIC for ofloxacin 1–2 μg/mL, ciprofloxacin 1–2 μg/mL, and levofloxacin 0.5–1 μg/mL) (Branger et al. 2004).
The fluoroquinolones have excellent oral bioavailability and are generally well-tolerated. Concomitant administration with divalent cations will inhibit the absorption of fluoroquinolones, so use of these medications in combination should be avoided. Fluoroquinolones are not recommended for routine use in children due to potential development of arthropathy as demonstrated in animal studies (Hooper and Strahilevitz 2015). Data regarding their use in SFGR and TGR are discussed in sections below. Ciprofloxacin is used at oral doses of 250–750 mg twice daily; ofloxacin is used at oral doses of 200–400 mg twice daily; and levofloxacin is used at oral doses of 250–750 mg once daily. Doses must be adjusted if there is renal impairment.
3.4 Macrolides/Ketolides
The macrolides consist of erythromycin, clarithromycin, and azithromycin . Josamycin, another macrolide, is available in other markets, but not in the U.S. These medications have in vitro activity against Rickettsia and Orientia. The oldest of these drugs, erythromycin, has MICs against SFGR and TGR of 4–8 μg/mL and 0.06–0.125 μg/mL, respectively (Raoult et al. 1988; Rolain et al. 1998). Clarithromycin and azithromycin are the newer macrolides available in the U.S. In regard to SFGR, MICs vary depending on the agent: R. rickettsii (2–8 μg/mL), R. conorii (1.0–16 μg/mL), and R. akari (0.25–2 μg/mL). The MIC of these drugs against TGR is 0.1–0.25 μg/mL (Maurin and Raoult 1993; Keysary et al. 1996; Ives et al. 1997). Macrolides are not active in vitro against E. caffeensis and A. phagocytophilum (Branger et al. 2004).
Both clarithromycin and azithromycin have excellent pharmacokinetic and pharmacodynamic properties, which result in high concentrations of the active drug in tissues and effector cells. They are safe in pregnancy. Although clarithromycin has many drug–drug interactions, azithromycin has much fewer. Use of either medication may be accompanied by gastrointestinal complaints (i.e., nausea, diarrhea, and abdominal pain), but they occur much less frequently than with erythromycin. The usual adult dose of clarithromycin is 500 mg twice daily. Azithromycin is typically started with a 500 mg loading dose on the first day, followed by 250 mg in subsequent days for indications such as community acquired pneumonia, but it has been studied using a variety of dosing schedules, including single dose regimens, depending on the infectious syndrome being treated (Sivapalasingam and Steigbigel 2015). When evidence is available, dosing schedules have been provided in the syndrome sections below.
Telithromycin is an erythromycin derivative classified in a separate antimicrobial class—the ketolides. Due to its enhanced pharmacokinetics, it is a once-daily medication. It has activity against several SFGR (R. rickettsii, R. conorii, R. africae) and TGR with in vitro MICs of 0.5–1 μg/mL (Rolain et al. 2000). Although not studied in clinical illness caused by SFGR and TGR, it has demonstrated clinical effectiveness in those with scrub typhus . Unfortunately, reports of severe liver toxicity have prompted the FDA to relabel the medication with additional warnings. For this reason, macrolide therapy seems to be a safer and more reasonable alternative.
3.5 Rifamycins
Although rifamycins are effective in vitro against a variety of SFGR (R. rickettsii, R. conorii, R. japonica, R. honei, R. sibirica, R. africae, R. parkeri, and R. slovaca) and the TGR, with MICs of 0.06–1.0 μg/mL (Rolain et al. 1998), there is no clinical data to support their effectiveness in humans. In a small study of those with Mediterranean spotted fever, a 5-day course of rifampin was associated with several treatment failures and was inferior to a 1-day course of doxycycline (Bella et al. 1991). Rifamycins are also effective against O. tsutsugamushi in susceptability studies. There is clinical data to support its use in those with scrub typhus (see below) (Watt et al. 2000). Rifampin has MICs against Ehrlichia chaffensis and Anaplamsa phagocytophilium of 0.03 μg/mL–0.125 μg/mL and 0.03 μg/mL, respectively (Brouqui and Raoult 1992; Klein et al. 1997; Horowitz et al. 2001; Maurin et al. 2003; Branger et al. 2004). Rifampin , the most available rifamycin, can be dosed at 300 mg twice daily or 600 mg daily in adults. It has excellent oral bioavailability and is well-tolerated (Maslow and Portal-Celhay 2015).
4 Antibiotic Treatment of Clinical Rickettsioses
4.1 Rocky Mountain Spotted Fever
There are no prospective randomized clinical trials comparing various antibiotic regimens for the treatment of RMSF. The excellent in vitro susceptibilities and overwhelming retrospective clinical evidence support tetracyclines as the antibiotic class of choice (Walker and Blanton 2015). As mentioned above, the ease of dosing, tolerability, and bioavailability with food favor doxycycline as the drug of choice in this class. Although the drug has excellent absorption via the oral route, RMSF is often accompanied by gastrointestinal symptoms such as nausea and vomiting, which may necessitate hospitalization and parenteral administration. When a patient with RMSF is presenting with severe or moderate illness, a 200 mg oral or intravenous loading dose of doxycycline should be given prior to continuation of 100 mg every 12 h. When parenteral therapy is used initially, it can be switched to oral therapy as soon as the patient is able to reliably tolerate oral medications. It should be noted that oral tetracyclines, including doxycycline, can induce or worsen nausea. The usual course of treatment for those with RMSF is to continue for 3–5 days after the resolution of fever. This typically results in a 7-day course.
Chloramphenicol has long been considered an alternative treatment for RMSF, but it does not seem as effective as drugs in the tetracycline class. Analysis of clinical data from confirmed and probable cases of RMSF collected by the Centers for Disease Control demonstrated a higher case fatality rate when patients were treated with chloramphenicol compared to those treated with tetracyclines (7.6 % compared to 1.5 % with an odds ratio of 5.5) (Holman et al. 2001). Although available in other parts of the Americas, where RMSF is reported, in the U.S. chloramphenicol is either not available (oral formulation) or difficult to obtain (parenteral formulation). Although fluoroquinolones and macrolides have been used successfully with other spotted fever group rickettsioses , there are no available clinical studies to support or guide the use of other antimicrobial agents for RMSF. If faced with a history of severe hypersensitivity to doxycycline , and considering the potential severity of RMSF, doxycycline desensitization should be considered. Doxycycline desensitization protocols have been published, but their initiation requires close patient monitoring in the intensive care unit (Fernando and Hudson 2013; Stollings et al. 2014). Fortunately, hypersensitivity reactions to doxycycline are infrequent.
4.2 Other Spotted Fever Group Rickettsioses
There are many spotted fever group rickettsioses distributed throughout the world. Rickettsia conorii , the agent responsible for MSF, is the second most pathogenic SFGR after R. rickettsii. Although most of the following discussion involves studies in patients with MSF, these same principles can be intuitively applied to other SFG rickettsioses. As with infection from all the other pathogens discussed in this chapter, doxycycline is the antibiotic of choice. Unlike other SFGR, prospective studies have been performed in MSF comparing various antibiotic regimens. Short courses of doxycycline therapy in those with milder forms of MSF appear effective. Adults treated with a single day of doxycycline (200 mg dosed twice on the day of treatment) had similar outcomes to patients treated with 10 days of tetracycline hydrochloride (Bella-Cueto et al. 1987). In children with MSF, continuing doxycycline for 1 day after patients became afebrile had similar outcomes to those treated with a 7-day course (Yagupsky et al. 1987). Similar to RMSF, chloramphenicol has long been considered an effective alternative, but the risks of potential adverse events must be weighed against the potential benefits. Fortunately, alternatives exist for the treatment of infection with these other less virulent spotted fever group organisms.
In patients with mild to moderate MSF, fluoroquinolones have been shown to be effective. A 7-day course of ciprofloxacin (750 mg oral twice daily) was no different than doxycycline in regard to duration of fever, but there were fewer gastrointestinal complaints in the group that took ciprofloxacin (Ruiz Beltran and Herrero Herrero 1992b). None of the patients in this study had a severe form of MSF. Another description of five patients treated with ciprofloxacin reported success in all but one. The failure occurred in a man with acquired immunodeficiency syndrome who had a very severe form of MSF (Raoult et al. 1986). Although fluoroquinolones looked to be an effective alternative in those with mild disease, there have been concerns regarding worse disease outcomes in those with MSF who have received fluoroquinolones (Botelho-Nevers et al. 2011). A proposed mechanism links the overexpression of a toxin-antitoxin system as demonstrated in cell culture experiments using R. conorii (Botelho-Nevers et al. 2012).
In those with milder forms of MSF, the newer macrolides may be an alternative in pregnant women, children, or those unable to take doxycycline . Although erythromycin has poor tolerability and has demonstrated disappointing results in those with MSF (Munoz-Espin et al. 1986), other macrolides such as clarithromycin and azithromycin appear effective for mild cases. In children, clarithromycin has been compared to chloramphenicol and has a shorter time to defervescence (Cascio et al. 2001). A 3-day course of azithromycin has been compared to a 5-day course of doxycycline in children and appears as effective (Meloni and Meloni 1996).
4.3 Typhus
The usual treatment of louse-borne epidemic typhus is doxycycline for 5–7 days. One caveat to consider is the setting in which the patient is being treated. Since typhus often occurs in large epidemics under conditions, which promote body louse infestations, short courses of mass treatment have been proposed. In these situations, a single 200 mg dose of oral doxycycline has been attempted (Perine et al. 1974; Raoult et al. 1998), but relapses have been documented (Huys et al. 1973). Therefore, such regimens should be used with great caution and should only be considered when resources are extremely limited. Chloramphenicol, where available, is an alternative therapy .
4.4 Murine Typhus
Tetracyclines are the treatment of choice for murine (endemic) typhus. Their successful use has been documented by a wealth of clinical experience that has been summarized elsewhere (Dumler 2012). Chloramphenicol is an alternative, but in a large retrospective analysis, it was associated with a longer time to defervesce when compared to doxycycline (4.0 days for chloramphenicol compared to 2.9 days for doxycycline). Murine typhus has also been successfully treated with fluoroquinolones, such as ciprofloxacin, but time to defervesce is even longer than the aforementioned antibiotics (4.2 days) (Gikas et al. 2004). It should be noted that treatment failures have also been reported with the use of fluoroquinolones (Laferl et al. 2002). The usual duration of treatment for murine typhus is 7 days.
4.5 Scrub Typhus
As with the organisms in the genus Rickettsia, there is overwhelming clinical experience supporting the use of tetracyclines for the treatment of infection with Orientia tsutsugamushi . Short courses of doxycycline have been used with some success. A single 200 mg dose of doxycycline was as effective as a 7-day course of tetracycline hydrochloride in one study (Brown et al. 1978). In another multicenter study performed in Korea, 3 days of doxycycline had similar outcomes to those treated with 7 days of tetracycline hydrochloride (Song et al. 1995). Because of reported relapses using abbreviated courses of doxycycline, duration of therapy as similar to those with RMSF should be used when possible. Chloramphenicol is an alternative treatment for scrub typhus, but it is associated with a longer febrile period and higher relapse rate as compared to patients treated with tetracyclines (Sheehy et al. 1973). In northern Thailand, where there are reports of patients who have had poor response to doxycycline (Watt et al. 1996, 1999), azithromycin has emerged as an alternative (Panpanich and Garner 2002; Fang et al. 2012). Both a single 500 mg dose of azithromycin and a 3-day dosing regimen have been shown to be as effective as a week course of doxycycline (Kim et al. 2004; Phimda et al. 2007). A 7-day course of rifampin has been studied in comparison to doxycycline with excellent results (Watt et al. 2000). As mentioned above, O. tsutsugamushi is intrinsically resistant to fluoroquinolones. They should not be used in scrub typhus (Tantibhedhyangkul et al. 2010; Jang et al. 2013).
4.6 Human Monocytotropic Ehrlichiosis
Tetracyclines are the treatment of choice for HME and infection with other Ehrlichia spp. Although no prospective clinical trials have been performed, case series show efficacy. With the use of tetracyclines, fever usually abates within 2 days of initiation (Fishbein et al. 1994). Early treatment with doxycycline has been associated with fewer complications and shorter hospital stays (Hamburg et al. 2008). Agents such as chloramphenicol, macrolides, and fluoroquinolones are not active and should not be used (Brouqui and Raoult 1992; Maurin et al. 2001; Branger et al. 2004). Although there is little published experience with rifampin in HME, its in vitro activity makes it an attractive alternative agent for HME during pregnancy .
4.7 Human Granulocytotropic Anaplasmosis
Treatment of HGA is similar to that of HME in that tetracyclines such as doxycycline are the treatment of choice. Rifampin is an alternative agent in pregnant women or children (see below). Chloramphenicol is not recommended. Although fluoroquinolones appear to have some in vitro activity, they are not recommended as a treatment option for HGA, as there is a lack of robust clinical experience and a documented case of relapse in a patient taking levofloxacin (Wormser et al. 2006).
5 Considerations in Childhood and Pregnancy
Tetracyclines are known to cause staining of permanent teeth in developing children. Therefore, many fear the use of doxycycline in children with suspected rickettsioses. Studies evaluating tooth shade in children who have taken courses of doxycycline note no appreciable change in tooth color after short courses of the drug (Grossman et al. 1971; Todd et al. 2015). Considering the morbidity and mortality associated with R. rickettsii infection, the American Academy of Pediatrics endorses the use of doxycycline in children suspected of having RMSF (“Rocky Mountain spotted fever” 2015).
Tetracyclines have a number of effects that raise concern in the developing fetus and child. Deposition of the drug in the fetal skeleton may result in a temporary inhibition of bone growth (Cohlan et al. 1963), and the drug can also cause discoloration of deciduous teeth in children whose mothers received tetracyclines (Cohlan 1977). They have also been associated with maternal hepatotoxicity and pancreatitis during pregnancy (Herbert et al. 1982). Chloramphenicol , long considered the alternative for RMSF during pregnancy, has availability issues in the United States (see above). It is also associated with gray baby syndrome , which is characterized by abdominal distention, pallor, cyanosis, and vasomotor collapse. This is a concern, as transplacental concentrations of chloramphenicol can be as high as 50 % of that in maternal blood (Ross et al. 1950). These issues must be strongly considered when faced with a case of RMSF during pregnancy. When chloramphenicol cannot be obtained, doxycycline may be the only viable treatment option.
In children and pregnant women with less severe spotted fever group rickettsioses or murine typhus, when the risk for severe morbidity and mortality is much less than that of RMSF, azithromycin seems to be a reasonable alternative based on in vitro susceptibilities and limited patient experience. Fluoroquinolones are generally contraindicated in these groups. Scrub typhus can be treated with azithromycin (a regimen backed by clinical studies), but with the limited evidence available, pregnancy and neonatal outcomes are still poor (McGready et al. 2014). Although there is very little clinical experience with rifampin use for HME and HGA, in vitro susceptibility data intuitively suggest that it would be effective. There are a few published cases of children and pregnant women who were successfully treated for HGA with rifampin (Buitrago et al. 1998; Krause et al. 2003; Dhand et al. 2007).
6 Prevention
Measures to prevent the aforementioned diseases are generally aimed at avoiding contact with potential vectors. In respect to preventing spotted fever group rickettsioses , ehrlichiosis , and anaplasmosis , protective clothing such as long sleeves, pants, and high socks should be worn to protect individuals from the bite of ticks. Treatment of clothing with permethrin is effective at reducing the number of tick bites (Miller et al. 2011; Vaughn et al. 2014). When in tick-infested areas, people should perform frequent body checks for the presence of ticks. Attached ticks should be promptly removed with forceps with care to remove the imbedded mouthparts. In areas where the brown dog tick ( Rhipicephalus sanguineus ) is responsible for spotted fever group rickettsioses , treatment of yards and dogs with ascaricides is beneficial in curbing the tick population (Drexler et al. 2014) and therefore may be helpful for the prevention of disease transmission.
In the case of typhus group rickettsioses , hygiene and vector control play a major role in disease prevention. During times of poor, overcrowded, and unhygienic conditions (e.g., mass migration, war, natural disasters), body lice may proliferate and ignite an epidemic of typhus. The washing of blankets and garments in hot water will kill lice and their eggs. If this is not possible on a large scale, the mass treatment of clothed individuals with permethrin by use of a compressed air duster is endorsed by the World Health Organization (Darby et al. 1988; “Epidemic typhus risk in Rwandan refugee camps” 1994). Murine typhus was controlled in the United States after aggressive vector control programs using dichlorodiphenyltrichloroethane affected the rat-flea population enough to break the cycle of transmission to humans (Strandtmann and Eben 1953; Pratt 1958). Measures to control rat infestations have also been beneficial in the control of murine typhus (Traub et al. 1978).
Prophylaxis for scrub typhus can be given to those who may be deployed or traveling to an endemic area. A weekly 200 mg oral dose of doxycycline is effective, but the regimen must be strictly followed, as failure to adhere will result in a loss of efficacy (Olson et al. 1980; Twartz et al. 1982). Currently, there are no available commercial vaccines for the prevention of infection caused by any of the organisms belonging to the order Rickettsiales.
7 Summary
The key to treatment of infections caused by organisms in the order Rickettsiales is clinical recognition. When a rickettsiosis is suspected, prompt empiric antibiotic therapy should be initiated. Early treatment can quickly abate illness and prevent severe complications. Tetracyclines are the drug class of choice for all these infections, with doxycycline being the preferred agent. Where available, chloramphenicol can be used for SFGR, TGR, and scrub typhus ; the drug is not effective for ehrlichiosis and anaplasmosis . Other alternative agents are available, but their efficacy against the different genera of this order is not as generalizable as that of tetracyclines.
References
Aguero-Rosenfeld ME, Horowitz HW, Wormser GP, McKenna DF, Nowakowski J, et al. (1996) Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med 125: 904-908.
Bakken JS, Dumler S (2008) Human granulocytic anaplasmosis. Infect Dis Clin North Am 22: 433-448.
Bella F, Espejo E, Uriz S, Serrano JA, Alegre MD, Tort J (1991) Randomized trial of 5-day rifampin versus 1-day doxycycline therapy for Mediterranean spotted fever. J Infect Dis 164: 433-434.
Bella-Cueto F, Font-Creus B, Segura-Porta F, Espejo-Arenas E, et al. (1987) Comparative, randomized trial of one-day doxycycline versus 10-day tetracycline therapy for Mediterranean spotted fever. J Infect Dis 155: 1056-1058.
Botelho-Nevers E, Edouard S, Leroy Q, Raoult D (2012) Deleterious effect of ciprofloxacin on Rickettsia conorii-infected cells is linked to toxin-antitoxin module up-regulation. J Antimicrob Chemother 67: 1677-1682.
Botelho-Nevers E, Rovery C, Richet H, Raoult D (2011) Analysis of risk factors for malignant Mediterranean spotted fever indicates that fluoroquinolone treatment has a deleterious effect. J Antimicrob Chemother 66: 1821-1830.
Branger S, Rolain JM, Raoult D (2004) Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother 48: 4822-4828.
Brouqui P, Raoult D (1992) In vitro antibiotic susceptibility of the newly recognized agent of ehrlichiosis in humans, Ehrlichia chaffeensis. Antimicrob Agents Chemother 36: 2799-2803.
Brown GW, Saunders JP, Singh S, Huxsoll DL, Shirai A (1978) Single dose doxycycline therapy for scrub typhus. Trans R Soc Trop Med Hyg 72: 412-416.
Buitrago MI, Ijdo JW, Rinaudo P, Simon H, Copel J, et al. (1998) Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis 27: 213-215.
Cascio A, Colomba C, Di Rosa D, Salsa L, di Martino L, Titone L (2001) Efficacy and safety of clarithromycin as treatment for Mediterranean spotted fever in children: a randomized controlled trial. Clin Infect Dis 33: 409-411.
Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, et al. (2006) Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis--United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep 55: 1-27.
Cohlan SQ (1977) Tetracycline staining of teeth. Teratology 15: 127-129.
Cohlan SQ, Bevelander G, Tiamsic T (1963) Growth inhibition of prematures receiving tetracycline. Am. J. Dis. Child 105: 453-461.
Darby WM, Boobar LR, Anderson LM (1988) An improved power duster for mass delousing of humans. J Med Entomol 25: 69-70.
Dhand A, Nadelman RB, Aguero-Rosenfeld M, Haddad FA, Stokes DP, Horowitz HW (2007) Human granulocytic anaplasmosis during pregnancy: case series and literature review. Clin Infect Dis 45: 589-593.
Drexler N, Miller M, Gerding J, Todd S, Adams L, et al. (2014) Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012-2013. PLoS One 9: e112368.
Dumler JS (2012) Clincial Disease: Current Treatment and New Challenges. In GH Palmer and AF Azad (Eds.), Intracellular Pathogens II: Rickettsiales (pp. 1-39). Washington, DC: ASM Press.
Dumler JS, Taylor JP, Walker DH (1991) Clinical and laboratory features of murine typhus in south Texas, 1980 through 1987. JAMA 266: 1365-1370.
Epidemic typhus risk in Rwandan refugee camps. (1994) Wkly Epidemiol Rec 69: 259.
Fang Y, Huang Z, Tu C, Zhang L, Ye D, Zhu BP (2012) Meta-analysis of drug treatment for scrub typhus in Asia. Intern Med 51: 2313-2320.
Fernando SL, and Hudson BJ (2013) Rapid desensitization to doxycycline. Ann Allergy Asthma Immunol 111: 73-74.
Fishbein DB, Dawson JE, Robinson LE (1994) Human ehrlichiosis in the United States, 1985 to 1990. Ann Intern Med 120: 736-743.
Gikas A, Doukakis S, Pediaditis J, Kastanakis S, Manios A, Tselentis Y (2004) Comparison of the effectiveness of five different antibiotic regimens on infection with Rickettsia typhi: therapeutic data from 87 cases. Am J Trop Med Hyg 70: 576-579.
Grossman ER, Walchek A, Freedman H (1971). Tetracyclines and permanent teeth: the relation between dose and tooth color. Pediatrics 47: 567-570.
Hamburg BJ, Storch GA, Micek ST, Kollef MH (2008) The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore) 87: 53-60.
Helmick CG, Bernard KW, D’Angelo LJ (1984) Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis 150: 480-488.
Herbert WN, Seeds JW, Koontz WL, Cefalo RC (1982) Rocky Mountain spotted fever in pregnancy: differential diagnosis and treatment. South Med J 75: 1063-1066.
Holman RC, Paddock CD, Curns AT, Krebs JW, McQuiston JH, Childs JE (2001) Analysis of risk factors for fatal Rocky Mountain Spotted Fever: evidence for superiority of tetracyclines for therapy. J Infect Dis 184: 1437-1444.
Hooper DC, Strahilevitz J (2015) Quinolones. In JE Bennett, R Dolin, MJ Blaser (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (8th ed., Vol. 1, pp. 419-439). Philidelphia: Elseveir Saunders.
Horowitz HW, Hsieh TC, Aguero-Rosenfeld ME, Kalantarpour F, Chowdhury I, et al. (2001) Antimicrobial susceptibility of Ehrlichia phagocytophila. Antimicrob Agents Chemother: 786-788.
Huys J, Kayhigi J, Freyens P, Berghe GV (1973) Single-dose treatment of epidemic typhus with doxycycline. Chemotherapy 18: 314-317.
Ives TJ, Manzewitsch P, Regnery RL, Butts JD, Kebede M (1997) In vitro susceptibilities of Bartonella henselae, B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R. akari, and R. prowazekii to macrolide antibiotics as determined by immunofluorescent-antibody analysis of infected Vero cell monolayers. Antimicrob Agents Chemother 41: 578-582.
Jabarit-Aldighieri N, Torres H, Raoult D (1992) Susceptibility of Rickettsia conorii, R. rickettsii, and Coxiella burnetii to PD 127,391, PD 131,628, pefloxacin, ofloxacin, and ciprofloxacin. Antimicrob Agents Chemother 36: 2529-2532.
Jang HC, Choi SM, Jang MO, Ahn JH, Kim UJ, et al. (2013) Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci 28: 667-671.
Kelly DJ, Salata KF, Strickman D, Hershey JN (1995) Rickettsia tsutsugamushi infection in cell culture: antibiotic susceptibility determined by flow cytometry. Am J Trop Med Hyg 53: 602-606.
Keysary A, Itzhaki A, Rubinstein E, Oron C, Keren G (1996) The in-vitro anti-rickettsial activity of macrolides. J Antimicrob Chemother 38: 727-731.
Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S (2004) A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis 39: 1329-1335.
Kirkland KB, Wilkinson WE, Sexton DJ (1995) Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis 20: 1118-1121.
Klein MB, Nelson CM, Goodman JL (1997) Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrob Agents Chemother 41: 76-79.
Krause PJ, Corrow CL, Bakken JS (2003) Successful treatment of human granulocytic ehrlichiosis in children using rifampin. Pediatrics 112: e252-253.
Laferl H, Fournier PE, Seiberl G, Pichler, H, Raoult D (2002) Murine typhus poorly responsive to ciprofloxacin: a case report. J Travel Med, 9: 103-104.
Maslow MJ, Portal-Celhay C (2015) Rifamycins. In JE Bennett, R Dolin, MJ Blaser (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Vol. 1, pp. 339-349). Philidelphia: Elseveir Saunders.
Maurin M, Abergel C, Raoult D (2001) DNA gyrase-mediated natural resistance to fluoroquinolones in Ehrlichia spp. Antimicrob Agents Chemother 45: 2098-2105.
Maurin M, Bakken JS, Dumler JS (2003) Antibiotic susceptibilities of Anaplasma (Ehrlichia) phagocytophilum strains from various geographic areas in the United States. Antimicrob Agents Chemother 47: 413-415.
Maurin M, Raoult, D (1993) In vitro susceptibilities of spotted fever group rickettsiae and Coxiella burnetti to clarithromycin. Antimicrob Agents Chemother 37: 2633-2637.
Maurin M, Raoult D (1997) Bacteriostatic and bactericidal activity of levofloxacin against Rickettsia rickettsii, Rickettsia conorii, ‘Israeli spotted fever group rickettsia’ and Coxiella burnetii. J Antimicrob Chemother 39: 725-730.
McClain JB, Joshi B, Rice R (1988) Chloramphenicol, gentamicin, and ciprofloxacin against murine scrub typhus. Antimicrob Agents Chemother 32: 285-286.
McDade JE (1969) Determination of antibiotic susceptibility of Rickettsia by the plaque assay technique. Appl Microbiol 18: 133-135.
McGready R, Prakash JA, Benjamin SJ, Watthanaworawit W, Anantatat T, et al. (2014) Pregnancy outcome in relation to treatment of murine typhus and scrub typhus infection: a fever cohort and a case series analysis. PLoS Negl Trop Dis 8: e3327.
Meloni G, Meloni T (1996) Azithromycin vs. doxycycline for Mediterranean spotted fever. Pediatr Infect Dis J 15: 1042-1044.
Miller NJ, Rainone EE, Dyer MC, Gonzalez ML, Mather TN (2011) Tick bite protection with permethrin-treated summer-weight clothing. J Med Entomol 48: 327-333.
Moffa M, Brook I (2015) Tetracyclines, Glycyclines, and Chloramphenicol. In JE Bennett, R Dolin, MJ Blaser (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (8th ed., Vol. 1, pp. 322-338). Philidelphia: Elsevier Saunders.
Munoz-Espin T, Lopez-Pares P, Espejo-Arenas E, Font-Creus B, Martinez-Vila I, et al. (1986) Erythromycin versus tetracycline for treatment of Mediterranean spotted fever. Arch Dis Child 61: 1027-1029.
Olano JP, Masters E, Hogrefe W, Walker DH (2003) Human monocytotropic ehrlichiosis, Missouri. Emerg Infect Dis 9: 1579-1586.
Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT (1980) Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg 29: 989-997.
Panpanich R, Garner P (2002) Antibiotics for treating scrub typhus. Cochrane Database Syst Rev (3) CD002150.
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, et al. (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657-702.
Perine PL, Krause DW, Awoke S, McDade JE (1974) Single-dose doxycycline treatment of louse-borne relapsing fever and epidemic typhus. Lancet 2: 742-744.
Phimda K, Hoontrakul S, Suttinont C, Chareonwat S, Losuwanaluk K, et al. (2007) Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother 51: 3259-3263.
Pratt HD (1958) The changing picture of murine typhus in the United States. Ann N Y Acad Sci 70: 516-527.
Raoult D, Drancourt M (1991) Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother 35: 2457-2462.
Raoult D, Gallais H, De Micco P, Casanova P (1986) Ciprofloxacin therapy for Mediterranean spotted fever. Antimicrob Agents Chemother 30: 606-607.
Raoult D, Ndihokubwayo JB, Tissot-Dupont H, Roux V, Faugere B, et al. (1998) Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 352: 353-358.
Raoult D, Roussellier P, Tamalet J (1988) In vitro evaluation of josamycin, spiramycin, and erythromycin against Rickettsia rickettsii and R. conorii. Antimicrob Agents Chemother 32: 255-256.
Rocky Mountain spotted fever (2015) In DW Kimberlin, MT Brady, MA Jackson, SS Long (Eds.), Red Book: 2015 Report of the Committee on Infectious Diseases (30th ed., pp. 682-684). Elk Grove Village, IL: American Acadamy of Pediatrics.
Rolain JM, Maurin M, Bryskier A, Raoult D (2000) In vitro activities of telithromycin (HMR 3647) against Rickettsia rickettsii, Rickettsia conorii, Rickettsia africae, Rickettsia typhi, Rickettsia prowazekii, Coxiella burnetii, Bartonella henselae, Bartonella quintana, Bartonella bacilliformis, and Ehrlichia chaffeensis. Antimicrob Agents Chemother 44: 1391-1393
Rolain JM, Maurin M, Vestris G, Raoult D (1998) In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother 42: 1537-1541.
Ross S, Burke FG, Sites J, Rice EC, Washington JA (1950) Placental transmission of chloramphenicol (chloromycetin). J Am Med Assoc 142: 1361.
Ruiz Beltran R, Herrero Herrero JI (1992) Deleterious effect of trimethoprim-sulfamethoxazole in Mediterranean spotted fever. Antimicrob Agents Chemother 36: 1342-1343.
Ruiz Beltran R, Herrero Herrero JI (1992) Evaluation of ciprofloxacin and doxycycline in the treatment of Mediterranean spotted fever. Eur J Clin Microbiol Infect Dis 11: 427-431.
Sheehy TW, Hazlett D, Turk RE (1973) Scrub typhus. A comparison of chloramphenicol and tetracycline in its treatment. Arch Intern Med 132: 77-80.
Sivapalasingam S, Steigbigel NH (2015) Macrolides, clindamycin, and ketolides. In J. E. Bennett, R. Dolin & M. J. Blaser (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Vol. 1, pp. 358-376). Philidelphia: Elseveir Saunders.
Song JH, Lee C, Chang WH, Choi SW, Choi JE, Kim Y, et al. (1995) Short-course doxycycline treatment versus conventional tetracycline therapy for scrub typhus: a multicenter randomized trial. Clin Infect Dis 21: 506-510.
Stollings JL, Chadha SN, Paul AM, Shaver CM, Hagaman D (2014) Doxycycline desensitization for a suspected case of ehrlichiosis. J Allergy Clin Immunol Pract 2: 103-104.
Strandtmann RW, Eben D J (1953) A survey of typhus in rats and rat ectoparasites in Galveston, Texas. Tex Rep Biol Med 11: 144-151.
Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, et al. (2010). Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents 35: 338-341.
Todd SR, Dahlgren FS, Traeger MS, Beltran-Aguilar ED, Marianos DW, et al. (2015) No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr 166: 1246-1251.
Traub R, Wisseman CL, Farhang-Azad A (1978) The ecology of murine typhus-a critical review. Trop Dis Bull 75: 237-317.
Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, Groves MG (1982) Doxycycline propylaxis for human scrub typhus. J Infect Dis 146: 811-818.
Vaughn MF, Funkhouser SW, Lin FC, Fine J, Juliano JJ, et al. (2014) Long-lasting permethrin impregnated uniforms: A randomized-controlled trial for tick bite prevention. Am J Prev Med 46:473-480.
Walker DH, Blanton LS (2015) Rickettsia rickettsii and Other Spotted Fever Group Rickettsiae (Rocky Mountain Spotted Fever and Other Spotted Fevers). In JE Bennett, R Dolin, MJ Blaser (Eds.), Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases (Vol. 2, pp. 2198-2205). Philadelphia: Elsevier Saunders.
Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, et al. (1996) Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet 348: 86-89.
Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D (1999) Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in Northern Thailand. Antimicrob Agents Chemother 43: 2817-2818.
Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D (2000) Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet 356: 1057-1061.
Wormser GP, Filozov A, Telford SR, Utpat S, Kamer RS, et al. (2006). Dissociation between inhibition and killing by levofloxacin in human granulocytic anaplasmosis. Vector Borne Zoonotic Dis 6: 388-394.
Yagupsky P, Gross EM, Alkan M, Bearman JE (1987) Comparison of two dosage schedules of doxycycline in children with rickettsial spotted fever. J Infect Dis 155: 1215-1219.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Blanton, L.S. (2016). Rickettsiales: Treatment and Management of Human Disease. In: Thomas, S. (eds) Rickettsiales. Springer, Cham. https://doi.org/10.1007/978-3-319-46859-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-46859-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46857-0
Online ISBN: 978-3-319-46859-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)