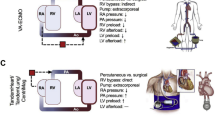
Abstract
Heart failure is a leading cause of death in developed countries, despite significant progress in medical management. Heart transplantation still remains the best therapy option for patients with end-stage heart failure, but it is limited by increased shortage of donor organs. Ventricular assist devices (VADs) with their variety (univentricular of biventricular support; extracorporeal or implantable; rotary or pulsatile) have become established surgical therapy for end-stage heart failure over the last decade. In patients with pronounced left ventricular failure, a left ventricular assist device support, where blood is bypassed from the left ventricular apex to the ascending aorta or descending aorta, is clinically efficient. When patients with advanced heart failure involving both ventricles become refractory to optimal medical therapy, biventricular mechanical circulatory support is required using biventricular VAD or a total artificial heart. According to INTERMACS data, overall survival in patients supported with implantable rotary blood pumps is approximately 80 % at 1 year and 70 % at 2 years. Current evidence suggests that rotary VAD support for prolonged duration is safe and effective. The incidence of complications (infection, stroke, bleeding, pump thrombosis, device failure) has decreased over the years. However, with longer time on support, many forms of interaction between the implanted device and the body have been recognized. The management of these interactions and complications during support remains challenging, but it is feasible, giving patients supported with a VAD the opportunity to return home and resume their normal lives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- End-stage heart failure
- Implantable ventricular assist devices
- Total artificial heart
- Patient–device interaction
1 Background
Advances in medical treatment and the use of implantable defibrillators and cardiac resynchronization therapy have significantly improved the outcome of patients with advanced heart failure (Slaughter et al. 2009).
However, the number of patients who become refractory to these forms of treatment is constantly growing. In the case of acute deterioration and onset of cardiogenic shock, despite optimal medical and device therapy, short-term circulatory support with temporary support devices has been shown to be a valuable tool for cardiopulmonary stabilization and bridging to the next therapy step: heart transplantation or implantation of a long-term device, with the possibility of myocardial recovery.
Heart transplantation is still the gold standard for the treatment of end-stage heart failure. Hence, the acute mismatch between the number of donor organs available and the number of patients on the waiting list has increased the demand for mechanical circulatory support for bridging to heart transplantation or transplant eligibility and long-term support.
The clinical appearance of advanced heart failure varies. Most patients suffer from systolic heart failure resulting in continuously decreasing systolic function mainly of the left ventricle. A significant proportion of patients develop diastolic heart failure, which results in an increase in filling pressure and a decrease in stroke volume.
In patients with isolated left ventricular dysfunction, a left ventricular assist device (LVAD) support, where blood is bypassed from the left ventricular apex to the ascending aorta or descending aorta, is usually sufficient. Prior to implantation, it must be ensured that right ventricular function is not severely impaired. In case of maintained right ventricular function, a left ventricular mechanical circulatory support device can deliver the whole volume that is reaching the left atrium to the systemic circulation. Mean blood pressure remains between 60 and 80 mmHg, with the pump producing flow of 4–7 l/min (full support pumps) or 2–4 l/min (partial support pumps). Pulsatile flow devices produce artificial ejection, mimicking the physiological heart cycle; hence, they work asynchronously with fixed stroke rate. The smallest pulsatile pump has a 10-ml blood chamber, and adult pulsatile pumps produce an artificial stroke volume of up to 80 ml.
Continuous flow VADs bypass the heart and pump blood volume independently of the heart cycle, producing a continuous flow profile in the aorta. In this case, there is no or little native ejection through the aortic valve (depending on the residual LV function), and the aortic valve leaflets may remain closed. Therefore, no pulse wave is present. Ultrasound devices or invasive methods are used for the assessment of mean arterial pressure. This kind of “unphysiological” pulseless circulation does not negatively affect end-organ function, cognitive function, or the microcirculation (Petrucci et al. 2012; Potapov et al. 2012), but may impair the structure of the aortic valve leaflets, and sometimes lead to aortic insufficiency during longer assist periods (Aggarwal et al. 2013).
Patients who present some contractility of the LV following LV unloading and/or myocardial recovery eject blood through the aortic valve. This additional cardiac output contributes little to the whole body perfusion, but it leads to an adequate blood wash-out phenomenon at the aortic root and diminishes the risk of thrombus formation in the valve area.
Early onset or late right ventricular failure in patients with an LVAD may lead to decreased pump output due to diminished transpulmonary flow and to low pump flow with subsequent hypoperfusion of the end organs and, ultimately, multiorgan failure. To avoid this dramatic complication, appropriate device selection and implanting strategy are crucial (Potapov et al. 2008a). Once the right ventricle is failing, temporary right ventricular support with short-term devices may be necessary as a bridge to RV recovery or to a permanent RVAD. If temporary RV dysfunction is expected, temporary RVAD support is started at the time of LVAD implantation and progressively weaned over weeks according to RV recovery.
When patients that present with advanced heart failure become refractory to therapy, with the involvement of both ventricles, biventricular mechanical circulatory support is required using biventricular VAD (BVAD) or a total artificial heart (TAH). BVAD support may be given with implantable pumps, using two continuous flow LVAD with one adapted for RV support, or with two extracorporeal pneumatic pumps connected to the major vessels and heart chambers by polyurethane cannulas (Berlin Heart Excor, Berlin Heart, Berlin, Germany; Thoratec, Thoratec Corporation, Pleasanton, CA, USA).
The total artificial heart is a pulsatile system containing two artificial ventricles with four valves: mechanical valves as in the temporary CardioWest total artificial heart TAH-t, Syncardia, Tucson, AZ, USA, or the Abiomed device, Abiomed Inc., Danvers, MA, USA; or biological valves as in the CARMAT TAH, CARMAT SA, France. There are some reports of the use of two adapted continuous flow LVAD connected to patients’ atria and major vessels used as a continuous-flow TAH.
2 Devices
2.1 Left Ventricular Devices (Extracorporeal, Implantable)
In the current era, over 90 % of the LVADs are implantable continuous-flow pumps.
These consist of the pump body with rotor and pump housing, inflow cannula (integrated or connected), outflow graft/cannula, driveline, and driver or controller with energy source.
-
Inflow cannula:
-
(a)
Material: titanium, sintered (HeartWare HVAD, M-VAD, HeartMate II, HeartMate III, Jarvik 2000), or polished (DuraHeart), complex (silicon/titanium, Incor), polyurethane cannula penetrating the skin (Berlin Heart Excor);
-
(b)
Insertion site: LV apex, LA in special cases (Excor, Abiomed, or Thoratec, e.g. in patients with restrictive cardiomyopathy);
-
(c)
Fixation to LV myocardium/sewing ring with interrupted or continuous felt-pledgeted sutures;
-
(d)
Optimal orientation toward the mitral valve, without contact to the LV wall.
Different devices have shown different kinds of integration into the internal surface of the LV chamber with “neo-intima” formation (Fig. 3.1).
-
(a)
-
Pump body:
-
(a)
Material: titanium (all implantable devices), polyurethane (Excor);
-
(b)
Blood propulsion: volume displacement or rotary pumping using impeller;
-
(c)
Generated flow profile: centrifugal, axial, radial, pulsatile (pneumatic, electromechanic);
-
(d)
Bearings: ruby (HM II), hydrodynamic/passive magnetic (HVAD, HM III), electromechanical (Jarvik 2000), electromagnetic (INCOR, DuraHeart, HM III);
-
(e)
Implant position: intrapericardial (HVAD, Jarvik 2000, HM III, INCOR, HeartAssist 5), requiring pump pocket within abdominal wall (HM II, DuraHeart) or extracorporeal (Excor) (Fig. 3.2).
-
(a)
-
Outflow cannula:
-
(a)
Dacron graft (HVAD, HM II, HM III, Jarvik 2000, HeartAssist 5, new version of Incor), silicon cannula (old version of Incor), polyurethane cannula penetrating the skin (Excor);
-
(b)
Kinking protection/strain relief (HM II, HVAD);
-
(c)
Anastomosis with ascending aorta or descending aorta using running or interrupted prolene sutures.
-
(a)
-
Driveline:
-
(a)
Subcutaneous tunneling (oblique or C-shaped) with abdominal fascia perforation in the epigastral region, neck tunneling with retroauricular pedestal fixation at temporal bone (Jarvik 2000)
-
(b)
Exit site: Right or left lateral abdominal quadrant. It is preferable to avoid crossing the linea alba (later need for abdominal surgery, interaction with chest tubes/infection)
-
(c)
Tissue integration: Dacron velour covered part, silicon, titanium pedestal (Jarvik 2000) (Fig. 3.3)
Fig. 3.3 Driveline incorporation at exit site. (a) HM II; (b) HeartWare LVAD; (c) Jarvik 2000 (postaurical pedestal); (d) HeartWare BVAD; (e) Excor (chronic irritation and local infection of LV-apex cannula, other three with good incorporation signs); (f) Incor (local infection with abscess formation along driveline)
The driveline is connected to a digital controller, lithium batteries (implantable LVADs) or to a pneumatic driver (Excor, Thoratec, CardioWest).
-
(a)
-
Controller and batteries:
-
(a)
Air compressor (Excor, CardioWest), polyurethane tubing connecting to pump chamber, manual pump for emergencies.
-
(b)
The controller runs the pump and also provides text messages and audible alarms to aid system operation management.
-
(c)
Lithium ion batteries, cable connection of the power unit to electricity from a wall or car outlet (Fig. 3.4).
-
(a)
-
Implantation techniques:
-
(a)
Using cardiopulmonary bypass or extracorporeal life support through a full sternotomy (HM II, HM III, HeartWare HVAD and MVAD, DuraHeart, Incor, Jarvik 2000, HeartAssist 5). After conventional full sternotomy and systemic heparinization, cannulation of the right atrium, using a two-stage cannula, and of the ascending aorta is performed. Cardiopulmonary bypass (CPB) with normothermia is initiated. The LV apex is exposed. The apical fixation tool is usually attached with 10–14 mattress sutures of 3–0 felt-reinforced polypropylene positioned around the apex. During a short period of induced ventricular fibrillation, a full-thickness cruciate incision inside the sewing ring is made using a special coring tool. At this time, the driveline is pulled from the pericardial space below the costal arch into the subcutaneous abdominal tissue and brought through the skin in the lower abdominal quadrant with a subcutaneous route of at least 10 cm. The aorta is partially clamped, and the distal anastomosis of the outflow graft and the ascending aorta is accomplished using a continuous polypropylene suture. Final de-airing of the outflow graft is done before the distal anastomosis is completed.
-
(b)
Minimally invasive (bilateral thoracotomy/partial sternotomy), CPB standby. The patient is placed in supine position and intubated with a double lumen tube. The first incision is made in the third right intercostal space close to the sternum, the aorta is partially clamped in its lateral portion, and anastomosis with the graft is performed. Optionally, an upper partial sternotomy may be used to approach the ascending aorta; the second incision is performed in the fifth or sixth left intercostal space, depending on the position of the LV apex. The fixation ring is attached to the apex, and the graft is passed intrapericardially under the sternum to the LV apex and connected to the pump. The driveline is tunneled out of the skin and connected to the controller. The LV apex is cored and the pump placed into the LV during continuous de-airing, fixed, and started. The procedure is done on the beating heart. If necessary, CPB can be used employing femorofemoral cannulation (Potapov and Krabatsch 2014; Hetzer et al. 2004; Cheung et al. 2011) (Fig. 3.5).
-
(c)
Implantation technique through left lateral thoracotomy after previous cardiac operations. In patients who have had previous cardiac operations via a median sternotomy severe adhesions may complicate intraoperative dissection. Additionally, open bypasses may cross the midline and be at increased risk for intraoperative damage. In such cases, approaching the left ventricle and descending aorta through a median sternotomy avoids these complications. In selected cases, the implantation may be performed with CPB on standby. After the patient is placed in the right lateral decubitus position with the left hip rolled back, the groin vessels are prepared and a left lateral thoracotomy is performed in the fifth intercostal space. The left lung is deflated, and the pulmonary ligament is transected up to the hilum. Placement of the sewing ring, pump insertion, and driveline tunneling are performed as described in (b). The proximal part of the descending aorta is partially excluded using a Satinsky clamp. A short longitudinal incision is made, and the distal anastomosis of the outflow graft and the descending thoracic aorta is accomplished using a continuous polypropylene suture. Tunneling of the driveline, final de-airing, and starting the LVAD are as described above (Hetzer et al. 2004) (Fig. 3.6).
-
(a)
2.2 Biventricular Devices (Extracorporeal, Implantable)
Up to 10 % of patients with end-stage heart failure experience biventricular failure that requires biventricular support. For these patients, only two long-term options are available: the total artificial heart (CardioWest, SynCardia, Tucson, AZ), which requires that the native heart be excised, and the biventricular assist device (BVAD), which uses either bulky extracorporeal or implantable displacement pumps (Krabatsch et al. 2011).
Due to improved patient selection, better understanding of right ventricular function, and early referral (preferably before RV failure), the number of individuals who require biventricular support has decreased in recent years (Kirklin et al. 2014). However, there are still patients in whom isolated LVAD support is insufficient and who are in need of biventricular support.
2.2.1 Pulsatile Extracorporeal BVAD (Berlin Heart Excor)
Since the first clinical implementation of the Berlin Heart Excor device in Berlin, Germany, in the late 1980s, thousands of adults and also children have been treated with it as a bridge to heart transplantation, recovery, or destination therapy all over the world (Potapov et al. 2008b). In the recent past, this system has been most widely used in the pediatric setting.
After conventional full sternotomy and systemic heparinization, the ascending aorta and right atrium are cannulated. Cardiopulmonary bypass with normothermia is initiated. First, the left ventricular cannula is inserted. The incision is made on the left ventricular apex in the coronary-free area, and the left ventricular cannula is inserted and secured by previously placed mattress sutures supported with Teflon pledgets. Next, a side-biting clamp is placed on the ascending cannula proximal to the previously inserted aortic cannula for CPB, the aorta is incised above the clamp, and the VAD aortic cannula is fixed with continuous or mattress sutures. Afterward, the cannulas are tunneled through the skin via tight-fitting skin incisions and the pump, filled with physiological solution, is connected to the cannulas. Residual air bubbles are evacuated through a de-airing nipple that is integrated into the blood chamber, assist circulation is begun, and CPB is stopped (Hetzer et al. 2006) (Fig. 3.7).
2.2.2 Continuous-Flow Implantable BVAD
Since the introduction of implantable rotary blood pumps into clinical practice, there have been many attempts to adapt these pumps, once developed as left ventricular assist devices, for support of the failing right ventricle. With minor modifications of the peripheral equipment that address the lower resistance of the pulmonary circulation and anatomical issues, rotary blood-pump technology may provide an alternative in destination therapy for biventricular failure (Krabatsch et al. 2011; Kirklin et al. 2014; Strueber et al. 2010; Hetzer et al. 2010). The implantation is performed on CPB. The technique for LVAD implantation is similar to that described above; the RVAD may by connected to the diaphragmatic part or to the free wall of the right ventricle. One of the attractive sites for insertion of the inflow cannula of the implantable RVAD is the right atrium. In this case, the pump is placed outside of the pericardium into the right pleural space. The outflow graft is connected to the pulmonary artery (Fig. 3.8).
(a) The narrowing of the right ventricular assist device (RVAD) outflow graft is produced before surgery by suturing 3 cm of the graft with 6-0 Prolene 2 cm from the outflow connector over a 5-mm Hegar dilator introduced into the graft. (b) Intraoperative view shows both pumps: 1, outflow graft of the left ventricular assist device (LVAD) connected to the ascending artery; 2, outflow graft of the RVAD connected to the pulmonary artery; 3, pump of the RVAD connected to the free wall of the right ventricle; 4, the pump of the LVAD is connected to the LV apex and is not shown (Hetzer 2010). (c) X-ray of two HVAD implanted as BVAD
2.3 Total Artificial Heart
In patients who have severe biventricular failure, or if conventional connection to the heart chambers is challenging due to technical, anatomical, or pathological issues, the total artificial heart (TAH) is one of the options. Indications for its use are irreversible biventricular failure, irreversible cardiac rejection, massive myocardial infarction with or without ventricular septal defect, and massive cardiac thrombosis.
Unlike the previously described devices, a pulsatile total artificial heart is implanted into the chest in orthotopic position after the diseased heart has been explanted leaving cuffs of both atria. Both artificial ventricles are connected to patients’ atria, and the major vessels produce one-way pulsatile flow (four mechanical valves). This allows the filling pressures to be kept low and the pump flow high, up to 8.4 L/min. Both drivelines are tunneled out of the skin in the epigastric region and connected to the pneumatic driver (Fig. 3.9). The only commercially available pulsatile TAH is the CardioWest-t (Copeland et al. 2004).
3 Survival, Long-Term Effects, and Complications
According to INTERMACS data, overall survival in patients supported with implantable rotary blood pumps is approximately 80 % at 1 year and 70 % at 2 years. Regarding biventricular support, recipients of implantable continuous flow BVAD had a better survival rate at 1 year than those with pulsatile systems: 57 % vs 45 %, respectively. Fifty-nine percent of recipients of the CardioWest-t total artificial heart survived at 1 year.
Since permanent support with ventricular assist devices has become a reality, an increasing number of patients are being supported for several years. Implantation of a VAD as destination therapy or for long-term support has almost doubled during the past 6 years and now represents 43 % of all implants (Kirklin et al. 2014).
3.1 Thrombo-embolic and Bleeding Complications
Ventricular assist devices affect the coagulation system. All pumps come into contact with blood, and through surface activation of the clotting cascade promote thrombosis and thromboembolic events. Freedom from any thrombembolic event at 1 year is currently 85–92 % (Slaughter et al. 2009; Najjar et al. 2014). Strict anticoagulation is therefore necessary, and may in turn cause bleeding complications.
Flow stagnation around the VAD cannula or diseased myocardium, exposure to foreign surfaces (VAD cannulas and pump body), and changes in coagulation balance (over-anticoagulation, onset of acquired von Willebrand factor disease, platelet deficiency, heparin-induced thrombocytopenia (HIT), and hypercoagulable blood status) are the most important factors that disturb coagulation, with the clinical consequence of bleeding or thromboembolism (Stepanenko et al. 2014).
The incidence of bleeding of any cause (requiring red blood cell transfusion or necessitating surgery) with different types of VAD ranges between 0.16 and 2.45 events per patient per year, while the incidence of thromboembolic events is 0.05–0.28 events per patient per year (Potapov et al. 2011).
3.2 Pump Malfunction
Pump malfunctions due to driveline damage or thrombosis are the most important pump-related complications and, if not treated immediately, are potentially fatal. Pump exchange is a valuable option, although patients with multiple pump exchanges carry a progressive increase in mid-term mortality. Some patients with pump thrombosis may be treated with fibrinolysis if pump exchange is not possible (Najjar et al. 2014). Exceptions are extracorporeal devices, where the exchange procedure is simple by clamping of the outflow cannulas, exchange of blood chambers, de-airing, and pump restart. This is performed without a general anesthetic and at the bedside.
3.3 Effects of Pulseless Continuous-Flow Circulation (Aortic Valve, End-Organs, Blood Components)
Several studies have shown that during long-term support with continuous-flow blood pumps, structural alterations of the aortic valve occur. The exact mechanisms and histopathological findings have been described elsewhere (Aggarwal et al. 2013). Briefly, new onset or late progression of preexisting aortic valve regurgitation with or without a commissural fusion phenomenon significantly diminishes the clinical success of VAD therapy. Some VAD recipients require surgical treatment (conventional replacement or AV closure, transcathether valve replacement, etc.) in the later follow-up (Dranishnikov et al. 2012; Robertson et al. 2015).
Under physiological conditions, the blood flow is pulsatile from the arterial tree up to the capillary bed. The role of pulsatility in the proper function of the human body is still unclear. The discussion started with the implementation of cardiopulmonary bypass, which was nonpulsatile and came into clinical practice 60 years ago and still continues. On the basis of experience with more than 5000 patients supported with long-term CF devices in the past 13 years, it is clear that continuous blood flow seems not to have disadvantages regarding neurocognitive function, end-organ recovery, and long-term function (Potapov et al. 2012; Slaughter 2010).
Severe hemolysis is a rare complication following VAD implantation. Postoperative hemolysis may be caused by the pump design (high rotor speed operation in continuous flow pumps, artificial surface properties, mechanical bearings, high shear stress, use of mechanical valves in pulsatile devices) or by postoperative complications (hypercoagulability status, onset of heparin-induced thrombocytopenia (HIT) II, malpositioning of the apical cannula, kinking of outflow grafts, pump thrombosis).
3.4 Exit Site Driveline or Cannula-Related Infection
Infection is a common problem of LVAD therapy. Drivelines or device infections are reported with incidence of 0.25–0.33 events per patient per year (John et al. 2014). The driveline tunneling procedure during implantation (C-shaped, subcutaneous, intra-abdominal), externalization techniques, and exit site driveline or cannula incorporation later play a large role as a source for postoperative morbidity and mortality in the case of the onset of local infection with potential generalization and sepsis (Stepanenko et al. 2010).
Excor cannulas are covered with Dacron velour and present acceptable incorporation if the cannula position is relatively stable, without chronic moving-related irritation (Fig. 3.3e)
Drivelines of modern implantable LVADs allow good incorporation and provide long-term exit site infection-free support (Fig. 3.3a–d).
During long-term support in some cases, there is an onset of local infection at the exit site that can ascend to the mediastinum along the driveline tunnel (Fig. 3.3f). Antibiotics and local antiseptics, together with driveline or cannula immobilization, are of great value in avoiding this complication. In the case of uncontrolled ascending infection, local vacuum-assisted closure treatment, driveline relocation, or listing for heart transplantation are possible solutions.
Conclusion
VADs with their variety (univentricular of biventricular support; extracorporeal or implantable; rotary or pulsatile) have become established surgical therapy for end-stage heart failure over the last decade.
With enormous progress made in VAD technology, the incidence of complications has decreased over the years. However, with longer time on support, many forms of interaction between the implanted pump and the body have been recognized. The management of these interactions and complications during support remains challenging, but it is feasible, giving patients supported with a VAD the opportunity to return home and resume their normal lives.
Key Points
-
VADs with their variety (univentricular of biventricular support; extracorporeal or implantable; rotary or pulsatile) have become established surgical therapy for end-stage heart failure.
-
All devices are connected to the heart and great vessels with the aid to bypass or replace failed pumping ventricle and provide antegrade blood flow with adequate body perfusion.
-
End-organ function is preserved and mild dysfunction is reversed in a large number of patients who have been supported for many years.
-
Device- and treatment-related effects (driveline exit site, altered blood flow patterns, and need for anticoagulation) together with patient factors (right ventricular function, age, sex, comorbidities) may be major drivers of adverse outcomes.
-
The rapid progress in VAD technology in recent years has improved survival and quality of life for patients with advanced heart failure.
References
Aggarwal A, Raghuvir R, Eryazici P, Macaluso G, Sharma P, Blair C, et al. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. Ann Thorac Surg. 2013;95(2):493–8.
Cheung A, Lamarche Y, Kaan A, Munt B, Doyle A, Bashir J, et al. Off-pump implantation of the HeartWare HVAD left ventricular assist device through minimally invasive incisions. Ann Thorac Surg. 2011;91(4):1294–6.
Copeland JG, Smith RG, Arabia FA, Nolan PE, Sethi GK, Tsau PH, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351(9):859–67.
Dranishnikov N, Stepanenko A, Potapov EV, Dandel M, Siniawski H, Mladenow A, et al. Simultaneous aortic valve replacement in left ventricular assist device recipients: single-center experience. Int J Artif Organs. 2012;35(7):489–94.
Hetzer R, Potapov EV, Weng Y, Sinawski H, Knollmann F, Komoda T, et al. Implantation of MicroMed DeBakey VAD through left thoracotomy after previous median sternotomy operations. Ann Thorac Surg. 2004;77(1):347–50.
Hetzer R, Alexi-Meskishvili V, Weng Y, Hubler M, Potapov E, Drews T, et al. Mechanical cardiac support in the young with the Berlin Heart EXCOR pulsatile ventricular assist device: 15 years’ experience. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:99–108.
Hetzer R, Krabatsch T, Stepanenko A, Hennig E, Potapov EV. Long-term biventricular support with the heartware implantable continuous flow pump. J Heart Lung Transplant. 2010;29(7):822–4.
John R, Aaronson KD, Pae WE, Acker MA, Hathaway DR, Najarian KB, et al. Drive-line infections and sepsis in patients receiving the HVAD system as a left ventricular assist device. J Heart Lung Transplant. 2014;33(10):1066–73.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64.
Krabatsch T, Potapov E, Stepanenko A, Schweiger M, Kukucka M, Huebler M, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation. 2011;124(11 Suppl):S179–86.
Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34.
Petrucci RJ, Rogers JG, Blue L, Gallagher C, Russell SD, Dordunoo D, et al. Neurocognitive function in destination therapy patients receiving continuous-flow vs pulsatile-flow left ventricular assist device support. J Heart Lung Transplant. 2012;31(1):27–36.
Potapov EV, Krabatsch T. Minimally invasive continuous-flow left ventricular assist device implantation: Avoiding a median sternotomy. J Heart Lung Transplant. 2014;33(11):1199–200.
Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y, et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant. 2008a;27(12):1275–81.
Potapov EV, Loforte A, Weng Y, Jurmann M, Pasic M, Drews T, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008b;23(3):185–94.
Potapov EV, Stepanenko A, Krabatsch T, Hetzer R. Managing long-term complications of left ventricular assist device therapy. Curr Opin Cardiol. 2011;26(3):237–44.
Potapov EV, Dranishnikov N, Morawietz L, Stepanenko A, Rezai S, Blechschmidt C, et al. Arterial wall histology in chronic pulsatile-flow and continuous-flow device circulatory support. J Heart Lung Transplant. 2012;31(11):1171–6.
Robertson JO, Naftel DC, Myers SL, Prasad S, Mertz GD, Itoh A, et al. Concomitant aortic valve procedures in patients undergoing implantation of continuous-flow left ventricular assist devices: an INTERMACS database analysis. J Heart Lung Transplant. 2015;34(6):797–805.
Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010;25(4):490–4.
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–51.
Stepanenko A, Pasic M, Potapov EV, Weng Y, Krabatsch T, Hetzer R. Accidental intraperitoneal tunneling of driveline of left ventricular assist device. Ann Thorac Surg. 2010;90(5):1690–1.
Stepanenko A, Potapov EV, Weng Y, Pasic M, Krabatsch T, Hetzer R. Anticoagulation assessment. Ann Cardiothorac Surg. 2014;3(5):538–40.
Strueber M, Meyer AL, Malehsa D, Haverich A. Successful use of the HeartWare HVAD rotary blood pump for biventricular support. J Thorac Cardiovasc Surg. 2010;140(4):936–7.
Acknowledgments
The authors thank Anne Gale, Editor in Life Sciences, for editorial assistance, and Ewald Hennig, PhD, and Friedrich Kaufmann, Dipl. Eng. for their technical support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stepanenko, A., Potapov, E., Falk, V., Krabatsch, T. (2016). Mechanical Circulatory Support in End-Stage Heart Failure: Bridge to Transplantation and Destination Therapy. In: Leone, O., Angelini, A., Bruneval, P., Potena, L. (eds) The Pathology of Cardiac Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-46386-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-46386-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46384-1
Online ISBN: 978-3-319-46386-5
eBook Packages: MedicineMedicine (R0)