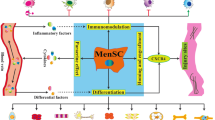
Abstract
Accumulating evidence has demonstrated that menstrual blood stands as a viable source of stem cells. Menstrual blood-derived stem cells (MenSCs) are morphologically and functionally similar to cells directly extracted from the endometrium, and present dual expression of mesenchymal and embryonic cell markers, thus becoming interesting tools for regenerative medicine. Functional reports show higher proliferative and self-renewal capacities than bone marrow-derived stem cells, as well as successful differentiation into hepatocyte-like cells, glial-like cells, endometrial stroma-like cells, among others. Moreover, menstrual blood stem cells may be used with increased efficiency in reprogramming techniques for induced Pluripotent Stem cell (iPS) generation. Experimental studies have shown successful treatment of stroke, colitis, limb ischemia, coronary disease, Duchenne’s muscular atrophy and streptozotocin-induced type 1 diabetes animal models with MenSCs. As we envision an off-the-shelf product for cell therapy, cryopreserved MenSCs appear as a feasible clinical product. Clinical applications, although still very limited, have great potential and ongoing studies should be disclosed in the near future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endometrium
- Regenerative medicine
- Endometrial stem cells
- Menstrual blood stem cells
- Pluripotent stem cells
- Immunomodulation
- Stem cell therapy
9.1 Introduction
In the past decades, several cell types from different sources have been investigated as therapeutic tools in experimental, translational and clinical research. Stem cells have been especially explored for their cytoprotective, regenerative and immunomodulatory capacities [1]. Adult, rather than embryonic stem cells , or those obtained by iPS-generating techniques, have been currently preferred in studies that aim clinical translation, despite their more restricted differentiation potential. Indeed, teratogenicity and ethical concerns, high costs and low efficiency stand as obstacles still to be surpassed before the more immature stem cells are enrolled in clinical trials .
Cells derived from bone marrow and cord-blood have been the focus of the initial studies on cell therapy , due to the already established experience with bone marrow transplantation . Later, easier access sources began to be examined, mainly in disposable tissues such as the placenta, amniotic fluid and membrane, umbilical cord tissue , dental pulp, and adipose tissue , among others [2–5]. Of particular relevance are the mesenchymal cells, which have been more extensively studied and hold good in vivo repairing potential. Mesenchymal cells do not spontaneously express class II major histocompatibility complex (MHC) molecules, what confers them low immunogenicity and ability to be tolerated by the host immune system [1]. Therefore, they have been tested as tools for treatment of autoimmune, inflammatory and degenerative diseases.
Successive studies have reported differences in mesenchymal cells from different sources and how they may be more or less suitable for treatment of specific disorders according to their tissue of origin [6–8]. In this scenario, menstrual blood has recently been investigated as source of stem cells for therapeutic purposes, the in vitro and preclinical studies revealing great potential, perhaps overcoming that of cells from other sources. This review addresses current and already established knowledge about in vitro and in vivo research on menstrual blood-derived stem cells, with emphasis on morphological and functional characteristics. Also discussed are the future perspectives of such cells, especially concerning clinical applications .
9.2 Endometrium and Menstrual Blood-Derived Stem Cells
More than 30 years ago, stem cells were first described to be present in the endometrium [9]. The intense tissue remodeling observed during menses and pregnancy mirrors the endometrial potential to renew itself, effect that has been ascribed to local stem cell activity [10]. In addition, endometrial cells from patients with endometriosis are remarkably able to migrate and graft to distantly located tissues [11]. In the endometrium, epithelial cells can be found in the surface epithelium as well as within the tubular glands, which anatomically continue to the interface with the myometrium.
The remaining tissue consists of stromal cells, smooth muscle cells, endothelial cells and leukocytes [12]. The functionalis, which can be considered the upper layer of the endometrium, contains glands that are held together loosely by stromal tissue. The basalis contains dense stroma, basal regions of the glands, supportive vasculature and lymphoid aggregates [13]. The functionalis is eliminated monthly during menstruation, while the remaining, basalis, is able to renew the endometrium, under hormonal influence. At each cycle, progenitor cells migrate from the basalis, proliferate, and regenerate the functionalis. In post-menopause women, progenitor cells can be found in the basalis, as a possible explanation to menstrual cycle reactivation through hormonal replacement [14].
The endometrium contains three types of stem cells: the epithelial progenitor cells, mesenchymal cells and endothelial progenitor cells [11]. Although multipotent stem cells may be isolated and cultured from endometrial biopsies and menstrual blood, it has been proposed that epithelial progenitor cells cannot be obtained from menstrual blood and most likely reside in the basalis layer [15, 16]. In fact, epithelial progenitors can be found in the endometrial basalis in post-menopause women, indicating a possible source of stem cells when menstrual blood is not any more available [14].
In 2004, Chan et al. isolated epithelial progenitors and stromal cells derived from the endometrium [17]. In the laboratory, both the isolated epithelial progenitors and the stromal cells were cloned and amplified, but phenotypic markers within the epithelial cells were lost and a feeder layer was needed over cell passages. Later, similar cells were isolated from menstrual blood [18–20] and named endometrial regenerative cells (ERCs). These cells differentiated into tissues from the three germ layers, indicating their in vitro multipotentiality. In 2008, Patel et al. isolated stem cells from menstrual blood (MenSCs) , showing in vitro clonogenic properties as well as the differentiation of tissues derived from mesoderm and ectoderm [21]. They also demonstrated markers of pluripotency, such as Oct-4, SSEA-4 and c-kit, which were expressed by the MenSCs and are usually found in immature cell types, such as embryonic stem cells . Furthermore, telomerase activity and human telomerase reverse transcriptase (hTERT) expression in those cells resembled that of embryonic stem cells [21, 22]. In a subpopulation of the MenSCs, unexpectedly high clonogenic and proliferative potential was confirmed, besides expression of pluripotency markers (e.g., SSEA-4) [20]. Also, MenSCs were efficiently and stably gene modified by retroviral transfection, indicating that these cells could become suitable tools for gene delivery. Finally, levels of matrix metalloproteases (MMPs) in MenSCs are several fold above those presented by mesenchymal cells derived from cord blood, indicating a specific ability for remodeling [18].
Studies have investigated the in vitro potential of the MenSC cell fate, in particular demonstrating the stem cells derived from menstrual blood differentiated into functional hepatocyte-like cells, expressing hepatic surface markers (e.g., albumin, cytokeratin-18 and alpha fetoprotein) and genes [23, 24]. Menstrual blood-derived cells were also differentiated into nucleus pulposus-like cells in a co-culture system, the final product confirmed by phenotypic and gene expression evaluations [25]. Similarly, menstrual blood progenitors were differentiated into glial-like cells [26]. Moreover, MenSCs have higher capability to be differentiated towards cardiomyocytes than bone marrow-derived cells and in vivo differentiation of menstrual blood cells into cardiomyocytes has been reported [27, 28]. Finally, chondrogenic differentiation of MenSCs seeded on a nanofibrous scaffold, strongly reactive to anti-collagen II antibodies, has been reported [29]. Menstrual blood-derived stem cells may also be used with increased efficiency in reprogramming techniques for iPS generation [30, 31].
Although numerous studies have been published on MenSC properties in the past years, some questions still remain unanswered. Since the initial investigations, it has been shown that more than one single cell population may be isolated from either endometrial tissue or menstrual blood, and the multiple available reports do not clearly indicate which is the most important cell type for regenerative purposes. Cervello et al. [32] isolated epithelial and stromal-cell enriched side populations through flow cytometry of Hoechst- stained endometrium cells [32]. In vitro, the cells were characterized and showed high proliferative potentials, especially when they were exposed to an environment containing hypoxic conditions, similar to that of the endometrial environment. However, when these cells were administered to immunodeficient mice, limited proliferation and differentiation were observed, demonstrating a divergence of in vitro and in vivo effects. Masuda et al. (2010) implanted similar cells in female mice under the kidney capsule, and observed human tissue development in some of the animals under estrogen stimulation [33]. Side-population cells successfully differentiated into glandular epithelial, stromal and, for the first time, endothelial cells, with presence of CD31 and human vimetin co-expressing in small and medium sized vessels. However, although they were detectable, the in vivo differentiation capacity was considered poor. For better proliferative results, cells were combined with the endometrial cells of the remaining population (i.e., main population.). Combined with reported data from literature, these findings suggest that the endometrium has multiple factors that can cooperate for therapeutic properties of this tissue, rather than from a single cell type. In fact, it has been previously proposed that even the endometrial niche itself may contribute to the quality of cell activity [11].
The angiogenic potential of the endometrium-derived cells is also pertinent for vascular growth and remodeling investigation. Disorders such as stroke, cardiac ischemia and chronic vascular insufficiency, among others, may stand as perfect targets for these cells. In fact, successful results have been achieved by preclinical studies on myocardial infarct, limb ischemia, and stroke [25, 34, 35]. Angiogenic effects are also proposed in the treatment of severe skin burns, perhaps associating cells to intelligent artificial films [34, 36].
Another question concerns the classification criteria for menstrual blood-derived stem cells. Murphy et al. [34] speculate that the ERCs they isolated share properties that overlap and may even be considered equivalent to cells reported by other studies, but may not be the same endometrial stromal cells described by Taylor, in 2004 [18, 21, 37, 38]. The ERCs show low concentrations of the STRO-1 mesenchymal cell marker, differentiate into a wider range of tissues and exhibit higher proliferative capacity than cord blood-derived cells [18]. When compared with bone marrow-derived mesenchymal cells, ERCs also presented higher lymphocyte proliferative inhibitory potential, and different gene expression profiles, which were mostly towards inflammatory and immune pathways in the ERCs, while stem cell and cancer signaling in the bone marrow-derived mesenchymal cells [39]. Taylor postulates that the stromal cells observed within the endometrium are derived from the bone marrow, as observed in female recipients of allogeneic bone marrow transplantation that have donor cells detected in tissues from endometrial biopsies. These findings were later duplicated in female rats, which presented bone marrow-derived GFP-positive cells in the endometrium long after transplantation [40]. In practical matters, however, although endometrial-derived cells are grouped through different phenotypical, differentiation and proliferative criteria, when applied in vivo they have similar effects and comparable therapeutic abilities to advance repair .
9.3 Experimental Applications of Stem Cells Derived from the Endometrium
Progenitor cells derived directly from the endometrium or indirectly, from menstrual blood, have been investigated for their in vivo immunomodulatory and restorative effects (see Table 9.1). In an aggressive glioma mouse model, ERCs injected either by intravenous or intratumoral routes successfully reduced tumor size in almost 50 % [43]. Among possible mechanisms, the authors considered secretion of inhibitory factors by the injected cells, selective angiogenesis in favor of benign and against malignant tissue, and cell-tumor interaction stimulating the differentiation of the tumoral tissue.
Borlongan et al. evaluated the effects of menstrual blood-derived cell transplantation in experimental stroke [35]. Stromal-like menstrual blood cells were selected for CD117 or C-kit receptors by isolating and expanding the stem cells. Those cells selected indicate a high probability of proliferation , migration and survival [44]. The embryonic-like stem cells expressed in vitro phenotypic markers, such as Oct4, SSeA and Nanog. Up to nine passages of cultures were maintained, which provided evidence of the safety and reliability of those cells, and part of the cells were induced to express neural markers (MAP2 and Nestin).
When menstrual blood cells were added to cultured rat neurons that had been exposed to a hypoxic insult, the menstrual blood cells exerted neuroprotective effects. Behavioral tests were administered and stroke animals that received menstrual blood cells displayed less neurological deficits, irrespective of the injection site, i.e. systemic or local administration into the striatum.
Analyses of the central nervous tissue in the transplanted stroke rats revealed that the grafted cells did not exhibit markers of cell lineage differentiation. Results showed that the human cells , although detected and some migrating to areas other than the injected, expressed their original stemness markers. Altogether, these results indicate that cell differentiation was not the main therapeutic operating mechanism and that other pathways may be involved in the observed functional recovery.
In addition, vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 were detected as possible neuroprotective trophic factors released by ERCs. These results may be supported by a recent in vitro study reporting that menstrual blood-derived stem cells express the neuronal marker β-III-tubulin and endogenous BDNF in higher levels than bone marrow and adipose tissue -derived cells [45].
In a different CNS disorder context, emphasizing more restorative than neuroprotective effects, Wolff et al. reported the use of endometrial-derived cells in a Parkinson’s disease immunocompetent mouse model [37]. In vitro differentiated dopamine-producing cells expressing neural markers nestin and tyrosine hydroxylase were injected into the brains of the animals. Labeled cells were detected in the brains up to 5 weeks after administered. Therapeutic potential was recognized in vivo when the migration, differentiation and production of dopamine were detected. These cells have the potential to functionally restore the damaged tissues, either through cell replacement or endogenous repair.
An experimental colitis mouse model improved after treatment with intravenous injections of human menstrual blood-derived ERCs [46]. Despite short follow-up of 14 days, treated animals presented decreased disease activity scores, reduced inflammatory cytokine levels and higher regulatory cell frequencies, indicating a systemic beneficial effect of cell infusions. In addition, histological evaluations evidenced significant modulation of the inflammatory infiltrate.
Streptozotocin-induced diabetes mice were treated with one single intravenous injection of human menstrual blood-derived stem cells [47]. Treated mice presented longer survival and better glycemic control when compared with untreated diabetic mice. In accordance with previous reports, MenSCs presented high proliferation , short doubling time, and increased expression of the immature cell marker Oct-4. Although migration of labeled MenSCs to the pancreata and islet beta-cell regeneration were detected, there were no evidence of cell transdifferentiation into pancreatic tissue, suggesting that paracrine mechanisms prevailed.
Menstrual blood-derived cells were also tested in a rat coronary ligation model [28]. In this study, menstrual blood-derived mesenchymal cells were co-cultured with fetal cardiomyocytes separated by an athelocollagen membrane (no cell-cell contact), and in vitro differentiated into beating cardiac precursor-like cells. Subsequently, non-differentiated menstrual blood-derived mesenchymal cells were injected into and marginal to the ischemic area of the heart, 2 weeks after coronary artery ligation. Clinical and histological effects were compared to bone marrow mesenchymal cell and culture medium or fibroblast injections. Animals treated with MenSCs presented higher heart function recovery rates, as well as smaller infarcted areas, than those from the other treatment groups. High in situ cardiomyogenic transdifferentiation was also observed. Also investigating the vessel-regenerating potential of the ERCs, Murphy et al. injected MenSCs into the hind-limb muscle just distal to the level of artery ligation, in a chronic limb ischemia rat model [34]. Multiple cell injections were administered shortly after the vascular obstruction – immediately, 2 and 4 days after ligation – and showed a protective effect, preventing limb necrosis and amputation. Finally, MenSC differentiated in vitro into hepatocyte-like cells were injected intrasplenically and restored liver function, normalizing albumin and transaminase levels [24].
9.4 The Advantages and Disadvantages of Cryopreservation of Menstrual Blood-Derived Stem Cells
Cryopreservation can be utilized to preserve previously expanded stem cells for future use, as shown in Fig. 9.1. MenSCs can be cryopreserved for extended period of time. There are both advantages and disadvantages of cryopreservation of stem cells. These pros and cons will eventually determine if the MenSCs should be cryopreserved. Table 9.2 summarizes the advantages and disadvantages of cryopreservation.
The first advantage of cryopreservation is that the stem cells can be stored for extended period of time. Hence, it can be readily available for expansion and use with relatively short period of time. This is extremely important for acute time sensitive disorders such as stroke and traumatic brain injury. Many preclinical studies have demonstrated that the earlier the injection of stem cells, the better the recovery is. Therefore, cryopreserved stem cells can shorten the waiting period so that they can be used within the critical therapeutic period. In addition, cryopreservation of stem cells allows autologous transplant. In clinical settings, autologous transplant is always more preferable than heterologous transplant. Another advantage of cryopreserved stem cells is that the quality of the stem cells can be ensured. The stem cells can be phenotyped, karyotyped and scanned for quality and any abnormality prior to transplantation. These tests can ensure the integrity of the stem cells for transplantation. Finally, cryopreservation of stem cells allows them to become ‘off the shelf’ products. These cells can be distributed to many small hospitals and clinics nationwide and even worldwide instead of concentrated in small number of major facilities, which have the equipment and the experience to harvest and process the stem cells.
Despite many advantages of cryopreserved stem cells, there are also disadvantages that are worth taken into consideration. Cryopreservation involves freezing and thawing processes of the stem cells. This raises the concern of the cells’ viability since many cells may not survive the processes. One of the solutions for this issue is to store a large numbers of cells, which in turn raises the cost associated with stem cell treatment. Another concern with cryopreservation of stem cells is that the function of the stem cells might change because of the freezing and thawing processes. Since freezing and thawing are forms of stress to the cells, this might change the properties of the stem cells. Further investigations might help to elucidate the effects of cryopreservation on the stem cells. Finally, the cost of processing and storing of the stem cells are high. This might create a bioethical issue that the majority of the population would not be able to afford the treatment and only a small niche of wealthy people could have access to this type of therapy. It is important for both scientists and clinicians to consider these issues so they can be addressed.
9.5 Clinical Applications of Menstrual Blood-Derived Stem Cells
Only in the last decade has the potential of menstrual blood-derived stem cells in regenerative medicine began to be explored. Therefore, very few clinical studies are available, most of them still ongoing (see Table 9.3). The only clinical study yet published evaluated safety aspects of endometrial-derived stromal cell administration in patients with multiple sclerosis [48]. Four patients were treated with intrathecal injections of 16–30 million cells and one of the patients also received an additional intravenous injection of the stem cells. No adverse events were registered, in accordance with the pre-clinical studies, and functional stabilization was reported. However, due to the nature of multiple sclerosis and the worsening progression of the illness, any conclusions about the effectiveness of the treatment could be considered premature due to the longest follow-up reaching only 12 months .
Ongoing trials aim to evaluate the effect of MenSC injections in several clinical conditions. Menstrual blood-derived stem cells have been tested in congestive heart failure patients, as a follow-up of the preclinical study published by Hida et al. [28, 49]. This double-blind, randomized phase II study intends to include 60 patients, divided into placebo and treatment groups. Allogeneic MenSCs (Medistem Inc, California, USA) are injected through a minimally invasive and previously described retrograde delivery technique [50]. The last communication, in 2013, reported that 17 patients had been enrolled, with safety being so far analyzed and confirmed [49].
In another trial, female liver cirrhosis patients receive intravenous injections of MenSCs twice a week, for 2 weeks [51]. Patients will be followed for 2 years and evaluated for safety and efficacy of the cell therapy upon liver function and progression of the cirrhosis. Another trial evaluates efficacy of MenSC intravenous injections in patients with acute lung injury due to H7N9 bird flu infections [52]. Effects of the cells upon lung injury are the main outcomes.
A phase I/II clinical trial investigating the effect of escalating doses of ERCs on limb ischemia has also been launched in 2012, aiming to enroll 15 patients with critical limb ischemia not eligible for invasive interventions [53]. Data on the study status, however, are not available. Effects of ERCs on type 1 diabetes are also under investigation in a phase I/II open label trial launched in 2012 [54]. In this study, multiple doses of ERCs are injected through the pancreatic artery or intravenously. Adverse events and effects on glucose metabolism and pancreatic beta cell function will be evaluated. Updated status of the study is also not available. Finally, the effects of ERCs on embryo implantation in the uterine cavity during in vitro fertilization will be investigated [55].
9.6 Conclusions
Menstrual blood-derived cells have great potential in regenerative medicine [56]. Ease of access, availability and safety are considered their main key to future clinical studies. In vitro investigations have shown the superiority of MenSC in proliferation and differentiation assays, as well as immunomodulatory potential, when compared with more traditionally used sources such as the bone marrow and cord blood. Moreover, preclinical studies have successfully shown their potential to modulate inflammation, prevent neuronal death and possibly increase angiogenesis, in vivo. Importantly, cell fusion may be a therapeutic mechanism in muscle and cardiac tissue injuries. These effects may be result of the more immature behavior of these cells, which although bringing them closer to embryonic stem cells , still retain a safe profile. Although clinical studies have already been started, further experimental studies are still required, as several questions about the full therapeutic potential and specific mechanisms of action persist. In particular, cryopreservation of MenSCs while feasible, may require further validation of the frozen-and-thawed stem cells, as well the cost-limiting ethical concerns that may be associated with such cell banking approach. These investigations may help to explore benefits of such cells and expand applications. Clinical practice bears specific challenges , such as the low yield and difficulty in expansion of ample supply of stem cells from this source, mainly low replication rate and risk of contamination. Additionally, stem cells derived from menstrual blood would only be applicable to the pre-menopausal female population, thereby limiting the target patient population when contemplating autologous menstrual blood cell therapy . A feasible solution would be to educate the female pre-menopausal population about the potential of the menstrual cells and, therefore, encourage this young adult population towards the anticipated harvesting and cryopreservation of the cells, for future autologous use. When such pre-menopausal collection and banking is not pursued in time, the use of stem cells derived from a post-menopausal endometrium may be a solution, though more invasive. For the male population, there remains the alternative of using allogeneic cells and of searching for alternative autologous sources.
Abbreviations
- MenSCs:
-
Menstrual blood-derived stem cells
- iPS:
-
Induced Pluripotent Stem cell
- MHC:
-
Major histocompatibility complex
- ERCs:
-
Endometrial regenerative cells
- hTERT:
-
Human telomerase reverse transcriptase
- MMPs:
-
Matrix metalloproteases
- VEGF:
-
Vascular endothelial growth factor
- BDNF:
-
Brain-derived neurotrophic factor
- DOPAC:
-
Dopamine and dihydroxyphenylacetic acid
References
Rammal H, Harmouch C, Lataillade JJ, Laurent-Maquin D, Labrude P, Menu P, Kerdjoudj H (2014) Stem cells: a promising source for vascular regenerative medicine. Stem Cells Dev 23:2931–2949
Hass R, Kasper C, Bohm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Brooke G, Rossetti T, Pelekanos R, Ilic N, Murray P, Hancock S, Antonenas V, Huang G, Gottlieb D, Bradstock K, Atkinson K (2009) Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol 144:571–579
Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM (2014) Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One 9, e115963
Duan B, Hockaday LA, Das S, Xu CY, Butcher JT (2015) Comparison of mesenchymal stem cell source differentiation towards human pediatric aortic valve interstitial cells within 3D engineered matrices. Tissue Eng Part C Methods 21(8):795–807
Lotfy A, Salama M, Zahran F, Jones E, Badawy A, Sobh M (2014) Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells 7:135–142
Prianishnikov VA (1978) A functional model of the structure of the epithelium of normal, hyperplastic and malignant human endometrium: a review. Gynecol Oncol 6:420–428
Gargett CE (2007) Uterine stem cells: what is the evidence? Hum Reprod Update 13:87–101
Gargett CE, Masuda H (2010) Adult stem cells in the endometrium. Mol Hum Reprod 16:818–834
Padykula HA (1991) Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci 622:47–56
Spencer TE, Hayashi K, Hu J, Carpenter KD (2005) Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 68:85–122
Nguyen HP, Sprung CN, Gargett CE (2012) Differential expression of Wnt signaling molecules between pre- and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinology 153:2870–2883
Musina R, Belyavski A, Tarusova O, Solovyova E, Sukhikh G (2008) Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull Exp Biol Med 145:539–543
Verdi J, Tan A, Shoae-Hassani A, Seifalian AM (2014) Endometrial stem cells in regenerative medicine. J Biol Eng 8:20
Chan RW, Schwab KE, Gargett CE (2004) Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 70:1738–1750
Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B, Riordan NH (2007) Endometrial regenerative cells: a novel stem cell population. J Transl Med 5:57
Van der Molen RG, Schutten JH, van Cranenbroek B, ter Meer M, Donckers J, Scholten RR, van der Heijden OW, Spaanderman ME, Joosten I (2014) Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum Reprod 29:303–314
Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C, Conte P, Paolucci P, Horwiz EM, Dominici M (2013) Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013:901821
Patel AN, Silva F (2008) Menstrual blood stromal cells: the potential for regenerative medicine. Regen Med 3:443–444
Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, Umezawa A (2007) Menstrual blood-derived cells confer human dystrophin expression in the murinemodel of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell 18:1586–1594
Khanjani S, Khanmohammadi M, Zarnani AH, Akhondi MM, Ahani A, Ghaempanah Z, Naderi MM, Eghtesad S, Kazemnejad S (2014) Comparative evaluation of differentiation potential of menstrual blood- versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS One 9, e86075
Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, Li CY, Ma JM, Xiang C (2013) Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B 14:961–972
Hu X, Zhou Y, Zheng X, Tian N, Xu C, Wu W, Li F, Zhu S, Zheng Y, Xue E, Yu Y, Zhang X, Xu H (2014) Differentiation of menstrual blood-derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine 39:754–760
Azedi F, Kazemnejad S, Zarnani AH, Behzadi G, Vasei M, Khanmohammadi M, Khanjani S, Edalatkhah H, Lakpour N (2014) Differentiation potential of menstrual blood- versus bone marrow-stem cells into glial-like cells. Cell Biol Int 38:615–624
Rahimi M, Zarnani AH, Mohseni-Kouchesfehani H, Soltanghoraei H, Akhondi MM, Kazemnejad S (2014) Comparative evaluation of cardiac markers in differentiated cells from menstrual blood and bone marrow-derived stem cells in vitro. Mol Biotechnol 56:1151–1162
Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, Kiyono T, Kyo S, Shimizu T, Okano T, Sakamoto M, Ogawa S, Umezawa A (2008) Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 26:1695–1704
Kazemnejad S, Zarnani AH, Khanmohammadi M, Mobini S (2013) Chondrogenic differentiation of menstrual blood-derived stem cells on nanofibrous scaffolds. Methods Mol Biol 1058:149–169
De Carvalho Rodrigues D, Asensi KD, Vairo L, Azevedo-Pereira RL, Silva R, Rondinelli E, Goldenberg RC, Campos de Carvalho AC, Urményi TP (2012) Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transplant 21:2215–2224
Li Y, Li X, Zhao H, Feng R, Zhang X, Tai D, An G, Wen J, Tan J (2013) Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev 22:1147–1158
Cervelló I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardó F, Higueras G, Sanz MA, Pellicer A, Simón C (2012) Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One 7, e30260
Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, Ito M, Yoshimura Y, Maruyama T, Okano H (2010) Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One 5, e10387
Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE, Riordan NH (2008) Allogeneic endometrial regenerative cells: an “Off the shelf solution” for critical limb ischemia? J Transl Med 6:45
Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR (2010) Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 19:439–452
Drago H, Marin GH, Sturla F, Roque G, Mártire K, Díaz Aquino V, Lamonega R, Gardiner C, Ichim T, Riordan N, Raimondi JC, Bossi S, Samadikuchaksaraei A, van Leeuwen M, Tau JM, Núñez L, Larsen G, Spretz R, Mansilla E (2010) The next generation of burns treatment: intelligent films and matrix, controlled enzymatic debridement, and adult stem cells. Transplant Proc 42:345–349
Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS (2011) Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell Mol Med 15:747–755
Taylor HS (2004) Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 292:81–85
Wang H, Jin P, Sabatino M, Ren J, Civini S, Bogin V, Ichim TE, Stroncek DF (2012) Comparison of endometrial regenerative cells and bone marrow stromal cells. J Transl Med 10:207
Bratincsák A, Brownstein MJ, Cassiani-Ingoni R, Pastorino S, Szalayova I, Tóth ZE, Key S, Németh K, Pickel J, Mezey E (2007) CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells 25:2820–2826
Peron JP, Jazedje T, Brandão WN, Perin PM, Maluf M, Evangelista LP, Halpern S, Nisenbaum MG, Czeresnia CE, Zatz M, Câmara NO, Rizzo LV (2012) Human endometrial-derived mesenchymal stem cells suppress inflammation in the central nervous system of EAE mice. Stem Cell Rev 8:940–952
Ulrich D, Edwards SL, Su K, Tan KS, White JF, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE (2014) Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A 20:785–798
Han X, Meng X, Yin Z, Rogers A, Zhong J, Rillema P, Jackson JA, Ichim TE, Minev B, Carrier E, Patel AN, Murphy MP, Min WP, Riordan NH (2009) Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle 8:606–610
Cho NH, Park YK, Kim YT, Yang H, Kim SK (2004) Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril 81:403–407
Zemel’ko VI, Kozhukharova IV, Kovaleva ZV, Domnina AP, Pugovkina NA, Fridlianskaia II, Puzanov MV, Anisimov SV, Grinchuk TM, Nikol’skiĭ NN (2014) BDNF secretion in human mesenchymal stem cells isolated from bone marrow, endometrium and adipose tissue. Tsitologiia 56:204–211
Lv Y, Xu X, Zhang B, Zhou G, Li H, Du C, Han H, Wang H (2014) Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. J Transl Med 12:344
Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C (2014) Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev 23:1245–1257
Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH, Drago H, Murphy MP, Minev B (2009) Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med 7:15
Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, Brown AA, Ichim TE, Patel AN (2013) Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med 11:56
Tuma J, Fernández-Viña R, Carrasco A, Castillo J, Cruz C, Carrillo A, Ercilla J, Yarleque C, Cunza J, Henry TD, Patel AN (2011) Safety and feasibility of percutaneous retrograde coronary sinus delivery of autologous bone marrow mononuclear cell transplantation in patients with chronic refractory angina. J Transl Med 9:183
(2012) Human menstrual blood-derived mesenchymal stem cells for patients with liver cirrhosis. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01483248. Accessed 22 Feb 2015
(2014) Using human menstrual blood cells to treat acute lung injury caused by H7N9 bird flu virus infection. Clinicaltrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02095444. Accessed 22 Feb 2015
(2012) Phase I/II trial of endometrial regenerative cells (ERC) in patients with critical limb ischemia. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01558908. Accessed 22 Feb 2015
(2012) Human menstrual blood-derived mesenchymal stem cells transplantation in treating type 1 diabetic patients. Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01496339. Accessed 22 Feb 2015
(2012) Role of stem cells in improving implantation rates in ICSI patients. Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01649752. Accessed 22 Feb 2015
Allickson JG, Sanchez A, Yefimenko N, Borlongan CV, Sanberg PR (2011) Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J 3:4–10
Acknowledgements
The authors thank Mr. Igor Passioura for excellent technical assistance in the manuscript preparation.
Conflict of Interest
PRS and CVB are founders and/or consultants of Saneron-CCEL, and hold patents and patent applications on stem cells and their applications.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Rodrigues, M.C.O., Lippert, T., Nguyen, H., Kaelber, S., Sanberg, P.R., Borlongan, C.V. (2016). Menstrual Blood-Derived Stem Cells: In Vitro and In Vivo Characterization of Functional Effects. In: Karimi-Busheri, F., Weinfeld, M. (eds) Biobanking and Cryopreservation of Stem Cells. Advances in Experimental Medicine and Biology, vol 951. Springer, Cham. https://doi.org/10.1007/978-3-319-45457-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-45457-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45455-9
Online ISBN: 978-3-319-45457-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)