Abstract
Solid organ transplantation is increasingly available in many westernized nations for end-stage cardiac, pulmonary, kidney, and liver disease. The number of overall organ transplants is increasing as is the number of transplant survivors, raising concerns about complications that develop in the posttransplant stage, notably osteoporosis and fractures. Bone loss after transplantation is a combined result of compromised bone density prior to transplantation, poor nutrition before and after receiving a new organ, hypogonadrotrophic hypogonadism developing before or after surgery, and medications prescribed to address chronic health conditions associated with organ failure, including loop diuretics and heparin-based products.
Some of these factors, combined with the negative effects of immunosuppressive medications, put patients at a heightened risk for bone loss and new fractures. Increasing attention has been given to evaluating and optimizing bone density prior to organ transplant, but as the prior chapter noted, attention to this critical issue often comes too late, long after bone loss has begun. This chapter considers the epidemiology of osteoporosis following solid organ transplantation. The authors subsequently discuss nonpharmacologic and pharmacologic interventions administered at the time of and following transplant, aimed specifically at preventing further bone loss while potentially increasing bone density.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Solid organ transplantation is increasingly available in many westernized nations for end-stage cardiac, pulmonary, liver, and kidney disease. The number of overall organ transplants is increasing as is the number of transplant survivors, raising concerns about complications that develop in the posttransplant stage, notably osteoporosis and fractures. Bone loss after transplantation is a combined result of compromised bone density prior to transplantation, poor nutrition before and after receiving a new organ, hypogonadrotrophic hypogonadism developing before or after surgery, and medications prescribed to address chronic health conditions associated with organ failure, including loop diuretics and heparin-based products. Some of these factors, combined with the negative effects of immunosuppressive medications, put patients at a heightened risk for bone loss and new fractures. Increasing attention has been given to evaluating and optimizing bone density prior to organ transplant, but as the prior chapter noted, attention to this critical issue often comes too late, long after bone loss has begun. This chapter considers the epidemiology of osteoporosis following solid organ transplantation as well as nonpharmacologic and pharmacologic interventions administered at the time of and following transplant which are specifically aimed at preventing further bone loss while potentially increasing bone density.
General Mechanisms of Bone Loss After Organ Transplantation
Kulak et al. [1] divide the process of bone loss into two phases: the first six months after organ transplantation constitute the early phase, and the subsequent year (approximately 6–18 months following transplant) represents the later phase. Steroids (glucocorticoids) at their highest doses, administered immediately following receipt of a new organ, inhibit osteoblast function as evidenced by decreased serum osteocalcin levels, while they simultaneously increase osteoclastogenesis, thereby creating an environment that favors bone breakdown over bone formation. Glucocorticoid doses, often 0.5 mg/kg/day for the first month and then 5 mg/day for 2–6 months following transplant, also disrupt the hypothalamic–pituitary adrenal axis in such a way as to decrease levels of sex hormones that assist in building bone [2]. Moreover, glucocorticoids inhibit intestinal calcium absorption as well as renal transport of calcium, both of which indirectly result in increased levels of PTH.
Immunosuppressive agents are central to survival of the transplanted organ in its new environment. Calcineurin inhibitors, specifically cyclosporine A and tacrolimus, are nephrotoxic. Detrimental to the bone in the same manner as glucocorticoid agents, they are often used in liver and cardiac transplant but are also administered after other types of transplants [3, 4]. Of the two agents, cyclosporine A has more commonly been associated with lower bone density and higher fracture rates than has tacrolimus [5]. These factors contributing to posttransplantation bone loss are influential in varying degrees, depending on the particular organ being transplanted. Four of the most frequent organ transplants—cardiac, lung, liver, and kidney—will be considered in this chapter.
Cardiac Transplant
While the number of transplants is increasing worldwide, organ availability, especially for those needing a heart transplant, remains scarce. An increasing number of patients opt to undergo an intermediate procedure by inserting a left ventricular assistive device (LVAD) [6]. The implantation of such a device is challenging, and many recipients have difficulties not only in achieving desired anticoagulation levels but also in dealing with such adverse effects as gastrointestinal bleeding, orthostatic hypotension, and physical debility that limits weight-bearing activities and general mobility. Patients who ultimately undergo heart transplantation after first receiving an LVAD have additional osteoporotic risk factors, including prolonged use of anticoagulants and high-dose proton pump inhibitors as well as nutritional compromise. Indeed, this group may have even more risk factors for osteoporosis than patients who receive a transplant directly, due to the use of the above medications for potentially an indefinite period of time.
Bone Loss and Osteoporosis (Posttranplant Immunosuppression)
Following cardiac transplant, patients experience increased rates of rapid bone loss in the spine and hip, particularly in the femoral neck. At the same time, osteoporosis may already exist prior to transplant: According to Shane et al., osteopenia or osteoporosis, as defined by the World Health Organization criteria, is present in approximately half of patients awaiting cardiac transplant, despite relatively normal mean T- and Z-scores [7]. In the six months following cardiac transplant, the rate of bone loss in the lumbar spine for patients with or without preexisting osteoporosis ranges between 6 and 10 %. Bone density declines by 6–11 % in the femoral neck during the same time interval [8]. Some studies suggest no further decline in the lumbar spine occurs beyond six months, but losses in the hip persist up to the end of the first year; there is limited evidence that partial recovery of lumbar spine BMD may take place in later years [9]. In a population of 41 cardiac transplant patients, Chou et al. found that 49 % were osteopenic and 17 % were osteoporotic [10]. Comparing two locations of common bone loss after a minimum of two years follow-up, the authors reported that 66 % of patients had either osteopenia or osteoporosis of the femoral neck and 26 % had one of these conditions in the lumbar spine. Osteoporosis can also exist in survivors of adolescent cardiac transplant as a result of not only preexisting osteoporosis and immunosuppressive therapy but also renal insufficiency, secondary hyperparathyroidism, and increased bone turnover [11].
Reduction in osteocalcin, a marker of bone formation, is found immediately following transplantation and continues to rise for the first year. In addition, levels of urinary telopeptides that reflect bone resorption are elevated shortly after transplantation but start to normalize after six months [12].
Fractures
The risk of vertebral fractures ranges from 22 to 35 % in the first three years after cardiac transplantation [2], with the percentage and timing of the fractures varying depending on the trial. A prospective study by Shane et al. [13] found that despite calcium and vitamin D supplementation, 36 % (7 women and 10 men) of 47 subjects sustained a fracture following cardiac transplantation; 85 % of this number did so in the first six months, with the highest risk found among those with low hip BMD prior to transplant. Similar trends for fracture risk in transplant recipients with pretransplant BMD below the fracture threshold were identified by Lee et al. [14] and Chou et al. [10]. These findings were supported by a European investigation five years later [15] that showed a 27 % incidence of vertebral fractures in the first two years after transplant among a group of 105 patients. During the investigation’s 7-year course, 33 % of subjects had vertebral fractures, and of those, two-thirds had multiple vertebral fractures. In an analysis of femoral neck fractures [13], only 4 % of patients experienced a fracture, all due to falls.
Treatment Options
Physical Therapy
Resistive exercises and other forms of weight-bearing exercise are helpful in maintaining overall mobility and bone density following LVAD implantation [16]. Physicians must take advantage of any therapy possible to keep the bones and muscles of these patients optimized should they require future medical treatments. Instituted in the early postoperative phase, a 2015 study of the effect of the exercise training phase in 12 patients with LVAD devices has demonstrated improved functional capacity, albeit in a small number of subjects [17]. The ability to walk on a treadmill continuously for six minutes was a key goal, followed by an increase in the treadmill’s speed to improve aerobic capacity. Subjects were necessarily limited by the need to carry the 2–2.5 kg batteries and controller, emphasizing the importance of close monitoring. Nonetheless, the researchers believe that the ability to achieve this objective by the time of discharge augurs well for adaptation to the daily needs of the posttransplant patient.
With regard to those who have undergone a full cardiac transplant, resistive training has been shown to maintain and, in some instances, restore bone mineral density losses following surgery. Braith and colleagues compared the 6-month outcomes of eight patients who underwent standard postoperative care, including a walking program, with another eight who performed structured resistance exercises (lumbar extension, duo decline chest press, and various other core muscle strengthening regimens); each group began two months after transplant and concluded six months posttransplant [18]. At two months posttransplant, both control and exercise groups demonstrated significant loss of bone density in the lumbar spine and femoral neck, relative to their baseline measurements immediately after surgery. In the resistance training group, BMD of the lumbar spine returned to within 1.9 % of immediate posttransplant levels, while the femoral neck improved to within 3.6 % of the original baseline level. By contrast, the control group saw no improvement in BMD between the 2-month and 6-month postoperative DXA scans.
Medications Alone and with Activity-Based Therapies
Over the last decade, combination therapies involving resistance training and alendronate or calcitonin have been investigated. Braith and colleagues performed a variant on the study described above by combining calcitonin and resistance exercises [19]. Six months of calcitonin therapy, initiated 48 h after transplant, was were compared with six months calcitonin therapy combined with resistance exercises beginning two months after transplant, a time when more aggressive activity is considered safe after sternotomy [20]. Although both groups lost 10–11 % of BMD in the first two months, femoral BMD in the calcitonin-alone group declined by 3.3 % at eight months post surgery, but BMD was maintained in the combined therapy cohort at the pretransplant BMD level. For the lumbar spine (L2 and L3), BMD in the group with calcitonin alone declined by 16.9 % eight months after transplant but only by 5 % in the mechanical loading group. Because both groups lost BMD early after transplant, it is unclear if the resistance group would have returned to baseline had the study extended further; however, once resistance training was permitted in the combination therapy group, subjects showed steady improvement. Although this study was limited by a small number of participants, it showed early promise of the value of physical exercise after transplant.
Alendronate has been examined in a similar manner by Braith et al. in a 2003 study, again combining resistance training with pharmacologic agents aimed to reduce bone loss [21]. Twenty-five participants were divided into three groups: The first was administered 10 mg daily alendronate beginning two months after surgery and the second received the same alendronate protocol but also participated in resistance training exercises (1–2 days/week). The third group was given no pharmacologic intervention or resistance exercises but did receive standard-of-care activities, including a walking program. DXA scans were performed at baseline, two months posttransplant, and eight months posttransplant. Results found that all three groups lost significant amounts of total body skeletal mass two months posttransplant (before interventions began) and showed regional bone losses of 5–6 % in the femoral neck and 11.2–12.5 % in the lumbar spine. While the total body BMD declined between months two (by 5.1 %) and eight (by 6.5 %) in the group receiving no pharmacologic intervention, the total body BMD loss of 5.3 % in the alendronate-only group at two months (prior to starting the drug) remained steady, neither improving nor declining by the end of the study. But when alendronate was combined with resistance training, the 5.6 % loss of BMD seen two months into the study had improved to a much smaller loss from baseline BMD of only 2.1 % by the end of the study, eight months following transplant.
In the lumbar spine, similar trends were found, except the decline at two months after transplant was in a significantly greater range of 12 %. Treatment with alendronate alone, given between months 2 and 8, only brought the BMD value up to a loss of 10.5 %. In contrast, patients receiving alendronate plus physical training involving lumbar extension exercises regained the majority of the lost bone, coming to within 3.4 % of the pretransplant BMD value.
Trials involving only bisphosphonates in the treatment of osteoporosis posttransplant have also been performed, with results measured over a longer time period. Again the studies were small and involved primarily pamidronate. For example, Shane et al. [13] examined the effect of a single intravenous infusion of pamidronate (60 mg) within two weeks of transplant, followed by oral etidronate and oral calcitriol in 18 patients compared with 52 subjects receiving only calcium and vitamin D supplementation. At 12 months after transplant, the pamidronate-etidronate therapy group experienced virtually no lumbar spine bone loss (0.2 %) in contrast to a significant decline in those without bisphosphonate therapy (6.8 %). Femoral neck fell by only 2.7 % in the therapy patients in contrast to 10.6 % in the non-therapy group. Whereas the therapy subjects experienced only three vertebral fractures, 17 patients in the non-bisphosphonate therapy group had 30 vertebral fractures, one hip fracture, and three episodes of rib fractures. Another trial by Krieg et al. [22] focused on the impact of a 3-year treatment of quarterly injections of 6 mg pamidronate; at the end point, the increase in BMD in the lumbar spine reached 18.3 % (14.3 % compared with BMD at the time of surgery), while bone loss in the femoral neck, which declined in the first posttransplant year by 3.4 %, completely recovered.
In cases where heart transplant patients cannot tolerate bisphosphonate treatment due to impaired renal function, denosumab may represent a viable alternative. A study of denosumab therapy in 46 kidney, liver, and heart transplant patients over a mean duration of 1.25 years indicates a mean increase of 9.8 % in the lumbar spine BMD in 97 % of patients and a mean increase of 8.0 % BMD in the hip in all patients [23]. However, these are very preliminary findings, and more extensive research focused solely on heart transplant patients is needed.
These various therapies appear to be valuable in preserving bone density in heart transplant patients. Yet, as with many other conditions, the emphasis is on preserving the overall health of the organ, limiting rejection episodes, and improving cardiac conditioning. Preventing bone fractures and excess rapid bone loss that could lead to hypercalcemia are essential components of an overall plan. In the early postoperative phase, coordination of care among physiatrists, rehabilitation therapists, cardiologists, and transplant surgeons remains essential to achieve optimal health of the patient in the months and years following cardiac transplant.
Lung Transplant
Osteoporosis Prior to Transplant
Although far fewer transplants occur in lung disease than in heart, liver, and kidney, osteoporosis is a prevalent complication, both before and after transplant. In a study of 48 patients awaiting lung transplant, Jastrzebski et al. determined that osteoporosis was present in half the study population, with osteopenia occurring in 40 % [24]. The greatest increase in bone loss was evident in COPD patients who are also affected by both pancreatic insufficiency and reduced vitamin D and calcium absorption [25]. Confirmed by other studies, preexisting osteoporosis has become a matter of increasing concern. Growing recognition of its importance should enable physicians to implement anti-osteoporotic strategies during the waiting period before transplantation, ensuring that patients are in the best possible health at the time of the procedure [26]. Evaluation of bone mineral density and counseling about the risk of fracture posttransplant are now routinely recommended for patients with low BMD or fracture pretransplant [27].
Osteoporosis and Fractures Post Lung Transplant
As in the case of other solid organ transplants, bone loss is accelerated in the early months following lung surgery, primarily as a result of high doses of immunosuppressive medications that induce increased bone turnover. However improvement is indicated in more recent trials.
Earlier studies pointed to a marked decrease in lumbar spine BMD of 3.5–24 % occurring in the first 3–6 months. In their 1996 analysis of bone loss post lung transplant, Aris et al. reported that 73 % of patients had BMDs of the spine and femur below a fracture level defined as two SDs beneath the age-matched mean. The fracture rate was approximately 225 fractures per 1000 person-years which is equal to or greater than that for women with postmenopausal osteoporosis who have already experienced fractures. Biochemical markers of bone resorption were also significantly higher [25]. Osteoporosis was attributed not only to the cumulative steroid dose but also to the degree of preexisting bone demineralization—a condition that was often not fully recognized.
Other studies confirmed bone loss and fracture occurrence in varying degrees. Spira et al. [28] reported that the prevalence of osteoporosis increased from 54% prior to lung transplant to 78% posttransplant. A lesser decrease in BMD of about 5 % at both lumbar spine and femoral neck, as well as a high incidence of fractures at 18 %, was recorded 6–12 months after transplant. On the basis of these findings, the authors postulated that the modest decrease in BMD was still sufficient to impel patients over the fracture threshold especially in those with preexisting low bone mass, again emphasizing the importance of earlier screening and treatment. Women with low pretransplantation BMD and a history of pretransplant glucocorticoid therapy are at greater risk of fracture in the first year following transplant [29].
In this context, it is interesting to note that more recent investigations have produced better outcomes. In a 2014 study of a cohort of 210 lung transplant patients [30], 17 subjects (8.0 %) experienced fractures after transplantation, with the median time to first fracture occurring at 12 months and the mean time for fracture incidence occurring at 18 months. Calcium and vitamin D supplementation as well as glucocorticoid use did not differ in the fracture and nonfracture groups. Of the 17 who fractured, eight had COPD. Comprehensive bone care, including DXA scans; vitamin D screening before and immediately after transplantation; early initiation of antiresorptive therapy, at both the pretransplant and immediate posttransplant phase; and improved clinician awareness could account for these improved results.
Treatment
Therapy for bone loss and fracture risk post lung transplant must necessarily involve both the nonpharmacological and pharmacological strategies customarily used for osteoporosis alone. Studies focusing specifically on lung transplant recipients are limited in number and size; however, they do demonstrate the greater efficacy of bisphosphonates, particularly intravenous pamidronate.
Nonpharmacologic Interventions
Calcium and Vitamin D Supplementation
Studies indicate that calcium and vitamin D supplementation in lung transplant recipients appears to have little impact on glucocorticoid-induced osteoporosis. In a comparison with the bisphosphonate pamidronate, Trombetti et al. [31] found that, at one year, patients receiving only calcium and vitamin D supplementation had a Z-scores of −0.4+/−0.1 at lumbar spine and −0.04+/−0.1 at femoral neck, in contrast to comparable scores of +0.2+/0.1 at lumbar spine and +0.2+/−01at femoral neck for bisphosphonate patients.
Exercise and Physical Therapy
In terms of exercise, weight-bearing and strengthening regimens are employed to improve and maintain bone density, while spinal posture and movement patterns may be beneficial in cases of vertebral fractures [32]. At the same time, exercise limitations, present before transplant, can persist well after transplant, suggesting chronic muscle deconditioning after lengthy pretransplant debilitation [33]. Cardiopulmonary exercise testing (CPET), incorporating maximal oxygen uptake, has been used for over two decades to assess aerobic capacity posttransplant, but results are mixed. Overall, Dudley et al. have shown that the absolute VO2 capacity after transplant appears to be fixed at 50 % of predicted VO2, regardless of pretransplant capacity [34]. Factors affecting exercise capacity include abnormalities in peripheral circulation and in skeletal muscle oxidative capacity as well as the effect of immunosuppressive medications.
As Seoane et al. have observed [35], the 6-min walk test (6-MWT) has essentially replaced CPET in evaluating lung disease itself, but only recently has it been applied to posttransplant patients. In their study of 49 lung transplant patients, they describe a normal distribution for the 6-MWT distance at six months following transplant, with improved distances continuing for a year. Although 6-MWT does not predict survival, it may have an as-yet undetermined predictive value for morbidity. However, its limitations, specifically the inability to determine peak oxygen uptake which, in turn, hinders assessment of the relative factors contributing to exercise capability, have led the American Thoracic Society to recommend that the 6-MWT be regarded as complementary to, but not a substitute for, CPET [34, 36].
To what extent is exercise beneficial for lung transplant patients? In a 2010 review of seven studies applying different forms of aerobic and resistance exercise, Wickerson et al. [37] concluded that a period of structured exercise training can have a positive effect on maximal and functional exercise capacity, skeletal muscle strength, and lumbar spine BMD. Several of the studies covered in the review and one completed more recently are noted here. A 6-month program of lumbar extension exercise [38] aimed at reversing vertebral osteoporosis post lung transplant revealed that lumbar BMD in the exercise group increased significantly (+9.25) over the period, whereas a control group lost bone mass, decreasing to 19.5 % less than pretransplantation levels. The lumbar spine was singled out because nearly all lung transplant patients have reduced BMD at that site, with lumbar spine compression fracture being the most debilitating consequence of glucocorticoid therapy and resulting in continued BMD loss. Another trial involving lung patients more than six months posttransplant reported that a period of normal daily activities exerted no impact on exercise performance in contrast to six weeks of aerobic endurance training which significantly improved submaximal and peak exercise performance [39]. A 2012 trial not only confirmed the benefits of exercise training in lung transplant patients but also added important information relating to broader health outcomes, specifically the impact of exercise on cardiovascular morbidity [40]. In patients between 40 and 65 years of age with an uncomplicated postoperative condition, those who underwent a 3-month structured exercise program (walking, stair climbing, cycling, and resistance training) immediately after the transplant, realized three major benefits:
-
1.
After one year, walking time averaged 85 min per day versus 54 min for a control group.
-
2.
Quadriceps force, 6MWT distance, self-reported physical functioning, and quality of life were improved in the intervention group.
-
3.
The average 24-hour diastolic and systolic blood pressure was significantly lower in the exercise group, with positive implications for cardiovascular health.
Although lung transplant patients may be unable to attain full exercise capacity or maximum skeletal muscle strength, the benefits of exercise are indisputable. Further studies of the safety and efficacy of more intensive training programs as well as of the impact of exercise on patients with a more complicated postoperative experience, possibly involving comorbidities, are anticipated [37].
Pharmacologic Treatment
Long regarded as a mainstay of osteoporosis treatment, bisphosphonates have not been extensively studied in the lung transplant population and have recently been the subject of several safety advisories from the FDA. In a highly cited trial involving posttransplant cystic fibrosis patients, Aris et al. [25] found that in contrast to controls, intravenous pamidronate combined with calcium and vitamin D supplementation produced an 8.8 % gain in BMD at the lumbar spine and an 8.2 % gain in femur BMD at the end of two years; however, there was no difference in fracture rates between the two groups. A pilot study directed at bone loss and osteoporotic fracture in lung transplant patients [41] demonstrated that aggressive therapy with pamidronate and supplements reduced incident symptomatic fracture in the first year, with only 4 % of the 45 patients evidencing fracture. Lumbar spine and hip bone density remained stable or improved in 65 % and 86 % of patients, respectively, but significant bone loss was still apparent in 42 % of patients in the year following transplant. It is unclear when or whether bone remodeling normalizes in this population, indicating that the need for therapy may persist indefinitely. For the present, however, bisphosphonate use for 12 months prior to and posttransplant is generally recommended.
Possible combination regimens have also been examined. An analysis of alendronate together with mechanical loading [42] has shown that the blended prophylaxis produced a significantly increased lumbar spine BMD, with values 10.8+/−2.3 % greater than that prior to transplant, thus demonstrating that an antiresorptive agent plus resistance exercise is more effective than the agent alone.
Other treatments have shown limited efficacy. Short-term therapy with calcitriol or cyclical etidronate may be partially effective in reducing bone loss after lung transplant, but their use requires monitoring of calcium levels [43]. The application of parathyroid hormone and denosumab requires further investigation as does the potential of immune tolerance, which may be particularly difficult to achieve in pulmonary transplant recipients, given such factors as lack of bronchial artery circulation posttransplant and an imperfect barrier against invading pathogens [44].
Liver Transplant
Statistics compiled by the US Department of Health and Human Services indicate that the number of patients actively awaiting liver transplant far exceeds those on the waiting list for heart and lung together and is surpassed only by the extremely large number, over 66,000, registered for a kidney transplant. Here again osteoporosis is apparent before surgery. As recently as 2012, Kaemmerer et al. reported that the number of patients evidencing osteoporosis before transplantation ranged from 12 to 55 % and those with bone fractures from 3 to 35 % [45]. Moreover, low BMD or bone fractures before transplantation increase the risk of BMD loss and particularly vertebral fractures after transplantation.
Post Liver Transplant Osteoporosis
As Eberling has observed [46], bone loss and fracture rates are highest in the first 6–12 months after transplant but at times can occur as early as the first three months. However, recent trials have shown conflicting results with regard to bone loss. A 2002 study by Ninkovic et al. demonstrated a significant absence of bone loss and reduced fracture occurrence of 8 %, although it still found significant bone loss at the femoral neck of 4 %, compared to baseline, during the first three months [47]. In the authors’ view, the decrease in both bone loss and fracture occurrence were most likely linked to the lower doses and reduced duration of glucocorticoids now in use, better bone health, and possibly the decision to implement the transplant option earlier in the course of chronic liver disease.
By contrast, a subsequent Mayo Clinic trial involving 360 posttransplant patients over a 16-year period (1985–2001) [5] continued to show a cumulative fracture incidence of 30 % in the first 12 months with an occurrence of almost 46 % by eight years. The greatest risk factors were pretransplant fracturing, primary binary cirrhosis, and corticosteroids. The researchers maintained that although other studies may have demonstrated an increase in the bone mass of liver recipients with fewer fractures, as of 2007, 25% of patients in their trial still developed new fractures in the posttransplant period. Another study focusing on BMD reported that women with primary binary cirrhosis had decreased BMD at three months post liver transplant followed by a subsequent increase, so that by 12 months, their median BMD was similar to that in the pretransplant stage and by 24 months was higher by 5 % [48].
Treatment
Nonpharmacologic Interventions
As in the case of other transplants, bone disease after a liver transplant was formerly attributable to the high dose of corticosteroids used as immunosuppressive agents. With corticosteroid administration now reduced to a minimum in favor of lower dosages and other immunosuppressive drugs, improvements in bone density over time are now being realized [49]. In addition to a decrease in the dose and duration of corticosteroid treatment, other nonpharmacologic measures including improved nutrition, cessation of smoking, and reduction of alcohol intake may improve bone health.
Vitamin D Supplementation
As Stein et al. have shown, severe vitamin D deficiency at the level of 250HD <25 nmol/L existed in 30 % of 23 liver transplant recipients examined, while vitamin D deficiency at the level of 25OHD <50 nmol/L was common in the remainder of the cohort [50]. Levels are particularly low in liver recipients who experience impaired hepatic 25-hydroxylation of vitamin D. At a time when bisphosphonates are increasingly used following transplant, physicians should be aware that these drugs are not optimally effective in cases of severe vitamin D deficiency and that IV bisphosphonates may actually precipitate hypercalcemia in a deficient state. Depending on individual vitamin D levels, liver recipients require supplementation (generally administered with 1000 mg/day calcium) in varying dosages that will enable them to achieve serum levels of vitamin D25 OH above 20 ng/mL.
The status of vitamin D levels at the time of transplantation is also a matter of concern. A 2015 trial involving 127 patients receiving a transplant between July 2010 and July 2011 found that 84 % had vitamin D deficiency at the time of transplant evaluation and 74 % remained deficient at the time of transplant [51]. While this study found no association between vitamin D deficiency pretransplant and decreased BMD or fracture risk posttransplant, further research is needed to determine what effect the restoration of serum levels of vitamin D25 OH pretransplant might have on such posttransplant outcomes as the level of immunosuppressive therapy needed.
Physical Activity
In the past 15 years, increasing attention has been focused on the role of exercise in treating osteoporosis post liver transplant, primarily with respect to its effect on health-related quality of life. In conducting some of the earliest studies on this interaction, Painter et al. [52] observed that posttransplant conditions such as pain, weakness, loss of range of motion, and osteoporosis, coupled with significant weight gain, contribute to physical inactivity and adversely affect levels of physical functioning. Just as exercise is important for the general population, it should not be ignored in transplant patients.
Limited data is available on the direct effect of exercise on osteoporosis in post liver transplant patients. Instead, generalized benefits of exercise following solid organ transplant are extrapolated, taking into account the specific limitations imposed by liver replacement surgery and its consequences. Initially, exercise in these patients must be limited and carefully monitored to prevent complications from both suture breakage and the fatigue that so often occur post liver transplant. Several analyses have been conducted on potential physical activity programs for this population, aimed primarily at improving exercise capacity, muscular strength, and cardiorespiratory fitness, but beneficial to bones as well. In one trial, the “exercise prescription” for home-based training included walking and cycling, with a frequency of at least three times per week for a duration increasing to 30 minutes per session [53]. Because subjects and their families fear damaging a new organ, noncompliance with exercise results, making professional guidance essential in developing and maintaining much-needed resistance and aerobic exercise programs [54]. Additional studies are needed to develop the optimal exercise intervention for osteoporosis in the post liver transplant period.
Pharmacologic Intervention
Bisphosphonates
Bisphosphonates are effective in treating post liver transplant bone loss, provided that vitamin D deficiency is also addressed. Thus far, investigations of the effect of bone-protective therapy in transplant patients have been hindered by their small size, short duration, and insufficient power to compare different medications and detect fractures. They do, however, demonstrate some benefits, particularly with regard to pamidronate, alendronate, zoledronic acid (ZA), and ibandronate. A randomized 12-month study of 30 mg of IV pamidronate, administered with supplemental calcium and vitamin D, resulted in a significant increase in lumbar spine BMD as compared to controls, as well as a decrease in bone turnover. In a 12-month follow-up, the efficacy of pamidronate appeared to be limited to trabecular bone, with no effect on femoral neck bone loss [55].
Studies involving alendronate have produced limited positive results. A placebo-controlled trial in patients with primary biliary cirrhosis showed that alendronate was able to increase BMD after one year, in comparison with placebo and independent of concomitant estrogen therapy [56]. A nonrandomized investigation also indicated that alendronate produced an increase in BMD within 24 months of transplant [57]; another trial revealed significant increases of BMD at the lumbar spine, femoral neck, and total femur at 12–24 months but did not appear to offer protection against fractures [58].
Examinations of the impact of ZA and ibandronate have shown somewhat greater efficacy but still inconsistent findings. Although Crawford et al. reported that zoledronic acid prevented bone loss by 3.8–4.7 % at the lumbar spine, femoral neck, and total hip within the first year of transplant. They also found that it induced temporary secondary hyperparathyroidism and hypocalcemia; the trial was insufficiently powered to assess fractures [59]. Similar results were reported in a subsequent, randomized controlled trial by Bodingbauer et al. who compared ZA, combined with calcium and vitamin D, to controls receiving only calcium and vitamin D. The end points of fracture and death occurred in 26 % of patients on ZA and 46 % in controls. In addition, 75 % event-free survival time was achieved for 360 days in the ZA group compared to 200 days in the control group [60].
Most recently, an examination of bone disease in liver transplantation involved both pre- and posttransplant treatment with ibandronate [45]. An oral monthly dose of ibandronate (150 mg), combined with calcium and vitamin D, was administered to patients awaiting transplant, with follow-ups at 3-, 6-, 12-, and 24-months post surgery. BMD of the lumbar spine was measured both before and after transplant: the percentage change from baseline to 3 months was 13.59, reaching 17.1 % at six months, 18.78 % at 12 months, and 24.26 % at 24 months. Femoral neck BMD increased by 3.1 % at 3 months and 5.1 % at 6 months in the same cohort. A secondary end point in this study, the prevalence of fracture occurrence, was 3.2 % posttransplant. The results of this study warrant further examination, but it appears that immediate postoperative bone loss after liver transplantation can be significantly reduced by pretreatment.
Although much research remains to be done, bisphosphonates, particularly the more potent ZA and ibandronate, have demonstrated the importance of early treatment, as well as the need for a spinal x-ray prior to transplant to identify the status of bone mass. Clinical risk factors should also be considered as an integral part of the transplantation process. Large multicenter, randomized clinical trials are needed to produce more definitive findings.
Renal Transplant
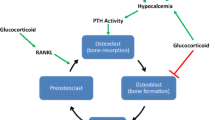
The kidney is the most commonly transplanted solid organ in the United States and throughout the world, resulting in bone disorders caused by posttransplant conditions as well as those persisting from the pretransplant phase. As Bia has observed, bone loss in kidney transplantation differs from that for the heart, lung, and liver because of several factors: the presence of renal osteodystrophy which leads to low BMD; the location of fractures, which are more frequent in appendicular sites (lower limbs, feet, and hip) in kidney transplants than in axial sites (spine and ribs) as in other solid organ transplants; and the potential adverse effects of bisphosphonates that tend to cause “oversuppression” of bone turnover in kidney recipients (Fig. 1) [61].
Factors which contribute to posttransplant bone disease. Posttransplant steroid use plays a major role in bone loss, although other metabolic derangements, especially in kidney transplant patients, may also contribute (Source: Bia [61])
Whereas patients with heart, lung, and liver disease tend to have preexisting osteoporosis, those with end-stage kidney diseases have what is termed renal osteodystrophy, which is an integral part of chronic kidney disease–metabolic bone syndrome (CKD–MBS) – also known as chronic kidney disease–mineral and bone disorder (CKD–MBD). This condition is marked by active vitamin D deficiency (low vitamin D-1.25-OH), hyperphosphatemia, secondary hyperparathyroidism, and excess aluminum levels—all of which may lead to reduced BMD and fractures but are distinct from osteoporosis as such [61]. While the magnitude of many of these metabolic abnormalities is lessened by kidney transplantation, some aspects of the CKD-MBS such as parathyroid hyperplasia are likely to remain [2]. Failure of enlarged parathyroid glands to involute means that PTH concentrations remain elevated after transplantation, a scenario true in 75 % of patients one year after renal transplant [62, 63]. In addition patients with end-stage kidney disease tend to be hypogonadal and thus could have already received treatment with immunosuppressive therapy (glucocorticoids or cyclosporine A) that will continue posttransplant [64]. Finally, transplant patients frequently still have chronic kidney disease with its attendant complications.
Posttransplant Osteoporosis, Bone Loss, and Fractures
In addition to the standard risk factors for osteoporosis including age and female gender, kidney transplant patients face other challenges, ranging from time since transplantation to the immunosuppressive regimen and graft dysfunction. Ahmadpoor et al. reported an incidence of osteoporosis at 26 % (20 of 77 patients who had undergone transplantation in the previous 6 months–2 years), with the most common sites being the hip and spine [65]. In a cohort of 44 patients followed up to 12 months posttransplant, Orzel et al. identified 43 % as osteopenic, 11 % as osteoporotic, and 46 % as normal, with younger age and high intact pretransplant parathyroid hormone levels as the principal risk factors [66].
As in the case of other transplants, improved results for BMD in kidney patients are evident in more recent studies. Whereas a 1991 investigation reported a BMD decline of 4–10 % in the first six months following transplant [67] due to the toxic effect of glucocorticoids, Bouquegneau et al. have pointed to newer trials that reveal a bone loss of only 0.1–5.7 % in the lumbar spine, reflecting reduced immunosuppressive therapy [68]. In 2014, a large trial (n = 326) to assess long-term changes in BMD following kidney transplant used extensive DXA measurements to demonstrate that BMD typically improved or remained stable over a period of 8.2 years, with baseline values only slightly above average for age and sex. It should be noted, however, that baseline measurements did not begin until six months after transplantation, allowing for the well-established decline in BMD within the early posttransplant period. Over the long term, the single factor leading to a significant increase in mean BMD at all sites was osteoporosis treatment [69].
Similarly the results of later trials on fracture occurrence demonstrate improvement over earlier findings, mainly attributable to a reduction in the use of immunosuppressive agents; time after transplantation and the presence of diabetes are other contributing factors. Data from the US Renal Data System (USRDS) demonstrate that the demographic-adjusted incidence of hip fractures in kidney transplant recipients has declined significantly to the point where it is 45 % lower in patients transplanted in 2010 than in 1997 [70]. Explanations for this decline include not only changes in immunosuppressive regimens but also an altered lifestyle (smoking cessation, reduced alcohol consumption, enhanced physical activity) and increased bisphosphonate use, which, as noted later, remains a matter of concern in kidney transplant patients.
In one of the largest studies of fracture incidence in kidney transplant patients yet undertaken (n = 4821) [71], Naylor et al. estimated the cumulative incidence of nonvertebral fractures at 3, 5, and 10 years in the period between 1994 and 2009 with the following results. The overall 3-year cumulative incidence of nonvertebral fractures was 1.6 %, with the number increasing over that time period; the hip fracture rate alone was 0.4 %. The overall 5- and 10-year cumulative incidence of nonvertebral fractures was 2.7 % and 5.5 %, respectively, with hip fractures alone at 1.7 % at the 10-year mark. The most common fracture site was the lower leg. These findings bear out the 2004 observation by Sprague et al. that “the more time since transplantation, the higher the reported fracture rate,” but with fracture occurrence at a much lower level than the rates of 5–44 % cited in this earlier study [72]. To explain the lower fracture incidence now observed, Naylor et al. [71] cite the fact that earlier studies could not take into account patients transplanted after the year 2000 when decreased prednisone doses, as well as the use of bisphosphonates and vitamin D supplementation, came into play.
Another approach to reducing fracture risk in kidney transplant is early corticosteroid withdrawal. A study of 430 patients receiving transplants between 2000 and 2006 demonstrated that 31 % of patients discharged from the hospital without corticosteroids had a decreased risk of fracture compared with those discharged on a corticosteroid regimen – a finding that became significant at 24 months posttransplant [73]. Despite these encouraging signs, kidney recipients still have a nonvertebral fracture rate of 1.6 % compared with 0.5 % for the comparable healthy population; women aged 50 and over sustain the highest cumulative 3-year increase of 3.1 % [71].
Is there an association between low BMD and fractures in kidney transplant? In a study conducted over 20 years ago, Grotz et al. [74] showed that many transplant recipients did not experience fractures, concluding that low BMD values at the lumbar spine could, at best, only partially explain fracture occurrence. DXA measurements at the femoral neck indicated no relation to fractures. Findings that BMD assessment does not discriminate between patients with fractures and those without have led to increasing interest in measurements of bone quality, achieved with newer three-dimensional imaging techniques such as quantitative computed tomography. Recent evidence that lower femoral neck BMD may be linked with increased fracture risk in chronic kidney disease [75] calls for increased efforts to develop simplified, less invasive, and more cost-effective ways to conduct bone biopsies [76].
Diabetes compounds the fracture risk for patients with kidney transplants. Epidemiologic studies show that pretransplantation diabetes can more than double the risk for fracture after a kidney transplant [73]. Hypoinsulinemia, hyperglycemia, and other diabetic complications including peripheral neuropathy can all decrease bone strength. However, new research finds that a simultaneous pancreas–kidney transplant, as opposed to a kidney transplant alone, can result in lower fracture rates in kidney transplants, particularly in men [77]. Apparent within three months of transplantation, overall fracture incidence was 31 % lower in men over a 5-year period but was not significantly different in women. Higher levels of circulating estrogen in women under the age of 50, less severe bone loss at the lumbar spine and femoral neck, and prior medication aimed at fracture prevention may, in part, account for this difference. As the authors emphasize, further studies to determine the mechanisms underlying coincident type 1 diabetes and chronic kidney disease are required to serve as the basis for new fracture prevention strategies that can be used in men, concurrently with the combined transplantation, as well as to advance other therapies that will help to prevent fractures in women.
Treatment
Both nonpharmacologic and pharmacologic therapies have been employed to prevent bone loss posttransplant. In cases where the effect of these therapies have not been examined in kidney recipients directly, findings have been extrapolated from relevant trials involving other types of transplants, keeping in mind the special circumstances that define bone loss in the post-kidney transplant population. Given the sharp decline in bone loss that occurs in kidney as well as in other transplants immediately after surgery, therapeutic measures should be initiated at the earliest point.
Nonpharmacologic Regimens
Calcium and Vitamin D Supplementation
Hyperparathyroidism, abnormal vitamin D metabolism, and the use of prednisone all lead to reduced calcium absorption and further contribute to bone loss post kidney transplant. At the same time, calcium supplementation alone is ineffective in maintaining BMD or reducing fracture risk [78]. A Cochrane Database Review of 24 trials found that no individual intervention with either vitamin D, calcitonin, or bisphosphonates was associated with reduced fracture risk but that when the results of all trials were combined, any one of these treatments proved effective against fracture risk and all had a beneficial effect on BMD at the lumbar spine [79]. This study supports the concurrent use of vitamin D (with or without calcium) and bisphosphonates to reduce the deleterious effects of immunosuppressive therapy on bone density after transplant and indicates that any intervention to alter bone metabolism can reduce fracture risk in the year following surgery. With respect to calcium supplementation, Torres et al. report that intermittent-dose calcitriol during the first three months posttransplant, followed by oral calcium supplementation during the first year, decreased the rate of bone loss at the total hip compared with calcium supplementation alone, without any adverse effect on hypercalcemia levels [80].
The relation between vitamin D and hypercalciuria admits of mixed findings. A recent study reported that hypercalciuria occurred more frequently in a vitamin D supplementation group, leading to a reduction in the dosage or treatment discontinuation in 30 % of patients; calcium supplementation was also cited as a possible cause of hypercalciuria. It is clear that additional randomized controlled trials are required to determine the most effective dose and optimal duration of supplementation as well as to assess the specific impact on fracture risk [81]. Until then, the level of calcium and vitamin D supplementation is determined on an individual basis taking into account regular screening results to determine the extent of bone damage, as well as the severity of the disease and existing comorbidities such as diabetes.
Physical Activity
As in the case of dietary supplementation, exercise programs must be tailored to individual needs of the individual and, to the extent possible, should encompass mechanical loading, stretching, and strengthening regimens. Although the primary goal of exercise in the posttransplant phase is to reduce cardiovascular risk and improve graft function [81], the benefits of exercise extend to increasing BMD and preventing fractures by advancing motor fitness, improving balance, and decreasing fall risk. However, analyses of the efficacy and effectiveness of exercise post renal transplant are few in number, and the lack of evidence, in itself, represents one of the principal factors contributing to low exercise rates [82]. Gordon et al. point to several other reasons underlying reduced physical activity: (1) lack of motivation and interest, coupled with fear of injuring the graft, (2) limited knowledge of the benefits of exercise on the part of healthcare professionals faced with what they consider to be more immediate and compelling concerns, and (3) inadequate reimbursement for physical activity programs and counseling on the part of insurance carriers. Exercise may be particularly difficult to initiate in older renal transplant recipients with reduced physical performance prior to transplant, as well as in younger patients who have been shown to be less physically active pretransplant by a measure of 25 % in comparison with healthy subjects [83].
The guidelines adopted by Kidney Disease: Improving Global Outcomes (KDIGO) recommend that at least 30 min of moderate-intensity exercise (walking, cycling, slow jogging) be undertaken on most, preferably all, days of the weeks, as adapted to the needs and capacity of the individual [84]. In a highly cited randomized clinical trial, Painter et al. found that one year after transplant, an exercise intervention group increased its regular physical activity from 50 to 67 %, whereas the “usual care” group experienced a decline from 47 to 36 %; in addition the exercise group realized significantly greater gains in peak oxygen uptake (VO2), muscle strength, and physical functioning [85]. Although guidelines and studies such as these have not directly addressed bone disease in renal transplant, they do raise awareness of the need to develop exercise regimens directed to individual needs as well as to conduct new empirical research on the safety and efficacy of specific exercise training programs with respect to the different outcomes of the transplant process including osteoporosis. Recent findings on the correlation between low physical activity and the risk of cardiovascular and all-cause mortality in renal transplant [86] may well stimulate further examinations of the impact of exercise on mineral and bone disorders as well as on fracture risk, leading to an improved quality of life.
Pharmacologic Measures
Bisphosphonates
As noted at the outset of this section, the value of bisphosphonates in kidney transplant is tempered by their potential to oversuppress bone metabolism. Given that concern, the general consensus is that bisphosphonates should not be used in kidney patients with low bone turnover that could be further exacerbated by these drugs, possibly increasing fracture risk. Studies of the effect of alendronate, pamidronate, and zoledronic acid have demonstrated benefits in terms of increased BMD.
In a 12-month analysis of kidney patients receiving 10 mg/day of alendronate plus calcitriol and calcium issued in 2001, BMD increased significantly by 5 % at the lumbar spine and 4 % at the femoral neck and bone turnover normalized; at the same time, bone continued to be lost due to prednisone treatment and persistent hyperparathyroidism [87]. Two intravenous doses of pamidronate given to male patients at the time of transplantation and one month later prevented early rapid bone loss, with no significant reduction at the lumbar vertebrae and femoral neck [88]. The regimen was well tolerated and easy to administer, with no detrimental effect on renal function and no discernible side effects. A subsequent study confirmed the efficacy of IV pamidronate in preserving bone mass but did observe an increased risk of low bone turnover [89].
A trial involving the third-generation bisphosphonate, zoledronic acid (ZA) reported that, at six months, two infusions of IV ZA increased trabecular calcium content significantly, with no change in BMD at the femoral neck. However, as Fratianni et al. have observed, the early bone-sparing effects of short-term ZA could not be sustained at three years after transplantation [90]. In addition, FDA warnings concerning the deterioration of renal function and renal failure resulting from ZA must also be taken into account, with dose reduction as recommended.
Still in their infancy, comparative analyses of these medications are impaired by the small sample sizes and the heterogeneous nature of the research, particularly with respect to time duration following transplant. Based solely on randomized controlled trials, a recent meta-analysis of bisphosphonates posttransplant [91] concluded that they were beneficial to BMD at the lumbar spine but not at the femoral neck. Although changes in vertebral and nonvertebral fractures or in adverse events were not associated with their use; bisphosphonates were not found to reduce fracture incidence. As a result of this study, the largest database on the use of bisphosphonates in patients undergoing renal transplantation is now in place and can serve as the basis for further analyses of their efficacy and safety as new information is obtained.
Other Medications
In general, bisphosphonates which are renally excreted are not recommended for kidney transplant patients with moderate to severe renal insufficiency. As an alternative, denosumab, the fully human monoclonal antibody against RANKL, has recently been investigated to determine its effect on BMD in renal transplant, with results indicating that it significantly increased areal BMD at vertebral and nonvertebral sites [92]. Unlike bisphosphonates it improved cortical volumetric BMD and thickness at the distal tibia and radius while decreasing levels of blood and urine biomarkers in bone turnover. Although associated with more frequent episodes of urinary tract infections, denosumab has the potential to improve bone health posttransplant and to sustain bone retention with long-term use. Synthetic parathyroid hormone (PTH) in the form of teriparatide does not improve BMD early after kidney transplantation, nor do histological analyses or bone markers provide evidence of improved bone turnover or mineralization [93].
The challenges of managing kidney recipients are many, emphasizing the importance of regular monitoring to determine the status of bone loss. Reduced doses of immunosuppressive therapy as well as early corticosteroid withdrawal, calcium and vitamin D supplementation as needed, increased physical activity, and the prudent use of bisphosphonates targeted at high-fracture risk recipients must be weighed carefully by an interdisciplinary team responsible for the care of transplant patients.
References
Kulak CAM, Barba VZC, Junior JK, Custodian MR. Bone disease after transplantation: osteoporosis and fracture risk. Arq Bras Endocrinol Metab. 2014;68(5):484–92.
Hawkins FG, Guadalix S, Sanchez R, Martinez. Post-transplantation bone disease. In: Dionyssiotis Y, editor. Osteoporosis. p. 299–322. ISBN: 978-953-51-0026-3. InTech, Available from: http://www.intechopen.com/books/osteoporosis/post-transplatation-bone-disease. Accessed 2 Mar 2016.
Giannini S, Nobile M, Cuiffreda M, Iemmolo RM, Dalle Carbonare L, Minicuci N. Long-term persistence of low bone density in orthotopic liver transplantation. Osteoporos Int. 2000;11(5):417–24.
Theibaud D, Krieg MA, Gillard-Berguer D, Jacquet AF, Goy JJ, Burckhardt P. Cyclosporine induces high bone turnover and may contribute to bone loss after heart transplantation. Eur J Clin Invest. 1996;26(7):549–55.
Guichelaar MM, Schmoll J, Malinchoc M, Hay JE. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46(4):1198–207.
Vanden Eynden F, Antoine M, El Oumeiri B, Chirade ML, Vachiery JL, Van Nooten GJ. How to cope with a temporarily aborted transplant program: solutions for a prolonged waiting period. Ann Transl Med. 2015;3(20):306. doi:10.3978/j.issn.2305-5839.2015.11.30.
Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103(3):197–207.
Cohen A, Shane E. Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int. 2003;14(8):617–30.
Stein E, Eberling P, Shane E. Post-transplantation osteoporosis. Endocrinol Metab Clin. 2007;36(4):937–63.
Chou NK, Su IC, Kuo HL, Chen YH, Yang RS, Wang SS. Bone mineral density in long-term Chinese heart transplant recipients: a cross-sectional study. Transplant Proc. 2006;38(7):2141–4.
Cohen A, Addonizio LJ, Lamour JM, Addesso V, Staron RB, Gao P, et al. Osteoporosis in adult survivors of adolescent cardiac transplantation may be related to hyperthyroidism, mild renal insufficiency, and increased bone turnover. J Heart Lung Transplant. 2005;24(6):696–702.
Sambrook PN, Kelly PJ, Fontana D, Nguyen T, Keogh A, Macdonald P, et al. Mechanisms of rapid bone loss following cardiac transplantation. Osteoporos Int. 1994;4(5):273–8.
Shane E, Rodino MA, McMahon DJ, Addesso V, Staron RB, Seibel MJ, et al. Prevention of bone loss after heart transplantation with antiresorptive therapy: a pilot study. J Heart Long Transplant. 1998;17(11):1089–96.
Lee AH, Mull RL, Keenan GF, Callegari PE, Dalinka MK, Eisen HJ, et al. Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med. 1994;96(1):35–41.
Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357(9253):342–7.
Oz M. Bridge experience with long term implantable left ventricular assist devices: are they an alternative to transplantation? Circulation. 1997;95(7):1844–52.
Blumberg Y, Kravits A, Dinkin D, Neimark A, Abu-Hatzira M, Shtein R, et al. Early physical rehabilitation after continuous flow left ventricular assist device implantation: suggested protocol and a pilot study. Int J Phys Med Rehabil. 2015;3(2):263–68. http://www/omicsonline.org/physical-medicine-rehabilitation.php. Accessed 10 Mar 2016.
Braith RW, Mills RM, Welsch MA, Keller JW, Pollock ML. Resistance exercise training restores bone mineral density in heart transplant recipients. J Am Coll Cardiol. 1996;28(6):1471–7.
Braith RW, Magyari PM, Fulton MN, Lisor CF, Vogel SE, Hill JA, et al. Comparison of calcitonin versus calcitonin +resistance exercise as prophylaxis for osteoporosis in heart transplant recipients. Transplantation. 2006;81(88):1191–5.
Braith RW, Welsch MA, Mills Jr RM, Keller JW, Pollock ML. Resistance exercise prevents glucocorticoid-induced myopathy in heart transplant recipients. Med Sci Sports Exerc. 1998;30(4):483–9.
Braith RW, Magyari MS, Fulton MW, Aranda J, Walker T, Hill JA. Resistance exercise training and alendronate reverse glucocorticoid-induced osteoporosis in heart transplant recipients. J Heart Lung Transplant. 2003;22(10):1082–90.
Krieg MA, Seydoux C, Sandino L, Goy JJ, Berguer DG, Thibeaux D, et al. Intravenous pamidronate as treatment for osteoporosis after heart transplantation: a prospective study. Osteoporos Int. 2001;12(2):112–6.
Brunova J, Kratochvilova S, Bruna J. Osteoporosis therapy with denosumab in patients after solid organ transplantation. Endocr Abs. 2016. doi:10.1530/endoabs.41.EP122.
Jastrzebski D, Lutogniewska W, Ochman M, Margas A, Kowalski K, Wyrwol J, et al. Osteoporosis in patients referred for lung transplantation. Eur J Med Res. 2010;15 Suppl 2:66–71.
Aris RM, Neuringer IP, Weiner MA, Egan TM, Ontjes D. Severe osteoporosis before and after lung transplantation. Chest. 1996;109(5):1176–83.
Shane E, Silverberg SJ, Donovan D, Papadopoulos A, Staron R, Addesso V. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101(3):262–9.
Dodd VA, Staron RB, Papadopoulos A, Evans L, Schulman LL, Jorgensen B, et al. Bone densitometry should be included in the evaluation of candidates for lung transplantation. J Transpl Coord. 1999;9(2):119–23.
Spira A, Gutierrez C, Chaparro C, Hutcheon M, Chan CKN. Osteoporosis and lung transplantation: a prospective study. Chest. 2000;117(2):476–81.
Shane E, Papadopoulos A, Staron RB, Addesso V, Donovan D, McGregor C, et al. Bone loss and fracture after lung transplantation. Transplantation. 1999;68(12):220–7.
Hariman A, Alex C, Heroux A, Camacho P. Incidence of fracture after cardiac and lung transplantation: a single center experience. J Osteoporos. 2014. doi:10.1155/2014/573041.
Trombetti A, Gerbase MW, Spiliopoulos A, Slosman DO, Nicod LP, Rizzoli R. Bone mineral density in lung-transplant recipients before and after graft: prevention of lumbar spine post-transplantation-accelerated bone loss by pamidronate. J Heart Lung Transplant. 2000;19(8):736–43.
Scherer SA, Bookstein NA. Clinical perspective: framework and rationale for physical therapy management of lung transplant patients with osteoporosis. Cardiovasc Pulm Ther. 2001;12(3):75–82.
Williams TJ, Patterson A, Clean PA, Zamel N, Mauer JR. Maximal exercise testing in single and double lung transplant recipients. Am Rev Res Dis. 1992;145(1):101–5.
Dudley KA, El-Chemaly S. Cardiopulmonary exercise testing in lung transplantation: a review. Pulmon Med. 2012; Article ID 2337852. http://dx.doi.org/10.1155/2012/237852.
Seoane L, Alex S, Pirtie C, Gupta M, Taylor DE, Valentine VG, et al. Utility of the 6-minute walk test following lung transplantation. Ochsner J. 2010;10(4):227–30.
Crapo RO, Enright PL, Zeballos RJ. (Writing Committee Members) ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Wickerson L, Mathur S, Brooks D. Exercise training after lung transplantation: a systematic review. J Heart Lung Transplant. 2010;29(5):497–503.
Mitchell MJ, Baz MA, Fulton MN, Lisor CR, Braith RW. Resistance training prevents vertebral osteoporosis in lung transplant recipients. Transplantation. 2003;76(3):557–62.
Stiebellehner L, Quittan M, End A, Wieselthaler G, Klepetko W, Haber P, et al. Aerobic endurance training program improves exercise performance in lung transplant recipients. Chest. 1998;113(4):906–12.
Langer D, Burtin C, Schepers L, Ivanoa A, Verleden G, Decramer M, et al. Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant. 2012;12(6):1584–92.
Cahill BC, O’Rourke MK, Parker S, Stringham JC, Karwande SV, Knecht TP. Prevention of bone loss and fracture after lung transplantation. Transplantation. 2001;72(7):1251–5.
Braith RW, Connor JA, Fulton MN, Lisor CF, Casey DP, Howe KS, et al. Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: a pilot study. J Heart Lung Transplant. 2007;26(2):132–7.
Henderson K, Eisman J, Keogh A, Macdonald P, Glanville A, Spratt P, et al. Protective effect of short-term calcitriol or cyclical etidronate on bone loss after cardiac or lung transplantation. J Bone Miner Res. 2001;16(3):565–71.
Jiang X, Nicholls MP. Working toward immune tolerance in lung transplantation. J Clin Invest. 2014;124(3):967–70.
Kaemmerer D, Schmidt B, Lehmann G, Wolf G, Settmacher U, Hommann M. Treatment of bone loss in patients with chronic liver disease awaiting liver transplantation. Transplant Res. 2012;1(1):7. doi:10.1186/2047-1440-1-7.
Eberling PR. Transplantation osteoporosis. In: Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Ames: Wiley; 2013. p. 495–507.
Ninkovic M, Love S, Tom BDM, Bearcroft PWP, Alexander GJM, Compston JE. Lack of effect of intravenous pamidronate on fracture incidence and bone mineral density after orthotopic liver transplantation. J Hepatol. 2002;37(1):93–100.
Eastell R, Dickson ER, Hodgson SF, Wiesner RH, Porayko MK, Wahner HW, et al. Rates of vertebral bone loss before and after liver transplantation in women with primary biliary cirrhosis. Hepatology. 1991;14(2):296–300.
Crippin JS. Bone disease after liver transplantation. Liver Transplant. 2001;7 Suppl 1:S27–35.
Stein EM, Cohen A, Freeby M, Rogers H, Kokolus S, Scott V, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant. 2009;23(6):861–5.
Chaney A, Heckman MG, Diehl NN, Meek S, Keaveny AP. Effectiveness and outcomes of current practice in treating vitamin D deficiency in patients listed for liver transplantation. Endocr Pract. 2015;21(7):761–9.
Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transplant. 2001;7(3):213–9.
Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, et al. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6(89):1896–905.
Surgit O, Ersoz G, Gursel Y, Ersoz S. Effects of exercise training on specific immune parameters in transplant patients. Transplant Proc. 2001;33(7–8):3298.
Pennisi P, Trombetti A, Giostra E, Mentha G, Rizzoli R, Flore CE. Pamidronate and osteoporosis prevention in liver transplant recipients. Rheumatol Int. 2007;27(3):251–6.
Zein CO, Jorgensen RA, Clarke B, Wenger DS, Keach JC, Angulo P, et al. Alendronate improves bone mineral density in primary biliary cirrhosis: a randomized placebo-controlled trial. Hepatology. 2005;42(4):762–71.
Millonig G, Graziadei IW, Eichler D, Pfeiffer KP, Finkenstedt G, Muehllechner P, et al. Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation: a prospective single-center study. Liver Transplant. 2005;11(8):960–6.
Atamaz F, Hepguler S, Akyildiz M, Karase Z, Kilic M. Effects of alendronate on bone mineral density and bone metabolic markers in patients with liver transplantation. Osteoporos Int. 2006;17(6):942–9.
Crawford BA, Kam C, Pavlovic J, Byth K, Handelsman DJ, Angus PW, et al. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144(4):239–48.
Bodingbauer M, Wekerle T, Paakrah B, Roschger P, Peck-Radpsavljevic M, Silberhumer G, et al. Prophylactic bisphosphonate treatment prevents bone fracture after a liver transplantation. Am J Transplant. 2007;7(7):1763–9.
Bia M. Evaluation and management of bone disease and fractures post-transplant. Transplant Rev. 2008;22(1):52–61.
Reinhardt W, Bartelworth H, Jockenhovel F, Schmidt-Gayk H, Witzke O, Wagner K, et al. Sequential changes of biochemical parameters after kidney transplantation. Nephrol Dial Transplant. 1998;13:436–42.
Parfitt AM. Hypercalcemic hyperparathyroidism following renal transplantation: differential diagnosis, management and implications for cell population control in the parathyroid gland. Miner Electrolyte Metab. 1982;8:92–112.
Rodino MA, Shane E. Osteoporosis after organ transplantation. Am J Med. 1998;104(5):459–69.
Ahmadpoor P, Reisi S, Ghafari MK, Sepehrvand N, Rahimi E. Osteoporosis and related risk factors in renal transplant recipients. Transplant Proc. 2009;4(7):2820–2.
Ozel L, Ata P, Ozel MS, Toros AB, Kara M, Unal E, et al. Risk factors for osteoporosis after renal transplantation and effect of vitamin D receptors Bsm I polymorphism. Transplant Proc. 2011;43(3):858–62.
Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544–50.
Bouquegneau A, Salam S, Delanaye P, Eastell R, Khwaja A. Bone disease after kidney transplantation. Clin J Am Soc Nephrol. 2016. doi:10.2215/CJN.11371015.
Naylor KL, Garg AX, Hodsman AB, Rush DN, Leslie WD. Long-term changes in bone mineral density in kidney transplant recipients. Transplantation. 2014;98(12):1279–85.
Sukumaran Nair S, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC. Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the United States. Am J Transplant. 2014;14(4):943–51.
Naylor KL, Jamal SA, Zou G, McArthur E, Lam NN, Leslie WD, et al. Fracture incidence in adult kidney transplant recipients. Transplantation. 2016;100(1):167–75.
Sprague SM, Josephson MA. Bone disease after kidney transplantation. Sem Nephrol. 2004;24(1):82–90.
Nikkel LE, Mohana S, Zhang D, McMahon J, Boutroy G, Dube G, et al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant. 2012;12(3):649–59.
Grotz WH, Munddinger FA, Gugel B, Exner V, Girste G, Schollmeyer AJ. Bone fracture and osteodensitometry with dual energy x-ray absorptiometry in kidney transplant recipients. Transplantation. 1994;58(89):912–5.
Ketteler M, Elder GJ, Evenepoel P, Ix JH, Jamal SA, Lafage-Proust M-H, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease—mineral and bone disorder: a commentary from a kidney disease: improving global outcomes controversies conference. Kidney Int. 2015;87(3):502–28.
Wong J, Tan MZ-W, Chandran M. Fifty shades of gray: bone disease in renal transplantation. Proc Singap Healthc. 2015;24(4):225–32.
Nikkel LE, Iyer SP, Mohan S, Zhang A, MacMahon DJ, Tanriover B, et al. Pancreas-kidney transplantation is associated with reduced fracture risk compared to kidney alone transplantation in men with type 1 diabetes. Kidney Int. 2013;63(3):471–8.
Chadban S, Chan M, Fry K, Parwardhan A, Ryan C, Trevillian P, et al. Nutritional interventions for the prevention of bone disease in kidney transplant recipients. Nephrology. 2010;15 Suppl 1:S43–7.
Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipient. Cochrane Database Syst Rev. 2007;18(3):CD005015.
Torres A, Garcia S, Gomez A, Gonzalez A, Barrios Y, Concepcion MT, et al. Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int. 2004;65(2):705–12.
Romano G, Lorenzon E, Montanaro D. Effects of exercise in renal transplant recipients. World J Transplant. 2012;2(4):46–50.
Gordon EJ, Prohaska T, Skminoff LA, Minich PJ, Sehgal AR. Needed: tailored exercise regimens for kidney transplant recipient. Am J Kidney Dis. 2005;45(4):769–74.
Bellizzi V, Cupisti A, Capitanini A, Calella P, D’Alessandro C. Physical activity and renal transplantation. Kidney Blood Press Res. 2014;39(2–3):212–9.
Kidney Disease: Improving Global Outcomes Work Group. Management of progression and complications of CKD. In: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3(Suppl 1):73–90.
Painter PL, Hector L, Ray K, Lynes L, Dibble S, Paul SM, et al. A randomized trial of exercise training after renal transplantation. Transplantation. 2002;74(1):42–8.
Zelle DM, Corpeleijn E, Stolk RP, de Greef MHG, Gans ROB, van der Heide JJH, et al. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(4):898–905.
Giannini S, D’Angelo A, Carraro G, Nobile M, Rigotti P, Bonfante L, et al. Alendronate prevents further bone loss in renal transplant patients. J Bone Miner Res. 2001;16(11):2111–7.
Fan SL-S, Almond MK, Ball E, Evans K, Cunningham KJ. Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int. 2000;57(2):684–90.
Coco M, Glicklich D, Faugere MC, Burris L, Bogner I, Durkin P, et al. Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol. 2003;1(10):2669–76.
Fratianni CM. Osteoporosis in solid organ transplantation: overview of treatment 2013. http://emedicine.medscape.com/article/128108-overview. Accessed 2 Mar 2016.
Kan S-L, Ning G-Z, Chen L-X, Zhou Y, Feng S-Q. Efficacy and safety of bisphosphonates for low bone mineral density after kidney transplantation: a meta-analysis. Medicine. 2016;95(5):2679. doi:10.1097/MD.0000000000002679.
Bonani M, Frey D, Brockmann J, Fehr T, Meuller TF, Saleh L, et al. Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: a randomized controlled trial. Am J Transplant. 2016;16(6):1882–91.
Cejka D, Benesch T, Krestan C, Roschger P, Klauschofer K, Pietschmann P, et al. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant. 2008;8(9):1864–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Oleson, C.V., Morina, A.B. (2017). Osteoporosis as a Complication of Transplant Medicine. In: Osteoporosis Rehabilitation. Springer, Cham. https://doi.org/10.1007/978-3-319-45084-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-45084-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45082-7
Online ISBN: 978-3-319-45084-1
eBook Packages: MedicineMedicine (R0)