Abstract
Introduction
The purpose of this study was to evaluate the effects of alendronate (ALN) on bone mineral density (BMD) and bone turnover markers in patients with orthotopic liver transplantation (OLT).
Methods
In the prospective, controlled, open study with 24 months of follow-up, 98 patients with OLT were randomised to receive ALN 70 mg weekly or no ALN; calcium (Ca) 1,000 mg daily and 0.5 mcg calcitriol daily were provided to all patients. Lumbar spine (LS) and hip BMDs were measured at 6-month intervals by dual-energy X-ray absorptiometry (DEXA). Spinal radiographs were obtained to assess vertebral fractures. Additionally, bone turnover markers, serum parathyroid hormone (PTH) and biochemical parameters were determined every 3 months.
Results
Compared with the control group, the ALN group showed significant increases in BMD of the LS (5.1±3.9% vs 0.4±4.2%, p<0.05 at 12 months, 8.9±5.7% vs 1.4±4.9%, p<0.05 at 24 months), femoral neck (4.3±3.8% vs −1.1±3.1%, p<0.05 at 12 months, 8.7±4.8% vs 0.6±4.5%, p<0.05 at 24 months) and total femur (3.6±3.8% vs −0.6±4.0%, p<0.05 at 12 months, 6.2±3.8% vs 0.3±4.6%, p<0.05 at 24 months). In the ALN group, osteocalcin and urinary deoxypyridinoline (DPD) decreased significantly at the sixth month, with no further change, by −35.6% and −63.0%, on average, respectively (p<0.05). In the control group, a significant increase in biochemical markers of bone turnover was observed in comparison to baseline values (p<0.05). PTH increased within reference levels without a difference between groups. Two nonvertebral fractures (4.2%) and nine vertebral fractures (18.8%) in the control group and three vertebral fractures (6.8%) in the ALN group occurred during the follow-up. The weekly ALN was well tolerated, and no severe side effects occurred.
Conclusion
This is the first randomised study including a control group to demonstrate that weekly ALN was able to significantly increase BMD in patients with OLT when compared with Ca and calcitriol alone. However, ALN did not appear to offer protection against fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthotopic liver transplantation (OLT) has become an effective and established therapy for end-stage liver disease. It has improved the life expectancy in these patients; however, the posttransplantation period is associated with complications such as osteoporosis [1–5]. Although the pathogenesis has not yet completely been elucidated, the use of high-dose corticosteroids and other immunosuppressive drugs appears to be the main factor in the development of osteoporosis after OLT [6, 7]. Furthermore, other risk factors for posttransplantation osteoporosis include immobility and preexisting diseases, including vitamin D deficiency, hypogonadism, cholestatic liver disease and alcoholism [1–4, 8, 9].
Most patients are affected by osteoporosis, particularly within the first 3–6 months [1, 8–10], and the prevalence of fracture can be as high as 65% in the first 6 months [11]. These fractures after OLT must be prevented since they are associated with a high level of morbidity and usually decrease the quality of life of those patients.
A variety of agents is now being used for management of posttransplantation osteoporosis. Bisphosphonates, which are analogues of inorganic pyrophosphate and inhibit bone resorption, have been shown to be safe and effective in treatment of postmenopausal and glucocorticoid-induced osteoporosis [12, 13]. Alendronate (ALN) is a potent bisphosphonate recently introduced into clinical practice for treatment of osteoporosis-related liver diseases [14]. It has a relatively high antiresorptive potency and can be more appropriate for high bone turnover after OLT. However, there is no controlled trial of this agent in the management of transplant recipients.
The primary objective of the present study was to evaluate the effects of ALN on bone mineral density (BMD) and bone turnover markers and fracture risk in patients with OLT. Secondly, we aimed to determine whether biochemical analyses and parathyroid hormone (PTH) are affected by 2 years of ALN treatment in these patients.
Materials and methods
Patients
In the prospective, controlled, open study with 24-months' of follow-up, 98 patients with cirrhosis awaiting their first OLT between January 2002 and January 2003 were recruited. Exclusion criteria were as follows: those taking any drug that can affect bone metabolism (e.g. bisphosphonates, oestrogen or oestrogen-related drugs, vitamin D supplements, calcium), including any treatment with glucocorticoids prior to the current study, presence of chronic renal failure defined as the presence of serum creatinine >3.0 mg/dl and/or creatinine clearance <30 ml/min. Female patients were at least 1 year postmenopausal, surgically sterile or would not give birth. All patients gave their written informed consent.
Immunosuppression
Immunosuppression was established with a combination of prednisone and calcineurin inhibitors (cyclosporine or tacrolimus). Prednisolone was given at a dosage of 500 mg intravenously (i.v.) in the operation room, followed by a taper from 100 mg to 20 mg for a period of 8 days and from 20 mg to 10 mg after 2 months. Steroids were stopped in a 6-month to 1-year period. Rejection episodes were treated by increasing the dose of calcineurin inhibitors; pulse steroid (500 mg/day) treatment was administered if that dose adjustment failed.
The dose of tacrolimus was adjusted according to blood levels, which aimed to be 5–10 ng/ml to a target a whole blood level of 5–10 mg/day. Cyclosporine A was administered to reach 200–300 ng/ml during the first month after operation and then tapered to maintain blood concentrations of 100–200 ng/ml. Patients with autoimmune disease or those who experienced severe rejection under cyclosporine A treatment received mycophenolate mofetil as the third immunosuppressive.
Study design
At the first visit, physical examination and clinical assessment were performed, and patients were asked to complete a comprehensive questionnaire, which focused on demographic data and liver-disease-related history. Patients were seen as a baseline visit and were randomised in a 1:1 ratio to receive either ALN 70 mg weekly or no ALN in the first month after OLT. All patients were given calcium 1,000 mg daily and calcitriol 0.5 mcg daily. Patients were instructed to take the ALN on an empty stomach 30 min before breakfast with two glasses of water and avoid lying down for 30 min after taking the tablet. All patients were required to take calcium (Calcium Sandoz, Novartis) daily with dinner and calcitriol (Rocaltrol- Roche) with breakfast.
After the baseline visit, patients were evaluated at 3-month intervals. At each clinic visit, patients were questioned about the occurrence and nature of adverse events. They were also questioned about the compliance with ALN. Patient-reported adverse events were recorded as mild, moderate and severe.
Methods
BMD of the lumbar spine (L1–L4) and proximal femur was measured by dual- energy X-ray absorptiometry (DEXA) using a Hologic QDR 4500A apparatus (Hologic, Waltham, MA, USA) at baseline and every 6 months for 2 years. In our laboratory, the coefficient variation is 1.0% for both lumbar spine and total femur sites. Results were expressed in g/cm2. Standard radiographs of the thoracic and lumbar spine were obtained at the first and second year to assess incident vertebral fracture and also when clinical symptoms suggested a vertebral fracture. The evaluation was done with two independent observers by using criteria from a previously described, visual, semiquantitative technique as a sensitive and reliable method [15–17]. Mild, moderate and severe fractures were defined as a relative height reduction in a vertebral body (anterior middle or posterior heights) of >20% and ≤25%, >25% and ≤40%, and >40%, respectively. Nonvertebral osteoporotic fractures were confirmed by a radiologist’s written report. The following fractures were excluded from the analysis: all pathologic fractures; fractures that clearly resulted from automobile accidents or other severely traumatic accidents; and fractures of the skull, face, metacarpals, fingers and toes.
Biochemical markers of bone turnover, PTH and biochemical assays were performed at baseline and at 3-month intervals. Blood and urine samples were collected from all patients after an overnight fast. Blood specimens were analysed for creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total alkaline phosphatase (TALP), albumin, serum calcium and phosphate, and 24-h urinary calcium and phosphate, which were all measured by standard automated techniques. Serum osteocalcin as a bone formation marker was measured by immunometric assay (IMMULITE Analyzer, DPC’s Technical Services Department, USA). Urine deoxypyridinoline (DPD) as an aid in monitoring type 1 collagen resorption changes was measured by chemiluminescent enzyme-labelled immunoassay (IMMULITE Analyzer, Metra Biosystems, DPC’s Technical Services Department, USA). Samples were stored at −70°C. Serum-intact PTH was measured by immunoradiometric assay (IDS, UK). The intra-assay and interassay variation coefficients were lower than 10% for all biochemical assays.
Statistical analysis
Baseline demographic and clinical characteristics were compared using independent samples t test (continuous data) and Fisher’s exact test (dichotomous data). The main efficacy analysis was the percentage of change from baseline in the lumbar spine BMD. Treatment groups were compared by independent samples t test. One-sample t test was used for within-group analysis for percentage of change from baseline in BMD. In addition, paired t tests were used to compare baseline BMD to follow-up BMD values within treatment groups. The repeated measurements analysis of variance was used to evaluate over the time of observation for biochemical and hormonal parameters. The Friedman test was done separately for each study group to test for changes over time with respect to abnormal distributed variables according to Kolmogow-Simirnov test. The Wilcoxon test was additionally performed when the Friedman test was significant. Incident vertebral and nonvertebral fractures between the groups were compared using Fisher’s exact test.
Statistical analyses were performed with the 10.0 Statistical Package for the Social Sciences (SPSS). All results were expressed as mean ± standard deviation (SD). A p value below 0.05 was considered to indicate statistical significance.
Results
Table 1 summarises patients’ characteristics at the start of the trial. There were no statistically significant differences in demographic and clinical data between the groups.
Two patients in the ALN group and one in the control group died in the first 3 month after the operation. In the ALN group, two patients (after 1 and 3 months) discontinued the study due to persistent gastrointestinal distress, and one was excluded from the study because of noncompliance. The remaining 44 patients in the ALN group and 48 patients in the control group completed the study.
Bone mineral density measurements
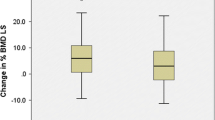
At 1 and 2 years, the mean percent increases in lumbar spine BMD in the patients treated with ALN, calcium and calcitriol were significantly greater than those in the patients treated with calcium and calcitriol alone (Fig. 1). The mean percent increases in the hip (neck and total femur) were also significantly greater in the ALN group (Fig. 1). At the 12th month, the mean percentage changes in BMD at lumbar spine, femoral neck and femoral total were 5.1±3.9% (p<0.05), 4.3±3.8% (p<0.05) and 3.6±3.8% (p<0.05) in the ALN group vs 0.4±4.2%, −1.1±3.1% (p<0.05) and −0.6±4.0% in the control group. At the 24th month, the ALN group showed significant increases in BMD by 8.9±5.7% (p<0.05), 8.7±4.8% (p<0.05) and 6.2±3.8% (p<0.05) at lumbar spine, femoral neck and femoral total, respectively, compared with an increase of 1.4±4.9% (p<0.05), 0.6±4.5% and 0.3±4.6% in the control group.
Biochemical markers of bone turnover and laboratory analyses
Table 2 shows the biochemical and hormonal parameters during the follow-up. In the ALN group, serum osteocalcin and urinary DPD decreased notably according to baseline values as −35.6% (p<0.05) and −63.0% (p<0.05), respectively. There was no further change after 6 months of ALN treatment during the 2-year period. On the other hand, in the control group, serum osteocalcin and urinary DPD increased 30% (p<0.05) and 15% (p<0.05), respectively, according to baseline values during the course of the study. All PTH levels were in normal range during the study. However, PTH significantly increased according to baseline after 3 months in both groups (p>0.05). During the study, there was no change in serum levels of calcium and phosphate in either group.
Although mean ALT and AST values were above the reference values after OLT in both groups, they decreased to normal values from 6 months of the follow-up (p <0.05 vs baseline). No additional difference was seen in the other biochemical parameters in either group.
Fractures
Two nonvertebral fractures (ankle and distal radius) were reported in two (4.2%) patients of the control group while there was no nonvertebral fracture in the ALN group. Seven (14.6%) patients had nine new vertebral fractures in the control group while three (6.8%) showed new vertebral fractures in the ALN group during the study (Table 3). Among these fractures, two in the control group and one in the ALN group were clinical; the others were seen on radiographs. There was no significant difference between the groups (Fisher’s exact test).
Adverse events
The most common adverse effects were musculoskeletal pain (38.6% of patients) and upper gastrointestinal adverse events (29.5% of patients) in the ALN group. The majority of adverse effects were reported as mild or moderate during the follow-up.
Discussion
This is the first randomised and controlled study that has demonstrated the weekly administration of ALN can prevent bone loss after OLT. During the follow-up, the ALN group showed significant increases in BMD at the lumbar spine that were greater than those seen in the control group who received calcium 1,000 mg/day and 0.5 mcg alone. Also, significant increases were seen in BMD of the hip (neck and total femur) in the ALN group. However, patients in the control group lost an average of 1.2% in BMD of hip at the sixth month of OLT (Fig. 1). These findings pointed to the rapid bone loss in the early posttransplantation period, as previously described [1, 8–10].
It has been reported that calcium is not sufficiently effective in preventing bone loss in patients initiating glucocorticoid therapy [18, 19]. Even though our patients received supplementation of calcitriol with calcium in the control group, these findings were similar with those of previous data, suggesting that potent antiresorptive agents may be needed in glucocorticoid osteoporosis [18–21]. Indeed, glucocorticoids play a major role in bone loss although pathogenesis of osteoporosis after solid organ transplantation is multifactorial [6, 7]. In a recent prospective study, early steroid withdrawal was reported to result in significant decrease in occurrence of osteoporosis after heart and kidney transplantation [22].
ALN is a potent antiresorptive agent that significantly increases BMD at each site. On the other hand, regarding the rate of fracture, we did not find a significant difference between the groups. However, a crucial point is that nonvertebral fracture resulting in significant pain and disability with a high morbidity did not develop in the ALN group while two nonvertebral fractures occurred in the control group. The small number of fractures limits the strength of the fracture analysis, which makes it difficult to assess the real magnitude of the difference between the groups suggesting whether or not calcium and calcitriol treatment without ALN is sufficient in the prevention of fractures in those patients. Also, it can be argued that fracture rate is less common than previously reported in the posttransplantation period. Ninkovic et al. [23] reported the same argument in their study showing no significant beneficial effects of pamidronate in the prevention of fractures at the first year after transplantation. These results suggest that evidence from randomised controlled clinical trials in a large series is needed to demonstrate the clinical efficacy of ALN on prevention of fractures in patients with liver transplantation.
On the other hand, supporting our results, previous studies with other bisphosphonates (pamidronate and etidronate) reported a significant increase in BMD by preventing bone loss in patients undergoing OLT [24–27]. A recent prospective, long-term study also reported antifracture effects of ALN during the first 2 years after OLT [14]. However, there was no control group in that study. Our experience prior to the present study, which also showed beneficial effects of ALN in these patients, prompted the present project since the control group increases the power of the study to demonstrate significant effects.
As expected, treatment with ALN reduced the rate of bone turnover, as was evidenced by a reduction on average of 35.6% and 63.0% of osteocalcin and DPD, respectively. On the other hand, a significant progressive increase was seen in bone turnover markers in the control group. Previous studies that included histomorphometric analysis of bone biopsies in the posttransplantation period demonstrated an increase in bone formation and resorption, reflecting the high rate of bone turnover in this population [8, 28, 29]. Also, it has been reported that osteocalcin levels increased significantly at the first month of the posttransplantation period and remained above reference values during the 3 years [28]. Our findings, in accordance with these reports, support that bisphosphonates seem to be more appropriate antiosteoporotic agents due to their high antiresorptive potency for these patients based on the known high bone turnover after transplantation.
Weekly ALN was a safe treatment and well tolerated in these patients and improved patient compliance with less frequent administration. The number of patients withdrawing due to adverse events was low. Although myalgia was reported as the most frequent adverse event, all of these were mostly mild and did not lead to drug withdrawal. More importantly, laboratory analyses did not reveal any significant difference between groups during follow-up suggesting a drug-related event. On the other hand, PTH progressively increased in all patients without any difference between groups, but this increase was not above the limit. It may be a result of secondary hyperparathyroidism due to cyclosporin-induced renal function impairment, as descrived previously [8, 30–32].
In summary, this study demonstrates that ALN provides an increase in BMD during the 2 years after liver transplantation and is well tolerated without deleterious effects on liver function tests. Nevertheless, these results have to be confirmed by further placebo-controlled studies on this population to establish definitive efficacy and safety.
References
Cheung AM (2001) Post-liver transplantation osteoporosis. J Hepatology 34:337–338
Delmas PD (2001) Osteoporosis in patients with organ transplants: a neglect problem. Lancet 357:325
Rodino MA, Shane E (1998) Osteoporosis after organ transplantation. Am J Med 104:459–469
Ramsey- Goldman R, Dunn JE, Dunlop DD, et al (1999) Increased risk of fracture in patients receiving solid organ transplant. J Bone Miner Res 14:456–461
Crippin JS (2001) Bone disease after liver transplantation. Liver Transpl 7:27
Epstein S (1996) Post-transplantation bone disease. The role of immunsuppressive agents on the skeleton. J Bone Miner Res 11:1–7
Smallwood GA, Wickman JM, Martinez E, et al (2002) Osteoporosis screening in an outpatient liver transplant clinic: Impact of primary immunsuppression. Transplant Proc 34:1569–1570
McDonald JA, Dunstan CR, Dilworth P, et al (1991) Bone loss after liver transplantation. Hepatology 14:613–619
Porayko MK, Wiesner RH, Hay JE, et al (1991) Bone disease in liver transplant recipients: Incidence, timing, and risk factors. Transplant Proc 23:1462–1465
Riemens SC, Oostdijk A, van Doormaal JJ, et al (1996) Bone loss after liver transplantation is not prevented by cyclical etidronate, calcium and alphacalcidiol. Osteoporos Int 6:213–218
Eastell R, Dickson ER, Hodgson SF, et al (1991) Rates of vertebral bone loss before and after liver transplanttaion in women with primary biliary cirrhosis. Hepatology 14:296–300
Hochberg MC, Ross PD, Black D, et al (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 42:1246–1254
Shiraki M, Kushida K, Fukunaga M, et al (1998) A placebo-controlled, single blind study to determine to appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis. The Alendronate Research Group. Endocr J 45:191–210
Millonig G, Graziadei IW, Eichler D, et al (2005) Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation: A prospective single-center study. Liver Transpl 11(8):960–966
Genant HK, Wu CY, van Kuijk C, et al (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Wu CY, Li J, Jergas M, et al (1995) Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 5(5):354–370
Genant HK, Jergas M, Palermo L, et al (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11(7):984–996
Sambrook P, Birmingham J, Kelly P, et al (1993) Prevention of corticosteroid osteoporosis-a comparison of calcium, calcitriol, and calcitonin. N Engl J Med 328:1747–1752
Cohen S, Levy RM, Keller M, et al (1999) Risedronate therapy prevents corticosteroid-induced bone loss. Arthritis Rheum 42(11):2309–2318
Adachi JD, Bensen WG, Brown J, et al (1997) Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 337:382–387
Adachi JD, Bensen WG, Bianchi F, et al (1996) Vitamin D and calcium in the prevention of corticosteroid induced osteoporosis: a 3 year followup. J Rheumatol 23:995–1000
van den Ham EC, Kooman JP, Christiaans ML (2003) The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transp Int 16(2):82–87
Ninkovic M, Love S, Tom BD, et al (2002) Lack of effect of intravenous pamidronate on fracture incidence and bone mineral density after orthotopic liver transplantation. J Hepatol 37(1):93–100
Shane E, Thys- Jacobs S, Papadopoulos A, et al (1996) Antiresoptive therapy prevents bone loss after cardiac transplantation (CTX). J Bone Miner Res 11:635
Fan S, Almond MK, Ball E, et al (1996) Randomised prospective study demonstrating prevention of bone loss by pamidronate during the first year after renal transplantation. J Am Soc Nephrol 7:A2714
Reeves HL, Francis RM, Manas DM, et al (1998) Intravenous bisphosphonate prevents symptomatic osteoporotic vertebral collapse in patients after liver transplantation. Liver Transpl Surg 4:404–409
Dodidon P, Bruckner T, Hosch S, et al (2003) Better late than never? Experience with intravenous pamidronate treatment in patients with low bone mass or fractures following cardiac or liver transplantation. Osteoporos Int 14:82–89
Monegal A, Navasa M, Guanabens N, et al (2001) Bone Disease after liver transplantation: A long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int 12:484–497
Vedi S, Greer S, Skingle SJ, et al (1999) Mechanism of bone loss after liver transplantation: A histomorphometric analysis. J Bone Miner Res 1999 14(2):281–287
Feller RB, McDonald JA, Sherbon KJ, et al (1999) Evidence of continuing bone recovery at a mean of 7 years after liver transplantation. Liver Transpl Surg 5:407
Crosbie OM, Freaney R, McKenna MJ, et al (1999) Predicting bone loss following orthotopic liver transplantation. Gut 44:430–434
Monegal A, Navasa M, Guanabens N, et al (1997) Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int 60:148–154
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was awarded the “Novartis Young Investigator Award” at the Second Joint Meeting of the European Calcified Tissue Society and the International Bone and Mineral Society, Geneva, 25–29 June 2005.
Rights and permissions
About this article
Cite this article
Atamaz, F., Hepguler, S., Akyildiz, M. et al. Effects of alendronate on bone mineral density and bone metabolic markers in patients with liver transplantation. Osteoporos Int 17, 942–949 (2006). https://doi.org/10.1007/s00198-006-0082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0082-5