Abstract
-
1.
To review the radiologic anatomy that is pertinent toward the safe performance of thoracic spine biopsy
-
2.
To review the indications and contraindications for thoracic spine biopsy
-
3.
To learn image-guided percutaneous thoracic spine biopsy approaches and techniques
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Anatomy, thoracic spine
- Biopsy, disk
- Biopsy, paraspinal soft tissues
- Biopsy, vertebrae
- Thoracic spine biopsy, coaxial technique
- Imaging guidance, computed tomography
- Imaging guidance, fluoroscopy
- Percutaneous spine biopsy, complications
- Percutaneous spine biopsy, indications
- Percutaneous spine biopsy, contraindications
- Spine biopsy, thoracic spine
-
1.
To review the radiologic anatomy that is pertinent toward the safe performance of thoracic spine biopsy
-
2.
To review the indications and contraindications for thoracic spine biopsy
-
3.
To learn image-guided percutaneous thoracic spine biopsy approaches and techniques
5.1 Introduction
Image-guided percutaneous thoracic spine biopsies are the second most commonly performed biopsy procedure of the spinal axis, second to lumbar spine biopsies (Rimondi et al. 2008; Heyer et al. 2008). The proximity to the lungs can be a source of major concern for operators who perform this procedure. Fortunately, the presence of consistent osseous landmarks, including the posterior ribs and their articulation with the vertebral body, can be used to avoid injuring the lung. A sound understanding of the radiologic anatomy of the thoracic spine can assist in enhancing the safety margin of this procedure. In general, posterior approaches are required to access the thoracic vertebrae, as the overwhelming majority of lesions are usually located within the vertebral body and/or pedicle, with a lesser proportion of lesions seen within the intervertebral disk and even fewer lesions in a paraspinal soft tissue location. The biopsy needle size and number of passes may be limited depending on the lesion size, extent, and location within the thoracic spine. While this may increase the challenge of the image-guided percutaneous spine biopsy procedure, thoracic spine biopsy is a procedure that is associated with a high diagnostic yield, 92% in one large series (Rimondi et al. 2008). Most thoracic spine biopsies are performed at the mid or lower thoracic spine levels. The majority of thoracic spine biopsies are requested to assess for the possibility of a neoplastic process. The suspected thoracic spine abnormality is often identified on an MRI examination. The increased use of post-treatment imaging surveillance with PET-CT has also resulted in the identification of spine lesions that may require additional evaluation. Whenever possible, these prior studies should be carefully scrutinized to determine if alternative, and possibly safer, biopsy sites within the lumbar spine, sacrum, or pelvis are present. When thoracic spine biopsy is indicated, an understanding of the pertinent radiologic anatomy, the possible approaches to the target lesion, and the available biopsy devices and techniques will increase the efficiency, safety, and success of the image-guided percutaneous thoracic spine biopsy procedure.
5.2 Anatomic Considerations
There are usually 12 rib-bearing thoracic vertebrae. In the presence of a transitional vertebral anatomy at the thoracolumbar junction, 11 or 13 thoracic vertebrae may occasionally be encountered. It is critical that the operator and the diagnostic radiologist be vigilant to the occurrence of these findings when present, in order to prevent the possibility of a wrong-level biopsy. Counting the vertebral bodies starting from the cervical spine can be helpful as there are seven cervical segments (Carrino et al. 2011). Alternatively, the counting scheme for the biopsy procedure should match the numbering scheme on the pre-procedure studies that identify the site and vertebral level of the lesion in question (Fig. 5.1). As occurs in the lumbar spine, the thoracic vertebrae share morphologic features in common from the first thoracic vertebra to the twelfth thoracic vertebra (Fig. 5.2). The major change that occurs within the thoracic spine as the vertebrae are studied from cranial to caudal is that the vertebral bodies get slightly larger within the lower thoracic spine. This also includes the size of the thoracic pedicles; for example, the T12 pedicles are larger than the T1 pedicles. This observation likely reflects the increased biomechanical load that the lower thoracic vertebrae are exposed to. Unlike the lumbar spine, where the pedicles are obliquely angled toward the vertebral body, the thoracic pedicles maintain a relatively tangential orientation toward the vertebral body. This orientation of the thoracic pedicles, as well as their size, must always be taken into account when considering a transpedicular approach for thoracic spine biopsy. Some thoracic spine lesions, therefore, especially lesions within the posterior median aspect of the vertebral body, may not be accessible with a transpedicular approach. The rib articulates with a vertebra at two junctures, posterior at the transverse process or costotransverse articulation and, more anteriorly, at the posterior aspect of the lateral vertebral body or costovertebral junction. Each vertebra consists of the posterior elements which form the neural arch and include the spinous process, lamina, articular facets, transverse process, and pedicles. The pedicles connect the posterior elements to the vertebral body.
Counting and labeling thoracic vertebral levels can be performed with scout MR or CT images or with fluoroscopy. This becomes particularly important when dealing with subtle thoracic lesions or when sampling thoracic disks. T2-weighted sagittal scout image (a) of the cervical and upper thoracic spine; C2 and T1 are labeled and a partial vertebral compression deformity is present at T7 (arrow). T2-weighted sagittal scout image (b) of thoracic and lumbar spine shows the T7 vertebral compression deformity (curved arrow) with marrow edema and three additional mild vertebral compression deformities (arrows) with marrow edema; T12, L5, and S1 (with marrow edema) are labeled
CT anatomy of the thoracic spine. Reformatted midline sagittal CT image (a) shows progressive enlargement of thoracic vertebrae moving in a caudal direction; compare T3 to T12. Axial CT image (b) from contrast enhanced CT study at the T4 level shows the vertebral body (T4), pedicle (p), lamina (L), spinous process (sp) and costovertebral junction (curved arrow). Key anterior structures include the lung, esophagus (E), trachea (T) and aortic arch (Aa). Axial CT image (c) from contrast-enhanced CT study at the T8 level shows the costotransverse (CTr) and costovertebral (CV) articulations. The aorta (A) is well visualized even on bone window algorithm. Note the tangential orientation (dashed line) of the pedicle relative to the vertebral body. Axial CT image (d) from contrast-enhanced CT study at the T12 level shows a more prominent pedicle and a larger vertebral body; the costovertebral articulation is noted (arrow); the transverse process is rudimentary; the aorta (A) is well visualized. Axial CT image (e) from same study in soft tissue algorithm at the T2 level shows the spinal cord (sc) within the spinal canal and the exiting nerve root (curved arrow) within the neural foramen; critical structures at the level of the cervicothoracic junction include the lung apex, the esophagus (E), and the trachea (T); the great vessels lie anterior and lateral to the latter structures
When counting the vertebrae within the spinal axis, the possible presence of a transitional vertebra at the thoracolumbar junction should be considered in order to prevent a wrong-level thoracic spine biopsy procedure.
The critical structures that partially surround the thoracic spine are best understood in the context of their anatomic relations. The posterior elements of the thoracic vertebra are surrounded by the posterior paraspinal musculature which includes the erector spinae muscles. The neural arch, medial pedicle cortex, and posterior vertebral body cortex form the margins of the spinal canal. The spinal canal includes the epidural space, which contains fat and a venous plexus, and the meningeal lined spinal cord which is surrounded by cerebrospinal fluid. Spinal nerves and vascular structures pass through the neural foramina that are located between the pedicles of adjacent vertebral bodies. These vascular structures include branches of the intercostal arteries, some of which provide blood supply to the spinal cord. The lateral relations of the thoracic spinal column include the lungs and pleura. The anterior relations of the spinal column include the posterior mediastinum and mediastinum. The aorta is located anterior and to the left with respect to the vertebral column. The sympathetic plexus lies anteriorly and laterally along the vertebral column. When deciding upon the feasibility of an image-guided percutaneous thoracic spine biopsy procedure, the operator must always factor these critical structures into their approach.
Critical structures at the thoracic spine level |
|---|
Spinal cord |
Lung |
Aorta |
Intercostal vessels |
5.3 Indications
Image-guided percutaneous thoracic spine biopsy is indicated for the evaluation of pathologic lesions that are located within the thoracic vertebrae, intervertebral disks, and/or adjacent paraspinal soft tissues (Table 5.1). The two most common indications for performing thoracic spine biopsy are evaluation of a neoplastic process and spine infection. Neoplastic processes within the thoracic spine are usually secondary lesions associated with metastatic disease, multiple myeloma, or lymphoma. Primary tumors within the thoracic spine, though uncommon, may also require a biopsy procedure. A biopsy may also be required in order to distinguish between a pathologic and an osteoporotic vertebral compression fracture (Figs. 5.3 and 5.4). As with all clinically indicated invasive interventions, the biopsy result should clearly influence the clinical management of the patient. This is the primary benefit of the biopsy procedure. If this benefit will not be achieved with the requested procedure, then it might not be necessary to subject the patient to an invasive procedure. The importance of reviewing all imaging studies prior to considering a thoracic spine biopsy is again emphasized. The remainder of the spinal axis and body should be studied in order to identify other possible sites, for example, within the lumbar spine, sacrum, or pelvis, which can be more safely sampled (Fig. 5.5).
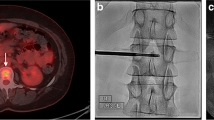
A 65-year-old female with history of breast cancer and back pain. Single posterior projection from a whole body bone scan (a) shows focal radionuclide uptake (arrow) within the mid-thoracic spine. T2-weighted sagittal image (b) shows a partial vertebral compression deformity with marrow edema (arrow) and bone retropulsion into the spinal canal. Focal inferior vertebral endplate edema and disk edema (curved arrow) are seen above the vertebral compression deformity. Reformatted sagittal CT image (c) in bone window algorithm shows a partial vertebral compression deformity (arrow) with loss of the trabecular striations and anterior bone formation. Axial CT image (d) from the bone biopsy procedure shows the tip of a bone needle (arrow) within the predominantly lytic vertebral body lesion; scant tissue was obtained with this needle. Axial CT image (e) shows a soft tissue cutting needle within the lesion. Multiple soft tissue cores confirmed the presence of metastatic breast cancer in this patient with a pathologic vertebral compression fracture
A 77-year-old female with chronic, severe low back pain. Reformatted sagittal CT image (a) in bone window algorithm shows a vertebra plana deformity of the T12 vertebral body (arrow) with small amounts of gas with the anterior vertebral body and adjacent disk space; mild bone retropulsion into the spinal canal is noted as is focal kyphosis. Marked osteopenia is seen. Biplane fluoroscopic images (b) in the frontal and lateral projection show coaxial advancement of a bone biopsy needle into the pedicle (arrow). Lateral fluoroscopic image (c) shows further advancement of the bone needle into the posterior vertebral body (curved arrow); the vertebral endplates (arrows) show the severity of this collapse, which limits advancement of the bone needle. The biopsy samples showed no evidence of malignant cells in this patient with an osteoporotic vertebral compression fracture
A 7-year-old female with back pain (same patient as in Fig. 5.1). T1-weighted sagittal scout image (a) of the thoracic and lumbar spine shows multiple thoracic vertebral compression deformities and hypointense vertebral body marrow signal within the T9 and S1 vertebra (arrows). Axial CT image (b) shows biopsy of the S1 vertebra (arrow); 5 bone cores were submitted to pathology. The biopsy was nondiagnostic
5.4 Contraindications
The major contraindication to performing a thoracic spine biopsy is uncorrected coagulopathy (Table 5.2). Special consideration is also given to patients with neurologic compromise and in whom the imaging findings are consistent with acute spinal cord compression. These patients will require immediate surgical decompression of the spine, and this important intervention should not be delayed by a spine biopsy procedure. As with image-guided percutaneous spine biopsy procedures in the other segments of the spinal axis, thoracic spine biopsy procedures should not be performed on unstable patients. Given the risk of spinal cord or lung injury, the procedure should be avoided in uncooperative patients. As there are 12 thoracic vertebrae, the possibility of occurrence of benign lesions with certain pathognomonic imaging features is not uncommon in the thoracic spine. Bone islands and Schmorl’s nodes may not require a biopsy procedure. For probably benign lesions, it may also be helpful to obtain and review the patient’s prior studies, if available, or clinically correlate the finding and perform follow-up imaging surveillance only if deemed clinically necessary.
5.5 Risks and Complications Associated with Thoracic Spine Biopsy and How to Minimize Them
The risks and complications that are associated with image-guided percutaneous thoracic spine biopsy are similar to those observed with lumbar spine biopsy (Olscamp et al. 1997; Tehranzadeh et al. 2007) (Table 5.3). There are, however, two major differences between thoracic spine biopsy and biopsy in other segments of the spinal axis. First, the risk of pneumothorax takes on a greater priority when performing a thoracic spine biopsy. There is much greater surface area of lung parenchyma at risk in thoracic spine biopsy as compared to cervical or lumbar spine biopsies where the lung apices and bases, respectively, are at risk. Second, the thoracic spinal cord is a structure at risk when performing a thoracic spine biopsy. Careful attention to technique and utilization of the appropriate osseous landmarks whether using CT or fluoroscopic guidance will help to reduce the likelihood of a lung or spinal cord injury. Similarly, the use of coaxial technique, by incurring only one single pass with a guide cannula, will decrease the likelihood of injuring normal anatomic structures. All biopsy instruments can then be passed through the guide cannula multiple times without disturbing the surrounding soft tissue structures. Active monitoring of the location and excursion of these biopsy instruments will also enhance the margin of safety for any biopsy procedure (Fig. 5.6). Procedure-related hemorrhage can be mitigated by adhering to appropriate coagulation status protocols, holding anticoagulant or antiplatelet medications for an appropriate period of time, and using coaxial technique. In the thoracic spine, it is important to be extremely careful navigating needles adjacent to the anterior neural foramina and near the inferior margins of the posterior ribs as these are anatomic locations where normal vascular structures are located. Utilization of standard thoracic biopsy techniques such as the transpedicular, costotransverse, and costovertebral approaches help to reduce the likelihood of injury to these vascular structures.
A 7-year-old female with back pain, 1 month later. Axial CT image (a) from a T9 biopsy procedure shows advancement of a bone needle via a costotransverse approach (arrow). Axial CT image (b) shows needle tip at lateral margin of vertebral body (arrow); because of the steep nature of this approach, the needle tip points at the vertebral body, not the lung. Axial CT image (c) shows entry of the bone needle (curved arrow) into the posterior vertebral body. The beam hardening artifact distal to the needle provides a good estimate of the needle trajectory within the remainder of the vertebral body (dashed lines); the needle trajectory was adjusted at the point of insertion within the lateral vertebral cortex (compare to image b). A reformatted sagittal image obtained with CT fluoroscopy (d) shows the needle tip’s (curved arrow) relationship to the vertebral endplates and anterior vertebral cortex. Axial CT image (e) shows coaxial advancement of the bone needle (arrow) through the guide cannula (curved arrow) in order to obtain additional bone cores (a total of 6 bone cores). Compare this final trajectory with that estimated in Figure c. Subsequent histopathologic analysis showed Langerhans cell histiocytosis
5.6 Imaging Guidance
CT and fluoroscopy are the two modalities that are used to perform image-guided percutaneous thoracic spine biopsy (Lis et al. 2004; Ortiz et al. 2010). The key osseous landmarks for imaging guidance are visualized with both modalities. CT does provide additional soft tissue resolution to identify, with better detail, the critical structures in the thoracic spine. In general, many operators tend to use fluoroscopic guidance for transpedicular approaches in the thoracic spine, especially when attempting to sample large or diffuse lesions within the thoracic vertebral body (Fig. 5.7) (Pierot and Boulin 1999). More experienced operators will also use fluoroscopy to perform percutaneous disk biopsies utilizing a posterior oblique approach that keeps the needle between the medial aspect of the rib and the lateral aspect of the pedicle. The ability of CT to visualize the lesion and its relation to the vertebra and to nearby critical structures tends to make CT the preferred modality for image-guided percutaneous thoracic spine biopsy. It is particularly helpful in sampling the posterior elements and the paraspinal soft tissues (Fig. 5.8). CT fluoroscopy increases the efficiency of the procedure by allowing faster monitoring of needle advancement and position. Since both CT and fluoroscopic guidance entail radiation exposure, adherence to sound radiologic imaging and shielding principles will assist in limiting radiation exposure not only to the patient but also to the operator and the operator’s staff.
An 80-year-old male with history of prostate, colon, and lung cancer. Fat-suppressed contrast-enhanced T1-weighted sagittal image (a) shows multiple hypointense foci (curved arrows) with mild peripheral enhancement; the largest lesion (arrow) is located within the T10 vertebral body. Axial CT image (b) from a T10 biopsy procedure shows the use of coaxial technique and a costovertebral approach (curved arrow) with subsequent placement of a bone needle (arrow) into a large sclerotic lesion. Five bone cores were obtained with this 12 gauge system; the biopsy showed no evidence of malignant cells. T1weighted sagittal image (c) performed 3 months later shows progression of a vertebral marrow replacement process, particularly at T10 (arrow). Frontal fluoroscopic projection (d) shows transpedicular coaxial placement (arrow) of a trephine bone needle into the sclerotic T10 vertebral body. Lateral fluoroscopic image (e) shows a guide cannula (arrow) within the distal pedicle and a biopsy needle (curved arrow) within the anterior vertebral body. Nine small bone cores were obtained in this procedure, and pathology revealed metastatic prostate cancer
A 56-year-old male with thoracic back pain. Single posterior projection (a) from a bone scan shows focal radionuclide uptake within a single thoracic vertebra (arrow), T6. Contrast-enhanced T1-weighted axial image (b) shows mild focal enhancement within the right T6 transverse process (double arrow) and pedicle (small arrow). Epidural (curved arrow) and paraspinal (large arrow) soft tissue enhancement is also seen. Axial CT image (c) from the biopsy procedure with skin grid in place shows a subtle mixed lytic and sclerotic pattern with the T6 posterior elements (dashed circle). Axial CT image (d) shows coaxial insertion of a bone needle (arrow) into the right transverse process. Axial CT image (e) shows repositioning of the guide cannula, avoiding another skin puncture, and transpedicular placement of the bone needle. The histopathology confirmed the presence of a hemangioma
5.7 Approaches
Posterior approaches are required to access the thoracic vertebrae, intervertebral disks, and paraspinal soft tissues. These posterior approaches are performed with the patient in the prone, prone oblique, or lateral decubitus position. The most optimal position, barring specific patient factors, is the prone position; however, some patients are unable to maintain this position due to pain or breathing issues. The prone position is the most common position with which spine interventions are performed at the thoracic spine level; hence, operators tend to have a comfort level with the imaging landmarks and with the angulations of their biopsy devices with the patient in this position. Each of the approaches to the thoracic spine is determined by the lesion location and size and by the local anatomic constraints that are seen with imaging guidance (Table 5.4) (also refer to Chap. 9). The two major approaches are either transpedicular or extrapedicular (Figs. 5.9 and 5.10) (Renfrew et al. 1991). Commonly used extrapedicular approaches within the thoracic spine include the costotransverse and costovertebral approaches. Intercostal approaches are occasionally required for posterior paraspinal soft tissue masses (Fig. 5.11). A major anatomic constraint within the upper thoracic spine tends to be the smaller size of the thoracic pedicle (Fig. 5.2). This may either limit the size of the instruments that can be used in the biopsy procedure or restrict the procedure to an extrapedicular approach, or both. It is imperative for the operator to review all pertinent pre-procedure imaging examinations in order to plan the most optimal approach to a thoracic spine lesion. Another decision to be made in thoracic spine biopsy is whether to approach the lesion from the right side or the left side of the thoracic spine. Certainly, if the lesion is located unilaterally, then this is the side that the posterior approach is initiated from. But, if the lesion is diffuse or large, and when anatomic structures permit, then a right-sided approach might be used in order to reduce the chance of injury to the aorta (Fig. 5.7).
A 50-year-old female. Reformatted parasagittal CT image (a) shows well-defined sclerotic lesion within the posterior T1 vertebral body and pedicle (arrow). Axial CT image (b) shows a transpedicular approach (arrow) to the sclerotic lesion. The biopsy showed normal bone formation and no evidence of malignant cells
A 29-year-old male with incidental lesion detected on outside study. Axial CT image (a) from the biopsy procedure with a skin grid in place shows a mixed sclerotic lesion within the posterior vertebral body (arrow). Axial CT image (b) shows tip of 20 gauge insert needle (arrow) adjacent to the transverse process. This needle was used to administer 2% lidocaine; the hub of the needle is removable so that it becomes a guidewire. Axial CT image (c) shows the hubless (arrow) 20 gauge needle and coaxial insertion of a 12 gauge introducer (curved arrow) and guide cannula (not seen on this image due to angled introduction of the coaxial system). Axial CT image (d) shows coaxial insertion of a trephine bone needle via a parapedicular approach (curved arrow). Axial CT image (e) shows the bone needle tip (arrow) within the lesion – a hemangioma
A 30-year-old male with incidental mass seen on chest radiograph. T1-weighted axial image (a) shows solid intermediate signal intensity soft tissue mass (arrow) adjacent to the upper thoracic spine. Axial CT image (b) shows placement of a guide needle with advancement of the needle tip to the margin of the lesion (arrow); the needle is purposely angled away from the lung. Axial CT image (c) shows coaxial insertion of a 22 gauge Chiba needle (small arrows) via the guide cannula (curved arrow) for the purposes of fine needle aspiration. Several FNA passes were performed and submitted for cytologic evaluation. Axial CT image (d) shows the subsequent placement of a 16 gauge cutting needle (arrows) via the same guide cannula (curved arrow). The cytopathologic evaluation showed that this was a schwannoma
It is imperative for the operator to review all pertinent pre-procedure imaging examinations in order to plan the most optimal approach to a thoracic spine lesion.
5.8 The Thoracic Spine Biopsy Procedure
5.8.1 General Considerations
5.8.1.1 Patient Factors
Image-guided percutaneous thoracic spine biopsy procedures should only be performed on cooperative patients. The patient should be evaluated to ascertain whether or not they can lie in the prone position. In rare instances, it is sometimes necessary to use the prone oblique position. The patient’s back should be examined in order to make sure that the skin is intact and that there are no pressure ulcers at the level of the intended biopsy procedure. Skin tattoos may also pose a barrier to biopsy, and these are often located in the upper back, near the cervicothoracic junction or the interscapular area. The patient’s medical history, pertinent laboratory values, allergies, imaging studies, and NPO status should be reviewed (Talac and McLain 2009). If certain medications, such as anticoagulants, antiplatelets, or antibiotics, require discontinuation prior to the procedure, then the details of these actions are confirmed. Informed consent is obtained from the patient or their designated representative. The risks and benefits of the biopsy procedure, and alternatives to this procedure including open biopsy and continued medical surveillance, should be discussed with the patient. The post-procedure recovery and any wound care instructions are briefly discussed and reinforced after the procedure.
5.8.1.2 Staff Factors
When needed, anesthesiology and pathology consultations should be obtained on a timely basis such that if the services of these medical consultants are required, then they will be able to assist with the performance of the procedure. The staff should be well trained and able to exercise the appropriate patient and procedure verification protocols. The staff is made aware that a thoracic spine biopsy will be performed. A procedure table has been prepared and sterilely draped prior to the procedure and can be positioned based upon the procedure logistics. The staff is instructed to place the patient in the prone position and to make sure that the patient is as comfortable as reasonably possible. The application of security straps around the lower body of the patient will help to prevent falls off of the procedure table. The patient is asked to place their arms up, whenever possible, with intravenous access in the forearm, wrist, or dorsum of the hand. Remember, antecubital intravenous catheters are problematic in these procedures as they tend to get occluded by a bent arm position. Monitoring equipment is placed on the patient, and the staff is reminded to keep leads, lines, and wires away from the intended sterile field and area of the biopsy. These wires can obscure the area of interest, especially with fluoroscopic procedures. The back is shaved, when necessary, with electric clippers.
5.8.1.3 Anesthesia
As with other percutaneous spine biopsy procedures, thoracic spine biopsy is performed with local anesthesia and either with intravenous sedation and analgesia or with intravenous anesthesia provided by an anesthesiologist or anesthetist. The level of anesthesia will be determined by the patient, the patient’s medical condition, the operator, and, when involved, the anesthesiologist. The patient is actively monitored, with respect to vital signs, oxygen saturation, and comfort level, by properly trained and qualified staff.
5.8.2 Patient Preparation
After the patient is positioned on the procedure table and the monitoring equipment placed, a time-out with the staff and patient is initiated so that the correct patient has the correct procedure at the correct level and, when applicable, on the correct side. For CT procedures a skin grid is placed on the back, and the intended level of skin entry is identified with CT imaging such that the skin can be marked with an indelible ink marker prior to prepping the skin. The skin is prepped and draped using strict aseptic technique. For fluoroscopy procedures, a clamp can be used to localize the level and side of interest in order to make a skin mark with a sterile marker pen. Once the monitoring equipment is recording the patient’s vital signs, oxygen saturation, and a continuous electrocardiogram, the patient can start to receive their sedation and analgesia or their intravenous anesthesia. In rare instances, for example, in patients who are immunocompromised, it may be necessary to provide intravenous antibiotic prophylaxis within one hour of starting the procedure (Santiago et al. 2014).
5.8.3 Technique
5.8.3.1 CT Guidance
After the operator reviews the pertinent prior examinations and decides upon the thoracic level of interest, a skin grid is placed upon the patient’s back. Scout frontal and lateral images of the thoracic spine are obtained; these may include the cervical spine for upper thoracic biopsies or the lumbar spine for lower thoracic biopsies in order to facilitate counting the vertebral body levels. This step takes on great importance in patients with transitional vertebrae or in patients in whom the pathology is difficult to visualize with CT (as compared to their initial MRI or nuclear medicine test). Having the prior examination available at the time of the biopsy procedure for additional review is also very helpful. After skin grid placement and scout CT images are obtained, the thoracic level of interest is scanned using serial axial sections. The preliminary axial CT study will often include the vertebral level above and below the level of interest. Additionally, the field of view should permit the visualization of the skin surface in order to identify the reference grid and the eventual skin entry site. These initial axial CT images are obtained with thin section technique (1–3 mm thick axial sections), often with bone algorithm when a vertebral biopsy is planned and occasionally with soft tissue algorithm when sampling large paraspinal soft tissue masses or fluid collections. The skin entry site is identified, and the skin is marked with an indelible ink marker prior to prepping with sterilizing solutions. The choice of trajectory and approach are at the operator’s discretion and will generally include a path that safely accesses the lesion and avoids normal structures using standard thoracic spine approaches. In general, a transpedicular approach can be used to access the pedicle and anterior and/or lateral vertebral body lesions. An extrapedicular approach tends to be used for some posterior and median vertebral body lesions and for sampling intervertebral disk or paraspinal soft tissue pathology (Fig. 5.12).
A 43-year-old female with history of intravenous drug abuse and back pain. T2-weighted sagittal image (a) shows a prevertebral fluid collection (large arrow) that communicates with an abnormal disk (small arrow) and a posterior epidural phlegmon (curved arrow). The adjacent vertebral bodies show marrow edema. T2-weighted axial image (b) shows large paravertebral fluid collection (large arrows) and spinal cord edema (curved arrow). Reformatted sagittal CT image (c) shows disk and vertebral endplate destruction (arrow) with sclerotic reaction and focal kyphosis. Axial CT image (d) shows insertion of a guide needle via a costotransverse approach (arrow). Axial CT image (e) shows placement of a guidewire (curved arrow) into the paraspinal fluid collection via the guide needle (arrow). Axial CT image (f) shows over the wire placement of a drainage catheter (arrows) into the collection. Abundant purulent material was drained via this catheter, and the specimens were positive for Streptococcus viridans
A small amount of anesthetic agent, such as 1 or 2% lidocaine is used to anesthetize the skin entry site as well as the deep soft tissues of the needle tract down to the level of the vertebral periosteum. The application of the deep tissue anesthetic is performed with imaging guidance in order to prevent injury to critical structures. This maneuver also will confirm that the chosen biopsy needle trajectory, with respect to safety, will be feasible (Fig. 5.10). A dermatotomy is performed with a #11 scalpel blade at the anesthetized skin entry site.
A coaxial biopsy system is often used for thoracic spine biopsies (Yaffe et al. 2003). This maintains access to the biopsy site via a guide cannula and allows the operator to use bone needles, soft tissue needles, or both. A bone needle can be advanced into the vertebra, and the bone needle stylet is then removed such that the bone needle cannula serves as a guide cannula for the coaxial insertion of other biopsy needles (Geremia et al. 1992). Bone needles tend to be used for vertebral lesions, if at a minimum, to penetrate the vertebral cortex and gain access to the lesion margin (Fig. 5.3). Another type of guide cannula system entails the advancement of a guided cannula over an introducer or blunt dissector. The introducer fits inside the guide cannula such that the guide cannula–introducer unit can be advanced over a guidewire. When the guide cannula reaches the margin of the vertebra, disk, or paraspinal soft tissue lesion, the introducer and guidewire are removed and the guide cannula is left in place. The guide cannula acts a safe conduit for sharp instruments and guards against soft tissue injury (Fig. 5.10). The guide cannula also facilitates patient comfort by minimizing trauma to the skin entry site. Once the guide needle is advanced to the cortical entry site, it may have to be held in place with one hand, while the insert needle and introducer/blunt dissector cannula are removed with the other hand. A trephine bone needle is then inserted through the guide cannula with the other hand and then rotated clockwise and counterclockwise with gentle forward pressure to dock the system into the vertebral cortex. The bone needle entry site is quickly scanned, this is optimized with CT fluoroscopy, and the trajectory and position of the needle is monitored during subsequent advancements (Fig. 5.6).
One difference between thoracic spine biopsy as compared to lumbar spine or sacral biopsy procedures is that bone needle excursions are shorter with thoracic spine biopsies due to the inherent relatively smaller size of the thoracic vertebrae and their proximity to the lungs and the aorta. Another difference in these types of procedures is that with thoracic spine biopsy the overall sample size may be limited depending on the lesion size and extent. It must be kept in mind that there are two key locations for optimizing the biopsy needle trajectory: first, at the skin insertion site and, second, at the vertebral cortex insertion site. This is based upon the fact that biopsy needles are metallic constructs that tend to move in a straight direction. The bone needle is usually advanced about 5 mm. Bone material accumulates within the bone needle lumen as the needle is advanced. The bone needle can be removed and replaced with a second or “fresh” bone needle. This will allow for the bone sample within the first bone needle to be expressed, with a metal pusher, into the appropriate biopsy container. This bone needle swapping maneuver will require stabilization of the guide cannula at the vertebral entry site with one hand, while the bone needle exchanges are performed with the operator’s other hand. Coaxial placement of the second bone needle will again require rotational movements with gentle forward pressure on this needle and with monitoring of needle progression with CT images. These procedural steps can be repeated as long as the biopsy needle tip is within the lesion and safely distant from critical structures. The operator should attempt to obtain at least three bone cores whenever possible. Another technique that can be used to obtain more tissue, after the initial traverse through the lesion, is to try to create a fresh biopsy tract by angling the bone needle slightly at the vertebral insertion site (Fig. 5.6). This latter maneuver also works very well with transpedicular technique using fluoroscopic guidance. Ultimately, however, the number of biopsy needle passes and trajectories are determined by the lesion location, size, and morphology.
For soft tissue masses, intraosseous lytic lesions, or disk space biopsies, the coaxial approach at the thoracic level may first include an attempt at FNA using small gauge needles that can be coaxially passed through a guide cannula (Fig. 5.13). FNA should be considered by the operator as an initial biopsy technique when the lesion to be sampled is potentially hypervascular (such as a renal or thyroid metastasis). Once the FNA passes are complete, whether or not abnormal cells have been detected, then a core soft tissue biopsy should be performed with a soft tissue cutting needle. The cutting needle is advanced coaxially to the margin of the lesion under CT guidance. The cutting compartment of the needle is exposed within the matrix of the lesion and this is confirmed with CT; the tip of the needle is monitored at all times with CT images, especially after any adjustments in needle position, and should not threaten critical structures (Fig. 5.14). Again the optimal goal is to obtain at least three soft tissue cores if possible. Another possible technique that can be used to sample lytic lesions is to sample the lesion margin with a trephine bone biopsy needle; this maneuver is sometimes able to sequester a small amount of cortical bone and the adjacent soft tissue abnormality (Figs. 5.15, 5.16, and 5.17). When sampling the disk or adjacent paraspinal soft tissues for possible infection, try to aspirate infected fluid first. If there is a definite fluid collection, but the viscosity of the collection is too thick, then try to use a larger gauge needle such as a 20 or an18 gauge needle and then aspirate with a 10 or 20 mL syringe. Alternatively, a small drainage catheter can be placed for larger collections (Fig. 5.12). If there is no fluid collection, then the disk-endplate complex can be biopsied with a trephine bone needle (Fig. 5.18). This latter maneuver usually yields tissue samples that can be submitted in their respective transport containers to microbiology and pathology. The other option is to use a percutaneous diskectomy device with coaxial technique and imaging guidance (Onik 1996; Chew and Kline 2001). Care should be taken in only advancing the diskectomy device slightly and with imaging guidance. Often after these types of deep tissue manipulation, there is a small amount of hemorrhage into the biopsy tract that can also be aspirated and submitted in a sterile container for microbiologic analysis.
A 67-year-old female with leukemia and fever; elevated CRP 76 and ESR 84. T2-weighted sagittal image (a) shows small anterior fluid collection (curved arrow) within the disk and superior endplate as well as prevertebral soft tissue swelling. Reformatted sagittal CT image (b) in bone window algorithm shows widening of the disk space (arrow) with endplate sclerosis and destruction. Axial CT image (c) shows coaxial placement of a 22 gauge needle (arrow) that was used to aspirate purulent material from the anterior disk; the microbiology was positive for coagulase-negative Staphylococcus
An 82-year-old female with back pain. T2-weighted sagittal image (a) shows two spinous process lesions (arrows); the large lesion impinges upon the spinal cord (curved arrow). Axial CT image (b) shows placement of a guide cannula (arrow); note the orientation of the guide cannula relative to the expansile lesion within the laminae and base of the spinous process (curved arrow). Axial CT image (c) shows coaxial placement, via the guide cannula (curved arrows), of a 22 gauge needle (arrows) into the lesion. Axial CT image (d) shows placement of a 16 gauge self-aspirating biopsy needle (arrows) via the guide cannula (curved arrows). Axial CT image (e) shows the coaxial (curved arrow) use of a 16 gauge cutting needle. The biopsy was positive for carcinoma cells; unknown primary
A 74-year-old female with incidental spinous process lesion. Axial CT image (a) in bone window algorithm with a skin grid in place shows trajectory (arrows) for lytic lesion within the spinous process. Axial CT image (b) shows use of coaxial technique with a guide cannula (arrow) and trephine bone needle (curved arrow); the trephine needle is carefully rotated with gentle forward pressure to avoid fracturing the spinous process. Pathologic analysis of the biopsy specimens showed metastatic papillary thyroid carcinoma
A 54-year-old male with back pain. Axial CT image (a) in soft tissue algorithm shows a lytic lesion that involves the left transverse process, pedicle, and posterior vertebral body (arrows). Axial CT image (b) shows coaxial placement of the biopsy needle (arrow) along the lateral margin of the lesion; this technique yielded both osseous and soft tissue material. The histopathology was consistent with an aneurysmal bone cyst
A 31-year-old female with back pain. Fat-suppressed contrast-enhanced T1-weighted axial image (a) shows enhancing lesions within the left side of the vertebral body (arrow) and left rib (curved arrow). Axial CT image (b) in bone window algorithm shows well-circumscribed lytic lesions with sclerotic margins within the left rib (curved arrow) and posterior vertebra body (arrow). Axial CT image (c) shows the use of coaxial technique to sample the margin of the vertebral body lesion. The biopsy showed epithelioid hemangioma
A 64-year-old male with right rib cage pain; normal WBC, ESR, and CRP. Single posterior projection (a) from a bone scan shows focal radionuclide uptake (arrow) within the mid-thoracic spine. T2-weighted sagittal image (b) shows focal hyperintense signal within the anterior vertebral body (arrow). Axial CT image (c) from the biopsy procedure shows the use of a costovertebral approach (curved needle) with coaxial insertion of a bone needle (arrow) into the disk-endplate complex. The biopsy, with respect to both pathology and microbiology, was negative
5.8.4 Fluoroscopic Guidance
Thoracic spine biopsies can also be performed with fluoroscopic guidance. In the case of vertebral body lesions, these biopsy procedures tend to be performed in cases where there are either large lesions or the vertebral body is diffusely infiltrated by tumor. Suspected pathologic vertebral compression fractures are often biopsied under fluoroscopic guidance (Fig. 5.4). In general, these biopsies tend to be performed with a transpedicular approach, but they can also be performed with an extrapedicular approach. With transpedicular technique, the operator aligns the pedicle in the superior third of the vertebral body of interest. This often entails aligning the vertebral endplates. Then the fluoroscopic is rotated in a medial to lateral fashion such that pedicle projects over the vertebral body. Think of the pedicle as a flashlight with a cylindrical beam that shines into the vertebral body – this will be the bone needle trajectory into the vertebral body and will represent the area of the vertebral body that will be sampled. In general, mild angulation of the fluoroscope facilitates bone needle access to the anterior and lateral aspect of the vertebral body, while steeper angulations of the fluoroscope will enable placement of the bone needle into more posterior and paramedian areas of the vertebral body.
The intended needle trajectory is anesthetized with a local anesthetic agent. A cross hair incision is made at the skin entry site with a #11 scalpel blade. A bone needle, anywhere from 10 gauge in diameter or smaller, at the operator’s discretion and preference, is advanced using a down-the-barrel approach with the pedicle of interest centered in the field of view of the fluoroscope. The bone needle is carefully advanced into the posterior vertebral body. The operator should check the needle position in the oblique, frontal, and lateral fluoroscopic projections during the advancement of the bone needle. This is done to ensure that the needle does not enter the spinal canal and stays within the confines of the vertebral body. In order to avoid entry into the spinal canal, the needle tip should not cross the medial pedicle margin on the frontal projection until the needle tip enters the posterior vertebral body on the corresponding lateral projection. Once the bone needle tip reaches the posterior vertebral body, the needle stylet is removed, and the bone cannula can now be used as a guide cannula for subsequent sequential coaxial bone biopsy needle placements and biopsies. With each coaxial bone needle pass, the position of the guide cannula and bone needle tip is monitored with fluoroscopy to ascertain that the needle has not extended beyond the vertebral body. An advantage of fluoroscopic technique is the opportunity that the operator has to slightly adjust the trajectory of the initial bone needle within the pedicle. This can be readily accomplished by slowly retracting the bone needle, with stylet in place, within the pedicle under fluoroscopic guidance and redirecting the needle tip slightly. This creates a new biopsy tract and a source of additional bone samples.
In certain situations, the operator may not be able to access the vertebral body through a transpedicular route. Extrapedicular approaches can be performed in the thoracic spine using fluoroscopic guidance, especially when the pedicles of a given vertebral body level are small and cannot accommodate the bone needle. The key principle with this type of approach is to stay medial to the rib and lateral to the pedicle at the initial parapedicular entry point; the needle tip is directed medially. It is important to note that these unique approaches can be utilized only if the operator is able to visualize the key osseous anatomic landmarks of the thoracic spine with fluoroscopy. These bony radiographic landmarks include the vertebral pedicle; the oval configuration of the posterior rib, or rib head, as it articulates with the vertebral body; the posterior and anterior vertebral body margins; the spinous process; and the superior and inferior vertebral endplates. This is essentially a costovertebral approach, and it can also be used to access the disk space by aligning the vertebral endplates at the level of interest. The disk space can be initially approached either with a 20 gauge spinal needle or an 18 gauge spinal needle using this oblique fluoroscopic approach. The operator should place the disk within the center of the fluoroscope’s field of view in order to facilitate advancement of the needle under fluoroscopy (Fig. 5.19). The spinal needle can also be used to inject a small amount of local anesthetic agent at the margin of the disk just prior to penetrating the annulus fibrosis. The needle tip position can be ascertained using a combination of oblique, frontal, and lateral projections. With experience the operator will note the tactile sensation of advancing a spinal needle into the disk. Once disk access is obtained, the operator can attempt needle aspirations under fluoroscopic guidance. The spinal needle in turn can be exchanged over a small guidewire in order to introduce a bone biopsy needle system for disk-endplate biopsy or to introduce a percutaneous diskectomy device. These options can be exercised at the operator’s discretion; however, each needle exchange and each needle pass require meticulous fluoroscopic surveillance.
A 30-year-old male with back pain and chronic vertebral compression deformity from prior car accident. Lateral fluoroscopic image (a) shows partial T8 vertebral compression deformity (arrow); the disk spaces above and below T8 have been aligned by slightly angling of the fluoroscope. Frontal fluoroscopic image (b) shows alignment of the disk spaces (arrows). Oblique fluoroscopic image (c) with the disk aligned (arrow) and centered in the field of view shows the approach to the aligned T8-9 disk between the rib (r) and the pedicle (p). A spinal needle would be advanced, using a down-the-barrel approach, directly into the disk at the point indicated by the curved arrow. In this case, staying medial and posterior to the rib avoids the lung, and staying lateral to the pedicle avoids the spinal canal
5.8.5 Post-procedure Care
Once the biopsy procedure is complete, then the guide cannula can be removed from the patient’s back, and hemostasis at the skin entry site can usually be achieved with hand compression. Longer periods of hand compression may be required in larger patients, in cases where a large gauge (e.g., 10 gauge) cannula has been utilized, in patients who have been on anticoagulant and/or antiplatelet therapy, or in cases where there is a possible hypervascular lesion. In some situations it may be necessary to inject a small amount of surgifoam into the deep soft tissues of the needle tract and up to the skin surface (also refer to Chap. 2). This may be helpful in patients in whom vascular lesions have been biopsied, in patients in whom antiplatelet and/or anticoagulant medication will be resumed, or in patients with blood oozing around the guide cannula. It is helpful to apply hand compression for about 3–5 min after the surgifoam is injected and to monitor the skin entry site afterward for an additional 3–5 min prior to moving the patient off the procedure table. A sterile bandage is placed on the skin entry site. Once the patient is moved into the supine position onto a stretcher, the skin entry site should be reexamined. After the biopsy procedure, the patient will be recovered for a minimum of 2 hours. It is important not only for the staff to monitor the patient’s pain level and vital signs but also to periodically look at the biopsy site for signs of active bleeding or swelling. Post-procedure care instructions are reviewed with the patient at the time of discharge (refer to Chap. 1). A contact telephone number is given to the patient should they have any further questions or should their condition deteriorate when they get home. Patients are reminded that it may take several days to receive the biopsy results and that they should follow up with the doctor who referred them for the procedure. It is very helpful for the operator to contact the requesting clinician and to update them with respect to the procedure and the patient’s clinical status after the procedure.
It is extremely important to take care of the biopsy specimens. Thoracic spine biopsy specimens should be placed in properly labeled containers. For bone biopsies, the transport media is usually 10% formalin. Certain suspected pathologic diagnoses require special transport media or handling at the time of acquisition, and this should be discussed with the pathologist ahead of time. Microbiology specimens are placed in sterile containers and are immediately transported to the microbiology laboratory. All specimens should be accounted for, labeled properly, and accompanied by appropriately completed requisitions, and promptly transported by trained and qualified personnel to the appropriate laboratories. The operator should address any clinical concerns with the pathologist or microbiologist before or at the time the specimens are submitted. The operator should follow up on the biopsy results.
Key Review Points
-
1.
Given the greater number of thoracic vertebrae, it is important to count vertebral levels carefully and to match the counting and labeling scheme with all prior studies in order to localize the correct level for a thoracic biopsy procedure.
-
2.
Critical anatomic structures to be aware of during an image-guided percutaneous thoracic spine biopsy include the spinal cord, the lungs, and the aorta and intercostal arteries.
-
3.
A sound radiologic understanding of osseous landmarks within the thoracic spine is a prerequisite to performing thoracic spine biopsy procedures with CT or fluoroscopic guidance.
-
4.
Common indications for thoracic spine biopsy include a clinical concern for neoplastic involvement of one or more thoracic vertebrae, evaluation of suspected pathologic vertebral compression fractures of the thoracic spine, and the assessment of possible spine infection.
-
5.
The approaches for thoracic spine biopsy are either transpedicular or extrapedicular; extrapedicular approaches include costotransverse, costovertebral, and intercostal techniques.
-
6.
The use of coaxial technique in the thoracic spine facilitates procedure efficiency and safety.
-
7.
Major determinants of specimen yield in thoracic spine biopsy procedures include lesion location relative to critical structures, lesion size, and lesion type (lytic, sclerotic, or mixed).
References
Carrino JA, Campbell Jr PD, Lin DC, Morrison WB, Schweitzer ME, Flanders AE, Eng J, Vaccaro AR. Effect of spinal segment variants on numbering vertebral levels at lumbar MR imaging. Radiology. 2011;259:196–202.
Chew F, Kline M. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218:211–4.
Geremia GK, Charletta DA, Granato DB, Raju S. Biopsy of vertebral and paravertebral structures with a new coaxial needle system. AJNR Am J Neuroradiol. 1992;13:169–71.
Heyer CM, Al-Hadari A, Mueller KM, Stachon A, Nicolas V. Effectiveness of CT-guided percutaneous biopsies of the spine: an analysis of 202 examinations. Acad Radiol. 2008;15:901–11.
Lis E, Bilsky MH, Pisinski L, Boland P, Healey JH, O’Malley B, Krol G. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol. 2004;25:1583–8.
Olscamp A, Rollins J, Tao SS, Ebraheim NA. Complications of CT-guided biopsy of the spine and sacrum. Orthopedics. 1997;20:1149–52.
Onik G. Automated percutaneous biopsy in the diagnosis and treatment of infectious discitis. Neurosurg Clin N Am. 1996;7:145–50.
Ortiz AO, Zoarski G, Brook A. Image-guided percutaneous spine biopsy. In: Mathis JM, Golovac S, editors. Image-guided spine interventions. 2nd ed. New York: Springer; 2010. p. 75–106.
Pierot L, Boulin A. Percutaneous biopsy of the thoracic and lumbar spine: transpedicular approach under fluoroscopic guidance. AJNR Am J Neuroradiol. 1999;20:23–5.
Renfrew DL, Whitten CG, Wiese JA, el-Khoury GY, Harris KG. CT-guided percutaneous transpedicular biopsy of the spine. Radiology. 1991;180:574–6.
Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M, Malaguti MC, Rossi G, Durante S, Mercuri M. Percutaneous CT-guide biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008;17:975–81.
Santiago FR, Kelekis A, Alvarez LG, Gilippiadis DK. Interventional procedures of the spine. Semin Musculoskelet Radiol. 2014;18:309–17.
Talac R, McLain RF. Biopsy principles and techniques for spinal tumors. Semin Spine Surg. 2009;21:70–5.
Tehranzadeh J, Tao C, Browning CA. Percutaneous needle biopsy of the spine. Acta Radiol. 2007;48:860–8.
Yaffe D, Greenberg G, Leitner J, Gipstein R, Shapiro M, Bachar GN. CT-guided percutaneous biopsy of thoracic and lumbar spine: a new coaxial technique. AJNR Am J Neuroradiol. 2003;24:2111–3.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ortiz, A.O. (2017). Thoracic Spine Biopsy. In: Image-Guided Percutaneous Spine Biopsy. Springer, Cham. https://doi.org/10.1007/978-3-319-43326-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-43326-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43324-0
Online ISBN: 978-3-319-43326-4
eBook Packages: MedicineMedicine (R0)