Abstract
Light pollution has become an important theme of both scientific research and policy-making. Although in recent years we have seen a boost of research on this topic, there is still surprisingly little knowledge on the levels of artificial light at night that wild animals really experience. I made use of miniature light loggers attached to individual free-living European blackbirds (Turdus merula) to measure the light intensity to which these birds are exposed to in forest and urban areas. I have first shown that male blackbirds living in a city are indeed exposed to higher levels of light at night compared to forest conspecifics, but these levels are substantially lower to what can be measured underneath typical street lamps. Recently I have offered new perspectives by estimating the subjective day length to which urban and rural blackbirds are exposed to and by analysing the overall light intensity to which blackbirds are exposed daily. In a series of studies, I have interpreted these data in the context of daily patterns of activity as well as seasonal biology. European blackbirds which were exposed to a longer photoperiod than their rural counterparts extended their activity into the night and showed reduced levels of melatonin production in the early morning, suggesting that this could be the biophysical process underlying the early onset of daily activity, but also the advanced breeding season observed in many avian species that successfully colonize urban areas. Indeed, I found a remarkable similarity between the difference in the photoperiod experienced by rural and urban blackbirds and the difference in timing of reproduction and onset of daily activity between my two study populations. I will discuss these findings and underlie several outstanding questions that still remain unresolved.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Light pollution is a global phenomenon of increasing importance. Indeed, as urban areas are the fastest growing land cover type around the world, the impact of artificial light at night on natural and anthropogenic ecosystems will likely increase, too. The dramatic change in the outdoor light environment recorded since the large-scale deployment of electric lights has the potential to impact a broad array of organisms. The increasing recognition of lightscapes as important environmental features has stimulated great scientific interest which has resulted in a unique amount and quality of ecological research published on this topic in the last decade (Rich and Longcore 2006). In this chapter, I will first highlight the importance of considering the light environment as a key driver of temporal biological processes, focusing on different levels of organismal responses, from genes to physiology to behaviour (Dominoni et al. 2016). I will then review the studies that have analysed how light pollution alters the natural lightscapes, including temporal and spatial global trends. Last, I will focus on my personal research, which has focused on analysing individual-based recordings of exposure to light at night using miniature light loggers mounted on free-living songbirds tagged at different urban and rural sites. In a series of papers, I used this information to define the light environment to which birds are exposed to in cities and compared it to the conditions of dark, forest areas. In addition, I also examined how the variation in exposure to light in and away from a city relates to the temporal (daily and seasonal) patterns of behaviour and physiology.
2 Natural and Artificial Changes in the Light Environment
To understand what the potential impacts of light pollution are on the biological functions of organisms, we must first understand how the presence of anthropogenic night light alters natural lightscapes. With respect to the physical properties of light, there are at least two important features that need to be considered.
Light Intensity
Huge changes (10 orders of magnitude) in the amount of light that reaches the ground occur between day and night, and this is the most obvious property of the light environment that organisms use (Roenneberg and Foster 1997). But more subtle, short-term variation in the light intensity detected by an organism can also occur, for instance, as a consequence of cloud cover, moving through thick vegetation or directly looking at the sun. During the night light intensity can also vary depending on the moon phase, which some organisms synchronize to, especially in tidal environment where moon phase can have a great influence on the time and amplitude of tides (Kronfeld-Schor et al. 2013). Thus, long-term light sampling or integration over time is required to bypass these small local fluctuations in the light environment. Light pollution can alter both spatial and temporal features of night light intensity in several ways. First, a recent study conducted in and around the city of Plymouth, UK, has shown that the average light intensity at night is six times higher in the city than in suburban and rural areas (Fig. 13.1) (Davies et al. 2013b). The major implication of such increase is that natural regimes of moonlight are masked. In particular, the amount of full-moon equivalent hours is increased, and the seasonal variation in nocturnal light intensity is almost lost. The consequences of such change for both diurnal and nocturnal organisms could be huge. In addition, several analyses of long-term satellite images have revealed a global trend in increasing night brightness overall the last 50 years (Bennie et al. 2015). However, recent work has highlighted that while the increasing trend is true for most regions in Europe, there are also areas in developed European countries that have experienced large decreases in night-time brightness, mostly because of a switch towards less industrial production and/or the implementation of new policies to contain the economic costs of night-time illumination (Bennie et al. 2014).
(A) Observed regimes of night-time sky brightness (grey points) recorded 19.7 km (a), 8.6 km (b) and 0 km (c) from Plymouth city centre are compared to the predicted natural regime of lunar sky brightness (d). Darker areas indicate denser aggregation of points. (B) Map of sampling locations and relative light pollution. (C) Annual variation in night length compared to the annual variation in the number of hours per night when sky brightness was observed to be greater than the third quartile sky brightness observed in each month at a dark sky location when the moon is close to full. Graph reproduced with permission from Davies et al. (2013b)
Spectral Composition
Sunlight covers a wide range of wavelength, but spectral changes occur during the day. For instance, twilights phases are normally enriched in short wavelengths (Roenneberg and Foster 1997). As moonlight is pretty much all reflected sunlight, a lunar spectrum looks very similar to a solar spectrum. However, the introduction of different sources of anthropogenic night light has altered not only the amount/intensity of light experienced at night but also its spectral properties (Gaston et al. 2012; Hale et al. 2013). As different wavelengths have the capacity to impact different biological functions and ecological processes to different extent, (Aubé et al. 2013; Davies et al. 2013a), this is an important issue to be considered if we want to understand the response of organisms to anthropogenic light, as well as its mechanistic underpinning. For instance, as the light action spectra for the circadian response of mammals and birds peak at mid- to low wavelengths (Davies et al. 2012), blue-rich light bulbs have been found to affect the expression of circadian behaviour and physiology the most, for instance, decreasing nocturnal levels of the hormone melatonin (Aubé et al. 2013).
While in the last 15 years several studies have quantified how light pollution affects lightscapes, we still have a limited understanding of how such changes in the light environment are perceived by wild organisms. For instance, as night-time light levels in urban areas might even be constantly above that of moonlight, are urban exploiters exposed to a 24-h day? How does the nocturnal light intensity that reaches an organism’s photoreceptors compare to that provided by anthropogenic light? And how does the light intensity perceived by an animal relate to its activity? In order to try to answer these questions, we need individual-based measurements of light intensity. Recent technological advances in telemetry have given us the possibility to tag individual organisms and follow them over a relative long period of time. After briefly reviewing the known effects of light pollution on the daily and seasonal activity patterns of animals, I will focus on my own work on wild European blackbirds (Turdus merula), where I used light loggers to record the amount of light intensity to which urban and rural blackbirds were exposed to at night, and relate it to several parameters of daily activity recorded with automated radiotelemetry.
3 Light Pollution and Biological Rhythms
All organisms have evolved in a rhythmic world and respond to periodic changes in the environment driven by cycles of light, temperature, food availability and many other parameters. However, the natural alternation of day and night is probably the most constant feature of the planet Earth, and photic information is used by virtually all organisms to synchronize their activity to the best time of day and year in order to maximize their fitness (Foster and Kreitzmann 2004). Moreover, organisms have evolved morphological structures and physiological mechanisms to detect and interpret light stimuli (Foster and Kreitzmann 2004). For instance, different organisms possess different photoreceptors in different tissues of their body, and the sensitivity of these photoreceptors varies greatly among taxa (Peirson et al. 2009; Dominoni 2015). At the same time, the most common visual reception organ, the eye, also shows great variability in size and structure which largely depends on whether the organisms are diurnal, nocturnal or crepuscular. As artificial light at night changes the temporal regimes of night-time light intensity (Davies et al. 2013b), it comes with no surprise that organisms living in light-polluted areas show a remarkable shift from their natural temporal patterns of daily and seasonal behaviour and physiology. Such changes have been documented in a vast array of species, from plants to invertebrates to birds and mammals (Rich and Longcore 2006; Kempenaers et al. 2010; Rotics et al. 2011; Dominoni et al. 2013b; van Geffen et al. 2014). Several reviews have already covered this topic; thus, my intention here is rather to highlight the general findings of these studies, their limitations and the promising areas for future research.
The response to light at night and its potential ecological consequences seem to depend not only on the temporal activity strategy of the species but also on its sensitivity to light and its feeding ecology. Many diurnal species, especially those which are also active at dawn and dusk, seem to be prone to extend their activity into the night. For instance, in songbirds, only those species that display a very early dawn chorus show a significant advance of its onset in light-polluted areas, suggesting that only species that are sensitive to low light intensity levels can afford to be active at night when anthropogenic light is present (Kempenaers et al. 2010). In nocturnal species, however, the response to light pollution seems to be largely species specific. In bats, while the common pipistrelle (Pipistrellus pipistrellus) has been found to increase its activity (Spoelstra et al. 2015), lesser horseshoe bats (Rhinolophus hipposideros) tend to avoid bright areas while commuting at night (Stone et al. 2009). However, although several studies in the last decade have reported these and similar effects, we still lack a deep understanding of the consequences of such temporal shifts in activity at basically all levels of biological organization, from the individual to the species, to populations and to ecosystems. A recent study has highlighted that the reproductive timing as well as the breeding success of wild songbirds is affected by an experimental manipulation of the night light environment and that this effect may also depend on the wavelength of light, with white lights being the more impacting light source (De Jong et al. 2015). The temporal shifts in activity timing will likely impact the time and modality of social encounters between individuals of the same species, but this has been rarely investigated. A landmark study on blue tits (Cyanistes caeruleus) has shown that a small advance in the time of male dawn song can increase annual reproductive success considerably by allowing males to gain more extra-pair youngs (Kempenaers et al. 2010). Thus, encounters between male and females of different breeding pairs of this species seem to be increased in the early morning under light pollution. In addition, interspecific relationships, for instance, the temporal overlap in activity timing between predators and preys, might also be affected, but these trophic effects are largely unknown. Spoelstra and collaborators have examined the activity of wood mice (Apodemus sylvaticus) and common pipistrelle under different light at night treatments in the wild, and they showed that mice activity was reduced and bat activity was increased under light pollution, with red light having the smallest impact (Spoelstra et al. 2015). However, the activity of mice’s main predators, howls, and pipistrelle’s main prey, mosquitoes, was unfortunately not monitored, leaving a black hole in our knowledge about how the change in temporal activity strategy caused by light pollution can cascade across trophic levels.
4 How Light Loggers Can Help Us in Understanding the Impact of Light Pollution on Animals’ Behaviour and Physiology
4.1 How Much Light Pollution Are Animals Exposed To?
The amount of artificial light that organisms are exposed to will greatly depend on their behaviour. For instance, male songbirds have been reported to perform dawn chorus directly under, or even on top, of streetlamps (Stephan 1985). Under these circumstances, light exposure can reach very high levels, even above 20 lux (Dominoni, unpublished data). Mammals that rely on visual cues to forage, such as mice, foxes and certain species of bats, are also likely to be exposed to considerably high levels of light pollution. These levels can vary depending on the type of bulbs used in streetlamps, but they are usually between 6 and 20 lux when measured at the ground and directly under the light source (Miller 2006; Kempenaers et al. 2010; Rotics et al. 2011; Dominoni et al. 2013b). When moving away from the direct light source, however, light intensity decreased exponentially and rapidly (Kempenaers et al. 2010). Thus, animals that spend the night in borrows or nest boxes, or that roost in very thick vegetation, are likely to be exposed to very low levels of light intensity (Dominoni et al. 2013b). Despite this evident link between behavioural modes and light exposure, most studies on the ecological effects of light pollution have measured light intensity in the environment surrounding the territory of an animal, but little consideration has been made on which part of the habitat the animal is more likely to use and when. Without detailed measurements at the individual level, it is difficult to make strong inference on the spatial and temporal variability in the exposure to light pollution.
I tried to overcome this limitation by deploying miniature light loggers on individual male European blackbirds breeding in the city of Munich, south-east Germany, and in a forest 40 km south-west from the city centre (Fig. 13.2). Blackbirds were caught at dawn using mist-nets, tagged and then immediately released in their territory. The tag I used was a combination of two devices: a light intensity micro-logger and a radio-transmitter (Fig. 13.3). Light loggers were used to record light intensity during day and night, while the radio-transmitters were used to record activity rhythms of the animals (see below). The locations where blackbirds were caught vary considerably in the amount of light intensity present in the environment (Dominoni et al. 2013a). Indeed, I used two light loggers as stationary loggers and deployed them on tree branches at the two extremes of the urban gradient. These loggers recorded data for approximately 3 weeks, and the data are shown in Fig. 13.4. While during daytime light intensity does not seem to vary significantly between urban and rural areas (panel a), night-time light intensity is considerably higher in the city centre of Munich (panel d). Interestingly, light intensity in the first half of the night is double as high as in the second half, probably due to the fact that fewer cars are around late at night, most bars close and many streetlights are turned off in the middle of the night.
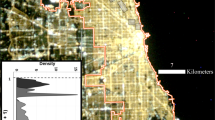
Study sites. (a) Overview map. Birds were sampled in the city of Munich and in the forest of Raisting, 40 km south-west of Munich. (b) Map of nocturnal light intensity in the city of Munich and locations where birds were caught (green circles, business district; red circles, urban parks). Notice the higher light intensity in the areas surrounding the business district compared to the other two locations. This picture is by courtesy of Franz Kurz, DLR, Oberpfaffenhofen, Germany
Bird tagging. (a, b) Blackbirds were tag using a “backpack”. This consisted of two devices, a light logger and radio-transmitter, stuck together using shrinking tubes and then attached to the animal with rub and cotton harnesses pulled through the legs. (c) Light logger (Sigma Delta Technologies, Australia). The loggers weighed 2.4 g and were composed of an electric circuit linked to a photodiode, whose spectral responsivity ranged from 300 to 1100 nm, with a peak around 780 nm. They recorded and stored light intensity every 2 min for the entire time. We calibrated the loggers against a photometer (LI-1400 and LI-2100, LI-COR, USA) in order to convert frequency into lux (illuminance) and watt/m2 (irradiance) values
24-h light profiles of the urban and forest study sites. Light was recorded between March 4 and 20, 2011, using stationary light loggers. Two loggers were placed at a height of ~2 m on tree branches at the edge of a forest patch (rural site) and a botanical garden (urban site). X-axis represents time, and Y-axis represents log-transformed light intensity. Lines depict mean light intensities for the entire recording period, shaded areas are s.e.m. (a) Complete 24-h profile. (b) Morning twilight phase (6:00–7:00). (c) Evening twilight phase (17:00–18:00). (d) Night (20:00–4:00)
But how much of this night light are blackbirds really exposed to? Figure 13.5 shows the median and maximum light intensity calculated from the light loggers retrieved from blackbirds captured in the city of Munich. Median light intensity was lower than 0.3 lux, while maximum light intensity was between 1 and 2.5 lux (Dominoni et al. 2013b). Thus, urban blackbirds are exposed on average to a night-time light intensity 20 times lower than what could be measured underneath a representative streetlamp in our study sites and around the level of a full-moon night. This suggests that although urban songbirds are only rarely exposed to very high levels of light pollution, nevertheless the nocturnal lightscape they live in masks the natural variation in night light due to the moon. As I will explain in the following section, this can potentially have important consequences for the photoperiod that birds experience in urban habitats. I showed this data expressed in lux the sake of consistency and comparison with most animal studies conducted in the wild and in captivity.
Natural exposure to light at night of free-living city European blackbirds. Light grey bars represent data obtained from loggers deployed on individual birds in their natural urban environment. For each city bird (N = 28), we calculated the median and maximum of the third quartiles of all nights and used the mean of those values from all birds (mean = 0.2 lux) for presentation in the figure. Data are represented as means ± SEM. White bar represents full-moon light intensity (0.1–0.3 lux). Dark grey striped bar represents the light intensity measured under a representative street lamp of our study site (~6 lux). Black bar represents the light intensity (0.3 lux) we used in the captive study to simulate urban exposure to light at night in the experimental treatment group (see text and Fig. 13.11)
However, lux is a measure of illuminance based on the sensitivity of the human eye and thus does not really apply to birds, as they are able also to see ultraviolet light. I have therefore calibrated the lux measurements using a pyranometer (Dominoni et al. 2014), an instrument able to record light radiance expressed as the amount of energy (radiant flux) incidence on a surface per unit area. Irradiance is often expressed as “intensity” in watt/m2. Figure 13.6 shows irradiance data during the early and late night in the three different study sites. I have split the urban habitat into two different subtypes depending on the size of the study area and human presence (see Dominoni et al. (2014) for more details). Although the data from stationary light loggers might have suggested that exposure to light pollution may vary between the early and late night, the data from loggers mounted on individual blackbirds did not show any significant differences between separate phases of the night in any of the study locations (Dominoni et al. 2014). Light intensity was, however, significantly higher in the business district compared to the rural forest and the suburban parks, justifying the splitting of the urban sites. Moreover, it is worth to highlight that not only absolute levels of light intensity were higher in the business district but also between-individual and within-individual variation was higher in the most urban locations (Dominoni et al. 2014). This suggests that (1) different individuals are exposed to a very different amount of light pollution within the same business district sites, and (2) individuals in the business district can be exposed to large differences in light pollution during subsequent nights. Therefore, variation at the level of the microhabitat occupied by each individual is crucial for the understanding of how avian behaviour and physiology might be influenced by light at night. On the other side, behaviour in the form of temporal activity patterns can obviously have produced the patterns of light exposure recorded by the light loggers. I will go in more details about this topic later in the chapter.
Variation in exposure to light at night in adult male European blackbirds recorded at three study sites differing in the degree of urbanization, using light loggers deployed on individual blackbirds. We calculated the median light intensity in the early night (10:00–00:30) and in the late night (00:30–3:00) and averaged these values for each bird. Box plots represent, from bottom to top, one standard deviation (SD) below the mean, lower quartile, median, upper quartile and one SD above the mean
4.2 Are Urban Birds Exposed to a Longer Day Length?
As I mentioned in the introduction, photoperiod is a crucial environmental cue that animals use to synchronize their daily and seasonal activities to the best time of the day and year, and its importance often overrides that of other cues such as temperature and food availability. For instance, many animals can be “tricked” to reproduce at any time of the year by subjecting them to long days, which simulate the approaching spring and summer and therefore the breeding season (Follett et al. 1967; Lambrechts and Perret 2000; te Marvelde et al. 2012). Therefore, one of the key questions when we aim at investigating the effects of light pollution on wild organisms is whether the presence of artificial lights might affect the detection of day length. More specifically, I hypothesized that in light-polluted areas, birds will be exposed to a longer day length than in rural areas free of artificial lights. I avoid using the word “perceive” because without proper physiological and neuroendocrinological measurements, it is not possible to state with certainty than birds perceive a longer day length. But it is logical to assume that if birds are exposed to a pattern and intensity of light at night that mimic those of a longer photoperiod, they will also physiologically perceive a longer day.
But what does define day length? In laboratory studies, photoperiod is easily defined by the fact that lights are usually turned on and off sharply. But during a natural dawn, light intensity increases gradually before reaching its maximum only around midday (Fig. 13.4). So when do animals begin realizing that the day is starting and they should get ready with their business? Within the same taxa, animals can have a considerably high variation in sensitivity to light (Thomas et al. 2002; Kempenaers et al. 2010). Thus, they likely have different thresholds of light intensity that need to be met to signal the onset or end of the day. This could depend on several ecological and physiological factors, including eye size (Thomas et al. 2002). Indeed, among songbirds, there is a clear separation between families based on when species start their dawn chorus relative to the beginning of twilight, with early rising species having on average larger eyes relative to body size (Thomas et al. 2002). Light pollution could therefore impact day-length detection differently depending on sensitivity to light. Indeed, Kempenaers and collaborators have shown that among five different species of songbirds, those belonging to the family Turdidae seem to be much more affected from light pollution than species of the families Paridae and Fringillidae (Kempenaers et al. 2010).
Based on these considerations and on how light pollution can alter the natural regimes of sky brightness at night (Fig. 13.1), my recent work has been devoted to understand whether birds in urban environment are exposed to patterns of light at night that may be perceived as a longer day length (Dominoni and Partecke 2015). In particular, assuming that the highest naturally occurring light intensity at night is produced by full moon, I defined “subjective day length”, or simply “day length”, the duration of time in minutes between the time that light intensity passes the level of full-moon light intensity in the morning and the time when that happens in the evening. This is exemplified in Fig. 13.7. I hypothesized that birds in light-polluted areas will be exposed to a longer subjective day length than rural conspecifics.
Schematic representation of the concept of “subjective day length”, which was used to test the hypothesis that urban birds are exposed to a longer day length than rural conspecifics. Briefly, full-moon light intensity (0.3 lux) was used as reference threshold level to which we compared each light intensity time series recorded on individual free-living blackbirds using light loggers. The times at which the series crossed full-moon intensity in the morning and evening were recorded, and the duration in minutes between these two time points was defined as subjective day length
I tested this hypothesis using the same data collected by the light loggers and presented in Fig. 13.6. As a threshold for the full moonlight intensity, I used a value of 0.3 lux, which corresponds to 0.0025 W/m2 as measured at ground level during a full-moon night in my forest study site. Based on this assumption and on the procedure to calculate subjective day length explained in Fig. 13.7, I analysed the variation in day length as well as in the onset and end of the subjective day across the different study sites. Subjective day length was significantly longer in the business district compared to both the rural forest and the urban park, which were not significantly different between each other. This difference was equally explained by an earlier onset and a later end of the subjective day in the business district compared to the other two sites (Fig. 13.8). Thus, birds breeding in highly light-polluted areas appear to be exposed to a longer day length than birds inhabiting darker areas. On average, this difference is 52 min. In the Munich region, this equals approximately to 19-day difference in photoperiod during the late winter/early spring, the time at which blackbirds start to reproduce (Partecke et al. 2005). I will get back to this concept later in the chapter, where I will discuss the effect of light pollution on the reproductive biology of urban blackbirds.
Full-moon light intensity (0.3 lux, or 0.0025 W/m2 at 500 nm in the rural forest) was used as threshold value to calculate the time at which the light intensity time series (recorded by light loggers deployed on individual male blackbirds) passed this value in the morning and evening and defined those times as onset (b) or end (panel c) of day. The “subjective day length” was calculated as the difference between the onset and end of day (a). We standardized the measurements to the natural variation in day length, the horizontal line at time 0 (a, total day-length hours; b, sunrise; c, sunset). Each dot represents one single day of recording for a specific bird, whereas box plots represent median and s.d. values for each study site. Sample sizes: rural forest N = 8, urban parks N = 11, business district N = 12
4.3 Does Variation in Exposure to Light at Night Relate to Activity Patterns?
Several studies in the last decade have linked light pollution to both an earlier onset of dawn song and a later offset of dusk chorus in birds. This has been shown not only in European blackbirds but also in several other songbird species such as great tit (Parus major), blue tit (Cyanistes caeruleus), European robin (Erithacus rubecula), American robin (Turdus migratorius) and song thrush (Turdus philomelos) (Miller 2006; Fuller et al. 2007; Dominoni et al. 2013b, 2014; Nordt and Klenke 2013; Da Silva et al. 2014, 2015). However, these studies have either measured the average night light intensity in the areas where the birds were recorded or record birds in areas with or without light pollution. Thus, our understanding of the relationship at the individual level between the timing of dawn and dusk song and the amount of night light was limited. I tried to overcome this using the data collected by both light loggers and automated radiotelemetry on the European blackbirds. Indeed, as the blackbirds carried radio-transmitters on their back, I was able to use automated receiving units (ARUs) to record the activity patterns of one specific bird. The ARUs recorded the signal strength emitted by the transmitters every minute. By looking at the variation in signal strength over time, these data were used to infer the activity state (active vs. nonactive) of the animal, although not its exact position. The basic assumption of this methodology is that variation in signal strength signals movement of an animal (Cochran et al. 1965). Conversely, when variation in signal strength is minimal, the animal is assumed to be inactive (Fig. 13.9). Together with Dr. Bart Kranstauber (Max Planck Institute for Ornithology, Germany, now at the University of Zurich, Switzerland), I have developed a statistical methodology to objectively quantify switches in activity state without using arbitrary threshold levels (Dominoni et al. 2014). I used this methodology to estimate the time at which a bird started its activity in the morning and ceased it in the evening and called these onset and end of activity.
Plots of raw light intensity and activity data. Data from one complete day are shown for one rural (a, c) and one urban bird from the business district (b, d). Light intensity data (a, b) was recorded by light loggers mounted on individual birds. Inlays show data used for the analyses, from 10 pm to 3 am. Activity data (c, d) was collected by automated telemetry stations (ARU, www.sparrowsystems.biz). ARU recorded the signal strength of radio-transmitters attached to individual birds. Switches between activity states (active/nonactive) were inferred by analysis of the change in signal strength between consecutive data points and are indicated by arrows in the graph. Light data is recorded on a 2 min basis, activity data on a minute basis
These data were collected in the three study sites on several individual blackbirds which simultaneously carried light loggers on their back, thus allowing a direct comparison between light exposure and activity patterns. As for light exposure, I used the average light intensity recorded during the true night, thus excluding the twilight phases. The reason behind this approach is that I wanted to avoid using periods of the night when the light intensity recorded by the light loggers could be highly influenced by the behaviour of the animals, as blackbirds are very active during twilights. Rather, I wanted to estimate the average nocturnal light environment to which blackbirds were exposed to, and the data were already shown in Fig. 13.6. I used these data as explanatory variable in different linear mixed models that aimed at testing the effect of several environmental variables (including noise, temperature, cloud cover, rainfall and season) (Dominoni et al. 2014). In all these models, light intensity at night was a significant predictor of the onset of activity in the morning: the higher the light intensity to which an animal was exposed to at night, the earlier the onset of its daily activity (Fig. 13.10). A trend was also found for the end of the daily activity (not shown), such as birds breeding in highly light-polluted areas were also prone to end their daily activity later than animals in darker areas. Among the other environmental variables considered, the only one found to be having a clear and significant effect was date (Dominoni et al. 2014). Indeed, birds started their activity earlier during the early spring (end of March and April), the time at which in our study sites male blackbirds have higher testosterone levels and females start to lay eggs. This result is in line with previous studies that have found that male dawn activity is stronger during the early breeding season, when males establish their territories and compete for access to females (Kunc et al. 2005). A recent work has confirmed that the effect of light pollution on dawn song in several songbird species is stronger in early spring (Da Silva et al. 2015).
Relationship between onset of activity and light at night (a) as well as season (b). Symbols (grey squares = rural; white circles = city parks; black triangles = business district) represent the mean for each individual. Error bars indicate s.e.m. Black line indicates a significant relationship between activity and light at night for the two urban sites pooled together. No significant relationship between the two explanatory variables and activity was found for the rural birds. The horizontal dotted lines represent the civil twilight, to which the onset of activity was standardized in order to control for seasonal changes in photoperiod
4.4 Experimental Demonstration of the Effects of Light Pollution on Daily and Seasonal Cycles of Birds
All studies conducted in the wild, even the experimental approaches that have been used in recent studies, suffer from the potential confounding factor of any non-measured environmental variable that can bias the results. Thus, in order to really demonstrate an effect of light at night on any biological process, captive studies are needed. I therefore took advantage of the fact that, through the use of light loggers, I had collected individual-based recordings of exposure to light intensity in urban and rural areas to set up realistic simulations of night-time urban and rural light intensity in captivity. In the winter of 2010/2011, I exposed 40 wild-caught male European blackbirds of mixed origin (20 from the rural forest and 20 from the business district/urban parks) to two different light treatments. Birds were equally divided into the two treatments based on their origin. They were exposed to the same natural variation in local photoperiod, but with different light intensity at night. The control group was exposed to 0.0001 lux at night, a light intensity very close to complete darkness mimicking a forest-like environment. The experimental group was exposed to 0.3 lux at night, a light intensity within the median night-time values recorded on male blackbirds in the city of Munich using light loggers (Fig. 13.5). The main aim of this experiment was to analyse the effect of the light at night treatment on the daily activity patterns and reproductive physiology of blackbirds, in order to experimental test the hypotheses originated from field studies. Birds were housed in individual cages in two rooms corresponding to the two different light treatments. Locomotor activity of blackbird was recorded around the clock using infrared sensors mounted on top of each cage. From January to July, I recorded monthly the singing activity of birds and the size of their gonads and collected a blood sample to analyse plasma concentration of testosterone. In addition, from May on I checked weekly the intensity of primary and body feathers moult to assess the onset of the moulting period for each bird.
The results clearly indicate that birds exposed to light at night advanced the onset of both their reproductive physiology (gonadal size and testosterone) and their moult (Fig. 13.11a, b) (Dominoni et al. 2013b). The effect size ranged between 2 and 4 weeks of early onset, a difference comparable to what found in the wild, where urban birds were shown to reach reproductive readiness approximately 19 days before their forest conspecifics (Partecke et al. 2005). This difference is interesting because it is exactly what found in the analysis of day-length exposure that I have shown earlier in this chapter (Fig. 13.8a) and in a recent manuscript (Dominoni and Partecke 2015). Thus, it appears that light pollution can explain most of the variation in the timing of reproductive physiology between urban and rural blackbirds. Similarly, the analyses of both locomotor activity and singing behaviour both indicate that birds exposed to light at night advanced their morning activity significantly compared to birds exposed to dark nights, and also this effect size closely mirrors the difference found between urban and forest blackbirds (Fig. 13.11c, d). Indeed, blackbirds under the light at night treatment started their activity considerably earlier, up to 2 h before dawn, than birds under dark nights, regardless of their origin (Dominoni et al. 2013c). In addition, as previously shown from field data (Fig. 13.10b), this difference appeared to be much more marked during the peak of the reproductive growth (Dominoni et al. 2013b), during the second half of March and the month of April (Fig. 13.11c). Taken all together, the results of this captive experiment clearly indicate that increasing the level of light at night to which birds are exposed to in the laboratory produces drastic changes in the reproductive physiology as well as in the timing of daily activity that confirms much of the variation in these processes found in birds breeding in urban habitats.
Experimental demonstration of the effects of light at night on daily and seasonal timing in European blackbirds. Male blackbirds of either urban (triangles) or rural (circles) origin were exposed to either dark nights (blue symbols) or light at night of 0.3 lux (red symbols). In all graphs, symbols represent means and bars s.e.m. (a) Variation in testicular width during the reproductive phase. Horizontal line at 5 mm represents the size at which testes were considered to produce fertile sperm (half of the maximal size). Horizontal box plots represent average onset of moult in the four different treatment groups. (b) Variation in plasma concentration of testosterone from blood collected on the same week that testes’ size was measured. (c) Onset of the first morning song in each treatment group. (d) Locomotor activity was recorded with infrared sensors attached on top of each individual cage. Grey areas indicate lights off and white area indicates lights on
5 Conclusion and Future Perspectives
The study of the ecological effects of light pollution is a growing field of research. During the last decade, we have seen an increasing number of studies investigating the responses of individual organisms, populations and communities to the presence of anthropogenic night light. Besides evident effects of light pollution on immediate mortality, such as collision against lit towers at night (Longcore et al. 2013), one of the best examples of how light pollution can affect wild organisms is the change in the daily and seasonal biology observed in several species. Such changes have been shown by both correlational and experimental studies in the wild and controlled experiments in captivity, which I reviewed in this chapter. Although some other environmental cues have likely the potential to impact the same timing processes (McNamara et al. 1987; McNamara and Houston 2008; Saggese et al. 2011; Dominoni et al. 2015), too, light pollution has consistently been shown to play a major role. Individual-based measurements of light exposure at night obtained using light loggers have confirmed such findings and revealed that light pollution can potentially alter the perception of day length, with important consequences for the way that organisms interpret the lightscape and therefore time their daily and seasonal activities. So what’s next?
An unresolved outstanding question in light pollution research is whether the observed changes in behaviour and physiology of individual animals come with fitness consequences. For instance, reproductive success of male songbirds that are able to sing earlier in the morning under light pollution seems to be enhanced due to increasing extra-pair paternity (Kempenaers et al. 2010). However, singing at night might come also with costs related to increased predation risk under poor visibility or decrease thermoregulatory capacity which could lead to higher energy expenditure. In addition, lack of synchronization between internal and external timing can also come with detrimental consequences, as extensive work in human shift workers has testified (Kantermann et al. 2010). Regardless of these specific examples, many studies have shown that the ecological effects of light pollution can be widespread and cause at the very least an increased in immediate mortality (Rich and Longcore 2006). Therefore, although it is imperative that future research will examine the different ways through which light pollution may affect fitness, we should already be discussing way for mitigating light pollution (Gaston et al. 2012). Besides the physical design of streetlamps, recent work has also stressed the necessity to include the wavelength of light as an important variable in light pollution research. As presented, short to mid-wavelengths of light seem to be impacting organismal responses the most, with long wavelengths in the red spectrum having minimal effects (van Geffen et al. 2014; De Jong et al. 2015; Spoelstra et al. 2015). However, more evidence-based knowledge is needed if we want to inform policy-makers about the best way to design artificial lights and implement new policies that look at keeping the impact of light pollution minimal as a priority for environmental planning.
References
Aubé M, Roby J, Kocifaj M (2013) Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS One 8:e67798
Bennie J, Davies TW, Duffy JP et al (2014) Contrasting trends in light pollution across Europe based on satellite observed night time lights. Sci Rep 4:3789. doi:10.1038/srep03789
Bennie J, Duffy J, Davies T et al (2015) Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sens 7:2715–2730. doi:10.3390/rs70302715
Cochran W, Warner D, Tester J, Kuechle V (1965) Automatic radio-tracking system for monitoring animal movements. Bioscience 15:98–100
Da Silva A, Samplonius JM, Schlicht E et al (2014) Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav Ecol 25:1037–1047. doi:10.1093/beheco/aru103
Da Silva A, Valcu M, Kempenaers B (2015) Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Philos Trans R Soc Lond B 370:20140126
Davies WIL, Turton M, Peirson SN et al (2012) Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol Lett 8:291–294
Davies TW, Bennie J, Inger R et al (2013a) Artificial light pollution: Are shifting spectral signatures changing the balance of species interactions? Glob Chang Biol 19:1417–1423. doi:10.1111/gcb.12166
Davies TW, Bennie J, Inger R, Gaston KJ (2013b) Artificial light alters natural regimes of night-time sky brightness. Sci Rep 3:1–6. doi:10.1038/srep01722
De Jong M, Ouyang JQ, Da Silva A et al (2015) Effects of nocturnal illumination on life history decisions and fitness in wild birds. Philos Trans R Soc Lond B 370:20140128
Dominoni DM (2015) The effects of light pollution on biological rhythms of birds: an integrated, mechanistic perspective. J Ornithol. doi:10.1007/s10336-015-1196-3
Dominoni DM, Partecke J (2015) Does light pollution alter daylength? A test using light-loggers on free-ranging European blackbirds (Turdus merula). Philos Trans R Soc Lond B 370:20140118
Dominoni D, Helm B, Lehmann M et al (2013a) Clocks for the city: circadian differences between forest and city songbirds. Proc R Soc Lond B 280:20130593
Dominoni D, Quetting M, Partecke J (2013b) Artificial light at night advances avian reproductive physiology. Proc R Soc Lond B 280:20123017
Dominoni DM, Goymann W, Helm B, Partecke J (2013c) Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front Zool 10:60. doi:10.1186/1742-9994-10-60
Dominoni DM, Carmona-Wagner EO, Hofmann M et al (2014) Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J Anim Ecol 83:681–692. doi:10.1111/1365-2656.12150
Dominoni DM, Van’t Hof TJ, Partecke J (2015) Social cues are unlikely to be the single cause for early reproduction in urban European blackbirds (Turdus merula). Physiol Behav 142:14–19. doi:10.1016/j.physbeh.2015.01.026
Dominoni DM, Borniger JC, Nelson RJ (2016) Light at night, clocks and health: from humans to wild organisms. Biol Lett 12(2):20160015. doi:http://doi.org/10.1098/rsbl.2016.0015
Follett BK, Farner DS, Morton ML (1967) The effects of alternating long and short daily photoperiods on gonadal growth and pituitary gonadotropins in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Biol Bull 133:330
Foster RG, Kreitzmann L (2004) Rhythms of life: The biological clocks that control the daily lives of every living thing. Yale University Press, New Haven, CT
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Lett 3:368–370. doi:10.1098/rsbl.2007.0134
Gaston KJ, Davies TW, Bennie J, Hopkins J (2012) Reducing the ecological consequences of night-time light pollution: options and developments. J Appl Ecol 49:1256–1266. doi:10.1111/j.1365-2664.2012.02212.x
Hale JD, Davies G, Fairbrass AJ et al (2013) Mapping lightscapes: spatial patterning of artificial lighting in an urban landscape. PLoS One 8:e61460. doi:10.1371/journal.pone.0061460
Kantermann T, Juda M, Vetter C, Roenneberg T (2010) Shift-work research: Where do we stand, where should we go? Sleep Biol Rhythm 8:95–105
Kempenaers B, Borgström P, Loës P et al (2010) Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr Biol 20:1735–1739
Kronfeld-Schor N, Dominoni D, de la Iglesia H et al (2013) Chronobiology by moonlight. Proc R Soc Lond B 280:20123088
Kunc HP, Amrhein V, Naguib M (2005) Seasonal variation in dawn song characteristics in the common nightingale. Anim Behav 70:1265–1271. doi:10.1016/j.anbehav.2005.02.010
Lambrechts MM, Perret P (2000) A long photoperiod overrides non-photoperiodic factors in blue tits’ timing of reproduction. Proc R Soc B Biol Sci 267:585
Longcore T, Rich C, Mineau P et al (2013) Avian mortality at communication towers in the United States and Canada: which species, how many, and where? Biol Conserv 158:410–419. doi:10.1016/j.biocon.2012.09.019
McNamara JM, Houston AI (2008) Optimal annual routines: behaviour in the context of physiology and ecology. Philos Trans R Soc B Biol Sci 363:301. doi:10.1098/rstb.2007.2141
McNamara JM, Mace R, Houston AI (1987) Optimal daily routines of singing and foraging in a bird singing to attract a mate. Behav Ecol Sociobiol 20:399–405
Miller MW (2006) Apparent effects of light pollution on singing behavior of American robins. Condor 108:130. doi:10.1650/0010-5422(2006)108[0130:AEOLPO]2.0.CO;2
Nordt A, Klenke R (2013) Sleepless in town – drivers of the temporal shift in dawn song in urban European blackbirds. PLoS One 8, e71476. doi:10.1371/journal.pone.0071476
Partecke J, Van’t Hof TJ, Gwinner E (2005) Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J Avian Biol 36:295–305
Peirson SN, Halford S, Foster RG (2009) The evolution of irradiance detection: melanopsin and the non-visual opsins. Philos Trans R Soc B Biol Sci 364:2849–2865. doi:10.1098/rstb.2009.0050
Rich C, Longcore T (2006) Ecological consequences of artificial night lighting. Island Press, Washington, DC
Roenneberg T, Foster RGG (1997) Twilight times: light and the circadian system. Photochem Photobiol 66:549–561
Rotics S, Dayan T, Kronfeld-Schor N (2011) Effect of artificial night lighting on temporally partitioned spiny mice. J Mammal 92:159–168
Saggese K, Korner-Nievergelt F, Slagsvold T, Amrhein V (2011) Wild bird feeding delays start of dawn singing in the great tit. Anim Behav 81:361–365. doi:10.1016/j.anbehav.2010.11.008
Spoelstra K, van Grunsven RHA, Donners M et al (2015) Experimental illumination of natural habitat – direct and indirect ecological consequences of artificial light of different spectral composition. Philos Trans R Soc Lond B 370:20140129
Stephan B (1985) Die Amsel-Turdus merula. Die Neue Brehm-Bücherei Bd. 95. Westarp Wissenschaften, Hohenwarsleben
Stone EL, Jones G, Harris S (2009) Street lighting disturbs commuting bats. Curr Biol 19:1123–1127
Te Marvelde L, Schaper SV, Visser ME (2012) A single long day triggers follicle growth in captive female great tits (Parus major) in winter but does not affect laying dates in the wild in spring. PLoS One 7, e35617
Thomas RJ, Székely T, Cuthill IC et al (2002) Eye size in birds and the timing of song at dawn. Proc Biol Sci 269:831–837. doi:10.1098/rspb.2001.1941
Van Geffen KG, van Grunsven RHA, van Ruijven J et al (2014) Artificial light at night causes diapause inhibition and sex-specific life history changes in a moth. Ecol Evol 4:2082–2089. doi:10.1002/ece3.1090
Acknowledgments
I first would like to thank Jesko Partecke and Barbara Helm for invaluable supervision and support throughout my years as a PhD student and young postdoc. I acknowledge the help, support and input originated from discussions with several people including Michaela Hau, Tim Greives, Catarina Miranda, Adam Fudickar, Till Roenneberg, Martin Wikelski, Bart Kranstauber, Marcel Visser, Kamiel Spoelstra and many others. Bruno Erne and Georg Heine have provided me with exceptional light loggers in a very brief time. Last but not least, I acknowledge that the initial idea of using light loggers on individual free-living birds is ownership of Prof. Eberhard Gwinner, whom I never met but whose legacy has profoundly influenced large part of my work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Dominoni, D.M. (2017). Ecological Effects of Light Pollution: How Can We Improve Our Understanding Using Light Loggers on Individual Animals?. In: Murgui, E., Hedblom, M. (eds) Ecology and Conservation of Birds in Urban Environments. Springer, Cham. https://doi.org/10.1007/978-3-319-43314-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-43314-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43312-7
Online ISBN: 978-3-319-43314-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)