Abstract
Tissue oxygenation and regulation is a critical feature for any organ function. The maintenance of an adequate supply of oxygen is required to maintain normal cellular function. This requires the coordinated action of the three major systems involved in oxygen transport: the cardiovascular system, the respiratory system, and the blood. The cardiovascular and respiratory systems are designed to carry the oxygen that is present in the atmosphere down to the mitochondria. The majority of oxygen is transported to the tissues bound to hemoglobin, and its equilibrium is a function of arterial oxygen content, hemoglobin level, arterial oxygen partial pressure, arterial oxygen saturation, and cardiac output. The interplay among these factors determines the transport of oxygen to the tissues defining its convection and diffusion properties. The resuscitation of patients with hemodynamic dysfunction is more than normalizing hemodynamics as an approach like that might prove to be inadequate, but also the therapies might have inherent negative effects. Therefore, the management of a patient in shock with the theoretical concepts of the main drivers for transport of oxygen and the subsequent delivery of oxygen to the tissues might lead to a structured approach that might benefit the patient more than using static clinical endpoints for these variables.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Tissue oxygenation and regulation is a critical feature for survival of any cell and, by extension, to any organism. The maintenance of an adequate supply of oxygen (O2) is required to maintain normal cellular function through the production of adenosine triphosphate (ATP) [1] mainly by oxidative phosphorylation in the mitochondrial Krebs cycle [2]. This requires the coordinated action of the three major systems involved in oxygen transport: the cardiovascular system, the respiratory system, and the blood. The cardiovascular and respiratory systems are designed to carry the oxygen that is present in the atmosphere down to the mitochondria.
2 Transport of Oxygen
The total amount of oxygen transported (DO2) can be calculated using the following formula:
-
CaO2 = arterial oxygen content
-
Hb = hemoglobin level
-
PaO2 = arterial oxygen partial pressure
-
SaO2 = arterial oxygen saturation
From this it is clear that the majority of oxygen is transported to the tissues bound to hemoglobin. Hemoglobin has an oxygenbinding capacity of 1.34 mL O2 per gram, where the oxygen content mainly depends on oxygen saturation and hemoglobin concentration, as the amount of dissolved oxygen in the blood is minimal. The oxygen partial pressure at sea level is approximately 160 mmHg. From this high initial pressure in the lungs, there is an abrupt fall of about 4–8 mmHg at the mitochondrial level (Fig. 2.1). The level of saturated hemoglobin (SaO2) is determined by the oxygen–hemoglobin dissociation curve, where the proportion of hemoglobin in its saturated form is plotted against the prevailing oxygen tension on the horizontal axis. This curve is an important tool for understanding how the blood carries and releases oxygen. This curve is such that when SaO2 drops to less than 90%, even small variations in PaO2 are associated to important changes on SaO2 [3]. Generally speaking, a SaO2 of about 50% (P50) associates to a PaO2 of 26 mmHg (Fig. 2.2, [4]). Shifts in the oxygen dissociation curve (resulting in changes in the P50) are related to changes in the off-loading of oxygen. A right shift of the curve (increase in P50) as seen in acidosis, hypercapnia, and fever facilitates oxygen off-loading. Normal DO2 is approximately 1000 mL/min or 500 mL/min.M2 if cardiac index is substituted for cardiac output:
Hemoglobin’s oxygen dissociation curve is sigmoidal. The four-subunit arrangement in hemoglobin (α1, α2, β1, β2) accomplishes a specific function when hemoglobin flows from high oxygen tension in the lungs to the low oxygen tension areas in the tissues and back to the lungs. Oxygen remains tightly bound to hemoglobin in the lungs but will be progressively released as partial oxygen pressure drops in the tissues of the body. The release of the second, and even more so the third, oxygen molecule requires a smaller drop in pressure as the erythrocyte moves farther from the lungs, whereas the reverse occurs when the erythrocyte moves to the lungs (figure constructed from [4])
Oxygen consumption (VO2) is the rate at which O2 is taken up from the blood and used by the tissues. It can either be directly measured or calculated. VO2 is defined by the Fick equation as the difference between the content of oxygen in the arterial and mixed venous compartment (equaling the amount of oxygen taken up by the periphery) multiplied by the cardiac output (the flow through the system).
-
CvO2 = arterial oxygen content
-
PvO2 = mixed venous oxygen partial pressure
-
SvO2 = mixed venous oxygen saturation
Oxygen extraction ratio (ERO2) is the relationship between DO2 and VO2, and it normally ranges from 0.25 to 0.30. When we reduce the formula for ERO2 to its main components, we are left with
Therefore, mixed venous oxygenation (or its surrogate, central venous oxygenation) is clinically used to estimate the balance between oxygen delivery and oxygen demand. Under normal conditions oxygen demand equals oxygen consumption. However, when central venous oxygenation falls, it reflects an imbalance between the demand and supply. This is not equal to inadequate oxygen consumption (as this would reflect a state of tissue hypoxia) but rather a compensation for a decrease in delivery either due to a decrease in oxygen content or cardiac output. The transport of oxygen does not equal the delivery of oxygen to the tissues. For this local blood flow is regulated by several tissue factors mainly related to the metabolic rate. So, cardiac output is redistributed among the tissues depending on their relative requirements, where this regulation occurs in the microcirculation [5]. Thus, under normal conditions, cardiac output is demand driven.
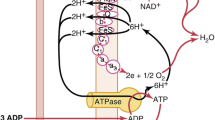
Once oxygen reaches the tissues, a part of it passes to the interstitial space and freely diffuses to the intracellular space and mitochondria. The site within the mitochondria at which oxygen is consumed is cytochrome c oxidase, the terminal electron acceptor in the electron transport chain. Mitochondria appear to be able to sustain normal oxygen consumption needed for generating ATP at a maximum rate, until the amount of oxygen in their immediate vicinity acutely falls below a critical value of 4–6 mmHg [6, 7]. In chronic hypoxemia conditions, this threshold is significantly higher, and suppression of oxygen consumption may already start below 40 mmHg [8].
Tissue oxygenation is typically described by one of the following three terms: first, normoxia, being a state where cellular PO2 is greater than the critical value; second, hypoxia, where some tissue regions have less than adequate oxygen levels and in consequence mitochondria produce ATP at a submaximal rate; and third, anoxia, which is the absence of oxygen in the tissue where mitochondria cease to produce ATP [9]. CO2 diffuses rapidly through the tissues and across peripheral capillary walls due to its greater solubility. Because of this CO2 elimination from tissues is seldom a concern of diffusion but rather dependent on the perfusion of the tissues. Therefore, changes in cardiac output relate to changes in central venous CO2 levels in many disease states [10,11,12].
Oxygen exchange occurs not only across the walls of capillaries but can be exchanged between any two regions in which a partial oxygen pressure difference occurs or where a gradient is present. Therefore, a significant transarteriolar O2 gradient is generally present. It was Krogh who presented a more accurate model and description of oxygen transport in tissues. Since all capillaries were assumed to be identical and uniformly spaced, he devised a simple tissue model for oxygen transport and consumption constituted by a single capillary with continuous blood flow, surrounded by a concentric cylinder of oxygenconsuming tissue. This model was refined over time to take into account the variations in capillary hematocrit, the low solubility of O2 in the plasma, and the resistance to oxygen diffusion between the blood and tissue due to the particulate nature of the blood [13]. Diffusion is the mechanism by which oxygen passes from blood to tissue cells. As red blood cells (RBC) pass through capillaries in single file due to their similar size to the capillary caliber, oxygen is continuously released from the RBC hemoglobin and eventually diffuses to the mitochondria where it is consumed. Although most (≈98%) of the oxygen in the blood is reversibly bound to hemoglobin, the vector or the “driving force” for oxygen movement from the blood to tissue is the PO2 difference that exists across the vascular wall, not hemoglobin level or arterial oxygenation levels [1, 2].
3 Some Clinical Considerations
From the formula for DO2, it may seem that manipulating oxygen content (oxygen saturation and hemoglobin levels) is as effective as manipulating cardiac output or its distribution. As already mentioned earlier, adaptation to the changing need for oxygen of tissues, these tissues do not influence oxygen content but rather change the flow. In addition, increasing oxygen levels have been associated with adverse effects on tissue oxygenation and outcome [14,15,16]. Therefore, the judicious use of oxygen has been challenged [17] and clinicians are increasingly willing to apply conservative supplemental oxygen strategies [18]. Although the same holds for blood transfusion given the results from older studies [19,20,21], more recent studies focusing on the microcirculation have shown beneficial in recruiting the microcirculation [22,23,24]. Therefore, a transfusion strategy should probably not focus on a static hemoglobin level but rather on the state of the microcirculation.
For almost three decades, DO2 optimization has been one of the fundamental strategies to improve tissue oxygenation during acute circulatory dysfunction, particularly in high-risk surgical or septic patients. And in the majority of studies, the main manipulated variable was cardiac output next to blood pressure. The pioneer studies by Shoemaker et al. identified an O2 debt in these patients that was related to organ failures and mortality [25]. In a subsequent study, Shoemaker et al. showed that a strategy of DO2 maximization to supranormal levels with fluids and vasoactive agents aimed at decreasing or preventing this O2 debt decreased mortality [10]. Other investigators confirmed that increasing DO2 to high levels not only increased VO2 but also improved survival in patients with severe sepsis [26,27,28]. However, other large studies showed no benefit where one study even showed increased mortality associated with this approach [29, 30].
Although not specifically targeting VO2 but incorporating all the elements of increasing DO2, Rivers et al. [31] showed that therapy aimed to improve cardiac output and oxygen content significantly increased survival in early severe sepsis in emergency department patients. A redo of the concepts of Rivers many years later did not show to have a survival benefit [32,33,34]. However, the patient population in these studies (among other characteristics) was markedly different from the original study [35]. Nevertheless, it seems obvious that in patients with a risk of under-resuscitation, like postsurgical patients, the concept of early hemodynamic optimization (that mainly manipulates DO2) is related to improved survival [36, 37].
The resuscitation of patients with hemodynamic dysfunction is more than normalizing hemodynamics as an approach like that might prove to be inadequate [38], but also the therapies might have inherent negative effects. More recently, the risk of fluid overload has been highlighted [39, 40], and it has been recognized that fluid resuscitation to fixed static hemodynamics might induce harm [41, 42]. Therefore, these static clinical endpoints of fluid resuscitation have been removed from the latest sepsis guidelines [43]. Like in the discussion on blood transfusion earlier, aiming for fixed endpoints for fluid resuscitation, cardiac output, and blood pressure, it seems more physiological to aim for the ultimate target: improving microcirculatory perfusion. Although some studies have shown that the microcirculation as the target of resuscitation might be a relevant endpoint [22, 44,45,46], larger clinical studies incorporating holistic protocols, covering all aspects of tissue perfusion, are necessary.
Another important aspect is that some therapies aimed at improving DO2 or VO2 in clinical practice could be harmful not only in terms of toxicity but also detrimental for the purpose for which they were indicated.
Especially the use of vasoactive agents (vasopressors, vasodilators, inotropes) may have unwanted side effects.
Dobutamine increases myocardial VO2 and might enhance maldistribution of flow between different organs due to unbalanced vasodilatory effects that could be associated with increased mortality [30, 47]. In general, the vasopressor load and the use of multiple vasopressors has been associated with adverse outcome [48,49,50]. Although vasodilators might improve the microcirculation and have been associated with an increase in oxygen consumption (as a marker of improved tissue perfusion) [51,52,53,54], there may be decreases in blood pressure [52] that may have negative [55] or even positive effects [56, 57] in some patients.
Therefore, the management of a patient in shock with the theoretical concepts of the main drivers for transport of oxygen and the subsequent delivery of oxygen to the tissues might lead to a structured approach that might benefit the patient more than using static clinical endpoints for these variables.
References
Wilson DF, Erecinska M, Drown C, Silver IA. The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys. 1979;195:485–93.
Wilson DF, Owen CS, Erecinska M. Quantitative dependence of mitochondrial oxidative phosphorylation on oxygen concentration: a mathematical model. Arch Biochem Biophys. 1979;195:494–504.
Barcroft J, Hill AV. The nature of oxyhaemoglobin, with a note on its molecular weight. J Physiol. 1910;39:411–28.
Gomez-Cambronero J. The oxygen dissociation curve of hemoglobin: bridging the gap between biochemistry and physiology. J Chem Educ. 2001;78:757.
Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. Compr Physiol. 2011;Suppl 9: Handbook of physiology, the cardiovascular system, microcirculation. https://doi.org/10.1002/cphy.cp020406.
Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem. 1988;263:2712–8.
Schlayer C. The influence of oxygen tension on the respiration of pneumococci (type I). J Bacteriol. 1936;31:181–9.
Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. Am J Phys. 1993;265:L395–402.
Pittman RN. Regulation of tissue oxygenation. In: Neil GD and Granger JP (eds). Colloquium series on integrated systems physiology: from molecule to function to disease. Morgan & Claypool Life Sciences, San Rafael (CA), 2011. p. 2009–11.
Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101:509–15.
Grundler W, Weil MH, Rackow EC. Arteriovenous carbon dioxide and ph gradients during cardiac arrest. Circulation. 1986;74:1071–4.
Vander Linden P, Bakker J, Schmartz D, Vincent JL. Arteriovenous PCO2 differences reflects tissue hypoxia during hemorrhagic shock in dogs. Circ Shock. 1991;34:87.
Ellis CG, Potter RF, Groom AC. The Krogh cylinder geometry is not appropriate for modelling O2 transport in contracted skeletal muscle. Adv Exp Med Biol. 1983;159:253–68.
Orbegozo Cortes D, Puflea F, Donadello K, Taccone FS, Gottin L, Creteur J, Vincent JL, De Backer D. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc Res. 2015;98:23–8.
Reinhart K, Bloos F, Konig F, Bredle D, Hannemann L. Reversible decrease of oxygen consumption by hyperoxia. Chest. 1991;99:690–4.
Cornet AD, Kooter AJ, Peters MJ, Smulders YM. Supplemental oxygen therapy in medical emergencies: more harm than benefit? Arch Intern Med. 2012;172:289–90.
Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care. 2011;15:305.
Eastwood GM, Peck L, Young H, Suzuki S, Garcia M, Bellomo R. Intensive care clinicians’ opinion of conservative oxygen therapy (SpO2 90-92%) for mechanically ventilated patients. Aust Crit Care. 2014;27:120–5.
Hebert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, Blajchman M. Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group. JAMA. 1995;273:1439–44.
Carson JL, Duff A, Berlin JA, Lawrence VA, Poses RM, Huber EC, O’Hara DA, Noveck H, Strom BL. Perioperative blood transfusion and postoperative mortality. JAMA. 1998;279:199–205.
Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17.
Yuruk K, Almac E, Bezemer R, Goedhart P, de Mol B, Ince C. Blood transfusions recruit the microcirculation during cardiac surgery. Transfusion (Paris). 2011;51:961–7.
Donati A, Damiani E, Luchetti M, Domizi R, Scorcella C, Carsetti A, Gabbanelli V, Carletti P, Bencivenga R, Vink H, Adrario E, Piagnerelli M, Gabrielli A, Pelaia P, Ince C. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study. Crit Care. 2014;18:R33.
Zafrani L, Ergin B, Kapucu A, Ince C. Blood transfusion improves renal oxygenation and renal function in sepsis-induced acute kidney injury in rats. Crit Care. 2016;20:406.
Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16:1117–20.
Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992;102:216–20.
Astiz ME, Rackow EC, Falk JL, Kaufman BS, Weil MH. Oxygen delivery and consumption in patients with hyperdynamic septic shock. Crit Care Med. 1987;15:26–8.
Gilbert EM, Haupt MT, Mandanas RY, Huaringa AJ, Carlson RW. The effect of fluid loading, blood transfusion, and catecholamine infusion on oxygen delivery and consumption in patients with sepsis. Am Rev Respir Dis. 1986;134:873–8.
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–32.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93.
Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506.
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–11.
Nguyen HB, Jaehne AK, Jayaprakash N, Semler MW, Hegab S, Yataco AC, Tatem G, Salem D, Moore S, Boka K, Gill JK, Gardner-Gray J, Pflaum J, Domecq JP, Hurst G, Belsky JB, Fowkes R, Elkin RB, Simpson SQ, Falk JL, Singer DJ, Rivers EP. Early goal-directed therapy in severe sepsis and septic shock: insights and comparisons to ProCESS, ProMISe, and ARISE. Crit Care. 2016;20:160.
Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699–707.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Kavanagh BP, Meyer LJ. Normalizing physiological variables in acute illness: five reasons for caution. Intensive Care Med. 2005;31:1161–7.
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, Schroeder ME, Marshall JC, Vincent JL, Intensive Care Over Nations Investigators. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45:386–94.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65.
Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol. 2013;13:17.
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
van Genderen ME, Engels N, van der Valk RJ, Lima A, Klijn E, Bakker J, van Bommel J. Early peripheral perfusion-guided fluid therapy in patients with septic shock. Am J Respir Crit Care Med. 2015;191:477–80.
Dubin A, Pozo MO, Casabella CA, Palizas F Jr, Murias G, Moseinco MC, Kanoore Edul VS, Palizas F, Estenssoro E, Ince C. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13:R92.
Tanaka S, Escudier E, Hamada S, Harrois A, Leblanc PE, Vicaut E, Duranteau J. Effect of RBC transfusion on sublingual microcirculation in hemorrhagic shock patients: a pilot study. Crit Care Med. 2016;45(2):e154–60.
Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, Florez J, Castro R, Aquevedo A, Pairumani R, McNab P, Ince C. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. 2013;39:1435–43.
Prys-Picard CO, Shah SK, Williams BD, Cardenas V Jr, Sharma G. Outcomes of patients on multiple vasoactive drugs for shock. J Intensive Care Med. 2013;28:237–40.
Dunser MW, Ruokonen E, Pettila V, Ulmer H, Torgersen C, Schmittinger CA, Jakob S, Takala J. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care. 2009;13:R181.
Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316.
Bihari D, Smithies M, Gimson A, Tinker J. The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med. 1987;317:397–403.
Lima A, van Genderen ME, van Bommel J, Klijn E, Jansem T, Bakker J. Nitroglycerin reverts clinical manifestations of poor peripheral perfusion in patients with circulatory shock. Crit Care. 2014;18:R126.
den Uil CA, Lagrand WK, Spronk PE, van der Ent M, Jewbali LS, Brugts JJ, Ince C, Simoons ML. Low-dose nitroglycerin improves microcirculation in hospitalized patients with acute heart failure. Eur J Heart Fail. 2009;11:386–90.
Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–6.
Preiser JC, De Backer D, Vincent JL. Nitroglycerin for septic shock. Lancet. 2003;361:880. (author reply 880).
Lamontagne F, Meade MO, Hebert PC, Asfar P, Lauzier F, Seely AJ, Day AG, Mehta S, Muscedere J, Bagshaw SM, Ferguson ND, Cook DJ, Kanji S, Turgeon AF, Herridge MS, Subramanian S, Lacroix J, Adhikari NK, Scales DC, Fox-Robichaud A, Skrobik Y, Whitlock RP, Green RS, Koo KK, Tanguay T, Magder S, Heyland DK, Canadian Critical Care Trials Group. Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med. 2016;42:542–50.
Duenser MW, Takala J, Brunauer A, Bakker J. Re-thinking resuscitation: leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion-based approach. Crit Care. 2013;17(5):326.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Castro, R., Hernández, G., Bakker, J. (2018). Oxygen Transport and Tissue Utilization. In: Pinto Lima, A., Silva, E. (eds) Monitoring Tissue Perfusion in Shock. Springer, Cham. https://doi.org/10.1007/978-3-319-43130-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-43130-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43128-4
Online ISBN: 978-3-319-43130-7
eBook Packages: MedicineMedicine (R0)