Abstract
Ductal Carcinoma in Situ is a non-invasive form of breast cancer, in which malignant ductal epithelial cells proliferate, but do not invade through the basement membrane. It is a heterogeneous disease, and is a non-obligate precursor to invasive carcinoma. With the advent of screening mammography the incidence of DCIS has greatly increased. MRI is the most sensitive examination for detecting DCIS. The most common presenting morphology of DCIS is nonmass enhancement, with a clumped internal enhancement pattern and with a segmental distribution pattern. There is great variety in the kinetic patterns of DCIS, and therefore assessment must be based on morphology. Additional tools, such as diffusion weighted imaging have been shown to be promising in helping detect clinically relevant DCIS.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ductal Carcinoma in Situ (DCIS)
- MRI
- Nonmass enhancement
- Clumped internal enhancement
- Clustered ring internal enhancement
- Segmental distribution
- Diffusion Weighted Imaging (DWI)
- Overdiagnosis
- Overtreatment
- Oncotype DX 12-gene assay (DCIS Score)
1 Background
Ductal carcinoma in situ (DCIS) is a noninvasive breast cancer, referred to as stage 0 breast cancer. At pathology DCIS shows the proliferation of malignant ductal epithelial cells that line a terminal ductal lobular unit without evidence of invasion through the basement membrane. DCIS represents a broad spectrum of disease, and is a non-obligate precursor to invasive breast cancer [1–3]. Some lesions may remain clinically quiescent, while others are precursors to invasive breast cancer.
DCIS is rarely symptomatic. With the advent of screening mammography, there has been a significant increase in the incidence of DCIS from 18.7 per 100,000 in 1973–1975 to 32.5 per 100,000 in 2005 [4], a 17-fold increase. DCIS now accounts for up to 25 % of screen detected breast cancers [5].
The term DCIS includes a markedly heterogeneous group of lesions which differ in genetic and molecular abnormalities, histopathologic features, and biologic markers [6]. DCIS is classified according to its tumor grade (high, intermediate, low) architectural pattern (solid, cribiform, papillary, micropapillary, comedo-type), and the presence or absence of necrosis.
Despite the fact that not all cases of DCIS progress to IDC, women who have been diagnosed with DCIS report similar psychologic morbidity as women with invasive cancer [7, 8]. With this similar psychologic morbidity as well as the great heterogeneity of DCIS, there is currently a significant controversy regarding the clinical significance of DCIS, as well as the possible overdiagnosis and overtreatment of DCIS.
The definition of overdiagnosis is “the detection of cancers that would never have been found were if not for the screening test” [9]. Overdiagnosis is considered a harm of screening mammography, and may be considered the most adverse outcome of screening mammography. Autopsy series of women not known to have breast cancer in their lifetime show a prevalence of DCIS of about 10–15 % [10–12].
It is not possible to recognize which cases of breast cancer are cases of overdiagnosis at the individual level; the number may only be estimated at a population level based on data from years of screening mammography. Puliti et al. conducted a literature review of observational studies providing estimates of breast cancer overdiagnosis in a European population based mammographic screening program. The analysis of the papers in the review and of the biases that may affect the estimates found that the most likely estimate of overdiagnosis, “expressed as a percentage of the expected incidence in the absence of screening”, was low, and ranged from 1 to 10 %. The authors found the much higher estimates of overdiagnosis in the literature they reviewed to be related to “the lack of adjustment for breast cancer risk and/or lead time bias” [13].
An additional factor in over diagnosis may be secondary to high inter-observer pathologist variability and discrepancies in the classification of DCIS [14–20]. Several of the discrepancies in this classification come from the criteria used to distinguish between atypical ductal hyperplasia (ADH) and DCIS [17]. The architectural appearance and extent of disease process are not simultaneously present in ADH: the diagnosis of ADH is therefore made when some features of DCIS are present, however others are absent [21, 22]. Rosai et al. reported an “unacceptably high” interobserver variation between experienced pathologists in the context of ADH versus DCIS categorization [19]. Thus the issue of over diagnosis, is one shared by both radiologists and pathologists.
Despite the heterogeneous nature of DCIS, it has been shown that almost all invasive cancers arise from DCIS [23]. Long term (30 year) follow-up of low grade DCIS treated only with biopsy without definitive excision or radiation therapy demonstrates a 30–60 % incidence of IDC, usually at or near the site of DCIS [24]. Half of recurrent cases of DCIS lesions manifest as invasive cancer, and 20 % of DCIS cases result in distant metastatic disease in 10 years [25]. It is therefore essential to have an accurate test for the diagnosis and detection of DCIS.
The goal of treating DCIS is to prevent the development of invasive cancer and to decrease the rate of local recurrence. Traditionally DCIS has been treated in most women with breast conservation therapy (lumpectomy) either with or without radiation therapy. Several randomized controlled trials have shown that adding radiation treatment following lumpectomy decreases the risk of local recurrence and invasive local recurrence by 50 % [26–31]. However, some patients at low risk of recurrence may not require radiation therapy.
A challenge in the treatment of DCIS is that clinical factors and pathologic features of DCIS have not been shown to consistently help clinicians determine patients at low risk. The Oncotype DX 12-gene assay (DCIS Score) is a multigene expression assay that generates individualized estimates of the 10-year risk of any local recurrence (LR). The score is generated from an algorithm that includes 12/21 genes in the Oncotype DX invasive assay. The Oncotype DX Score has been shown to predict local recurrence in patients who have undergone breast conservation therapy alone [32]. Therefore, the Oncotype DX score in combination with other well-established risk factors has the potential to be a useful tool in decreasing the over-treatment of DCIS.
2 Sensitivity of Imaging Modalities
An accurate assessment of the extent of DCIS is required for successful breast conservation therapy, as patients with positive margins after surgery and patients with residual synchronous foci of DCIS have an increased risk of recurrence. Several studies have shown that MRI is the most sensitive imaging examination for the detection of DCIS. The overall sensitivity of MRI for DCIS has been shown to be approximately 92 %, versus 56 % for mammography [33]. MRI detection is related to contrast uptake. Contrast uptake, or enhancement, is secondary to tumor vascularity, vessel density and permeability. Therefore, MRI, unlike mammography may detect not just calcified DCIS, but also noncalcified DCIS.
Over the past several years, studies have demonstrated the increasing sensitivity of MRI for detecting DCIS. Early studies looking at the sensitivity of MRI for DCIS were performed at a higher temporal resolution and lower spatial resolution, focused on mass lesions, and were also performed in patients with a new diagnosis of breast cancer, generally diagnosed by either mammogram or ultrasound [34]. More recent studies have used higher spatial resolution MRI technique, focused on nonmass enhancement distinct from background parenchymal enhancement (BPE), and evaluated a high risk screening population. These more recent studies have shown a greatly increased sensitivity of MRI for detecting DCIS [33, 34].
Not only is MRI the most sensitive examination for the detection of DCIS but it’s sensitivity increases with increasing histologic grade. MRI has been shown to have a sensitivity of 80 % for low grade DCIS, 91 % for intermediate grade, and 98 % for high grade DCIS [33]. Thus MRI is the most sensitive at detecting the type of DCIS that is most likely to progress to invasive carcinoma and to recur.
In addition, contrast enhancement is a biomarker of protease and angiogenic activity. There is increasing vascularity with increasing grades of DCIS. Protease activity is required to penetrate into the basement membrane and beyond it [35]. These factors suggest that DCIS detected on MRI may be more clinically relevant. Therefore, MRI may prove a useful tool in the evaluation of DCIS and may help to allay criticism of mammographic overdetection.
3 MRI Features of DCIS
3.1 Morphology
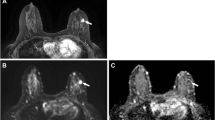
Given that MRI has been shown to be the most sensitive imaging modality for the detection of DCIS, it is important to recognize the various MR imaging presentations of DCIS. The most common presenting morphology of DCIS is nonmass enhancement, seen in 60–81 % of cases [36–38]. Nonmass enhancement (NME) is defined as enhancement of an area that is not a mass. The term NME has replaced nonmass like enhancement in the BI-RADS lexicon [39]. Nonmass enhancement is further defined by its internal enhancement pattern as well as its distribution. The most common internal enhancement pattern seen when DCIS presents as NME is a clumped pattern, defined as cobblestone-like enhancement with occasional confluent areas. This internal enhancement pattern is seen in approximately 41–64 % of cases presenting as NME (Fig. 9.1). Less frequently when DCIS presents as NME, it may present with a heterogeneous (16–29 % of cases) or homogenous (0–16 % of cases) internal enhancement pattern [36–38].
High nuclear grade DCIS in a 47 year old found on screening mammography. (a) CC spot magnification view demonstrates segmental pleomorphic calcifications (b) MRI performed for extent of disease, maximum intensity projection (MIP) images demonstrates regional nonmass enhancement with a clumped internal enhancement pattern in the inner right breast
In the second edition of BI-RADS for MR, the internal enhancement pattern “clustered ring” has been added. This is defined as small rings of enhancement, which are clustered together (Figs. 9.2 and 9.3) [39]. A study by Tozaki et al. showed that this finding was seen in 63 % of cases of malignancy (including both invasive and non-invasive), versus only 4 % of benign cases. The specificity for malignancy of the finding of clustered ring enhancement was 96 % [40].
28-year-old female with a palpable lump in the right breast. Given the patient’s age she had an ultrasound examination to start. (a) Ultrasound images show an irregular mass containing multiple echogenic foci (b) Subsequently performed CC mammogram demonstrates fine pleomorphic calcifications. (c) MRI performed for extent of disease demonstrates regional NME with a clustered ring internal enhancement pattern, consistent with biopsy proven DCIS
When DCIS presents as NME, the most common distribution pattern is a segmental distribution, seen in approximately 14–77 % of cases [36–38, 41]. This is defined as a triangular region of enhancement, apex pointing to the nipple, suggesting a duct or its branches (Fig. 9.4) [39]. It may also present less commonly in a linear, focal, regional, or diffuse enhancement pattern [36–38, 41]. The MR BI-RADS 2nd edition has removed the distribution ductal from the lexicon [39].
34 year old with left bloody nipple discharge, normal mammogram and ultrasound. (a) LM and (b) CC views from a ductogram show an intraductal-filling defect in a slightly lower, slightly outer duct. Left duct surgical excision revealed IDC and DCIS on pathology. (c) MRI post-contrast subtracted MIP demonstrated extensive clumped NME with a segmental distribution (d) Kinetic image demonstrates mixed, predominantly Type 2 and Type 3 delayed kinetics. MR guided biopsy revealed DCIS and IDC. Kinetic key: Type 1 = blue, Type 2 = green, Type 3 = red
DCIS may also present as a mass morphology on MRI. A mass is defined as a 3D space occupying structure with convex outward contour, which may or may not displace or otherwise affect the surrounding normal breast tissue [39]. This morphology has been seen in approximately 14–41 % of cases of DCIS on MRI [36–38]. Masses are further defined by shape, margin, and internal enhancement patterns. When DCIS presents as a mass on MRI it most commonly is an irregular mass, seen in 14–83 % of cases (Fig. 9.5). Less frequently it may present as an oval or round mass [36, 37, 42]. Of note, the 2nd edition of the MR BI-RADS lexicon has removed the shape lobular for mass lesions to be consistent with the mammogram and ultrasound sections. Masses with up to 3 lobulations now simply are described as “oval” [39]. The literature describes various mass margins when DCIS presents as a mass on MRI, including irregular (14–92 % of cases) and spiculated (0–92 % of cases) (Fig. 9.5). Infrequently DCIS presenting as a mass may have smooth margins (4–8 % cases) [36, 37, 42]. DCIS manifesting as a mass on MRI, may have various internal enhancement patterns. The most common pattern is heterogeneous (9–67 %), followed by homogenous (9–25 %), and less commonly rim enhancement (0–8 %) (Fig. 9.6) [36, 37]. To our knowledge, there has not been a report of non-enhancing dark internal septa in reports of DCIS seen as a mass on MRI. Of note, the terms central enhancement and enhancing septations have been removed from the new BI-RADS lexicon [39].
The least common morphologic appearance of DCIS is a focus [36–38]. A focus is defined as a lesion <5 mm, which is too small to further characterize (Fig. 9.7) [39]. The new BI-RADS edition has removed the term foci from the lexicon [39]. Rosen et al. found that pure DCIS manifests as a focus in 12.5 % of cases while 3.0 % of invasive carcinomas manifest as a focus [38]. Van Goethem et al. found that a focus was seen in 20 % of DCIS cases versus 2.8 % invasive cancers [44]. Factors suggesting that a focus is malignant on MRI include: no T2 hyperintensity, lack of fatty hilum, washout kinetics, new or enlarging in size. Signs of benignity of a focus include: T2 hyperintensity, presence of a fatty hilum, persistent kinetics, and stability [39].
Intermediate-grade DCIS in a 44 year old woman with negative mammographic findings who underwent screening MR imaging because of a strong family history of premenopausal breast cancer. Sagittal postcontrast subtraction image demonstrates a 4 mm focus that demonstrated type 3 (washout) kinetics (Reprinted with permission from Greenwood et al. [43], with permission from Radiology Society of North America (RSNA®))
Jansen et al. found that there was no statistically significant difference between MR morphology in low, intermediate, and high nuclear grade DCIS lesions [36]. Additional studies by Chan et al., and Rahbar et al., also found no significant difference in MR morphology between high grade and non-high grade DCIS [37, 45]. At this point, no study to our knowledge has shown that MR morphology is able to predict nuclear grade of DCIS.
3.2 Kinetics
The kinetic pattern of DCIS varies widely. The initial enhancement phase is defined as occurring within the first 2 minutes after contrast injection or until peak enhancement is reached [39]. In the initial phase, the most common kinetic pattern for DCIS is a fast uptake, seen in 49–68 % of cases, less commonly an intermediate (<20 % of cases) or slow pattern (<20 % of cases) [36, 38, 46]. Of note, in the new BI-RADS 5th edition the term fast has replaced rapid [39]. The delayed enhancement phase is defined as following 2 minutes after contrast injection or after peak enhancement is reached and is used to described the shape of the curve [39]. There is a wide variety of delayed phase kinetic patterns seen in DCIS. The most common pattern is a plateau (type 2), seen in 20–52 % of cases (Fig. 9.8) followed by a washout pattern (type 3), in 28–44 % of cases, and persistent enhancement pattern is seen in 20–30 % of cases [36, 38, 46]. Given the significant variation in the kinetic patterns of DCIS, it is very important to base the assessment for DCIS on MRI primarily on morphology rather than kinetics.
High grade DCIS in a 56-year-old woman with a palpable lump (a) Ultrasound image demonstrates a hypoechoic mass with indistinct margins and echogenic foci, corresponding to calcifications on mammography. (b) MRI performed for extent of disease demonstrates corresponding segmental clumped NME (c) with predominantly Type 2 (plateau) delayed kinetic pattern, compatible with biopsy proven high grade DCIS (Kinetic key: Blue = type 1, yellow = type 2, red = type 3)
Additional studies have looked at whether kinetic patterns may predict grade of DCIS. Jansen et al. found no significant difference in kinetic patterns, both initial and delayed, and grade of DCIS [36]. A study in 2012 by Rahbar et al. found no significant association between nuclear grade and delayed phase of enhancement. They did find a non-significant trend (p = .05) towards higher peak initial enhancement in high-grade DCIS lesions, compared to non-high grade, at 1.5 T [47]. However, a subsequent study by Rahbar et al. in 2015, found no statistically significant difference in kinetic patterns, initial and delayed, of various grades of DCIS when done at 3 T MR imaging [45].
3.3 1.5 T Versus 3 T
There has been increasing use of 3 T MRI for clinical dynamic contrast enhanced breast imaging over the last decade. As it has become apparent that high spatial resolution allows for more accurate detection of DCIS, it follows that 3 T MRI may have increased sensitivity for DCIS, as a benefit of 3 T imaging is higher signal to noise ratio, which allows for higher spatial resolution [48]. Rahbar et al. did a prospective study in patients newly diagnosed with pure DCIS. Each patient underwent a pre-operative breast MRI at both 1.5 T and 3 T imaging. They found that maximum DCIS lesion size on 3 T had a higher correlation with maximum size found on pathology than did 1.5 T [49]. 3 T may therefore be clinically helpful in pre-operative planning for DCIS lesions and further research in this area may be of clinical significance.
4 Diffusion Weighted Imaging
As discussed earlier, DCIS has a variable morphologic and kinetic presentation at MRI, which may present diagnostic challenges. No statistically significant difference in morphology has been shown to predict high grade versus non-high grade DCIS [36, 37, 45]. As the concerns for overdiagnosis and overtreatment grow, this becomes a challenge. In addition, another challenge is that breast MRI requires gadolinium administration with may limit accessibility. Diffusion weighted imaging (DWI) is a valuable MRI technique that may better be able to predict grade of DCIS and in addition it does not require any intravenous contrast. DWI quantifies the random motion of water in biologic tissue. The apparent diffusion coefficient (ADC) is the most common quantification of this water transport. Cancers are often more cellular than normal tissue, therefore restrict the diffusion of free water, and this forms the basis of DWI in oncology [50]. In breast cancer, a restricted ADC is widely accepted as a marker of cellularity [51–57].
Rahbar et al. looked at 74 pure DCIS lesions and found that quantitatively these lesions demonstrated higher DWI and lower ADC than normal tissue in the same patient, with a statistical significant difference [58]. In a subsequent study Rahbar et al. studied whether 3 T MRI was able to identify low risk DCIS. This study looked at the features of 36 DCIS lesions on MRI, 8 classified as low risk and 28 high risk. Again no statistically significant differences were found for morphologic features and kinetics between low risk and high risk DCIS. However, low risk DCIS lesions showed different DWI features, such as higher contrast to noise ratio and lower normalized ADCs than high-risk DCIS lesions [45].
Iima et al. studied 22 patients with pure DCIS and found that the ADC of high and intermediate grade DCIS were significantly lower than those of low-grade DCIS, and there was a significant negative trend between mean ADC and tumor grade. These preliminary results suggest that possibly DWI may be able to identify patients with low grade DCIS, which if confirmed could decrease patient anxiety and decrease invasive approaches [59].
An additional study by Rahbar et al., suggests that the combination of findings on DCE MRI and DWI may be able to predict low grade from high grade DCIS, with up to 81% accuracy. Larger size lesions corresponded with higher grade DCIS. A higher contrast to noise ratio (CNR), between each lesion and normal tissue on DWI (b = 600 s/mm^2) was seen in non-high grade DCIS which was thought to be related to greater T2-shine through, as no significant difference in ADC values between high grade and non-high grade lesions [45]. This lack of difference between ADC values and grade of DCIS is different than the results of Iima et al., as ADC values are technique-dependent, and further research is required in this area.
5 MRI Features Suggestive of Occult Invasion
Microinvasive DCIS is a subtype of disease which shows 1 mm or less of extension of cancer cells through the basement membrane. Hahn et al. found that microinvasive DCIS showed more suspicious MR imaging characteristics than pure DCIS. These findings included spiculated mass-type lesion, segmental distribution, and clustered ring enhancement of nonmass enhancement, and strong initial enhancement kinetics with washout kinetics [60].
The early identification of an invasive cancer along with DCIS, which is different than microvinasive cancer, is important because it results in changes to surgical management, including a sentinel node biopsy [61]. Wisner et al. looked at whether certain MRI BI-RADS criteria or radiologist perception correlated with invasive cancer after initial diagnosis of DCIS on core-biopsy. 13/51 patients with core-biopsy proven DCIS had invasion at excision. There was a significant positive correlation between the presence of a mass and invasion while nonmass enhancement had a significant negative correlation with invasion [62]. Goto et al. found that that certain MR findings of breast lesions, particularly in NME lesions, including large size of lesion and relatively higher signal intensity on fat-saturated-T2 W images, were suggestive of invasion in biopsy proven DCIS [63]. However, Nori et al. did not find MRI morphologic features to be significantly associated with prediction of DCIS plus invasive cancer when looking at cases of DCIS diagnosed on percutaneous biopsy [64]. This is an area where future research attention may be helpful.
6 Summary
With the advent of screening mammography the incidence of DCIS has increased significantly. MRI has been shown to be the most sensitive examination in the detection of DCIS. Not only is MR the most sensitive imaging modality but it is likely the one to detect the most clinically relevant cases of DCIS, and it is therefore essential to recognize the various presentations of DCIS on MR imaging. The most common morphology of DCIS is nonmass enhancement, and the most common distribution for the NME is in a segmental pattern. The most common kinetic pattern of DCIS is a fast initial uptake, however there is great variation in the delayed phase. It is thus, essential to evaluate lesions based on the morphologic pattern.
Given the broad spectrum of disease that DCIS represents, and the significant current controversies regarding both overdiagnosis and overtreatment of DCIS, additional research evaluating MR and its various techniques, including DWI, may be extremely useful in helping increase the detection of clinically relevant cases of DCIS and improving prediction of nonprogressive DCIS.
References
Mossa-Basha M, Fundaro GM, Shah BA, Ali S, Pantelic MV. Ductal carcinoma in situ of the breast: MR imaging findings with histopathologic correlation 1. Radiographics. 2010;30:1673–87.
Ozzello L. Ultrastructure of intra-epithelial carcinomas of the breast. Cancer. 1971;28:1508–15.
Recht A, Rutgers E, Fentiman I, Kurtz J, Mansel R, Sloane J. The fourth EORTC DCIS consensus meeting (Château Marquette, Heemskerk, The Netherlands, 23–24 January 1998)—Conference report. Eur J Cancer. 1998;34:1664–9.
Virnig BA, Wang S-Y, Shamilyan T, Kane RL, Tuttle TM. Ductal carcinoma in situ: risk factors and impact of screening. JNCI Monographs. 2010;2010:113–6.
Lynge E, Ponti A, James T, Májek O, Euler-Chelpin MV, Anttila A, et al. Variation in detection of ductal carcinoma in situ during screening mammography: a survey within the International Cancer Screening Network. Eur J Cancer. 2014;50:185–92.
Colin C, Devouassoux-Shisheboran M, Sardanelli F. Is breast cancer overdiagnosis also nested in pathologic misclassification? Radiology. 2014;273:652–5.
Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol J-P, Paszat L, et al. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77:285–93.
De Morgan S, Redman S, White KJ, Cakir B, Boyages J. ‘Well, have I got cancer or haven’t I?’ The psycho-social issues for women diagnosed with ductal carcinoma in situ. Health Expect. 2002;5:310–8.
Gotzsche PC. Breast cancer screening. International Agency for Research on Cancer (IARC) handbooks of cancer prevention. Vol. 7 Radiographics, October special issue 2013.
Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2005;97:135–44.
Nielsen M, Thomsen J, Primdahl S, Dyreborg U, Andersen J. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer. 1987;56:814–9.
Welch HG. Using autopsy series to estimate the disease “Reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med. 1997;127:1023–8.
Puliti D, Duffy SW, Miccinesi G, Koning HD, Lynge E, Zappa M, et al. Overdiagnosis in mammographic screening for breast cancer in Europe: a literature review. J Med Screen. 2012;19:42–56.
Costarelli L, Campagna D, Mauri M, Fortunato L. Intraductal proliferative lesions of the breast—terminology and biology matter: premalignant lesions or preinvasive cancer? Int J Surg Oncol. 2012. doi:10.1155/2012/501904.
Douglas-Jones A, Gupta S, Attanoos R, Morgan J, Mansel R. A critical appraisal of six modern classifications of ductal carcinoma in situ of the breast (DCIS): correlation with grade of associated invasive carcinoma. Histopathology. 1996;29:397–409.
Elston C, Sloane J, Amendoeira I, Apostolikas N, Bellocq J, Bianchi S, et al. Causes of inconsistency in diagnosing and classifying intraductal proliferations of the breast. Eur J Cancer. 2000;36:1769–72.
Ghofrani M, Tapia B, Tavassoli FA. Discrepancies in the diagnosis of intraductal proliferative lesions of the breast and its management implications: results of a multinational survey. Virchows Arch. 2006;449:609–16.
Jain RK, Mehta R, Dimitrov R, Larsson LG, Musto PM, Hodges KB, et al. Atypical ductal hyperplasia: interobserver and intraobserver variability. Mod Pathol. 2011;24:917–23.
Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991;15:209–21.
Sloane JP, Amendoeira I, Apostolikas N, Bellocq J, Bianchi S, Boecker W, et al. Consistency achieved by 23 european pathologists in categorizing ductal carcinoma in situ of the breast using five classifications. Eur J Cancer. 2220(36):1769–72.
Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. European Commission Working Group on Breast. Hum Pathol. 1992;23:1095–7.
Tavassoli FA, Norris HJ. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990;65:518–29.
Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–41.
Cady B. How to prevent invasive breast cancer: Detect and excise duct carcinoma in situ. J Surg Oncol. 1998;69:60–2.
Bjiker N, Peterse JL, Julien JP, Fentiman IS, Duval C, Di Palma S, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of Eurpoean Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–71.
Bijker N, Meijen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, et al. Breast-conserving treatment with or without radiotherapy for ductal carcinoma in situ (DCIS): ten-year results of European Organisation for Research and Treatment of Cancer (EORTC) randomized phase III trial 10853. Eur J Cancer Suppl. 2006;4:108.
Correa C, McGale P, Taylor C, Want Y, Clarke M, Davies C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. JNCI Monographs. 2010;2010:162–77.
Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–9.
Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: An update of the national surgical adjuvant breast and bowel project experience. Semin Oncol. 2001;28:400–18.
Solin LJ. The impact of adding radiation treatment after breast conservation surgery for ductal carcinoma in situ of the breast. JNCI Monographs. 2010;2010:187–92.
Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. JNCI J Natl Cancer Inst. 2011;103:478–88.
Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. JNCI J Natl Cancer Inst. 2013;105:701–10.
Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–92.
Lehman CD. Magnetic resonance imaging in the evaluation of ductal carcinoma in situ. JNCI Monographs. 2010;2010:150–1.
Jansen SA, Paunesku T, Fan X, Woloschak GE, Vogt S, Conzen SD, et al. Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model 1. Radiology. 2009;253:399–406.
Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade 1. Radiology. 2007;245:684–91.
Chan S, Chen J-H, Agrawal G, Lin M, Mehta RS, Carpenter PM, et al. Characterization of pure ductal carcinoma in situ on dynamic contrast-enhanced MR imaging: do nonhigh grade and high grade show different imaging features? J Oncol. 2010;2010:1–9.
Rosen EL, Smith-Foley SA, Demartini WB, Eby PR, Peacock S, Lehman CD. BI-RADS MRI enhancement characteristics of ductal carcinoma in situ. Breast J. 2007;13:545–50.
Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston: American College of Radiology; 2013.
Tozaki M, Igarashi T, Fukuda K. Breast MRI using the VIBE sequence: clustered ring enhancement in the differential diagnosis of lesions showing non-masslike enhancement. Am J Roentgenol. 2006;187:313–21.
Yamada T, Mori N, Watanabe M, Kimijima I, Okumoto T, Seiji K, et al. Radiologic-pathologic correlation of ductal carcinoma in situ 1. Radiographics. 2010;30:1183–98.
Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005;11:382–90.
Greenwood HI, Heller SL, Kim S, Sigmund EE, Shaylor SD, Moy L. Ductal carcinoma in situ of the breasts: review of MR imaging features. Radiographics. 2013;33:1569–88.
Van Goethem M, Schelfout K, Kersschot E, Colpaert C, Weyler J, Verslegers I, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. Clin Imaging. 2006;30:225–32.
Rahbar H, Parsian S, Lam DL, Dontchos BN, Andeen NK, Rendi MH, et al. Can MRI biomarkers at 3 T identify low-risk ductal carcinoma in situ? Clin Imaging. 2015;40:125–9. doi:10.1016/j.clinimag.2015.07.026.
Vag T, Baltzer PAT, Dietzel M, Benndorf M, Gajda M, Camara O, et al. Kinetic characteristics of ductal carcinoma in situ (DCIS) in dynamic breast MRI using computer-assisted analysis. Acta Radiol. 2010;51:955–61.
Rahbar H, Partridge SC, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263:374–82.
Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3 T versus 1.5 T. Magn Reson Imaging Clin N Am. 2007;15:277–90.
Rahbar H, Demartini WB, Lee AY, Partridge SC, Peacock S, Lehman CD. Accuracy of 3 T versus 1.5 T breast MRI for pre-operative assessment of extent of disease in newly diagnosed DCIS. Eur J Radiol. 2015;84:611–6.
Koh D-M, Padhani AR. Diffusion-weighted MRI: a new functional clinical technique for tumour imaging. Br J Radiol BJR. 2006;79:633–5.
Chen X, Li W-L, Zhang Y-L, Wu Q, Guo Y-M, Bai Z-L. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer 2010; doi:10.1186/1471-2407-10-693/.
Fornasa F, Pinali L, Gasparini A, Toniolli E, Montemezzi S. Diffusion-weighted magnetic resonance imaging in focal breast lesions: analysis of 78 cases with pathological correlation. Radiol med La radiologia medica. 2010;116:264–75.
Kul S, Cansu A, Alhan E, Dinc H, Gunes G, Reis A. Contribution of diffusion-weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. Am J Roentgenol. 2011;196:210–7.
Partridge SC, Demartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. Am J Roentgenol. 2009;193:1716–22.
Partridge SC, Demartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging. 2010;31:562–70.
Sinha S, Lucas-Quesada FA, Sinha U, Debruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson. 2002;15:693–704.
Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Non-mass-like enhancement on contrast-enhanced breast MR imaging: Lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. Eur J Radiol. 2010;75:126–32. doi:10.1016/j.ejrad.2009.09.013.
Rahbar H, Partridge SC, Eby PR, Demartini WB, Gutierrez RL, Peacock S, et al. Characterization of ductal carcinoma in situ on diffusion weighted breast MRI. Eur Radiol. 2011;21:2011–9.
Iima M, Bihan DL, Okumura R, Okada T, Fujimoto K, Kanao S, et al. Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology. 2011;260:364–72.
Hahn SY, Han B-K, Ko EY, Shin JH, Hwang J-Y, Nam M. MR features to suggest microinvasive ductal carcinoma of the breast: can it be differentiated from pure DCIS? Acta Radiol. 2013;54:742–8.
Intra M, Rotmensz N, Veronesi P, Colleoni M, Iodice S, Paganelli G, et al. Sentinel node biopsy is not a standard procedure in ductal carcinoma in situ of the breast. Ann Surg. 2008;247:315–9.
Wisner DJ, Hwang ES, Chang CB, Tso HH, Joe BN, Lessing JN, et al. Features of occult invasion in biopsy-proven DCIS at breast MRI. Breast J. 2013;19:650–8.
Goto M, Yuen S, Akazawa K, Nishida K, Konishi E, Kajihara M, et al. The role of breast MR imaging in pre-operative determination of invasive disease for ductal carcinoma in situ diagnosed by needle biopsy. Eur Radiol. 2011;22:1255–64.
Nori J, Meattini I, Giannotti E, Abdulcadir D, Mariscotti G, Calabrese M, et al. Role of preoperative breast MRI in ductal carcinoma in situ for prediction of the presence and assessment of the extent of occult invasive component. Breast J. 2014;20:243–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Greenwood, H.I., Joe, B.N. (2017). In Situ Disease on Breast MRI. In: Heller, S., Moy, L. (eds) Breast Oncology: Techniques, Indications, and Interpretation. Springer, Cham. https://doi.org/10.1007/978-3-319-42563-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-42563-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42561-0
Online ISBN: 978-3-319-42563-4
eBook Packages: MedicineMedicine (R0)