Abstract
Breast magnetic resonance imaging (MRI) is increasingly used in conjunction with mammography as a screening tool to detect breast cancers in asymptomatic high-risk women. Conventional dynamic contrast-enhanced (DCE) breast MRI has a high sensitivity but only moderate specificity for the detection of breast cancer. The primary goal of developing and applying advanced breast MRI techniques that can assess tissue biology is to improve lesion specificity. This review provides a summary of the advances in DCE-MRI techniques and the use of diffusion-weighted imaging and magnetic resonance spectroscopy for breast cancer detection. Publications on the use of these advanced MRI techniques are largely single-institution studies with small numbers of patients, which limits the generalization of this data to a wider screening population. In their current forms, these adjunctive techniques require further research, incorporating an expanded patient population, to validate their utility for breast cancer screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequently diagnosed cancer and ranks as the leading cause of cancer death in women worldwide [1]. Breast cancer death rates in the USA have dramatically declined over the last three decades, which is in part due to early detection afforded by the widespread adoption of mammography as the standard screening tool. Recently, breast magnetic resonance imaging (MRI) and breast ultrasound have been used to supplement mammographic screening in asymptomatic high-risk women and women with dense breast tissue. In women with elevated risk, adding breast MRI resulted in a supplemental cancer detection yield of 14.7 per 1000 women screened, and adding ultrasound yielded an additional 3.7 per 1000 women screened [2].
Dynamic contrast-enhanced (DCE) breast MRI has an extremely high sensitivity for breast cancer detection, reported in the literature to be between 89 and 100 % [3, 4]. DCE-MRI is the most sensitive imaging tool currently in use for the screening of high-risk women [5]. The specificity of breast MRI, however, is modest, and its positive predictive value is variable, ranging between 24 and 89 % [4, 6]. A meta-analysis performed in 2008 revealed an overall sensitivity of 90 % and specificity of 72 % [7]. Unfortunately, this modest specificity may translate into patients enduring more biopsies that ultimately yield benign pathology. A systematic review of the effectiveness of adding MRI to mammography and ultrasound screening of young high-risk women found an estimated 7 to 46 additional benign biopsies are performed per 1000 screening rounds when MRI is added to the screening regimen [8]. Improving specificity through the use of advanced MRI techniques such as diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS), and supplemental methods of DCE-MRI has been the primary aim of many studies.
Dynamic Contrast-Enhanced Imaging
The clinical performance of breast MRI has traditionally emphasized anatomic and morphologic detail as provided by high spatial resolution imaging. Supplemental methods of kinetic assessment have been adopted clinically to only a limited degree, usually encompassing only the basic curve descriptions of persistent, plateau, or wash-out patterns. Attempts at more advanced kinetic analysis usually lengthen both the imaging and interpretation time, which directly increases the cost of the exam and reduces the feasibility of MRI to function as a universal screening tool. Kuhl and colleagues recently reported the results of a streamlined screening protocol that acquired axial T1-weighted gradient echo images before and immediately after contrast injection with an acquisition time of only 3 minutes [9]. This protocol resulted in a high negative predictive value of 99.8 % and an additional cancer yield of 18.2 per 1000 patients screened [9]. Yet, for those patients who had lesions identified with this limited protocol, review of additional dynamic MR images is still necessary to complete the interpretation. Eliminating the dynamic contrast enhancement data may hamper lesion characterization and fails to address the need to improve specificity.
Spatial Versus Temporal Resolution
Accurate clinical assessment of DCE-MRI demands an imaging acquisition with high spatial resolution for precise morphologic assessment of enhancing lesions of any size, large or small, and with a scan time short enough to permit accurate kinetic or pharmacokinetic analysis of the time-course data [10]. Unfortunately, spatial and temporal resolution compete with one another, since one cannot be altered without adversely affecting the other [11]. Ultimately, standard pulse sequences are a compromise between these two opposing demands.
Lesion morphology is generally considered a more important criterion than determination of enhancement kinetics in categorizing a lesion as suspicious for malignancy or not [11, 12]. Higher spatial resolution permits the visualization of fine spicules, meant to convey suspicion, as well as low signal intensity internal septations, which are encountered more frequently with benign fibroepithelial lesions. High spatial resolution is a primary contributor to the diagnostic performance of DCE-MRI, improving both diagnostic confidence and accuracy [11]. Thus, an in-plane resolution less than 1 × 1 mm is recommended when performing clinical imaging [13•]. To achieve this, the temporal resolution is typically limited to 1–2 min, even with the adoption of various parallel imaging acceleration methods [14]. Higher temporal resolution necessarily decreases in-plane resolution, which may adversely affect lesion conspicuity and morphologic assessment.
Novel strategies of sampling (or, more aptly, undersampling) k-space are being developed to subvert these technical constraints in an effort to provide the highest spatiotemporal resolution dynamic images possible. Time-resolved MR angiography sequences such as TRICKS (time-resolved imaging of contrast kinetics) developed by General Electric, TWIST (time-resolved angiography with stochastic trajectories) developed by Siemens, and 4D-TRAK (4D-time-resolved MRA with keyhole) developed by Philips are examples of this new approach to imaging. These sequences partially undersample K-space, particularly at the periphery, but employ sophisticated sampling patterns and view-sharing techniques that share data between successive time points to improve spatial resolution. In a small cohort of 31 patients, TWIST was comparable to conventional GRE images in terms of SNR, quantitative kinetic analyses, and morphologic assessment [14].
Altering factors such as the method of k-space sampling or the degree of flip angle applied in the GRE sequence has been shown to change the shape of the kinetic uptake curve generated, which could affect the overall diagnostic interpretation [15]. Rather than alter these physical parameters, some investigators have focused on blending various high temporal sequences with other high spatial sequences to find the best combination for cancer detection. Grovik and colleagues describe a split dynamic MRI technique where high spatial resolution 3D T1-weighted turbo field echo sequences (THRIVE) are interleaved with high temporal resolution 3D T1-weighted EPI sequences with two echoes following a single-bolus contrast injection [16, 17]. Although there was improvement in diagnostic accuracy for one reader using this protocol, there was overall no significant improvement in sensitivity or specificity.
Other investigators have focused on performing very high temporal resolution sequences early in the wash-in phase. One such study used a TurboFLASH sequence with temporal resolution of 2–3 s to assess lesions within the first 2 min following contrast administration [18]. Their study yielded a 95 % sensitivity, 86 % specificity, and overall accuracy of 93 % when lesions enhancing within 11.5 s after opacification of the aorta are considered suspicious for malignancy. More recently, Mann and colleagues used a bi-temporal protocol interleaving 20 ultra-fast TWIST acquisitions (with a temporal resolution of 4.32 s, total 102 s) at the time of contrast administration to capture the inflow of contrast in breast lesions [19•]. Evaluation of the maximum slope of contrast enhancement achieved a higher accuracy in the differentiation of benign versus malignant lesions than BI-RADS assessment of the wash-out phase. Unfortunately, the adoption of ultra-fast MRI sequences such as these has been limited in clinical practice, most likely because a standard protocol has yet to be adopted and reliably show improvement in specificity. So far, only a few studies reporting on small case series have achieved mixed results.
Pharmacokinetic Modeling
Attainment of high spatiotemporal resolution images should improve utilization of more advanced pharmacokinetic analyses in routine clinical breast MRI applications. Higher temporal resolution imaging permits more elegant quantitative assessments of contrast agent compartmental exchange to better discriminate benign from malignant lesions [4]. By providing estimations of tumor blood flow and capillary permeability, this more quantitative approach mitigates the typical clinical approach of qualitatively evaluating curve shapes or empirically assessing time-course data (e.g., wash-out ratios), which can lack reproducibility and limit overall specificity [20, 21].
Several of these pharmacokinetic parameters, such as Ktrans (a volume transfer coefficient reflecting vascular permeability) and Kep (a flux rate constant for the movement of contrast agent from the extracellular extravascular space into the plasma compartment), offer improved specificity and prediction of biologic aggressiveness by serving as potential biomarkers of tumor angiogenesis and cellular proliferation in breast cancer [4, 22]. A recent study reported that both invasive and in situ ductal carcinomas exhibited significantly higher Ktrans and Kep values compared to normal glands, benign lesions, and ductal dysplasias [22]. Another parameter, Ve, describes the fractional volume of the extravascular extracellular space (a ratio that reflects tumor cellularity). Kim and colleagues have correlated higher Ve values with higher levels of glucose metabolism, as measured by SUVmax values on PET/CT imaging [23]. Higher Ktrans, higher Kep, and lower Ve values have been shown to correlate with poor prognostic factors and the triple-negative phenotype [24], while another study showed no statistical significance between conventional enhancement or pharmacokinetic parameters and immunohistochemical subtypes of breast cancer [25].
Pharmacokinetic modeling so far lacks evidence to suggest that it reliably adds diagnostic information beyond what is obtained with the analysis of enhancement rates and visual assessment of curve types. Moreover, there is a shortage of commercially available software packages to easily extract and manipulate the kinetic data for modeling purposes. Although quantitative DCE kinetics may augment morphologic assessment to improve specificity, pharmacokinetic modeling still remains an emerging technology and has yet to be used on a broad clinical scale.
Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) is an MRI technique that utilizes unenhanced sequences to measure the movement of water molecules in vivo. DWI assesses the ability of water molecules to randomly diffuse across extracellular barriers, which provides details regarding tissue structure and provides insight into the characteristics of cellularity and cell membrane integrity.
The MRI signal from diffusion weighting is described by the monoexponential equation: S D = S 0 e −b*ADC [26]. S D is the signal intensity with diffusion weighting; S 0 is the signal intensity without diffusion weighting; b is the diffusion sensitization factor (s/mm2), and ADC is the apparent diffusion coefficient. This equation shows the relationship of signal intensity reduction proportional to the movement of water molecules. In other words, signal intensity is inversely proportional to the freedom of water molecules to diffuse across a cellular membrane and directly proportional to molecular restriction [27]. ADC is a quantitative measurement that is directly proportional to the mobility of water [3, 4]. The ADC value is obtained from acquiring two MRI signals and using the following formula: ADC = ln(S 1/S 2)/(b 2 − b 1) with b 1 as the minimum b value and b 2 as the maximum b value; S 1 is the signal intensity at b = b 1 and S 2 is the signal intensity at b = b 2.
The potential usefulness of DWI in breast cancer screening is rooted in the differences in DWI signal and ADC values for malignant and benign breast lesions. Breast malignancies tend to exhibit high cellular density due to cellular proliferation, which results in increased diffusion restriction (decreased water mobility) and decreased ADC values. Therefore, malignant breast lesions typically demonstrate DWI signal hyperintensity and lower ADC values compared to normal breast tissue and benign lesions, which is depicted in Fig. 1 [3, 6, 28]. Unfortunately, DWI signal and ADC values overlap for benign and malignant lesions. The results of a meta-analysis of 13 studies evaluating the diagnostic performance of DWI in 964 breast lesions reported mean ADC values for malignant lesions ranging from 0.87 to 1.36 × 10−3 mm2/s and benign lesions ranging from 1.00 to 1.82 × 10−3 mm2/s [29]. Recommended ADC threshold cut-off values to differentiate between malignant and benign lesions also ranged from 0.9 to 1.76 × 10−3 mm2/s [29]. This meta-analysis also indicated a pooled sensitivity of 84 % (95 % CI: 0.82, 0.87) and specificity of 79 % (95 % CI: 0.75, 0.82) for differentiating between malignant and benign breast lesions based on DWI [29].
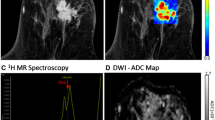
Fifty-seven-year-old woman with left breast invasive ductal carcinoma. a–c Axial 1.5 T magnetic resonance images depict a 12-mm irregular mass in the 3:00 left breast, which is the patient’s biopsy-proven malignancy. The mass (arrows) enhances on axial post-contrast T1-weighted GRE imaging with fat saturation (a), demonstrates high signal intensity with supplemental diffusion-weighted imaging (b = 600 s/mm2) (b), and has low ADC values on the corresponding ADC map (c)
DWI has the potential to be valuable in breast cancer screening, particularly when consideration is given to the fast imaging acquisition time and lack of reliance on an injection of intravenous contrast. A recent study of asymptomatic women showed that a combination of two non-contrast MRI sequences, DWI and T2-weighted images, had a higher sensitivity for detecting non-palpable breast cancer than mammography alone [30]. The same study also reported that a combination of mammography with these two non-contrast sequences yielded an even higher sensitivity, suggesting a possible supplemental role for DWI in breast cancer screening [30]. A recent meta-analysis of 14 studies encompassing 1140 patients with 1276 breast lesions demonstrated that a higher diagnostic accuracy is achieved for breast cancer detection when DWI is used in conjunction with DCE-MRI [31•].
There are challenges that prevent DWI from being implemented in a widespread breast cancer screening protocol. Excellent image quality is inconsistently achieved. Fat suppression and shimming are important technical factors that must be optimized to provide meaningful information and limit magnetic susceptibility effects and chemical shift artifact [26]. Misregistration of the DWI sequences can also result in inaccurate ADC calculations [26]. Perhaps more importantly, no standard protocol exists for diffusion weighted image acquisition. Selected b values vary widely between studies, since no optimal b values or number of b value acquisitions have been identified to best distinguish benign from malignant lesions [27, 29]. Selected b values across studies have often ranged from 0 to 1000 s/mm2. Images acquired with low b values exhibit higher ADC values and vice versa. Although Pereira and colleagues note that the calculated ADC values from the combination of b = 0 and b = 750 s/mm2 performed slightly better than other b value combinations, there was no statistical difference in the differentiation of benign and malignant breast lesions for calculated ADCs of different b value combinations [3]. Clearly, further investigation is warranted before DWI can serve as either a stand-alone imaging tool or as an adjunctive MRI sequence for widespread screening of breast cancer.
Magnetic Resonance Spectroscopy
Breast proton magnetic resonance spectroscopy (MRS) is another non-contrast MRI technique that provides information regarding the biochemical nature of tissue. MRS currently serves as an adjunct to breast DCE-MRI to add metabolic information about a lesion and improve specificity.
MRS creates signal spectra with peaks that represent the chemical composition of a specified region of tissue. One of the major spectral resonance peaks of interest is total choline concentration (tCho). Choline metabolites are involved in cell membrane turnover, so tCho may function as another imaging biomarker for cellular proliferation. The tCho peak occurs at approximately 3.2 ppm and represents a combination of free choline and several derivative compounds, including phosphocholine and glycerophosphocholine.
While the biochemical mechanism is still being investigated, tCho has been shown to be elevated in malignancies, due to both increased intracellular phosphocholine concentration and increased cell density [4, 32]. Shin and colleagues have also documented higher choline levels in invasive breast malignancies compared to in situ disease [33, 34]. Multiple MRS studies have reported elevated levels of tCho in malignant breast lesions compared to benign lesions [35–38]. For instance, one study found a mean tCho of 1.90 mmol/kg for malignant lesions and 0.39 mmol/kg for benign lesions [38]. A recent meta-analysis of 19 MRS studies, encompassing 773 malignant and 452 benign breast lesions, resulted in a pooled sensitivity of 73 % and a pooled specificity of 88 % for lesion diagnosis [39].
Various techniques have been utilized for the acquisition and analysis of breast MRS. Most MRS protocols are performed with a 1.5 T magnet with single-voxel MRS that samples a single lesion of interest. Single-voxel MRS is usually performed following DCE-MRI, to facilitate lesion localization and accurate voxel placement. Accurate placement of the voxel, which includes as much of the lesion of interest as possible and excludes surrounding normal tissue, is important for improved diagnostic characterization. Adipose tissue reduces shim quality, despite the presence of fat suppression [40]. Also, there is a 3.2 ppm spectral peak in adipose tissue that is impervious to fat suppression and can be misinterpreted as tCho [40]. Unfortunately, single-voxel MRS allows only one lesion to be evaluated at a time, and the labor intensiveness greatly limits its utilization for screening purposes.
Multi-voxel MRS, also known as chemical shift imaging, samples an array of spectra from a large volume of the breast. There are several advantages of multi-voxel imaging compared to the single-voxel technique, including assessment of tumor infiltration into the surrounding tissues, characterization of tumor and tissue heterogeneity, and the ability to evaluate multiple lesions simultaneously [41–43]. The ability of multi-voxel MRS to provide extended coverage of the breast decreases the need for prior lesion localization [4]. However, multi-voxel imaging is more technically challenging and the larger spatial coverage necessitates longer acquisition times compared to the single-voxel technique.
It is thought that higher field strength magnets improve the performance of MRS, since signal-to-noise ratios and spectral resolution are improved, which help better delineate the choline peak [32]. However, a potential pitfall has been described in recent studies using higher field strength devices and higher sensitivity breast coils, which have also shown detectable choline levels in normal fibroglandular breast tissue [4, 40, 44–46]. Perhaps more discouraging is that some breast cancers fail to exhibit elevated choline levels at all [33, 47], and MRS exhibits a low sensitivity in detecting choline levels in lesions less than 1 cm in size [36, 40, 48]. These limitations may prove to be significant barriers for the adoption of implementing MRS into a general breast cancer screening protocol.
Conclusion
Multiple novel MRI techniques are being investigated for clinical use in breast cancer detection and screening. While the current role of these techniques is limited in practice, ultra-fast dynamic MRI and DWI are both relatively quick to perform and may have the greatest potential for future use in the screening population. DWI also has the added benefit of imaging without the need for intravenous contrast administration, which further strengthens its appeal as a screening tool. Continued research into these advanced MRI techniques is needed to validate their widespread utilization and adoption in breast cancer screening.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Torre LA et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Berg WA et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–404.
Pereira FP et al. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am J Roentgenol. 2009;193(4):1030–5.
Rahbar H, Partridge SC. Multiparametric MR imaging of breast cancer. Magn Reson Imaging Clin N Am. 2016;24(1):223–38.
Lehman CD et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381–8.
Partridge SC et al. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193(6):1716–22.
Peters NH et al. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116–24.
Lord SJ et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43(13):1905–17.
Kuhl CK et al. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–10.
Kuhl CK et al. Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology. 2006;239(3):666–76.
Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology. 2005;236(3):789–800.
Gutierrez RL et al. Dynamic breast MRI: does lower temporal resolution negatively affect clinical kinetic analysis? AJR Am J Roentgenol. 2012;199(3):703–8.
Aribal E et al. Multiparametric breast MRI with 3T: effectivity of combination of contrast enhanced MRI, DWI and 1H single voxel spectroscopy in differentiation of Breast tumors. Eur J Radiol. 2016;85(5):979–86. Multiparametric imaging that incorporated DWI and MRS into a DCE MRI protocol failed to improve diagnostic accuracy of breast MRI in this single-center prospective study of 129 patients. DCE MRI showed the highest individual sequence sensitivity and specificity compared to DWI and MRS. Highest specificity overall was attained when all three exams were positive, although at the expense of diminished sensitivity.
Tudorica LA et al. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn Reson Imaging. 2012;30(9):1257–67.
Ledger AE et al. Investigating the influence of flip angle and k-space sampling on dynamic contrast-enhanced MRI breast examinations. Acad Radiol. 2014;21(11):1394–401.
Grovik E et al. Split dynamic MRI: single bolus high spatial-temporal resolution and multi contrast evaluation of breast lesions. J Magn Reson Imaging. 2014;39(3):673–82.
Grovik E et al. Single bolus split dynamic MRI: is the combination of high spatial and dual-echo high temporal resolution interleaved sequences useful in the differential diagnosis of breast masses? J Magn Reson Imaging. 2015;42(1):180–7.
Boetes C et al. MR characterization of suspicious breast lesions with a gadolinium-enhanced TurboFLASH subtraction technique. Radiology. 1994;193(3):777–81.
Mann RM et al. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49(9):579–85. This single institution study of 160 patients utilized ultrafast DCE MRI to detect and classify breast lesions with high accuracy using the maximum slope of contrast enhancement rather than washout phase characterization. This substantially shortened the scan time, which would be conducive for cost-effective MRI screening.
Huang W et al. Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology. 2011;261(2):394–403.
Jansen SA et al. Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR Am J Roentgenol. 2009;193(3):832–9.
Li L et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–82.
Kim TH et al. Correlation between F-18 fluorodeoxyglucose positron emission tomography metabolic parameters and dynamic contrast-enhanced MRI-derived perfusion data in patients with invasive ductal breast carcinoma. Ann Surg Oncol. 2015;22(12):3866–72.
Koo HR et al. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging. 2012;36(1):145–51.
Kim JY et al. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging. 2015;33(1):72–80.
Partridge SC, McDonald ES. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn Reson Imaging Clin N Am. 2013;21(3):601–24.
Brandao AC, Lehman CD, Partridge SC. Breast magnetic resonance imaging: diffusion-weighted imaging. Magn Reson Imaging Clin N Am. 2013;21(2):321–36.
Cabuk G et al. The diagnostic value of diffusion-weighted imaging and the apparent diffusion coefficient values in the differentiation of benign and malignant breast lesions. J Med Imaging Radiat Oncol. 2015;59(2):141–8.
Chen X et al. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693.
Yabuuchi H et al. Detection of non-palpable breast cancer in asymptomatic women by using unenhanced diffusion-weighted and T2-weighted MR imaging: comparison with mammography and dynamic contrast-enhanced MR imaging. Eur Radiol. 2011;21(1):11–7.
Zhang L et al. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol. 2016;57(6):651–60. This meta-analysis of 14 studies demonstrated that combined DCE MRI and DWI achieved a higher diagnostic accuracy for the detection of breast cancer than either imaging technique alone.
Haddadin IS et al. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22(1):65–76.
Baek HM et al. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009;251(3):653–62.
Shin HJ et al. Evaluation of breast cancer using proton MR spectroscopy: total choline peak integral and signal-to-noise ratio as prognostic indicators. AJR Am J Roentgenol. 2012;198(5):W488–97.
Roebuck JR et al. Human breast lesions: characterization with proton MR spectroscopy. Radiology. 1998;209(1):269–75.
Yeung DK, Cheung HS, Tse GM. Human breast lesions: characterization with contrast-enhanced in vivo proton MR spectroscopy--initial results. Radiology. 2001;220(1):40–6.
Begley JK et al. In vivo proton magnetic resonance spectroscopy of breast cancer: a review of the literature. Breast Cancer Res. 2012;14(2):207.
Meisamy S et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0 T. Radiology. 2005;236(2):465–75.
Baltzer PA, Dietzel M. Breast lesions: diagnosis by using proton MR spectroscopy at 1.5 and 3.0 T--systematic review and meta-analysis. Radiology. 2013;267(3):735–46.
Bolan PJ. Magnetic resonance spectroscopy of the breast: current status. Magn Reson Imaging Clin N Am. 2013;21(3):625–39.
Dorrius MD et al. The added value of quantitative multi-voxel MR spectroscopy in breast magnetic resonance imaging. Eur Radiol. 2012;22(4):915–22.
Jacobs MA et al. Combined dynamic contrast enhanced breast MR and proton spectroscopic imaging: a feasibility study. J Magn Reson Imaging. 2005;21(1):23–8.
Sijens PE et al. Quantitative multivoxel proton chemical shift imaging of the breast. Magn Reson Imaging. 2010;28(3):314–9.
Hu J et al. A high spatial resolution in vivo 1H magnetic resonance spectroscopic imaging technique for the human breast at 3 T. Med Phys. 2009;36(11):4870–7.
Zhao CG et al. Quantitative mapping of total choline in healthy human breast using proton echo planar spectroscopic imaging (PEPSI) at 3 Tesla. J Magn Reson Imaging. 2012;36(5):1113–23.
Konyer NB et al. Comparison of MR imaging breast coils. Radiology. 2002;222(3):830–4.
Bathen TF et al. In vivo MRS of locally advanced breast cancer: characteristics related to negative or positive choline detection and early monitoring of treatment response. MAGMA. 2011;24(6):347–57.
Cecil KM et al. The evaluation of human breast lesions with magnetic resonance imaging and proton magnetic resonance spectroscopy. Breast Cancer Res Treat. 2001;68(1):45–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jessica H. Porembka, Stephen J. Seiler, and Pooja B. Sharma declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Screening and Imaging
Rights and permissions
About this article
Cite this article
Porembka, J.H., Seiler, S.J. & Sharma, P.B. Advanced Breast MRI Techniques: Helpful for Screening Breast Cancer?. Curr Breast Cancer Rep 8, 236–241 (2016). https://doi.org/10.1007/s12609-016-0226-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-016-0226-3