Abstract
In integrated steel mills, the sinter plant is one of the major sources of emissions to the atmosphere, whereas the emissions of waste water and solid residues from the sinter plant are usually less significant. Therefore, state-of-the-art emission control technology for off-gas treatment is essential in order to comply with stringent emission limits. In the first part of this chapter, the sinter process is described, the resulting emissions are characterized and primary measures to reduce the off-gas volume and the emission of various pollutants are presented. The second part gives an overview of the state-of-the-art emission control technologies applied in sinter plants for the reduction of particulate emissions, emissions of SO2 and other acid gases, NOx emissions and emissions of dioxins. In the third part, methods of treating and recycling the residues from sinter plant off-gas cleaning are described. The last part describes some possible future developments in air pollution control for sinter plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In an integrated steel mill, the sinter is an important feed material for the production of pig iron in the blast furnace. The sinter is produced in the sinter plant by agglomeration of the charge which consists of iron ore fines, fluxes and fine-grained recycled iron-containing materials such as dusts, sludge and scale. Due to the high volume of off-gas from the sintering process , sinter plants are responsible for a significant proportion of the atmospheric emissions from integrated steelworks.
The process off-gas from a sinter plant is usually de-dusted in an electrostatic precipitator (ESP) . At some sinter plants, a subsequent process for off-gas desulphurization (DeSOx), reduction of dioxin emissions and/or further de-dusting has been installed in order to comply with tighter emission limits. In a few sinter plants, a process for the reduction of NOx emissions (DeNOx) is also applied.
Other sources of dust emissions in sinter plants are the handling and conveying of the feed material, the sinter cooler and the crushing and screening of the sinter produced. These emissions are extracted by a ventilation system, often referred to as the room de-dusting system (Remus et al. 2013).
2 Emissions from the Sintering Process and Primary Methods of Emission Reduction
The limits for emissions to the atmosphere from sinter plants have been tightened in recent years. The current emission limits for Europe and China are summarized in Table 18.1. The best available technique-associated emission levels (BAT-AELs) for air emissions from sinter plants are defined in the commission implementing decision of 28 February 2012, establishing the best available technique (BAT ) conclusions under Directive 2010/75/EU of the European Parliament and of the council on industrial emissions for the iron and steel production. The BAT-AELs in Europe depend on the applied BAT technology for emission reduction.
For China, fixed emission limits are valid. The limit values are within the range of the European limits. For dust and polychlorinated dibenzo(p)dioxins and furans (PCDD/Fs) , the emission limits are somewhat higher than the European limits, while the limit for SOx is lower with the exception of that for the wet DeSOx process and the regenerative activated carbon process.
BAT for the secondary emissions, the emissions from sinter strand discharge, sinter crushing, cooling, screening and conveyor transfer points, is to achieve an efficient extraction and subsequently to reduce dust emissions by using a bag filter or an ESP. The respective BAT-AEL for dust is <10 mg/Nm3 for a room de-dusting bag filter and <30 mg/Nm3 for de-dusting with an ESP. The Chinese emission limit for the secondary dust emissions is also 30 mg/Nm3.
2.1 The Sintering Process
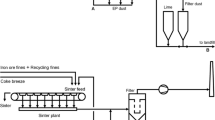
The feed material of the sintering process—iron ore, fluxes, recycling fines and a solid fuel, usually coke breeze—is mixed and then placed on a continuous, travelling grate called the sinter strand. A burner at the beginning of the strand ignites the fuel in the mixture after which the combustion is self-supporting. Underneath the strand, there are several wind boxes to draw air and combustion gases down through the sintering bed into a duct which is connected to the induced draft (ID) fan . As the sinter mixture proceeds along the sinter strand, the combustion front is drawn downwards through the mixture.
The combustion of the coke—approximately 4–6 % of the charge—creates sufficient heat (1300–1480 °C) to sinter the fine particles together into porous sinter. The off-gas from the sinter strand contains considerable amounts of entrained particulate matter and various gaseous pollutants. It is usually de-dusted in an ESP before it is released into the air via the main stack. The ESP is installed upstream of the ID fan to protect the fan impeller. In some older sinter plants, cyclones are still used for this purpose.
The fused sinter is discharged at the end of the sinter strand and subsequently crushed and screened. The undersize material is recycled back to the feed. The remaining sinter product is cooled in a cooler where air is used for the cooling. The cooled sinter is crushed and screened again. The fines are recycled and the product is sent to the blast furnace (Remus et al. 2013).
2.2 Emissions from the Sintering Process
The particulate matter contained in the off-gas from the sinter strand originates from two sources. Firstly, fine-grained feed material can be carried by the gas flow out of the lower parts of the bed. Secondly, some components like alkali chlorides can be volatilized in the high-temperature zone of the bed. These components recondense and form very fine particles when the off-gas cools down. Typical particle size distributions of the particulate matter from the de-dusting of various sinter plants can be found in the literature. The mass median diameter of the ESP dust is in the range of 2.8–40 μm, while cyclone dust is coarser (Kasama et al. 2006; Lanzerstorfer 2015a). The dust remaining in the off-gas downstream of the de-dusting stage is considerably smaller. Its mass median diameter is typically less than 1 μm (Fleischanderl et al. 2007).
The chemical composition of dust emitted from sinter plants has been investigated in several studies (Xhrouet et al. 2002; Ahn and Lee 2006; Tsai et al. 2007; Sammut et al. 2010; Sinha et al. 2010; Lanzerstorfer and Steiner 2015). The main components of the dust are Fe, C, K, Ca, Cl and S. The dust also contains increased amounts of volatile heavy metals, especially Pb and to a lesser extent Cd and Zn (Remus et al. 2013). In one study, no difference was found in the Hg concentration in the off-gas before and after the ESP (Park et al. 2008). Therefore, it can be assumed that at sinter off-gas temperature nearly all Hg is gaseous.
In the sintering process, coke breeze is usually used as the solid fuel to supply the thermal energy for sintering. Due to the short residence time of the gas in the combustion zone and the rapid cooling of the combustion off-gas when it flows through the bed of feed material below the flame front, some products of incomplete combustion remain in the off-gas. The main component from incomplete combustion is CO. Its concentration in sinter off-gas is in the range of 0.5–1.5 % (vol) (Leuwerink and van der Panne 2001; Eisen et al. 2004; Kasama et al. 2006; Remus et al. 2013).
The S contained in the fuel is oxidized in the combustion zone, and some of it is discharged with the off-gas as SO2. In sinter pot tests, it was found that approximately 20–50 % of the S was emitted as SO2 depending on the basicity of the sinter mixture and the specific S input (Lanzerstorfer et al. 2015a). Typical SO2 concentrations in the sinter off-gas are between approximately 100 and 500 mg/Nm3 dry (Remus et al. 2013). However, S-containing recycled material in the feed can significantly increase the SO2 emissions.
The emitted NOx consists mainly of NO. Usually the dominating source is the N contained in the coke breeze (Mo et al. 1997, Remus et al. 2013). The typical NOx concentration in the sinter off-gas is between approximately 150 and 500 mg/Nm3 dry (Remus et al. 2013).
The emissions of HF and HCl depend on the F and Cl content of the feed material and on the basicity of the sinter mixture. The reported sinter off-gas concentrations for HF are in the range of 0.2–4 mg/Nm3 dry and for HCl 0.7–400 mg/Nm3 dry (Remus et al. 2013).
Residues which contain oil are the major source of hydrocarbon emissions from sinter strands. Hydrocarbons can evaporate before they are reached by the combustion front moving downwards through the bed. Additionally, some hydrocarbon emissions result from incomplete combustion of the fuel. The concentration of non-methane volatile organic compounds (NMVOC) in the sinter off-gas is in the range of 0.7–120 mg/Nm3 dry (Remus et al. 2013). In another study, the composition of the NMVOC emissions was reported (Tsai et al. 2008).
Due to the incomplete combustion process in the sinter bed, some polycyclic aromatic hydrocarbons (PAH) can be found in the sinter off-gas. A PAH concentration in the off-gas after the ESP of 0.01 mg/Nm3 was reported (Guerriero et al. 2008).
Iron ore sintering is one of the most important emission source types for PCDD/Fs (Quaß et al. 2004) and other organohalogen compounds. An overview on the formation of PCDD/F in the sinter process is given in several articles. For sintering, it is generally accepted that PCDD/Fs are formed through the de novo reaction (Ooi and Lu 2011; Wang et al. 2003b). The typical emissions concentration of PCDD/F in the off-gas is 0.07–2.86 ng I-TEQ/Nm3 (Remus et al. 2013). Measurements showed that the congener pattern of PCDD/Fs and polychlorinated biphenyls (PCBs) is substantially the same for all sinter plants. The contribution of the PCDFs to the I-TEQ emission is 80–90 % (Hofstadler et al. 2000; Kasai et al. 2001a; Wang et al. 2003b; Chang et al. 2007).
PCBs are essentially precursors of the formation of PCDD/F. They are relatively volatile and may be driven off ahead of the combustion zone as the sinter bed is heated by the gaseous combustion products. PCDD/Fs contained in the recycled material can also contribute to PCDD/F formation in the off-gas. Even as they are destroyed in the sinter strand while decomposing, the resulting compounds can contribute as precursors to the de novo synthesis. The contribution of PCBs to the total TEQ emission (PCDD/Fs and PCBs) is in the range of 5–7 % (Aries et al. 2006; Grochowalski et al. 2007).
The concentration of polychlorinated naphthalenes (PCNs) was found to be in the range of 0.4–23.3 pg TEQ/Nm3 (Liu et al. 2012).
The steps for crushing, raw material handling, belt charging and discharging from the screens all involve the generation of considerable amounts of entrained particulate matter. The emissions are normally extracted by the room de-dusting system and discharged through a separate stack. The dust concentration after de-dusting is in the range of 7–50 mg/Nm3 depending on the type of de-dusting equipment installed (Remus et al. 2013). The PCDD/F concentration in this gas was measured in the range of <0.001–0.060 (Anderson and Fisher 2002).
The composition of the off-gas extracted from individual wind boxes varies considerably along the sinter strand. Characteristic curves are available for the temperature and CO2, CO, O2, H2O, HCl, SO2, NOx and PCDD/F content (Kasai et al. 2001a; Brunnbauer et al. 2006; Remus et al. 2013). These profiles are especially important for selective off-gas recirculation.
2.3 Primary Measures for Emission Reduction
There are different ways of reducing the emissions produced in the sintering process. The addition of recycling materials to the sinter feed usually increases the emissions. Therefore, limits for the quality and the quantity of these recycled materials lead to a reduction of the emissions produced.
Another way of reducing the amount of pollutants emitted is partial recirculation of the off-gas. By this technique, the amount of off-gas produced is reduced while the concentration of the pollutants in the off-gas stays the same or is increased by a factor which is smaller than the reduction factor for the off-gas volume.
The emissions of PCDD/F can be reduced by the use of inhibitors like urea, monoethanolamine (MEA) and triethanolamine (TEA) . Usually, the inhibitors are added to the charge, but there are reports of tests where an aqueous solution of the inhibitor was sprayed into the off-gas in the wind boxes.
Increasing the basicity of the sinter to reduce the emissions of acid gases would be another primary measure for emission reduction. However, the basicity of the sinter is usually a requirement set by the blast furnace operation.
Another measure to reduce emissions can be the optimization of the operation conditions of the sinter plant. The Taguchi experimental design was used for sinter pot tests to optimize sinter plant operation with respect to PAH and PCDD/F emissions (Chen et al. 2008, 2009). A multi-objective optimization tool was employed for sinter plant process optimization targeting emission reduction and productivity (Cavaliere et al. 2011; Cavaliere and Perrone 2013). Numerical and experimental analyses allowed the definition of the optimal conditions for the minimization of pollutants at acceptable productivity of the sinter plant.
2.3.1 Control of Recycling Material
In integrated steel mills, fine-grained materials rich in iron and carbon-like dust, sludge and mill scale are usually recycled through the sinter plants (Hansmann et al. 2008). The recycling is generally controlled depending on the analysis of the material because the recycled residues also have some effect on sinter quality, strength and productivity. For example, an increased input of Zn into the blast furnace has to be avoided because Zn can cause operational problems there (Esezobor and Balogun 2006), and the sintering process is typically not very efficient in reducing the Zn content (Lanzerstorfer et al. 2015a). There are further constraints on the materials that can be recycled to avoid operational problems in the sinter plant. In particular, the concentration of hydrocarbons must be controlled to avoid glow fires in the ESP (Remus et al. 2013), and the concentration of alkali metals (Na and K) has to be limited because of their adverse impact on the removal efficiency of the ESP (Schuster et al. 2003; Remus et al. 2013).
2.3.2 Off-Gas Recirculation
The recirculation of off-gas is an efficient technique to reduce the amount of off-gas discharged through the main stack and to save some fuel at the same time. The CO contained in the off-gas recycled to the sinter strand is oxidized when it passes the flame front. The concentration of CO in the off-gas discharged from the sinter process remains more or less unchanged because of the reaction equilibrium between O2, CO and CO2 prevailing in the flame front. However, the total amount of emitted CO is reduced by the same factor as the off-gas volume. Also the organic pollutants (VOC, PAH, PCDD/F) are destroyed when the recycled off-gas passes the high-temperature zone of the flame front. When recirculated off-gas passes through the sinter bed, most of the dust is filtered out, and the acid gases SO2, HCl and HF can react with base components in the sinter bed.
Fuel saving results from two effects: firstly, when CO is oxidized, the heat of reaction of 283 kJ/mol (Haynes 2012) is released and secondly, from the sensible heat of the recycled off-gas.
There are various process designs used for off-gas recirculation systems . In the EOS system, a part of the off-gas collected in the wind boxes is recirculated after de-dusting into a hood covering the whole sinter strand. The required O2 content in the hood atmosphere is achieved by the controlled addition of fresh air. Thus, only a reduced amount of off-gas has to be discharged through the main stack (Leuwerink and van der Panne 2001). Reported results for the fuel saving and the emission reduction achieved by the EOS system at Tata Steel, Ijmuiden, are summarized in Table 18.2.
The LEEP process at the sinter plant of HKM, Duisburg aims to recirculate the off-gas with the highest pollutant concentrations and energy content. Therefore, the off-gas from the wind boxes of the second half of the sinter strand is recirculated into hoods covering the whole length of the sinter strand, while the off-gas from the wind boxes of the first half of the sinter strand is discharged. As the temperature of the off-gas from the first half is substantially lower than the temperature of the off-gas from the second half, a Ljungström heat exchanger is installed to reduce this difference. Heating-up the off-gas from the first half to a temperature above the acid dew points is required to prevent corrosion. Both off-gas streams are de-dusted in ESPs before they enter the ID fans (Eisen et al. 2004).
In the Eposint system (voestalpine Stahl, Linz, and Dragon Steel, Taichung), the recirculated off-gas is also from the second half of the sinter strand. In contrast to the LEEP system, the off-gas from the last two wind boxes is added to the off-gas from the first half of the sinter strand thus increasing the temperature of this gas without a heat exchanger. The hood above the sinter strand for off-gas recycling does not cover the whole strand but leaves approximately the last quarter of the strand uncovered. The air added to the recycled off-gas is collected at the sinter cooler outlet because of its higher temperature (Brunnbauer et al. 2006; Reidetschläger et al. 2012).
Reported results for the fuel saving and the emission reduction achieved are summarized in Table 18.2. When the reduction of the emission of a pollutant is lower than the reduction of the off-gas volume, the concentration of this pollutant in the off-gas is increased due to the off-gas recirculation . With selective off-gas recirculation for some pollutants, the reduction can be even higher than the reduction of the off-gas volume.
A selective off-gas recirculation using four different off-gas zones is installed at a sinter plant of NSSC, Yawata Works (Sakuragi et al. 1994). The off-gas volume was reduced by 28 %, and the reported reduction in coke breeze consumption was 7 %. The reductions in dust and SO2 emissions are not really comparable because of the existing wet DeSOx system for the off-gas from one of the zones.
2.3.3 Suppression of PCDD/F Formation
Primary abatement of PCDD/Fs can be achieved through process optimization (Chen et al. 2008; Cavaliere et al. 2011; Cavaliere and Perrone 2013) and feed material selection (Xhrouet and De Pauw 2004; Nakano et al. 2009). A significant increase in the discharged amount of PCDD/Fs was found in the case of the recycling of ESP dust to the sinter feed (Kasai et al. 2001b).
The formation of PCDD/Fs can be suppressed by the addition of substances which have an inhibiting effect on the formation. Two different methods are described in the literature. In the first method, nitrogen compounds are added to the solid sinter mix in order to inhibit catalytic reactions on the surfaces involved. Tests with the addition of MEA, TEA or urea were carried out in different sinter plants. With an addition of 0.02 % urea to the charge, a reduction in PCDD/F emissions of approximately 50 % was achieved (Schofield et al. 2004; Anderson et al. 2007). Also CaO was found to be a PCDD/F suppressing agent when added to the charge (Nakano et al. 2009).
The second method was tested in one sinter plant. The inhibitors MEA and TEA were dissolved in water and introduced into the wind boxes by way of spraying nozzles. After some time of operation in which the inhibitor was injected continuously, the PCDD/F concentration was measured and compared with the results of reference tests without inhibitor injection. MEA as well as TEA were both effective in preventing PCDD/F formation, up to 90 % inhibition was reached using MEA (Xhrouet et al. 2002; Xhrouet and De Pauw 2003).
3 Emission Reduction Processes
In the last 25 years, a second-stage off-gas cleaning system was installed at several sinter plants to comply with lower emission limits for dust, SO2 and PCDD/F. This additional cleaning stage can be a wet system or a dry system (Menad et al. 2006; Delwig et al. 2007; Guerriero et al. 2009). However, dry systems using a sorption process are predominantly used for the separation of acid gases and PCDD/F and a fabric filter for final de-dusting (Weiss 1998; Leroy et al. 2007; Lanzerstorfer et al. 2008; Yu et al. 2009). In some sinter plants, a DeNOx process is also applied (Wang et al. 2009; Remus et al. 2013; Putz 2015).
An overview of the state-of-the-art technology for sinter plant off-gas cleaning is summarized in the “Best Available Techniques (BAT) Reference Document for Iron and Steel Production” (Remus et al. 2013).
3.1 Advanced Dry De-dusting of Sintering Off-Gas
In several sinter plants, advanced ESPs were installed to achieve lower dust emissions. The main problem in sintering dust separation with an ESP arises from the high specific resistivity of the dust (Lee et al. 2001; Lanzerstorfer and Steiner 2015). There are two systems in use at sinter plant ESPs to deal with this problem.
The first is pulse energization using microsecond pulses. With pulse energization, the peak voltage is higher, providing better particle charging in the precipitator. Reported clean dust concentrations after ESP are in the range of 20–75 mg/Nm3 (Kim et al. 1997; Grass et al. 2004; Remus et al. 2013).
The second is a moving electrode electrostatic precipitator (MEEP). In this ESP type, the movable precipitation electrodes are cleaned externally by brushes. Thus back corona effects can be reduced (Bastürk et al. 2009). Reported clean gas dust concentrations are in the range of 25–50 mg/Nm3 (Buchwalder et al. 2008; Ando et al. 2011; Remus et al. 2013).
Bag filters are usually applied downstream of the ID fan. The removal of dust can be easily combined with the removal of SO2 and other acidic gases (DeSOx) by injection of a base chemical and the removal of PCDD/F and other persistent organic pollutants by injection of an adsorbent. All the dust, the un-reacted reagents and the reaction products as well as the adsorbent are filtered off by means of the filter. A significant proportion of the removed dust is recirculated to the off-gas in order to increase the utilization of the consumables and thus reduce the costs. The rest is discharged out of the system for disposal. When DeSOx is applied, the solid residues are usually not recycled to the sinter strand due to the release of the SO2 at sinter temperature. The clean gas dust concentration after the filter is generally <10 mg/Nm3 (Remus et al. 2013).
3.2 Reduction of SOx Emissions
The first DeSOx systems installed at sinter plants applied wet processes. The off-gas was desulphurized by scrubbers using Ca(OH)2, Mg(OH)2, NaOH or a solution of NH3 as reactants. Most of these systems were installed in Japan (Remus et al. 2013). In 1998 a semidry DeSOx process was installed at the sinter plant of DK, Duisburg. Lime milk is atomized in a spray absorber. After evaporation of the water, the dry residue is separated from the off-gas in a fabric filter (Moore et al. 2003).
The new DeSOx systems comprise a dry sorption process in combination with a fabric filter (Weiss 1998; Leroy et al. 2007; Hartig et al. 2007; Lanzerstorfer et al. 2008; Remus et al. 2013; Wisse 2014). In some systems, entrained flow sorption is applied (Fleischanderl and Aichinger 2009), while other systems use various kinds of reactors (Schuster et al. 2003; Yu et al. 2009). When Ca(OH)2 is used as base reactant, some water is added to the off-gas or the reactant because SO2 separation efficiency is higher at lower temperatures (Fleischanderl et al. 2006). When NaHCO3 is used, no cooling is required. This is especially advantageous in the case of a subsequent DeNOx system (Fleischanderl and Aichinger 2009). The ranges for the stoichiometric factor for Ca(OH)2 and NaHCO3 consumption are 2–4 and 1.1–1.4, respectively. Reported clean gas SOx concentrations after dry sorption processes are in the range of 225–400 mg/Nm3 (Remus et al. 2013). By mixing a carbon-containing sorbent to the reactant for acid gas separation, the PCDD/F emissions can be reduced simultaneously.
3.3 Reduction of PCDD/F Emissions
At some sinter plants, adsorbent is injected prior to the ESP to reduce the PCDD/F emissions by an entrained flow adsorption process. Usually, lignite coke or activated carbon is used as adsorbent. To minimize the risk of glow fires in the ESP, the carbon content of the captured dusts typically has to be limited to <20 %. Thus, limestone or hydrated lime is added as an inert material. In order to minimize the risk of fires, the off-gas temperature has to be <180 °C. With increasing dosing rate the separation efficiency improves, dosing rates up to 300 mg/Nm3 lignite coke are reported. However, a PCDD/F concentration of <0.1 ng/Nm3 cannot be safely achieved because of the limited injection rate of the carbon-containing adsorbent due to the risk of an explosive mixture in the ESP (Bastürk et al. 2009). The adsorbent and the inert material are dosed into the turbulent stream of the off-gas and dispersed by a static mixer. During the flow in the ducts, the PCDD/Fs and other organohalogen compounds are adsorbed. The residence time between the injection point and the gas cleaning device is typically in the range of some seconds. In the ESP, the adsorbent and the inert material are collected together with the sinter dust. Usually, this ESP dust is recycled to the sinter strand where the PCDD/Fs are cracked in the high-temperature zone.
At ArcelorMittal, Ghent, a yearly average concentration of PCDD/Fs of 0.5 ng I-TEQ/Nm3 is achieved with a typical dosing rate of 80 mg/Nm3 of activated coal and 200 mg/Nm3 of limestone. Additionally, only strictly limited amounts of grease, oil and chloride are added to the raw mix with recycled materials (Bonte et al. 2003; Remus et al. 2013). At ArcelorMittal, Eisenhüttenstadt, 80 mg/Nm3 of pulverized lignite coke is injected. The PCDD/F concentrations were 0.115–0.255 ng I-TEQ/Nm3 (Buchwalder et al. 2008). At Thyssen Krupp, Duisburg, zeolite and lignite coke is injected. The average PCDD/F concentrations were 0.152–0.22 ng I-TEQ/Nm3 (Remus et al. 2013).
For dry sorption processes in combination with a fabric filter, reported PCDD/F concentrations were <0.05–0.23 ng I-TEQ/Nm3 (Remus et al. 2013).
Activated carbon adsorption systems compete with coupled DeNOx and dioxin destruction systems gases (Finocchio et al. 2006). The removal efficiency for PCDD/Fs in a tail-end DeNOx system (SCR) on an ng I-TEQ basis was approximately 70 % (Kasai et al. 2001a; Wang et al. 2003a). Laboratory studies show that the reduction efficiency increases with temperature (Chang et al. 2007, 2009).
3.4 Reduction of NOx Emissions
For the reduction of NOx emissions , selective catalytic reduction (SCR) plants were installed at some sinter plants. In the SCR process, the NOx reacts with the added NH3 to N2 and H2O. This reaction takes place in a reactor that contains a catalyst which is usually V2O5–WO3/TiO2. In sinter plants, SCR systems are tail-end installations, where the SCR is installed as the last cleaning stage before the off-gas is discharged through the stack. Because of the required reaction temperature, the off-gas has to be heated from the discharge temperature (typically 120–180 °C) to the operation temperature of the catalyst, which is in the range of 250–425 °C (Heck 1999) depending on the SOx concentration in the off-gas (Institute of Clean Air Companies 2009). The off-gas is heated in two steps: firstly, some of the heat contained in the off-gas after the catalyst is transferred to the off-gas before the catalyst in a heat exchanger. Secondly, a gas-fired burner is used to finally reach the required temperature. Before the off-gas enters the catalyst, NH3 is added and mixed as homogeneously as possible into the off-gas by a static mixer.
The reported reduction efficiency of the SCR systems at the CSC, Kaohsiung, sinter plants is approximately 80 % (Lee et al. 1999). The catalyst operation temperature is 350 °C (Wang et al. 2003a). At voestalpine Stahl, Linz, the sinter plant was equipped with a SCR system a few years ago. The sinter off-gas is heated from 140 °C to about 260 °C in a heat exchanger. Heating to the catalyst operation temperature of 280 °C is done by burners fired with coke oven gas. The required clean gas concentration for NOx of <100 mg/Nm3 is reached at a NH3 slip of <0.1 mg/Nm3. After 12,000 operating hours, the measured activity of the catalyst was 98.6 % of the original value (Putz 2015).
3.5 Simultaneous Reduction of SOx and NOx Emissions
Simultaneous reduction of SOx and NOx emissions can be achieved by the regenerated activated carbon (RAC) process . SO2 is adsorbed on activated carbon (AC) which is thermally regenerated. Sulphuric acid is yielded in the regeneration process as a by-product. By this process, HCl, HF, Hg, PCDD/F and optionally NOx are also removed from the off-gas. The system can be designed as a single-stage or a two-stage process. In the single-stage process, the off-gas is led through a bed of AC where the pollutants are adsorbed. NOx removal only occurs when NH3 is injected into the gas stream before the AC bed. In the two-stage process, the off-gas is led through two beds of AC. The NH3 for NOx reduction is injected before the second bed.
The reported DeSOx efficiency of a one-stage system is 95 % and the DeNOx efficiency is 15–20 % (Kasama et al. 2006). RAC systems are operated at sinter plants in Japan, Korea and Australia (Remus et al. 2013).
3.6 Advanced Wet Emission Reduction Processes
Typical wet scrubbers show limited efficiency in the collection of fine dust. The reported clean gas dust concentrations after a two-stage gas cleaning system consisting of cyclones and a subsequent venture scrubber was 70–90 mg/Nm3 (Shvets et al. 2003). In the 1990s, some advanced wet second-stage off-gas cleaning systems were installed at sinter plants (Hofstadler et al. 1999; Leuwerink and van der Panne 2001; Brunnbauer et al. 2006). In an AIRFINE scrubber system, circulating water is sprayed into the off-gas by compressed air. The reported dust separation efficiency was 80–90 %, and the dust concentration after the scrubber was in the range of 30–40 mg/Nm3. A simultaneous reduction of the PCDD/F emissions (Smit et al. 1999) as well as a reduction of acid gases emissions was reported (Hofstadler et al. 1999). However, both installations of this technique were replaced by bag filter systems in the recent years.
At another sinter plant, a wet ESP was installed as second-stage dust separator. The reported de-dusting efficiency is 83 % (Boscolo et al. 2008), and the reduction of PCDD/F emissions is approximately 70 % (Guerriero et al. 2009).
4 Residues from Dry Off-Gas Cleaning at Sinter Plants
In dry sinter off-gas de-dusting and DeSOx, the residues are fine-grained dusts; no waste water is produced. The dry consistency of the residues and the absence of waste water are major advantages in comparison with wet off-gas cleaning systems.
4.1 Residue from De-dusting of Sintering Off-Gas
The solid residues from de-dusting with ESPs or cyclones are usually recycled to the charge of the sinter plant. In some sinter plants, the dust collected in the last electrical field of the ESP is excluded from recycling because of the high concentration of chloride (Remus et al. 2013). The reason for the exclusion is that chloride is volatilized to a great extent in the sintering process in the form of alkali chlorides and discharged with the off-gas (Debrincat and Loo 2007). The chlorides are present as KCl and to a lesser extent as NaCl (Ahn and Lee 2006; Peng et al. 2008, 2009; Zhan and Guo 2013). When the off-gas temperature decreases , the alkali chlorides condense on the dust and are collected as dust in the ESP. Thus, higher recycling rates of chlorides lead to an up-cycling of chloride in the sintering process resulting in a higher alkali chloride concentration in the off-gas which negatively influences the performance of the ESP (Schuster et al. 2003). Therefore, ESP dust with a higher chloride concentration is not recycled in the sintering process but has to be disposed of in landfill sites (Eisen et al. 1996).
For reduction of the amount of chloride-containing dust which has to be excluded from recycling, air classification of ESP dust was investigated (Lanzerstorfer 2015b). The results showed that this would be a feasible process for the treatment of the ESP dust. By separating off the finest fraction with the highest chloride content, the amount of recycled dust can be increased while keeping the recycled amount of chloride constant.
4.2 Residues from Desulphurization of Sintering Off-Gas
The residue from dry second-stage off-gas cleaning is a fine-grained powder. Its flowability is poor, especially when NaHCO3 is used instead of Ca(OH)2 as reagent (Lanzerstorfer 2015c). The residue has to be disposed of in landfills because of the high concentrations of S and Cl which are the result of the separation of SO2, HCl and the finest dust fraction (Peng et al. 2008, 2009). Recycling of this residue in the sinter process would result in increased SO2 emissions (Remus et al. 2013). In order to avoid landfill disposal, leaching of this residue with water was investigated. The yielded material can be reutilized in the cement industry (Xu et al. 2012) or can be recycled to the sinter plant (Lanzerstorfer et al. 2015b). However, a significant amount of salty water, which has to be discharged, is generated by these processes. Therefore, such a process is only feasible for sinter plants located near the sea.
Guo et al. (2009) investigated the utilization of the residue from semidry desulphurization as cementing material. After thermal treatment of the residue at 450 °C, the addition of up to 20 % of the residue did not negatively influence the properties of the produced samples.
5 Future Developments
Some developments for improved sinter plant off-gas cleaning are currently under investigation. If the technical reliability and the economy of these technologies are proved, their application could influence state-of-the-art off-gas cleaning of sinter plants.
5.1 Reduction of NOx Emissions in Sinter Plants with Catalytic Bags Filters
This technology combines de-dusting and DeSOx with entrained flow injection in a fabric filter with membrane filter bags and DeNOx with an additional catalytic membrane placed inside the fabric membrane. The fabric membrane provides the protection of the catalytic support by the removal of dust and acid gases. Tests with off-gas from a sinter plant were performed in a small pilot unit. The off-gas was heated to 220 °C before it entered the bag filter. The reported results from small-scale pilot tests at a sinter plant showed up to 80 % reduction of NOx when the SOx concentration was kept below 15 mg/Nm3. The destruction efficiency for PCDD/F was approximately 90 %. However, maintaining such a low SOx concentration by a dry sorption process required a high injection rate of the sorbent (Iosif et al. 2015).
5.2 Single-Stage Gas Cleaning with a Bag Filter
The use of a fabric filter for sinter plant de-dusting upstream of the ID fan did not gain widespread acceptance (Kazyuta et al. 2004; Remus et al. 2013). The recently installed fabric filters with a dry sorption process for removal of acid gases are all installed downstream of the ID fan. There are two reasons for this arrangement. Firstly, the static pressure difference for the filter casing is lower because upstream of the ID fan there is negative pressure of 15–20 kPa. Secondly, the two-stage design enables the separate collection of the residues. Usually, the residue from the first de-dusting stage can be recirculated to the sinter process, while the residue from the second cleaning stage has to be disposed of in landfill sites to avoid a build-up of Cl and S in the system.
A single-stage off-gas cleaning concept for new sinter plants was suggested (Lanzerstorfer and Neuhold 2015). It comprises an entrained flow sorption process and a fabric filter, installed upstream of the suction fan. In this way, the investment costs for the off-gas cleaning system could be reduced. Air classification of the residue was investigated to avoid landfilling of the whole residue. In laboratory tests the residue was split into two fractions: one fraction with a low Cl and S content for recycling to the sinter feed and a second fraction that contains most of the Cl and S.
5.3 Metal Mesh Dust Filter
A new off-gas filtration process was tested in pilot tests using metallic filter screens. A filter cake is built on the mesh from the particles in the off-gas which then acts as a filter medium. This cake has to be formed in a recirculation operation mode. A dust outlet concentration of <10 mg/Nm3 was achieved (Briggs et al. 2004; Schofield et al. 2004).
6 Conclusions
Off-gas cleaning at iron ore sinter plants is still an area of importance for integrated steel mills. The emission limits for sinter plants have been tightened in recent years. Several competing technologies are available to reduce the emission of dust, SO2, NOx, HCl, HF and PCDD/Fs to these limits. Due to different operating conditions and raw materials, the emissions and, therefore, the requirement for emission control systems differ from sinter plant to sinter plant. Thus, the selection of the appropriate technology is a demanding task. The overview of the state of the art in air pollution control for sinter plants given in this chapter attempts to present a survey of off-gas cleaning technologies applied in sinter plants.
References
Ahn YC, Lee JK (2006) Physical, chemical, and electrical analysis of aerosol particles generated from industrial plants. J Aerosol Sci 37:187–202. doi:10.1016/j.jaerosci.2005.04.008
Anderson DR, Fisher R (2002) Sources of dioxins in the United Kingdom: the steel industry and other sources. Chemosphere 46:371–381
Anderson DR, Fisher R, Johnston S, Aries E, Fray TAT, Ooi TC (2007) Investigation into the effect of organic nitrogen compounds on the suppression of PCDD/Fs in iron ore sintering. Organohalogen Compd 69:2470–2473
Ando H, Shiromaru N, Mochizuki Y (2011) Recent technology of moving electrode electrostatic precipitator. Int J Plasma Environ Sci Technol 5:130–134
Aries E, Anderson DR, Fisher R, Fray TAT, Hemfrey D (2006) PCDD/F and “Dioxin-like” PCB emissions from iron ore sintering plants in the UK. Chemosphere 65:1470–1480. doi:10.1016/j.chemosphere.2006.04.020
Bastürk S, Delwig C, Ehler W, Hartig W, Hillmann C, Lüngen HB, Richter J, Schneider H, Zirngast J (2009) Technologien und Trends zur Abgasreinigung an Sinteranlagen. Stahl Eisen 129(5):51–59
Bonte L, Buttiens K, Fournelle R, Merchiers G, Pieters M (2003) New coal injection plant for dioxin reduction at the Sidmar sinter plants. Stahl Eisen 123(1):47–50
Boscolo M, Padoano E, Tommasi S (2008) Identification of possible dioxin emission reduction strategies in pre-existing iron ore sinter plants. Ironmak Steelmak 35:146–152. doi:10.1179/174328107X247815
Briggs AMW, Carcedo FG, Sedeno JV, Estrela MA (2004) Reductions in dust and gaseous emissions from sinter strands. Final Report. Office for Official Publications of the European Communities, Luxembourg
Brunnbauer G, Ehler W, Zwittag E, Schmid H, Reidetschläger J, Kainz K (2006) Eposint – a new waste gas recirculation system concept for sinter plants. Szahl Eisen 126(9):41–46
Buchwalder J, Hensel M, Richter J, Lychatz B (2008) Verminderung der Staubemissionen an der Sinteranlage von ArcelorMittal Eisenhüttenstadt. Stahl Eisen 128(9):111–117
Cavaliere P, Perrone A (2013) Analysis of dangerous emissions and plant productivity during sintering ore operations. Ironmak Steelmak 40:9–24. doi:10.1179/1743281212Y.0000000019
Cavaliere P, Perrone A, Tafuro P, Primavera V (2011) Reducing emissions of PCDD/F in sintering plant: numerical and experimental analysis. Ironmak Steelmak 38:422–431. doi:10.1179/1743281211Y.0000000034
Chang MB, Chi KH, Chang SH, Yeh JW (2007) Destruction of PCDD/Fs by SCR from flue gases of municipal waste incinerator and metal smelting plant. Chemosphere 66:1114–1122. doi:10.1016/j.chemosphere.2006.06.020
Chang SH, Chi KH, Young CW, Hong BZ, Chang MB (2009) Effect of fly ash on catalytic removal of gaseous dioxins over V2O5-WO3 catalyst of a sinter plant. Environ Sci Technol 43:7523–7530
Chen Y-C, Tsai P-J, Mou J-L (2008) Determining optimal operation parameters for reducing PCDD/F emissions (I-TEQ values) from the iron ore sintering process by using the Taguchi experimental design. Environ Sci Technol 42:5298–5303
Chen Y-C, Tsai P-J, Mou J-L (2009) Reducing PAH emissions from the iron ore sintering process by optimizing its operation parameters. Environ Sci Technol 43:4459–4465
Debrincat D, Loo CE (2007) Factors influencing particulate emissions during iron ore sintering. ISIJ Int 47:652–658
Delwig C, Hartig W, Hoffmann M, Lüngen HB (2007) Developments in sinter technology. Stahl Eisen 127:S51–S66
Eisen HP, Groß J, Hüsig K-R, Kersting K, Stedem K-H (1996) Reduction of dust emissions in German sinter plants. In: Proceedings of 3rd international ironmaking congress, Gent, pp 165–169
Eisen HP, Hüsig K-R, Köfler A (2004) Construction of the exhaust recycling facilities at a sintering plant. Stahl Eisen 124(5):37–40
Esezobor DE, Balogun SA (2006) Zinc accumulation during recycling of iron oxide wastes in the blast furnace. Ironmak Steelmak 33:419–425
European Commission (2012) Commission implementing decision of 28 February 2012 establishing the best available techniques (BAT) conclusions under Directive 2010/75/EU of the European Parliament and of the Council on industrial emissions for iron and steel production; notified under document C(2012) 903, (2012/135/EU)
European Parliament and Council of the European Union (2010) Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control)
Finocchio E, Busca G, Notaro M (2006) A review of catalytic processes for the destruction of PCDD and PCDF from waste gases. Appl Catal B Environ 62:12–20. doi:10.1016/j.apcatb.2005.06.010
Fleischanderl A, Aichinger C (2009) New developments to achieve environmentally-friendly sinter production. In: Proceedings of the iron & steel technology conference 2009, vol I. Association for Iron & Steel Technology, Warrendale, pp 191–200
Fleischanderl A, Neuhold R, Meierhofer G, Lanzerstorfer C (2006) MEROS® - improved dry-type gas-cleaning process for the treatment of sinter offgas. In: Proceedings of iron & steelmaking conference 2006, Linz, Paper No. 11.4, pp 1–6
Fleischanderl A, Plattner T, Lanzerstorfer C (2007) Efficient reduction of PM 10/2.5 emissions at iron ore sinter plants. In: Proceedings of DustConf2007, Maastricht, S2/3, pp 1–12
Grass N, Hartmann W, Klöckner M (2004) Application of different types of high-voltage supplies on industrial electrostatic precipitators. IEEE Trans Ind Appl 40:1513–1520
Grochowalski A, Lassen C, Holtzer M, Sadowski M, Hudyma T (2007) Determination of PCDDs, PCDFs, PCBs and HCB emissions from the metallurgical sector in Poland. Environ Sci Pollut Res 14:326–332. doi:10.1065/espr2006.05.303
Guerriero E, Lutri A, Mabilia R, Tomasi Scian MC, Rotatori M (2008) Polycyclic aromatic hydrocarbon emission profiles and removal efficiency by electrostatic precipitator and wetfine scrubber in an iron ore sintering plant. J Air Waste Manag Assoc 58:1401–1406. doi:10.3155/1047-3289.58.11.1401
Guerriero E, Guarnieri A, Mosca S, Rosetti G, Rotatori M (2009) PCDD/F removal efficiency by electrostatic precipitator and wetfine scrubber in an iron ore sintering plant. J Hazard Mater 172:1498–1504. doi:10.1016/j.jhazmat.2009.08.019
Guo B, Ren A, Gao J, Fang Z, Zhu T (2009) Sintered flue gas semidry processing desulphurization ash as cementing materials. In: Proceedings 3rd international conference on bioinformatics and biomedical engineering, Beijing, IEEE. doi:10.1109/ICBBE.2009.5163404
Hansmann T, Fontana P, Chiappero A, Both I, Roth J-L (2008) Technologies for optimum recycling of steelmaking residues. Stahl Eisen 128(5):29–36
Hartig W, Hoffmann M, Reufer F, Weissert H (2007) Commissioning and first operational results of the new gas cleaning installation with the Paul Wurth Entrained Flow Absorber (EFA) at ROGESA No 3 sinter strand. In: Proceedings METEC Congress, Düsseldorf, Germany, pp 322–330, 11–15 Jun 2007
Haynes WM (ed) (2012) CRC handbook of chemistry and physics, 93rd edn. Taylor & Francis, Boca Raton
Heck RM (1999) Catalytic abatement of nitrogen oxides–stationary applications. Catal Today 53:519–523
Hofstadler K, Lanzerstorfer C, Gebert W (1999) Fine dedusting and waste gas cleaning of ore sintering plants with AIRFINE. Revue Metallurgie-CIT 96:1191–1196
Hofstadler K, Friedacher A, Gebert W, Lanzerstorfer C (2000) Dioxin at sinter plants and electric arc furnace – emission profiles and removal efficiency. Organohalogen Compd 46:66–69
Institute of Clean Air Companies (ed) (2009) White paper – selective catalytic reduction (SCR) control of NOx emissions from fossil fuel-fired electric power plants. Institute of Clean Air Companies, Arlington
Iosif AM, Havelange O, DeMontard B, Ebert J (2015) Reduction of NOx emissions in sinter plants with catalytic bags filter. In: Proceedings METEC & 2nd ESTAD conference, Düsseldorf, Germany, 15–19 Jun 2015
Kasai E, Aono T, Tomita Y, Takasaki M, Shiraishi N, Kitano S (2001a) Macroscopic behaviors of dioxins in the iron ore sintering plants. ISIJ Int 41:86–92
Kasai E, Hosotani Y, Kawaguchi T, Nushiro K, Aono T (2001b) Effect of additives on the dioxins emissions in the iron ore sintering process. ISIJ Int 41:93–97
Kasama S, Kitaguchi H, Yamamura Y, Watanabe K, Umezu A (2006) Analysis of exhaust gas visibility in iron ore sintering plant. ISIJ Int 46:1027–1032
Kazyuta VI, Mantula VD, Shvets MN (2004) Ecology and resource conservation: bag filters for cleaning sintering gases. Steel Translat 34(11):68–73
Kim JR, Lee KJ, Hur NS (1997) Reduction of dust emission in sinter plant at Kwangyang Works. Curr Adv Mater Process 10:799
Lanzerstorfer C (2015a) Mechanical properties of dust collected by dust separators in iron ore sinter plants. Environ Technol 36:3186–3193. doi:10.1080/09593330.2015.1055821
Lanzerstorfer C (2015b) Application of air classification for improved recycling of sinter plant dust. Resour Conserv Recycl 94:66–71. doi:10.1016/j.resconrec.2014.11.013
Lanzerstorfer C (2015c) Mechanical and flow properties of residue from dry desulphurization of iron ore sinter plant off-gas. Environ Eng Sci 32:970–976. doi:10.1089/ees.2015.0180
Lanzerstorfer C, Neuhold R (2015) Residues from single-stage dry de-dusting and desulphurization of sinter plant off-gas: enabling partial recirculation by classification. Int J Environ Sci Technol 12:2939–2946. doi:10.1007/s13762-014-0709-6
Lanzerstorfer C, Steiner D (2015) Characterization of sintering dust collected in the various fields of an electrostatic precipitator. Environ Technol. doi:10.1080/09593330.2015.1120787
Lanzerstorfer C, Fleischanderl A, Plattner T, Ehler W, Zwittag E (2008) Emissionsminderung bei Eisenerz-Sinteranlagen. In: VDI-Bericht 2035. VDI Verlag, Düsseldorf, pp 161–170
Lanzerstorfer C, Bamberger-Straßmayr B, Pilz K (2015a) Recycling of blast furnace dust in the iron ore sinter process: investigation of coke breeze substitution and the influence on off-gas emissions. ISIJ Int 55:758–764. doi:10.2355/isijinternational.55.758
Lanzerstorfer C, Xu Q, Neuhold R (2015b) Leaching of the residue from the dry off-gas de-dusting and desulphurization process of an iron ore sinter plant. Int J Miner Metall Mater 22:116–121. doi:10.1007/s12613-015-1051-9
Lee C-S, Charng C-T, Hong G-W (1999) DeNOx system in sinter plant at CSC. SEAISI Q 1999:44–50
Lee JK, Hyun OC, Lee JE, Park SD (2001) High resistivity characteristics of the sinter dust generated from the steel plant. KSME Int J 15:630–638
Leroy J, Ravier E, Wajs A (2007) New abatement technique of the atmospheric emissions of large sinter plant. First results of industrial pilot in Arcelor’s Fos-sur-mer. In: Proceedings DustConf2007, Maastricht, p.S2/2,1
Leuwerink T, van der Panne A (2000) Reduced emissions from Hoogovens sinter and pellet plants. In: Seminar on sinter and pellets 1999, International Iron and Steel Institute, Committee on Raw Materials, Brussels, pp 176–183
Leuwerink T, van der Panne A (2001) Operation results of emission optimized sintering with Airfine gas cleaning. Stahl Eisen 121:29–34
Liu G, Zheng M, Du B, Nie Z, Zhang B, Liu W, Li C, Hu J (2012) Atmospheric emission of polychlorinated naphthalenes from iron ore sintering processes. Chemosphere 89:467–472. doi:10.1016/j.chemosphere.2012.05.101
Menad N, Tayibi H, Carcedo FG, Hernández A (2006) Minimization methods for emissions generated from sinter strands: a review. J Clean Prod 14:740–747. doi:10.1016/j.jclepro.2004.03.005
Ministry of Environmental Protection of the People’s Republic of China (2012) Emission standard of air pollutants for sintering and pelletizing of iron and steel industry, GB28662-2012
Mo C-L, Teo C-S, Hamilton I, Morrison J (1997) Admixing hydrocarbons in the raw mix to reduce NOx emission in iron ore sintering process. ISIJ Int 37:350–357
Moore C, Deike R, Hillmann C (2003) Minimization of dioxin emissions during sintering of iron residues. In: Proceedings 3rd international conference on science & technology of ironmaking (ICSTI), Düsseldorf, Germany, pp 578–581, 16–20 Juni 2003
Nakano M, Morii K, Sato T (2009) Factors accelerating dioxin emission from iron ore sintering machines. ISIJ Int 49:729–734
Ooi TC, Lu L (2011) Formation and mitigation of PCDD/Fs in iron ore sintering. Chemosphere 85:291–299. doi:10.1016/j.chemosphere.2011.08.020
Park KS, Seo Y-C, Lee SJ, Lee JH (2008) Emission and speciation of mercury from various combustion sources. Powder Technol 180:151–156. doi:10.1016/j.powtec.2007.03.006
Peng C, Guo Z-C, Zhang F-L (2008) Discovery of potassium chloride in the sintering dust by chemical and physical characterization. ISIJ Int 48:1398–1403
Peng C, Zhang F-L, Guo Z-C (2009) Separation and recovery of potassium chloride from sintering dust of ironmaking works. ISIJ Int 49:735–742
Putz B (2015) Operational experience in the field of sintering with the new installed DeNOx plant at voestalpine Linz. In: Proceedings METEC & 2nd ESTAD conference, Düsseldorf, Germany, 15–19 Jun 2015
Quaß U, Fermann M, Bröker G (2004) The European dioxin air emission inventory project––final results. Chemosphere 54:1319–1327. doi:10.1016/S0045-6535(03)00251-0
Reidetschläger J, Stiasny H, Hötzinger S, Aichinger C, Fulgencio A (2012) Selective waste gas recirculation system for sintering plants. Stahl Eisen 132(1):25–30
Remus R, Aguado-Monsonet MA, Roudier S, Sancho LD (2013) Best available techniques (BAT) reference document for iron and steel production, industrial emissions Directive 2010/75/EU, Integrated Pollution Prevention and Control. Publications Office of the European Union, Luxembourg
Sakuragi J, Kubo S, Terada J, Mochida J (1994) Operation results of the exhaust gas recirculation system in Tobata No. 3 sinter plant. Revue Metallurgie-CIT 94:899–908
Sammut ML, Noack Y, Rose J, Hazemann JL, Proux O, Depoux M, Ziebel A, Fiani E (2010) Speciation of Cd and Pb in dust emitted from sinter plant. Chemosphere 78:445–450. doi:10.1016/j.chemosphere.2009.10.039
Schofield N, Fisher R, Anderson DR (2004) Environmental challenges for the iron- and steelmaking process. Ironmak Steelmak 31:428–432
Schuster E, Zirngast J, Zellner H, Pössler J (2003) Improved flue-gas cleaning by bag filter at the sinter strand of voestalpine Stahl Donawitz. In: Proceedings METEC Congress, Düsseldorf, Germany, pp 574–577, 16–21 Jun 2003
Shvets MN, Brekhunov AV, Kachmarchik YA, Yurchenko VN, Savenchuk SV (2003) Reconstruction of gas-purification systems for sintering machines. Steel Translat 33(2):9–12
Sinha M, Ramna RV, Sinha S, Bose G (2010) Characterization of ESP dust sample from sinter plant. ISIJ Int 50:1719–1721
Smit A, Leuwerink THP, van der Panne ALJ, Gebert W, Lanzerstorfer C, Riepl H, Hofstadler K (1999) Reduction of dioxin emissions from Hoogovens sinter plant with the AIRFINE® system. Organohalogen Compd 40:441–444
Tsai J-H, Lin K-H, Chen C-Y, Ding J-Y, Choa C-G, Chiang H-L (2007) Chemical constituents in particulate emissions from an integrated iron and steel facility. J Hazard Mater 147:111–119. doi:10.1016/j.jhazmat.2006.12.054
Tsai J-H, Lin K-H, Chen C-Y, Lai N, Ma S-Y, Chiang H-L (2008) Volatile organic compound constituents from an integrated iron and steel facility. J Hazard Mater 157:569–578. doi:10.1016/j.jhazmat.2008.01.022
Wang L-C, Lee W-J, Tsai P-J, Lee W-S, Chang-Chien G-P (2003a) Emissions of polychlorinated dibenzo-p-dioxins and dibenzofurans from stack flue gases of sinter plants. Chemosphere 50:1123–1129
Wang T, Anderson DR, Thompson D, Clench M, Fisher R (2003b) Studies into the formation of dioxins in the sintering process used in the iron and steel industry. 1. Characterisation of isomer profiles in particulate and gaseous emissions. Chemosphere 51:585–594. doi:10.1016/S0045-6535(02)00784-1
Wang JB, Hung CH, Hung CH, Chang-Chien GP (2009) Polychlorinated dibenzo-p-dioxin and dibenzofuran emissions from an industrial park clustered with metallurgical industries. J Hazard Mater 161:800–807. doi:10.1016/j.jhazmat.2008.04.026
Weiss W (1998) Maßnahmen zur Verbesserung der Entstaubung einer Eisenerzsinteranlage mit nachfolgenden Untersuchungen zur Minderung der PADD/PCDF-Emissionen, Stahlwerke Bremen
Wisse AM (2014) Installation von Gewebefiltern an der Sinteranlage von Tata Steel in IJmuiden. Stahl Eisen 134(3):41–50
Xhrouet C, De Pauw E (2003) Prevention of dioxins de novo formation by ethanolamines. Environ Chem Lett 1:51–56
Xhrouet C, De Pauw E (2004) Formation of PCDD/Fs in the sintering process: influence of the raw materials. Environ Sci Technol 38:4222–4226
Xhrouet C, Nadin C, De Pauw E (2002) Amines compounds as inhibitors of PCDD/F de novo formation on sintering process fly ash. Environ Sci Technol 36:2760–2765
Xu S, Liu J, Song M (2012) Water-washing of iron-ore sintering gas cleaning residue for beneficial reutilization as secondary construction material. Procedia Environ Sci 16:244–252
Yu Z, Li Q, Xu H, Lin C (2009) Design and application of the Dry-FGD process in sanming steel No. 2 sintering plant. In: Yan K (ed) Electrostatic precipitation - 11th international conference on electrostatic precipitation. Springer, Berlin, pp 620–623
Zhan G, Guo Z-C (2013) Water leaching kinetics and recovery of potassium salt from sintering dust. Trans Nonferrous Met Soc China 23:3770–3779
Acknowledgements
Translation of the document GB28662-2012 from Chinese by Mrs. Xu Qi and proofreading by Mr. Peter Orgill is gratefully acknowledged. The author also gratefully acknowledges the helpful suggestions of the reviewers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lanzerstorfer, C. (2016). State of the Art in Air Pollution Control for Sinter Plants. In: Cavaliere, P. (eds) Ironmaking and Steelmaking Processes. Springer, Cham. https://doi.org/10.1007/978-3-319-39529-6_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-39529-6_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39527-2
Online ISBN: 978-3-319-39529-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)