Abstract
Using social information can be an efficient way to respond to changing situations or learn skills. Most bat species (Order Chiroptera) are gregarious and could theoretically benefit from socially obtained information about food or roosts. Many bats experience opportunities for social learning, and recent years have seen a variety of studies addressing this phenomenon in the Chiroptera. Because bats are aerial, small, nocturnal, and emit calls outside the range of human hearing, they are notoriously difficult to study, and distinguishing between individuals when multiple bats are present can be especially challenging. Recent advances in technology, including high-quality synchronized video and audio recordings, and the use of passive integrated transponder (PIT) tags and radio-tracking, have allowed for detailed information to be obtained about individuals in multi-bat settings. Recent studies have shown that bats can learn from one another about food type, food location, and other food-related cues. In addition, social information can play a role in roost site selection and the acquisition and modification of vocalizations. Here, I review recent research documenting vocal learning in bats, as well as interactions between individuals in foraging and roosting contexts and the impact of these interactions on bats’ behavior and success. I also report on novel findings wherein individuals of a frugivorous bat species display decreased foraging success in the presence of other naïve individuals and discuss possible reasons for this result. Finally, future directions for research on social learning in bats, which could employ such technologies as thermal imaging cameras, GPS tracking, and on-board microphones, are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Relatively long-lived animals, particularly those whose food sources or roosts change seasonally and over the course of a lifetime, should benefit from the ability to learn new skills and gather new information throughout their lives. Flexibility, innovation, and learning abilities should be especially important for animals with these characteristics. Animals that are able to use social information (e.g., watching, listening, following, and imitating), in addition to individual learning, can respond more appropriately in unpredictable environments (Cavalli-Sforza and Feldman 1983; Boyd and Richerson 1985).
Group living animals, especially, may benefit from gaining information based on the behavior of others (Cavalli-Sforza and Feldman 1983; Boyd and Richerson 1985). This might include obtaining social information in a variety of ways, such as learning which food sources are safe to consume based on olfactory or taste cues from roostmates (e.g., Galef 1988), or learning a novel method of foraging or accessing food through interactions with, or observations of, a knowledgeable conspecific(s) (e.g., Lachlan et al. 1998; Rapaport and Ruiz-Miranda 2002; May and Reboreda 2005; Thornton and McAuliffe 2006). Obtaining social information in these scenarios might benefit the observer by preventing it from ingesting unpalatable items or increasing its foraging efficiency, respectively.
Bats, with their propensity for spending time in the company of conspecifics (and sometimes heterospecifics), relatively long lifespans, and challenges such as migrating, ephemeral roosts, and changing food sources, are ideal for addressing questions about social learning and information transfer. The term “social learning” has been defined in a variety of ways. It can be difficult to define and confine what behaviors can be categorized as social learning, and there can sometimes be overlap between social learning and other phenomena, such as communication. For the purposes of this chapter, I use this term in a broad sense, to encompass examples of information transfer and to describe any time an individual uses direct observation of or information from another animal gain a skill (e.g., how to handle prey, how to make a specific vocalization) or acquire information (e.g., where to find food, where to roost), i.e., “when individuals learn from information generated by the behavior of other individuals” (Giraldeau and Caraco 2000, p. 254).
While most bat species spend time in the company of conspecifics, there is a vast array of social structures represented in this group (Bradbury 1977; McCracken and Wilkinson 2000; Kerth 2008). Social organization can have an impact on several aspects of social learning. For example, if individuals roost or forage with kin or other stable groups wherein cooperation or reciprocity is likely, transferring information about food or roosts to individuals (or at least not behaving aggressively toward naïve individuals) should be favored (e.g., Kerth and Reckardt 2003; Ratcliffe and ter Hofstede 2005). Likewise, if stable groups use vocalizations for group recognition, social modification of calls (a form of vocal learning) is necessary for convergence of group members’ calls (e.g., Boughman 1998). In addition, if young bats stay with their mothers for a relatively long duration, learning foraging-related skills from one’s mother may be more important and practical (e.g., Wilkinson 1985). In contrast, bats without stable groups or that mostly forage alone should experience fewer opportunities for learning from others.
While social learning by bats has received relatively little study compared to some other aspects of bat social behavior, there has been an increase in this line of research in recent years. Wilkinson and Boughman (1999) summarize the research on foraging-related social influences on bats conducted prior to 2000; therefore this chapter will focus on foraging-related research that post-dates Wilkinson and Boughman (1999), and on non-foraging-related studies concerning social learning in bats. First, I will discuss roosting-related social learning and information transfer in bats, followed by the mounting evidence of vocal learning in various bat species. I will then outline more recent studies of social influences on foraging in bats, including previously unpublished data investigating the ability of Artibeus jamaicensis to learn socially about food location.
2 Roosting-Related Information Transfer
Species of the Order Chiroptera occupy a diverse range of roosts, including caves, mines, tree cavities, foliage, and the outside and interiors of buildings. While roost type varies, individuals of most species live in close proximity with conspecifics and sometimes with other species as well (e.g., Twente 1955; Swift and Racey 1983; Graham 1988). Questions regarding exactly how bats select specific roosts have not been fully answered, and the social aspect of this phenomenon is even more challenging to understand. However, there is evidence from multiple species that bats exchange information about roost location and suitability. For example, Wilkinson (1992) found that young evening bats (Nycticeius humeralis) follow experienced bats to a new roost site when excluded from their previous one, possibly by eavesdropping on the calls of the older bats.
Similarly, female Bechstein’s bats (Myotis bechsteinii) exchange information about roost suitability (Kerth and Reckardt 2003). This fission-fusion species forms stable colonies of 15–40 individuals (Kerth et al. 2000), yet uses 50 or more day roosts during one reproductive season (Kerth and König 1999). These factors make information transfer related to roosts beneficial for maintaining group cohesion. Over the course of 2 years, the researchers presented maternity colonies with both suitable (accessible) and unsuitable (interior entranced blocked with mesh wire) roosts and recorded bat presence at each roost using passive integrated transponder (PIT-tag) readers. They found that significantly more bats were recruited to the suitable roost boxes. Naïve bats arriving at suitable roosts were significantly more likely to arrive within 3 min of an experienced bat compared with those arriving at unsuitable roosts, and recruited bats often arrived in groups with more than one experienced bat. The authors found no evidence of reciprocity or relatedness being factors in recruiting behavior, but postulated that the benefits of group living may drive the behavior. They did not think that bats at accessible roosts were using calls to recruit naïve bats, but they did not conduct audio recordings.
In contrast to the study described above, an experiment focusing on noctule bats (Nyctalus noctula) revealed that eavesdropping on conspecific echolocation calls emitted from cavity roosts was a crucial component for bats searching for cavities (Ruczynski et al. 2007). In this laboratory experiment, the researchers provided naïve bats with various social and nonsocial cues in a task requiring bats to find a cavity roost in a tree trunk. Bats found the roost opening significantly more quickly when playbacks of conspecific echolocation calls were emitted from the roost compared with searching with no extra cues. Subsequent research showed that bats of this species are also attracted to social calls being played back at potential roost sites (Furmankiewicz et al. 2011). For this experiment, which took place in the field with freely behaving bats, the authors recorded social calls from pregnant females in maternity roosts, and then played the calls back at artificial roosts. Bats responded by flying near or inspecting the roost from which social calls were emitted significantly more frequently than they inspected or approached roosts emitting background noise or no sound. Considering that this is a migratory species that changes roosts every few days and prefers roosts with fairly specific microclimate conditions (e.g., Ruczynski and Bogdanowicz 2005), individuals should benefit greatly by capitalizing on the roost discovery of others and may also benefit from keeping track of familiar individuals. Considering the high amplitude of the social calls and other factors, the authors (Furmankiewicz et al. 2011) suggest that bats might be using social calls from within the roost to help maintain group cohesion.
While cavity-roosting noctule bats respond to conspecific social calls from within roosts, a sophisticated system of using social calls to transfer information about roosts has been documented in another species. Spix’s disk-winged bat (Thyroptera tricolor) roosts in furled Heliconia leaves and thus must change roosts as often as daily (Findley and Wilson 1974; Vonhof et al. 2004) yet maintains small cohesive groups for as long as almost 2 years (Chaverri 2010). Chaverri et al. (2010) discovered that to keep track of roosting locations (and the roostmates within), bats play a version of “Marco, Polo,” with flying/searching bats emitting an “inquiry call,” and the bat in the roost responding with a different call. A follow-up study revealed that flying bats (but not bats inside a roost) discriminate between the calls of group members and other bats, and respond preferentially to group members (Chaverri et al. 2012). These findings further support the idea that this call and response system promotes group cohesion as well as roost-finding. These studies highlight the intersection between roost selection and information transfer in bats. Learning about roost location and quality from others serves the dual purposes of finding a high-quality roost and ensuring that other bats will be present there, thus allowing individuals to continue to reap the benefits of group living, and in some cases, cohesion of a stable group.
3 Vocal Learning
Vocal learning can be defined as animals modifying existing vocalizations or acquiring new vocalizations based on conspecific influence. Though widely documented in birds, vocal learning is thought to be much less common in mammals, having been found in only a handful of mammalian groups (Janik and Slater 1997; Boughman and Moss 2003). Considering the strong reliance of many bat species on echolocation and the ever-mounting records of vocal communication from many species (e.g., Fenton 1985; Pfalzer and Kusch 2003; Chaverri et al. 2010; Knörnschild et al. 2010a; Carter et al. 2012; Wright et al. 2013), it is not surprising that at least some bat species utilize vocal learning. Researchers have found evidence of vocal learning for both echolocation and social calls, and it is important to remember that the two call types may not always be mutually exclusive. Echolocation pulses can convey information about attributes such as sex, age, familiarity, or individual identity (e.g., Masters et al. 1995; Kazial and Masters 2004; Voigt-Heucke et al. 2010; Jones and Siemers 2011), and bats may be able to extract information from the echoes returning from calls emitted primarily for a communicative purpose. Some evidence for vocal learning related to echolocation calls comes from horseshoe bats (Rhinolophus ferrumequinum). This species displays changes in its echolocation calls over the course of a lifetime, and Jones and Ransome (1993) discovered that pups’ developing calls bore similarities to the calls of their mothers. In addition, there is evidence that Taiwanese leaf-nosed bats (Hipposideros terasensis), a CF-FM species, change the resting frequency of their calls based on the call frequency used by conspecifics, with call frequency convergence observed (Hiryu et al. 2006). While the two studies above provide evidence of a social aspect of echolocation call features, learning involved in the development or use of communicative calls has been observed in four bat species to date (see review by Knörnschild 2014; Prat et al. 2015). Newborn bats of many species emit isolation calls when separated from their mothers (e.g., Gould 1975; Thomson et al. 1985; Balcombe 1990), and in some species, such as lesser spear-nosed bats (Phyllostomus discolor), mothers reply with a directive call, thus aiding in mother–pup reunions. Esser and Schmidt (1989) found that in most of the pups they observed (6/8), the features of the isolation call converged on the acoustic features of their mothers’ directive calls. However, this was an observational study, and genetic or maturation effects could not be ruled out. A follow-up, controlled study including one group of hand-reared bats that was acoustically isolated from conspecifics and a second group of bats that was exposed to playbacks of a maternal directive call supported these findings (Esser 1994). Specifically, pups exposed to auditory playbacks altered the call structure of their isolation calls to resemble that of the played back directive calls, while pups in the control group did not (Esser 1994).
Female greater spear-nosed bats (P. hastatus) roost in stable groups and use group-specific social calls to coordinate foraging (Wilkinson and Boughman 1998). Characteristics of these group-specific “screech calls” are the product of vocal learning. When young bats are switched between groups, both resident and new bats modify their social calls to converge on a new group-specific screech call (Boughman 1998). Similar to P. hastatus, the greater sac-winged bat (Saccopteryx bilineata) has been found to display learned group signatures (Knörnschild et al. 2012). This species displays resource-defense polygyny, with harem males attempting to retain their females (see Voigt et al. 2008) through various means. As S. bilineata pups mature, the isolation calls of pups within a group—regardless of relatedness—display a convergence of acoustic features, which is indicative of vocal learning (Knörnschild et al. 2012). This species also provides the first known example of a bat species displaying vocal imitation, wherein new vocalizations are acquired socially (compared with modifying innate vocalizations; Knörnschild et al. 2010b; Boughman and Moss 2003). Adult males use a complex song as a means of defending their territories (Behr and von Helversen 2004; Davidson and Wilkinson 2004), and pups of both sexes imitate the song emitted by their harem male (Knörnschild et al. 2010b).
Recent research on the Egyptian fruit bat (Rousettus aegyptiacus) shows a greater role of vocal learning than was previously understood in the development of this bat’s vocal repertoire (Prat et al. 2015). While this species does not use laryngeal echolocation (it uses tongue clicks instead; Kulzer 1956), it emits a rich vocal repertoire. Prat et al. (2015) recorded vocalizations from young bats who either matured in a colony or were acoustically isolated from older bats (each pup in this group was alone with its mother, who remained silent with no other adults present) but were exposed to playbacks of conspecific calls. They found that pups raised with other bats developed the adult call repertoire. Isolated bats did not develop the full repertoire but mimicked the playbacks to which they were exposed. As vocal learning is demonstrated in an increasing number of bat species, questions arise about how prevalent the phenomenon might be amongst animals that rely so heavily on audition. With the evidence of vocal learning across a variety of bat species and involving both echolocation and communicative calls, this phenomenon may prove to be widespread within Chiroptera.
4 Social Learning of Food-Related Information
4.1 Overview and Previous Research
As discussed in the two previous sections, bats can learn vocal production and information about roosts from conspecifics. Unsurprisingly, evidence also continues to mount that bats can learn about food sources, types, and locations form one another, though still relatively few species have been tested for this capacity. Several studies have found that bats are attracted to the echolocation calls, particularly feeding buzzes, of foraging conspecifics, which can indicate the presence of food nearby (e.g., Barclay 1982; Balcombe and Fenton 1988; Fenton 2003; Gillam 2007). Gaudet and Fenton (1984) demonstrated that three species of captive insectivorous bats (Myotis lucifugus, Epteiscus fuscus, and Antrozous pallidus) learned a novel foraging task (taking food from an alligator clip) significantly faster via interaction with a knowledgeable conspecific compared with training by humans. In addition, a study of Myotis myotis and M. oxygnathus demonstrated cross-species social learning (Clarin et al. 2014). Bats in this study were trained to associate a light cue with a food reward, and naïve bats learned the task more quickly when allowed to interact directly with a knowledgeable bat than by merely observing a knowledgeable individual or without a knowledgeable bat present at all (Clarin et al. 2014). Considering that many bat species regularly roost with and/or forage near heterospecifics, other cases of interspecific social learning seem likely.
Wilkinson (1987) showed that naïve lesser spear-nosed bats (P. discolor) found a single accessible food cup among sixteen faster when they were searching with a knowledgeable bat (versus searching alone). In addition, evening bats (N. humeralis) have been shown to exchange information by following conspecifics to foraging sites and roosts (Wilkinson 1992). For more details about research on social influences on foraging in bats conducted prior to 2000, please see Wilkinson and Boughman (1999). More recent years have seen an increase in experimental (versus observational) studies of social learning about food by bats. For example, Page and Ryan (2006) found that the frog-eating bat, Trachops cirrhosus, acquired a novel foraging behavior more quickly in the presence of a trained conspecific than alone or with another naïve bat. This experiment involved training bats to respond to an acoustic cue that signified food availability. A later study with the same species (Jones et al. 2013) found that bats’ tendency to copy conspecifics depended on the success of individual foraging. Bats were presented with either reliable or unreliable feeding cues, and some individuals were paired with a trained tutor. Bats presented with unreliable cues and a knowledgeable tutor were significantly more likely to respond to the cue demonstrated by the tutor than bats presented with reliable cues or those without a tutor (Jones et al. 2013). These results indicate that bats are more likely to use information from others when individually-obtained information is not reliable.
One would expect that young animals are likely to benefit from social learning, and a lab study of big brown bats (Eptesicus fuscus) supports this idea. When young (<2 months old) and adult (≥1 year old) naïve bats were paired either with bats who were experienced with a novel foraging task (taking a tethered insect while flying) or with other naïve bats, the bats who interacted with experienced individuals were significantly more likely to learn the foraging task (Wright et al. 2011). Furthermore, we found evidence that close following flight and attention to feeding buzzes was positively related to bats’ social learning of the task (Wright et al. 2011).
As described above, young insectivorous bats may learn foraging skills from others (e.g., Wilkinson 1992; Wright et al. 2011), and vampire bat pups have been known to share feeding wounds with their mothers (Wilkinson 1985). While multiple studies demonstrate that bats learn about food from one another, evidence for actual teaching is more elusive. To qualify as teaching, the following criteria must be met: (1) the “teacher” must change its behavior in the presence of the naïve individual, (2) there is an initial cost to the teacher’s behavior modification (e.g., loss of food), and (3) the naïve individual learns the behavior faster than it otherwise would have (Caro and Hauser 1992). Thus far, evidence of teaching in bats is scarce, but there is report of a single instance of teaching in pallid bats (Antrozous pallidus; Bunkley and Barber 2014). In this case, all three criteria of teaching were met when an adult female familiar with a laboratory feeding task approached and called to a naïve juvenile male before accessing the food source. This male subsequently learned the foraging task much more quickly than other naïve individuals.
While this observation could be indicative of more widespread behaviors, it is, of course, important to use caution when interpreting the results of a single observation. Controlled studies with a larger sample size could determine if this behavior is anomalous or common.
In addition to the interaction involving pallid bats, a study of free-living common big-eared bats (Micronycteris microtus) found that mothers provision their young with insect prey following weaning (Geipel et al. 2013). As the pups matured and became more successful in their own hunting attempts, provisioning, which was observed for 5 months, decreased in frequency. The authors propose that such provisioning introduces young bats to adult prey, allows them to learn acoustic images of the prey, and lets them practice prey-handling. Indeed, research with other mammals (e.g., meerkats; Thornton and McAuliffe 2006) has found that post-weaning provisioning serves to teach young animals how to handle (and perhaps recognize) prey (see Caro and Hauser 1992; Thornton and Raihani 2008). Young bats of various species have been shown to follow other bats to foraging and roosting sites (Wilkinson 1992), learn a foraging task via interaction with experienced bats (Wright et al. 2011), and potentially learn prey-handling skills through adult provisioning (Bunkley and Barber 2014; Geipel et al. 2013), yet the topic of how young bats learn to forage, hunt, and/or handle prey has scarcely been studied. Further research into this topic, especially conducted on free-living animals like the Geipel et al. (2013) study, would offer insight into how bats with diverse feeding habits become proficient at finding and consuming their food.
Most of the research described above involves animal-eating bats, but predatory species are not alone in being influenced by conspecifics in a foraging context. Short-tailed fruit bats (Carollia perspicillata) tested individually and in groups in a laboratory setting found food more quickly when flying with other bats (Wright 2012). This effect was seen regardless of whether any other bat present was knowledgeable of the food’s location in advance, and the results indicate that social facilitation (enhanced feeding behavior influenced by mere presence of conspecifics) was at play (Wright 2012). Bats were presented with multiple mesh feeders, only one of which contained accessible banana. Five individuals tested alone and in a group found the food more quickly in a group. While the presence of a knowledgeable bat had no measureable effect on bats’ performance (and the bat that knew of the food’s location was not always first to feed), the results showed that the same few individuals fed first more often than expected by chance (Wright 2012). Considering that someone must be the “leader” if animals are following one another to a food source, it is possible that some individuals are more prone to being the putative leader by often finding food first. Additional research could help rule out other possible explanations for the same bats often feeding first.
While animals often learn about food from conspecifics during foraging, information exchanged in the roost can also be beneficial. Ratcliffe and ter Hofstede (2005) demonstrated that captive C. perspicillata are more likely to eat a novel flavor of food if they have been exposed to a bat that has recently consumed this food. Presumably, bats smelled and tasted the food flavor on their roostmates’ fur or breath. Likewise, tent-making bats (Uroderma bilobatum) preferred food their roostmates had recently consumed (O’Mara et al. 2014) in both a captive and natural roost setting. This study also went a step further and found that bats preferred food their roostmates had actually consumed over food whose odor was present on the fur but had not been eaten. This indicates that bats selectivity use odor cues from conspecifics’ breath (O’Mara 2014).
4.2 (Mis) Information Transfer in Jamaican Fruit-Eating Bats
When deciding whether to rely on social versus individual information, animals should consider the reliability of the available information. If individually-obtained information (e.g., trial-and-error, searching alone) is unreliable, individuals should use socially-obtained information (e.g., Jones et al. 2013). While social learning can be and often is beneficial to naïve individuals, basing one’s behavior on the actions of others can instead have negative impacts if, for example, the “demonstrator” is not knowledgeable or reliable. In such situations, which could also include a rapidly changing environment, relying on social information could result in suboptimal or maladaptive behavior (Giraldeau et al. 2002; reviewed by Rieucau and Giraldeau 2011). Research experimentally showing that animals socially acquire suboptimal behavior is scarce, and in some cases, the risk of being alone versus in a group must be weighed against the advantages of an otherwise preferred behavior (Rieucau and Giraldeau 2011). My research with Jamaican fruit-eating bats (Artibeus jamaicensis) provides an example of individuals tending toward suboptimal behavior, apparently based on the behavior of conspecifics.
In March–May 2008, I conducted a study examining the effects of conspecifics on foraging behavior and success in A. jamaicensis. This species roosts in harems and can be found foraging in large numbers at fruiting fig trees, but there is mixed evidence regarding whether this bat forages in cohesive groups (see Ortega and Castro-Arellano 2001), and it is not known if A. jamaicensis exchange information about food. I wanted to find out if these bats were helped or hindered by the presence of others in a foraging setting, and what related mechanisms might be at play. To address these questions, I captured (in mist-nets) and tested 31 adult, non-lactating A. jamaicensis on Barro Colorado Island in Panama.
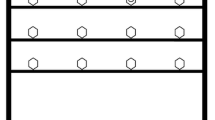
Bats were presented with three food-finding tasks of increasing complexity (Fig. 10.1) either in groups of four or five bats (n = 19) or individually (n = 12). Bats were tested nightly in a screen tent (3.4 × 4 m with 2.4 m center height) after at least 9 h of food deprivation. Individuals were marked wtih reflective tape cut into individually-distinct shapes and temporarily affixed to their backs.
Schematic of experimental setup for A. jamaicensis foraging study. Bats were tested individually or in groups. The location of the accessible food (banana) was not always the same. Bats only showed a difference in performance based on conspecific presence for the most complex task (level 3). Bats flying in level 3 took longer to access the food when flying in groups versus alone
The food-finding task was as follows: In level 1, in one corner of the tent, a mesh partition with a single opening large enough for bats to fly through (~50 cm diameter) led to a single food cup containing banana. In level 2, the feeding area was divided into two parts, one with an accessible food, and a second cup containing banana but covered in mesh such that bats could smell but not consume it. In level 3, the feeding area was divided into four parts, with three sections containing inaccessible food and one containing the accessible food cup (Fig. 10.1). In levels 2 and 3, bats could not move from one compartment to another without first exiting the hole through which they had entered (Fig. 10.1).
Each bat or group of bats was presented with all three levels (one level per night, with two exceptions when bats were given the same level for a second night). No individual was trained or given any information regarding the accessible food’s location prior to testing. I tested bats for 4 h, or until every bat had accessed the food, with a break after 2 h of testing. The location of the accessible food was not the same for each group/individual. Based on real-time observations and subsequent analyses of video recordings, I assessed the time taken for each bat to feed, time spent at the food source, time spent at inaccessible (closed) food, attempts to access the inaccessible food, and interbat interactions. Due to the non-normal distribution of the time data, data were categorized as follows (multinomial distribution): bat accessed food within 30 min; between 31 and 120 min; or did not feed within 120 min (this includes bats that did not access the food at all). A generalized linear mixed model was used (GLMM), and levels 1, 2, and 3 were analyzed separately.
For each of the three levels, the trend was toward lone bats accessing the food more quickly than those flying with conspecifics. For levels 1 and 2, there was no significant difference in time to access the food between individual/lone bats (control; n = 12) and bats flying in groups (n = 19 for level 1 and 18 for level 2; P > 0.05 for both). However, in the most complex task (level 3), bats tested alone (n = 12) accessed the food significantly faster (mean ± SD = 66 ± 86 min) than bats tested in groups (n = 19; mean ± SD = 150 ± 96 min; F 1.28 = 6.07; P = 0.02; Fig. 10.2).
These findings show that social context had a significant impact on bats’ foraging behavior. Bats’ foraging speed was unaffected by the presence of conspecifics when the task was relatively simple, but bats fared better alone when the foraging task was complex. To understand why presence of conspecifics had a negative impact on bats’ food-finding speed in level 3, I drew additional information from the video recordings and tested measures allowing me to address two hypotheses. If bats were taking longer to access the food because they wished to avoid leading other bats to the food source or because they feared aggression from other bats, I expected bats to leave the foraging area quickly when other individuals are present. In contrast, if bats in groups take longer to access the food because they are confused or distracted by conspecifics’ search behavior, I expected bats to spend more time at the inaccessible food when conspecifics are nearby (and/or to visit the inaccessible food more frequently than lone bats).
To quantitatively address these hypotheses, I calculated how often bats flying in groups or alone visited closed feeding cups (inaccessible food), how long bats spent at the accessible food source, and how long bats spent at closed feeding cups overall and when another bat was nearby (multinomial distribution; GLMM). There was a high level of individual variation regarding time spent at the accessible food, and there was no consistent trend or significant difference in time spent at the food source for bats flying in groups versus alone at any of the three levels. Likewise, there was no significant difference in the frequency with which lone vs. group bats visit closed feeding cups, nor the overall time bats in groups versus alone spend at closed feeding cups. However, the data show that bats flying in groups spent significantly more time at closed feeding cups if another bat is in the same section of the “maze” (n = 17) compared with time spent at closed cups with no other bat in the same section (n = 59; F 1.70 = 8.57; P < 0.01). On average, bats spent five times longer near/trying to access closed feeding cups when another bat was nearby (25 s vs. 125 s.; Fig. 10.3). This finding supports the hypothesis that individuals are confused or distracted by one another’s search behavior.
In a roundabout way, the results of this experiment support the idea of social learning in A. jamaicensis, since bats spent more time trying to consume inaccessible food when another bat was nearby. Bats appeared to look to conspecific behavior for clues about where and how to access food. However, given the nature of the task and that all bats were initially naïve, bats were slower, not faster, to find the accessible food source when flying with others, but only in the most complex task (level 3). In the simpler tasks (levels 1 and 2), there was no difference in food-finding speed between bats flying alone and those flying in groups, perhaps indicating less of a need for social information when the task is relatively straightforward. It was only when the chances of finding inaccessible food outweighed those of finding accessible food three to one that social context became relevant.
These findings highlight the importance of a reliable demonstrator for animals relying on social information. Because none of the bats in this experiment were trained or had prior experience with the accessible food’s location, there were no knowledgeable “leaders,” and individuals’ decision to rely on social information was more likely to lead them to closed food cups versus the single cup with accessible food. The idea that naïve “bystanders” can actually hinder foraging success (“tutor dilution”; Giraldeau and Caraco 2000) has also been found in experiments involving flock-foraging birds (e.g., Lefebvre and Giraldeau 1994). In addition, experiments with guppies found that fish will choose a longer route to food when exposed to demonstrators exhibiting this behavior (Laland and Williams 1998), but choose the shorter route when swimming alone (Bates and Chappell 2002). This difference indicates that the benefits of staying with the group must be weighed against otherwise optimal or efficient behavior (e.g., a shorter route). My experiment did not allow me to distinguish between bats believing that conspecifics could lead them to food versus choosing to forage near other individuals for other reasons (e.g., anti-predatory measures). In situations like those described above, the tendency of individuals to choose social information over individual information becomes a hindrance rather than a benefit.
In addition to being confused or distracted by the behavior of unknowledgeable conspecifics, other factors that either played a more minor role and/or were difficult or impossible to quantify may have had an impact on the bats’ behavior. Such factors, evidence of which were observed in real time and/or in video recordings, include kleptoparasitism (food-stealing) or aggression, scrounging (consuming food other bats dropped), following behavior, and pre-existing inter-individual relationships. Bats were occasionally observed squabbling audibly or taking food from one another. When one bat landed at the food source, the resident bat sometimes fled but often stayed, and bats sometimes landed at the food source in quick succession. I occasionally saw bats eat food that others had dropped. Because bats were wild-caught in the forest just before testing, I had no information about bats’ relatedness, familiarity with each other, or any dominance hierarchies among individuals. Additionally, bats are unlikely to encounter a situation in the wild wherein they can smell food but not access it. In summary, this experiment revealed that complexity/difficulty of a task affects whether animals rely on social information, as well as the importance of a reliable demonstrator/leader if social learning is to be beneficial.
5 Conclusions and Future Directions of Research
As described in this chapter, a variety of bat species have shown the capacity for learning from others in a variety of contexts (Table 10.1), yet the vast majority of bat species have not been tested for this phenomenon. Because of bats’ small size, nocturnal lifestyle, rapid movements, aerial nature, and propensity for roosting and flying in groups that can number hundreds or thousands of individuals, behavior of individuals is notoriously difficult to study in a natural environment. Traditionally, research involving social learning has included two individuals at a time—one knowledgeable about a given task and one naïve—in a controlled laboratory setting. While important information can be gained from this set up, recent advances in technology have allowed for more experiments involving more than two individuals in more naturalistic settings.
In a captive setting, synchronized high-speed video cameras and microphones offer detailed re-creation of bat flight paths and interactions coupled with their vocalizations. This allows researchers to study exactly how bats are interacting with one another, both physically and acoustically, as they engage in foraging or other behaviors. As advances in technology continue to shrink the size of electronic components, on-board microphones for bats are being developed and have already been used for studies focusing on echolocation (Hiryu et al. 2008; Boonman et al. 2013). Such microphones allow for detailed information about the echolocation (Cvikel et al. 2014) and social calls emitted by multiple individuals flying together and can give researchers insight into what each bat says and hears as it interacts with conspecifics.
Additionally, the use of PIT-tags lets researchers know which bat passes through a certain point at a certain time, as well as allowing for detailed records of the behavior of many individuals freely behaving together. Studying free-living bats in the wild offers special challenges, but technologies such as thermal cameras and PIT-tags are useful tools. Radio-tracking has long been used to track the location of individual bats from roost to foraging site and back, and more recent efforts using GPS trackers affixed to bats (Tsoar et al. 2011; Cvikel and Yovel 2014; Cvikel et al. 2015) further enhance the available data. As the devices described above continue to be improved upon and perhaps shrunk even more, opportunities for studying social learning and information transfer among free-living bats in roosts and at foraging sites should expand.
While evidence for vocal learning exists for only a handful of Chiropteran representatives thus far (see above), this phenomenon has been tested in only a tiny fraction of bat species, and there is a growing number of studies showing group-specific signature calls in bats (e.g., P. hastatus—Boughman 1998; S. bilineata—Knörnschild et al. 2012; T. tricolor—Gillam and Chaverri 2012). This, combined with the fact that social vocalizations have been reported for a wide variety of species (e.g., see Fenton 1985; Pfalzer and Kusch 2003), provides fertile ground for additional experiments investigating vocal learning in bats. As a taxonomic group with over 1200 representatives, many of whom rely heavily on acoustic information for orientation and/or communication, vocal learning is likely much more common among Chiroptera than is currently known. A final direction of research with relatively few representative studies is the intersection of social learning and vocal communication. As discussed previously, it is known that some bat species modify or acquire vocalizations based on the calls of conspecifics. In addition, social calls can inform other bats of roosting locations, including helping them to find new roosts.
While the inherent intent of vocal communication is to convey information to other individuals, little is known about the importance of social calls for bats learning about food from others. Phyllostomus hastatus use social calls to coordinate foraging (Wilkinson and Boughman 1998), but this is arguably as much about group cohesion as foraging itself. Social calls have been recorded from other species during foraging (e.g., Pipistrellus pipstrellus—Barlow and Jones 1997; E. fuscus—Wright et al. 2013, 2014), but none of these calls are known to facilitate learning prey location or handling by other bats. Additionally, there have been anecdotes of bats emitting apparent social calls in a social learning context (e.g., during potential teaching in A. pallidus—Bunkley and Barber 2014). During the time that young bats are learning foraging or prey-handling skills, or when stable groups of bats are foraging together, it seems reasonable that social calls might be used to convey information about resource location or prey capture/handling skills. Future studies focusing on the potential value of communicative calls for social learning may yield fascinating results.
Literature Cited
Balcombe JP (1990) Vocal recognition of pups by mother Mexican free-tailed bats, Tadarida brasiliensis mexicana. Anim Behav 39:960–966
Balcombe JP, Fenton MB (1988) Eavesdropping by bats: the influence of echolocation call design and foraging strategy. Ethology 79:158–166
Barclay RMR (1982) Interindividual use of echolocation calls: eavesdropping by bats. Behav Ecol Sociobiol 10:271–275
Barlow KE, Jones G (1997) Function of pipistrelle social calls: field data and a playback experiment. Anim Behav 53:991–999
Bates L, Chappell J (2002) Inhibition of optimal behavior by social transmission in the guppy depends on shoaling. Behav Ecol 13:827–831
Behr O, von Helversen O (2004) Bat serenades—complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav Ecol Sociobiol 56:106–115
Boonman A, Bar-On Y, Cvikel N, Yovel Y (2013) It’s not black or white—on the range of vision and echolocation in echolocating bats. Front Physiol 4:248
Boughman JW (1998) Vocal learning by greater spear-nosed bats. Proc R Soc B 265:227–233
Boughman JW, Moss CF (2003) Social sounds: vocal learning and development of mammal and bird calls. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic communication. Springer, New York, xiv + 404 pp, pp 138–224
Boyd R, Richerson PJ (1985) Culture and the evolutionary process. University of Chicago Press, Chicago, IL, 331 p
Bradbury JW (1977) Social organization and communication. In: Wimsatt WA (ed) Biology of bats, vol. 3. Academic Press, New York, 651 p, pp 1–72
Bunkley JP, Barber JR (2014) An observation of apparent teaching behavior in the pallid bat, Antrozous pallidus. Western North Am Naturalist 74:249–252
Caro TM, Hauser MD (1992) Is there teaching in nonhuman animals? Q Rev Biol 1992:151–174
Carter GG, Logsdon R, Arnold BD, Menchaca A, Medellin RA (2012) Adult vampire bats produce contact calls when isolated: acoustic variation by species, population, colony, and individual. Public Libr Sci One 7:e38791
Cavalli-Sforza LL, Feldman MW (1983) Cultural versus genetic adaptation. Proc Natl Acad Sci USA 80:4993–4996
Chaverri G (2010) Comparative social network analysis in a leaf-roosting bat. Behav Ecol Sociobiol 64:1619–1630
Chaverri G, Gilliam EH, Kunz TH (2012) A call-and-response system facilitates group cohesion among disc-winged bats. Behav Ecol ars188
Chaverri G, Gillam EH, Vonhof MJ (2010) Social calls used by a leaf- roosting bat to signal location. Biol Lett 6:441–444
Chaverri G, Gillam EH (2016) Acoustic communication and group cohesion in Spix’s disc-winged bats. In: Ortega J (ed) Sociality in Bats. Springer, Berlin, pp 161–178
Clarin TM, Borissov I, Page RA, Ratcliffe JM, Siemers BM (2014) Social learning within and across species: information transfer in mouse-eared bats. Can J Zool 92:129–139
Cvikel N, Yovel Y (2014) Full night, on-board audio and GPS monitoring of bat behavior in the wild. J Mol Neurosci 53:S133–S133
Cvikel N, Levin E, Hurme I, Borissov A, Boonman E Amichai, Yovel Y (2014) On-board recordings reveal no jamming avoidance in wild bats. Proc R Soc London B 282:20142274
Cvikel N, Berg KE, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y (2015) Bats aggregate to improve prey search but might be impaired when their density becomes too high. Curr Biol 25:206–211
Davidson SM, Wilkinson GS (2004) Function of male song in the greater white-lined bat, Saccopteryx bilineata. Anim Behav 67:883–891
Esser K-H (1994) Audio-vocal learning in a non-human mammal: the lesser spear-nosed bat Phyllostomus discolor. Neuroreport 5:1718–1720
Esser K-H, Schmidt U (1989) Mother-infant communication in the lesser spear-nosed bat Phyllostomus discolor (Chiroptera, Phyllostomidae)—evidence for acoustic learning. Ethology 82:156–168
Fenton MB (1985) Communication in the Chiroptera. Indiana University Press, Bloomington, IN, 161 p
Fenton MB (2003) Eavesdropping on the echolocation and social calls of bats. Mammal Rev 33:193–204
Findley JS, Wilson DE (1974) Observations on the neotropical disk-winged bat, Thyroptera tricolor Spix. J Mammal 55:562–571
Furmankiewicz J, Ruczynski I, Urban R, Jones G (2011) Social calls provide tree-dwelling bats with information about the location of conspecifics at roosts. Ethology 117:480–489
Galef BG Jr (1988) Communication of information concerning distant diets in a social, central-place foraging species: Rattus norvegicus. In: Zentall TR, Galef BG Jr, (eds) Social learning: psychological and biological perspectives. Lawrence Erlbaum Associates, Inc., Mahwah, NJ, pp 119–139, 368 p
Gaudet CL, Fenton MB (1984) Observational learning in three species of insectivorous bats (Chiroptera). Anim Behav 32:385–388
Geipel I, Kalko EKV, Wallmeyer K, Knörnschild M (2013) Postweaning maternal food provisioning in a bat with a complex hunting strategy. Anim Behav 85:1435–1441
Gillam EH (2007) Eavesdropping by bats on the feeding buzzes of conspecifics. Can J Zool 85:795–801
Gillam EH, Chaverri G (2012) Strong individual signatures and weaker group signatures in contact calls of Spix’s disc-winged bat, Thyroptera tricolor. Anim Behav 83:269–276
Giraldeau L-A, Caraco T (2000) Social foraging theory. Princeton University Press, Princeton, NJ, 362 p
Giraldeau L-A, Valone TJ, Templeton JJ (2002) Potential disadvantages of using socially acquired information. Philos Trans R Soc B, 357:1559–1566
Gould E (1975) Neonatal vocalizations in bats of eight genera. J Mammal 56:15–29
Graham GL (1988) Interspecific associations among Peruvian bats at diurnal roosts and roost sites. J Mammal 69:711–720
Hiryu S, Katsura K, Nagato T, Yamazaki H, Lin L-K, Watanabe Y, Riquimaroux H (2006) Intra-individual variation in the vocalized frequency of the Taiwanese leaf-nosed bat, Hipposideros terasensis, influenced by conspecific colony members. J Comp Physiol A 192:807–815
Hiryu S, Shiori Y, Hosokawa T, Riquimaroux H, Watanabe Y (2008) On-board telemetry of emitted sounds from free-flying bats: compensation for velocity and distance stabilizes echo frequency and amplitude. J Comp Physiol A 194:841–851
Janik VM, Slater PJB (1997) Vocal learning in mammals. Adv Study Behav 26:59–100
Jones G, Ransome RD (1993) Echolocation calls of bats are influenced by maternal effects and change over a lifetime. Proc R Soc London B 252:125–128
Jones G, Siemers BM (2011) The communicative potential of bat echolocation pulses. J Comp Physiol A 197:447–457
Jones PL, Ryan MJ, Flores V, Page RA (2013) When to approach novel prey cues? Social learning strategies in frog-eating bats. Proc R Soc London B 280:20132330
Kazial KA, Masters WM (2004) Female big brown bats, Eptesicus fuscus, recognize sex from a caller’s echolocation signals. Anim Behav 67:855–863
Kerth G (2008) Causes and consequences of sociality in bats. Bioscience 58:737–746
Kerth G, König B (1999) Fission, fusion and nonrandom associations in female Bechstein's bats (Myotis bechsteinii). Behaviour 136:1187–1202
Kerth G, Reckardt K (2003) Information transfer about roosts in female Bechstein’s bats: an experimental field study. Proc R Soc London B 270:511–515
Kerth G, Mayer F, König B (2000) Mitochondrial DNA (mtDNA) reveals that female Bechstein’s bats live in closed societies. Mol Ecol 9:793–800
Knörnschild M (2014) Vocal production learning in bats. Curr Opin Neurobiol 28:80–85
Knörnschild M, Glockner V, von Helversen O (2010a) The vocal repertoire of two sympatric species of nectar-feeding bats (Glossophaga soricina and G. commissarisi). Acta Chiropterologica 12:205–215
Knörnschild M, Nagy M, Metz M, Mayer F, von Helversen O (2010b) Complex vocal imitation during ontogeny in a bat. Biol Lett 6:156–159
Knörnschild M, Nagy M, Metz M, Mayer F, von Helversen O (2012) Learned vocal group signatures in the polygynous bat Saccopteryx bilineata. Anim Behav 84:761–769
Kulzer E (1956) Flughunde erseugen Orientierungslaute durch Zungenschlag. Naturwissenschaften 43:117–118
Lachlan RF, Crooks L, Laland KN (1998) Who follows whom? Shoaling preferences and social learning of foraging information in guppies. Anim Behav 56:181–190
Laland KN, Williams K (1998) Social transmission of maladaptive information in the guppy. Behav Ecol 9:493–499
Lefebvre L, Giraldeau L-A (1994) Cultural transmission in pigeons is affected by the number of tutors and bystanders present. Anim Behav 47:331–337
Masters WM, Raver KA, Kazial KA (1995) Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age, and family affiliation. Anim Behav 50:1243–1260
May D, Reboreda JC (2005) Conspecific and heterospecific social learning in shiny cowbirds. Anim Behav 70:1087–1092
McCracken GF, Wilkinson GS (2000) Bat mating systems. In: Crichton EG, Krutzsch PH (eds)Reproductive biology of bats. Academic Press, San Diego, CA, pp 321–362, 510 p
O’Mara MT, Dechmann DK, Page RA (2014) Frugivorous bats evaluate the quality of social information when choosing novel foods. Behav Ecol aru120
Ortega J, Castro-Arellano I (2001) Artibeus jamaicensis. Mamm Species 662:1–9
Page RA, Ryan MJ (2006) Social transmission of novel foraging behavior in bats: frog calls and their referents. Current Biol 16:1201–1205
Pfalzer G, Kusch J (2003) Structure and variability of bat social calls: implications for specificity and individual recognition. J Zool (London) 261:21–33
Prat Y, Taub M, Yovel Y (2015) Vocal learning in a social mammal: demonstrated by isolation and playback experiments in bats. Sci Adv 1:e1500019
Rapaport LG, Ruiz-Miranda CR (2002) Tutoring in wild golden lion tamarins. Int J Primatol 23:1063–1070
Ratcliffe JM, ter Hofstede HM (2005) Roosts as information centres: social learning of food preference in bats. Biol Lett 1:72–74
Rieucau G, Giraldeau LA (2011) Exploring the costs and benefits of social information use: an appraisal of current experimental evidence. Philos Trans R Soc B 366:949–957
Ruczynski I, Bogdanowicz W (2005) Roost cavity selection by Nyctalus noctula and N. leisleri (Vespertillionidae, Chiroptera) in Bialowieza Primeval Forest, eastern Poland. J Mammal 86:921–930
Ruczynski I, Kalko EKV, Siemers BM (2007) The sensory basis of roost finding in a forest bat, Nyctalus noctula. J Exp Biol 210:3607–3615
Swift SM, Racey PA (1983) Resource partitioning in two species of vespertilionid bats (Chiroptera) occupying the same roost. J Zool 200:246–259
Thomson CE, Fenton MB, Barclay RMR (1985) The role of infant isolation calls in mother-infant reunions in the little brown bat, Myotis lucifugus (Chiroptera: Vespertilionidae). Can J Zool 63:1982–1988
Thornton A, McAuliffe K (2006) Teaching in wild meerkats. Science 313:227–229
Thornton A, Raihani NJ (2008) The evolution of teaching. Anim Behav 75:1823–1836
Tsoar A, Nathan R, Bartan Y, Vyssotski A, Dell'Omo G, Ulanovsky N (2011) Large-scale navigational map in a mammal. Proc Natl Acad Sci USA 108(37):E718–E724
Twente JW Jr (1955) Some aspects of habitat selection and other behavior of cavern-dwelling bats. Ecology 36:706–732
Voigt CC, Behr O, Caspers B, von Helversen O, Knörnschild M, Mayer F, Nagy M (2008) Songs, scents, and senses sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J Mammal 89:1401–1410
Voigt-Heucke SL, Taborsky M, Dechmann DK (2010) A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim Behav 80:59–67
Vonhof MJ, Whitehead H, Fenton MB (2004) Analysis of Spix’s disc-winged bat association patterns and roosting home ranges reveal a novel social structure among bats. Anim Behav 68:507–512
Wilkinson GS (1985) The social organization of the common vampire bat. I. Pattern and cause of association. Behav Ecol Sociobiol 17:111–121
Wilkinson GS (1987) Altruism and co-operation in bats. In: Racey PA, Fenton MB, Rayner JMV (eds) Recent advances in the study of bats. Cambridge University Press, Cambridge, UK, pp 299–323, 484 p
Wilkinson GS (1992) Information transfer at evening bat colonies. Anim Behav 44:501–518
Wilkinson GS, Boughman JW (1998) Social calls coordinate foraging in greater spear-nosed bats. Anim Behav 55:337–350
Wilkinson GS, Boughman JW (1999) Social influences on foraging in bats. In: Box HO, Gibson KR (eds) Mammalian social learning: comparative and ecological perspectives. Cambridge University Press, Cambridge, UK, pp 188–204, 424 p
Wright GS (2012) Communication and social influences on foraging in bats. PhD dissertation, University of Maryland, College Park, MD
Wright GS, Wilkinson GS, Moss CF (2011) Social learning of a novel foraging task by big brown bats (Eptesicus fuscus). Anim Behav 82:1075–1083
Wright GS, Chiu C, Xian W, Wilkinson GS, Moss CF (2013) Social calls of flying big brown bats (Eptesicus fuscus). Front Physiol 4:214
Wright GS, Chiu C, Xian W, Wilkinson GS, Moss CF (2014) Social calls predict foraging success in big brown bats. Curr Biol 24:885–889
Acknowledgments
For the Artibeus jamaicensis study: I thank J. Nelson for her assistance with field work. J. Wright, R. Spanjer, J. Kalkavage, K. Heer, I. Wagner, L. Albrecht, E. Kalko, K. Barboza Marquez, and K. O’Grady provide help with field work, logistical help, or data analysis. I thank G.S. Wilkinson, C.F. Moss, and members of their labs for helpful feedback on the design, execution, and analysis of the study. Funding was provided by the Animal Behavior Society, the American Society of Mammalogists, and while the author was supported by training grant DC-00046 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health. Thanks to C. Chiu, G. Chaverri, and J. Ortega for their feedback on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Wright, G.S. (2016). Social Learning and Information Transfer in Bats: Conspecific Influence Regarding Roosts, Calls, and Food. In: Ortega, J. (eds) Sociality in Bats. Springer, Cham. https://doi.org/10.1007/978-3-319-38953-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-38953-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-38951-6
Online ISBN: 978-3-319-38953-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)