Abstract
We have incorporated LiNc-BuO, an oxygen-sensing paramagnetic material, in polydimethylsiloxane (PDMS), which is an oxygen-permeable, biocompatible, and stable polymer. We fabricated implantable and retrievable oxygen-sensing chips (40 % LiNc-BuO in PDMS) using a 20-G Teflon tubing to mold the chips into variable shapes and sizes for in vivo studies in rats. In vitro EPR measurements were used to test the chip’s oxygen response. Oxygen induced linear and reproducible line broadening with increasing partial pressure (pO2). The oxygen response was similar to that of bare (unencapsulated) crystals and did not change significantly on sterilization by autoclaving. The chips were implanted in rat femoris muscle and EPR oximetry was performed repeatedly (weekly) for 12 weeks post-implantation. The measurements showed good reliability and reproducibility over the period of testing. These results demonstrated that the new formulation of OxyChip with 40 % LiNc-BuO will enable the applicability of EPR oximetry for long-term measurement of oxygen concentration in tissues and has the potential for clinical applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Electron paramagnetic resonance (EPR) oximetry
- Oxygen sensor
- Partial pressure of oxygen (pO2)
- Skeletal muscle
1 Introduction
Measurement of partial pressure of oxygen (pO2) in an accurate, reliable, and repeatable fashion is crucial to the understanding, diagnosis, and treatment of a number of pathophysiological conditions, including ischemic disease, reperfusion injury, oxygen toxicity, cancer, peripheral vascular disease, and wound healing [1]. Of the several methods available for measuring pO2 in tissues [2], electron paramagnetic resonance (EPR) oximetry has some distinct advantages, including the ability to make minimally invasive, real-time (in vivo), and repeated measurements.
In addition, EPR oximetry is unique in terms of its ability to provide absolute values of pO2 [1, 2]. Repeated measurements of pO2, from a single location in tissue, are made possible by the use of water-insoluble crystalline probes, such as lithium phthalocyanine (LiPc) [3], lithium naphthalocyanine (LiNc) [4], or lithium octa-n-butoxynaphthalocyanine (LiNc-BuO) [5]. Of these crystalline probes, LiNc-BuO has some advantages over others, including high EPR sensitivity (spin density and narrow lineshape) and oxygen sensitivity, and long-term stability and responsiveness to oxygen in vivo [1].
Although, LiNc-BuO can be used in its raw (bare) crystalline form to measure oxygen, its in vivo application may be limited by particle migration within tissue leading to loss of EPR signal intensity over time, and potential biocompatibility concerns due to direct exposure of tissue to the crystals. Encapsulation of crystalline probes in a bio-inert matrix is a strategy, employed in the past, to overcome the limitations associated with the use of bare material, especially for clinical application [6, 7]. Recently, we have encapsulated LiNc-BuO in polydimethylsiloxane (PDMS) , a well-characterized, biocompatible, and highly oxygen-permeable polymer, for the development of implantable, and surgically retrievable, EPR probe formulations (denoted as LiNc-BuO:PDMS and referred to hereafter as ‘OxyChip ’) [8]. Fabrication, by cast-molding/polymerization method, and physical characterization of the OxyChips demonstrated that encapsulation in the PDMS matrix did not have any significant effect on the oxygen-sensing ability of the embedded LiNc-BuO microcrystals. The cast-molding procedure also facilitated the fabrication of OxyChips with different shapes, sizes, and spin densities [8].
In the present study, we report the biological evaluation and functional testing of OxyChips, including in vitro/in vivo biocompatibility and oxygen-sensing performance. The results establish that the encapsulation of LiNc-BuO microcrystals in PDMS, while enhancing their biocompatibility and suitability for direct in vivo application, does not interfere with their oxygen-sensing ability.
2 Methods
2.1 Polymer-Encapsulation of LiNc-BuO Microcrystals
The synthesis and physicochemical characterization of LiNc-BuO microcrystals have been described previously [5]. Medical grade PDMS base, namely MED-4210 Platinum Silicone Elastomer, was obtained from Factor II, Inc. (Lakeside, AZ). 40 % LiNc-BuO:PDMS chips (w/w) were fabricated in the form a thin wire (diameter, 0.6 mm) using a modification of previously reported procedure [8]. Briefly, the PDMS base and catalyst/crosslinker (supplied with the PDMS elastomer) were mixed in a 10:1 ratio, as recommended by the manufacturer, after which the LiNc-BuO crystals were added. The heterogeneous dispersion/mixture was outgassed using a vacuum desiccator connected to a vacuum pump. One end of a 20-G PTFE tube was dipped into the PDMS mixture (LiNc-BuO + PDMS + Catalyst/Crosslinker) and negative pressure was applied from the other end with a 10-ml syringe to draw the PDMS mixture into the tubing to the desired length, usually about 5 cm. The PTFE tubing with PDMS mixture was cured in an oven for at least 8 h at 70 °C, followed by withdrawing (by gentle pulling) the cured PDMS chip out from the tubing. The chip (LiNc-BuO in PDMS) was further cured at 70 °C overnight. The cured chip (referred to as OxyChip) in the form of a wire (0.6-mm diameter) was cut into small segments of 5-mm lengths for use in the present study. In order to verify that mechanical stress or tearing of PDMS coating does not occur resulting in leaching of LiNc-BuO crystals, the OxyChips were suspended in water or ethanol and subjected to 72 h of continuous stirring at room temperature. After removing the OxyChips , the solution was analyzed using EPR spectroscopy, which did not show any detectable absorption due to LiNc-BuO suggesting the absence of any detectable paramagnetic debris in solution (data not shown).
2.2 In Vitro EPR Measurements
Calibration curves and the time course of response of the OxyChips were performed by measuring the EPR linewidth while the equilibrating gas content was changed from 0 to 21 % (160 mmHg) oxygen. Measurements were carried out using an L-band (1.2 GHz) EPR spectrometer (custom-built) equipped with a surface-loop resonator [9]. Gases with known concentrations of oxygen and nitrogen or air, equilibrated at 37 °C, were flushed over the samples, and the spectra were recorded every minute until equilibration, as evidenced by a steady linewidth, was achieved.
2.3 In Vivo EPR Measurements
Measurements of muscle oxygenation were performed using the same L-band (1.2 GHz) EPR spectrometer (see Sect. 2.2) using a surface-loop resonator. Animals were placed on a plastic bedplate and the previously implanted OxyChip was positioned just beneath the loop, i.e., approximately centered within the active volume of the loop resonator. Body temperature was monitored using a thermistor rectal probe, and maintained at 37 ± 1 °C using a heating pad and an infrared lamp. In order to verify that the implanted OxyChip was sensitive to changes in tissue oxygenation, the muscle tissue was subjected to temporary constriction of blood flow after baseline EPR measurement, thereby reducing the oxygen supply to the hind limb. This was achieved by gently tying the limb above the location of chip implantation using an elastic band. The constriction was maintained in place until EPR measurements were made (usually less than 5–8 min) and then removed. All animals were subjected to constriction on every other week during the course of the measurements.
2.4 Animal Preparation
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Geisel School of Medicine at Dartmouth. Eight adult male Wistar rats (200–220 g, Charles River Laboratories, MA, USA) were used. One rat died on day 56 due possibly to anesthesia, but it was excluded from data analysis. For all surgical procedures, 3.0–3.5 % isoflurane in 70 % N2:30 % (228 mmHg) O2 was used for anesthesia induction, and 1.5–2.5 % for anesthesia maintenance. Physiologic monitoring during the procedure comprised measurement and maintenance of core (rectal) temperature at 37 ± 1 °C using a heating pad.
2.5 Evaluation of the Stability of OxyChips in the Skeletal Muscle Tissue
Prior to implantation, each piece of 0.6-mm × 5.0-mm OxyChip was suspended in 5 ml of distilled water and subjected to 72 h of continuous stirring at room temperature using a tiny magnetic stirrer pellet followed by sterilization by autoclaving at 121 °C for 30 min. Calibration curves were made before and after the washing and autoclaving procedures. For implantation of OxyChips, the animals were anesthetized by nose-cone breathing of 2–2.5 % of isoflurane in 30 % (228 mmHg) oxygen. One OxyChip was implanted in the biceps femoris of the right limb using an 18-G angiocatheter. The injected depth was 3–4 mm from the surface of the skin. Normal tissue pO2 (baseline) was measured for 30 min when rats were anesthetized with 1.5 % isoflurane in 30 % (228 mmHg) O2 at the following time points: day 7, day 14, day 21, day 28, day 42, day 56, day 70 and day 84 after OxyChip implantation. Normal, ischemic and recovery tissue pO2 (baseline pO2 for 25 min, ischemic muscle pO2 induced by muscle compression with a rubber band for 5–8 min and recovery from ischemia induced by release pressure for 15 min) were measured.
2.6 Statistical Analysis
Data were analyzed by Student’s t-test. Paired t-test was used to compare pO2 changes within the same group. The tests were two-sided, and a change with a p-value <0.05 was considered statistically significant. N is the number of rats and n is the number of OxyChips. All data are expressed as mean ± SEM.
3 Results and Discussion
Figure 46.1 shows the oxygen calibration of the OxyChip that was measured before and after autoclaving. The calibration remained linear, with no significant difference in oxygen sensitivity up on autoclaving. The results demonstrated the stability of the OxyChip, with no effect of autoclaving on the intended functionality of the OxyChip.
Calibration plot of OxyChip before and after sterilization by autoclaving procedure. The response of the chip to different concentrations of perfused oxygen is shown with a linear fitting and regression coefficient (R 2). Mean ± SEM, n = 4 OxyChips. Inset: Molecular formula of lithium octa-n-butoxy-naphthalocyanine (LiNc-BuO) (left) and samples of 0.6-mm × 0.6-mm chips used in the oxygen measurements (right)
In our previous study, histological analysis of excised muscle tissue surrounding the implant after 4 weeks of implantation showed that the chip elicited a characteristic wound-healing response, with the recruitment of inflammatory cells and the formation of a thin fibrous capsule [11]. However, the fibrous coating of the chip did not have any significant effect on the in vivo oxygen-sensing ability of the implant. In the present study, the OxyChip remained intact in the muscle and enabled repeated measurements of tissue pO2 for 11 weeks from the same rats. The pO2 measurements established the ability of the OxyChip to provide repeated measurements of in vivo tissue oxygenation over the 11-week duration, and possibly longer. Further, in order to verify if the implanted OxyChip was responsive to changes in tissue pO2, blood flow to the muscle was temporarily constricted using an elastic band and subsequently released. The decrease in blood flow, during the constriction, leads to a decrease in oxygen supply to the muscle.
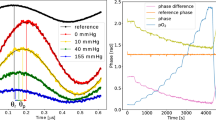
Figure 46.2a shows that the implanted chip was capable of reporting the dynamic change in tissue oxygenation induced by the muscle constriction in an individual rat. The muscle pO2 also returned to normal levels when the constriction was removed. Comparison of constricted-state pO2 readouts with normal muscle pO2 revealed a significant difference between the two, confirming that the constriction was effective and that the OxyChip was able to report the changes in muscle pO2 (Fig. 46.2b). The results showed the mean baseline muscle pO2 ranged from 18 to 27 mmHg over the 11-week period, which is within the range of rat gastrocnemius muscle pO2 values reported previously [4, 10]. The differences in the baseline pO2 values on different weeks may be due to the differences in the local physiology of the muscle.
Time course of pO2 in biceps femoris of right limb in a single rat (a) and group mean (b) prior to, during and post leg muscle compression. Baseline is the average pO2 from measurements of the last 15 min of 30-min baseline period. Compression is the average pO2 in the 5–8 min period of the muscle constriction. Recovery is the average pO2 in the last 10 min of a 20-min recovery period of measurements when the constriction was removed. The black arrow indicates the beginning of compression and the gray arrow indicates start of pressure release. Data represent Mean ± SEM; N = 7 rats. *p < 0.01, compared to baseline on the same day (paired t-test)
4 Conclusion
We have successfully carried out biological evaluation and functional testing of a new EPR oximetry sensor, the OxyChip, containing 40 % LiNc-BuO in PDMS. Calibration of the OxyChips was not affected by sterilization procedures. The suitability and applicability of the chip for long-term in vivo oximetry was established by monitoring the oxygenation of rat muscle tissue for an extended period. Overall, we have established that the new OxyChip is a promising choice for clinical EPR oximetry.
References
Khan N, Williams BB, Hou H et al (2007) Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal 9:1169–1182
Swartz HM, Hou H, Khan N et al (2014) Advances in probes and methods for clinical EPR oximetry. Adv Exp Med Biol 812:73–79
Liu KJ, Gast P, Moussavi M et al (1993) Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A 90(12):5438–5442
Ilangovan G, Manivannan A, Li H et al (2002) A naphthalocyanine-based EPR probe for localized measurements of tissue oxygenation. Free Radic Biol Med 32(2):139–147
Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P (2003) Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med 35(9):1138–1148
Dinguizli M, Jeumont S, Beghein N et al (2006) Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron 21(7):1015–1022
Eteshola E, Pandian RP, Lee SC, Kuppusamy P (2009) Polymer coating of paramagnetic particulates for in vivo oxygen-sensing applications. Biomed Microdevices 11(2):379–387. doi:10.1007/s10544-008-9244-x
Meenakshisundaram G, Eteshola E, Pandian RP et al (2009) Fabrication and physical evaluation of a polymer-encapsulated paramagnetic probe for biomedical oximetry. Biomed Microdevices 11(4):773–782
Swartz HM, Walczak T (1998) Developing in vivo EPR oximetry for clinical use. Adv Exp Med Biol 454:243–252
Hou H, Khan N, Lariviere J et al (2014) Skeletal muscle and glioma oxygenation by carbogen inhalation in rats: a longitudinal study by EPR oximetry using single-probe implantable oxygen sensors. Adv Exp Med Biol 812:97–103
Meenakshisundaram G, Eteshola E, Pandian RP et al (2009) Oxygen sensitivity and biocompatibility of an implantable paramagnetic probe for repeated measurements of tissue oxygenation. Biomed Microdevices 11:817–826
Acknowledgments
These developments were supported by National Institutes of Health grants R01 EB004031 and P01 CA190193.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Hou, H. et al. (2016). Skeletal Muscle Oxygenation Measured by EPR Oximetry Using a Highly Sensitive Polymer-Encapsulated Paramagnetic Sensor. In: Luo, Q., Li, L., Harrison, D., Shi, H., Bruley, D. (eds) Oxygen Transport to Tissue XXXVIII. Advances in Experimental Medicine and Biology, vol 923. Springer, Cham. https://doi.org/10.1007/978-3-319-38810-6_46
Download citation
DOI: https://doi.org/10.1007/978-3-319-38810-6_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-38808-3
Online ISBN: 978-3-319-38810-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)