Abstract
Osteoimmunology describes the cross-talk of cells of the musculoskeletal and the immune system during the pathogenesis of various diseases; among the most prevalent ones is periodontitis, a chronic infectious inflammatory disease of the tooth-supporting structures, i.e., the periodontium consisting of the gingiva, alveolar bone, periodontal ligament, and root cementum. Periodontal disease is initiated by oral pathogens that accumulate at the gingival margin of the teeth and thereby trigger a response of the innate and adaptive immunity. In contrast to other osteoimmunological disorders (e.g., osteoporosis, rheumatoid arthritis), the immune system plays a two-sided role in the pathogenesis of periodontitis: it controls the infection and protects the organism from bacterial invasion but also propagates the destruction of the soft and hard tissues surrounding the tooth. Periodontitis, if left untreated, finally results in tooth loss.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Osteoimmunological Aspects of Periodontal Diseases
Osteoimmunology describes the cross-talk of cells of the musculoskeletal and the immune system during the pathogenesis of various diseases; among the most prevalent ones is periodontitis, a chronic infectious inflammatory disease of the tooth-supporting structures, i.e., the periodontium consisting of the gingiva, alveolar bone, periodontal ligament, and root cementum. Periodontal disease is initiated by oral pathogens that accumulate at the gingival margin of the teeth and thereby trigger a response of the innate and adaptive immunity. In contrast to other osteoimmunological disorders (e.g., osteoporosis, rheumatoid arthritis), the immune system plays a two-sided role in the pathogenesis of periodontitis: it controls the infection and protects the organism from bacterial invasion but also propagates the destruction of the soft and hard tissues surrounding the tooth. Periodontitis, if left untreated, finally results in tooth loss.
The present chapter discusses, in a concise manner, the interplay of pathogens, mediators, and enzymes, and host cells involved in the pathogenesis of periodontitis. Further, a short overview on some of the potential “osteoimmunological targets” for the treatment of periodontitis is included.
12.1.1 Anatomy of the Periodontium
The term “periodontium” is derived from the Greek terms “peri-” (around) and “-odous” (tooth); it consists of four main components, which differ in their histological structure but act functionally as a single unit:
-
Gingiva
-
Periodontal ligament
-
Alveolar bone
-
Root cementum
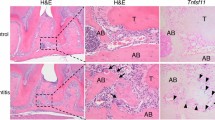
The gingiva is via the junctional epithelium attached to the tooth surface and thereby forms a barrier against external factors (e.g., bacterial invasion). The other three components of the periodontium are forming the “attachment apparatus”; the periodontal ligament consists of a rich vasculofibrous connective tissue, with collagen fibers incorporated in the root cementum and in the alveolar bone, and provides tooth support and tooth function (Fig. 12.1a–c).
Thin ground section of the periodontium of two adjacent teeth, stained with toluidine blue, in health (a) and disease (b). E enamel, CEJ cementoenamel junction, P plaque (due to overhanging crown margins), G gingival fibers, D dentin, L periodontal ligament, B alveolar bone, C root cementum. (c) Thin ground section of the lateral teeth of the upper jaw (By courtesy of late Prof. Dr. mult. K. Donath and Prof. DDr. Ulm)
12.1.2 Periodontitis: Disease Initiation
The oral cavity provides a habitat for about 750 different microbial species and is one of the most complicated microbial ecosystems in the human body (Jenkinson and Lamont 2005). It provides huge amounts of nutrients through the saliva, gingival crevicular fluid, and food remnants, particularly those containing sugar. The first steps of dental plaque and biofilm formation occur within minutes after tooth brushing. A pellicle, consisting mainly of salivary proteins, attaches first at surface pits and fissures of the tooth and at the smooth tooth surfaces of the interdental area and the gingival margin, which are not affected much by the constant movement of the cheek and tongue. Thereafter, early bacterial colonizers such as Streptococci species, Actinomyces species, Capnocytophaga species, and Veillonella species are able to attach to salivary pellicle receptors (Foster and Kolenbrander 2004; Listgarten 1994; Ritz 1967; Teles et al. 2013). The primary bacterial attachment may still be reversible, but if not interrupted, the bacteria start to build up a biofilm by multiplying, forming multilayers, and secreting extracellular matrix. This extracellular matrix helps the bacteria to stick together, form the biofilm architecture, and provide each other nutrients. Further, the early colonizers provide binding sites for the next colonizers (e.g., Fusobacterium nucleatum). F. nucleatum, which is an obligate anaerobic bacterium, plays a highly relevant role in biofilm maturation. F. nucleatum co-aggregates with multiple other bacteria, including early as well as late colonizers, and aerobic as well as anaerobic species and is present in both periodontally healthy sites and in diseased sites, however, in larger amounts in diseased sites. The co-aggregation with F. nucleatum allows late colonizers, which are mainly gram-negative facultative and obligate anaerobic bacteria (e.g., Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans), to survive in an aerated environment due to oxygen gradients in the biofilm structure (Bradshaw et al. 1998; Holt and Ebersole 2005).
Biofilm formation at the gingival margin causes in the beginning gingivitis, which is a reversible condition affecting only the soft tissues (Löe et al. 1965). In susceptible patients, however, increasing biofilm accumulation provokes progression to periodontitis, which involves periodontal attachment and bone loss, mostly irreversible. Recently the “polymicrobial synergy and dysbiosis” model was proposed for periodontal disease pathogenesis (Hajishengallis et al. 2012; Hajishengallis and Lamont 2012). Herein, single pathogens, so-called keystone pathogens, are able to initiate a shift of the entire microbial colonization from homeostasis to dysbiosis, i.e., the imbalance of a microbial flora in an ecosystem associated with the presence of a disease, thereby causing a strong immune response. Thus far, P. gingivalis is the best-documented keystone pathogen. Its presence, although not necessarily in high amounts, elevates the pathogenicity of the entire oral microbial community, e.g., by an increased expression of various virulence factors (Hajishengallis 2014; Hajishengallis and Lamont 2012). An experimental periodontitis model with mice demonstrated that presence of P. gingivalis in less than 0.01 % of the total bacterial amount was still enough for periodontal disease initiation (Hajishengallis et al. 2011). However, after this initiating step, the major portion of periodontal tissue degradation is not caused by the biofilm; in fact, it is the host immune response that propagates the loss of periodontal structures.
12.1.3 Periodontitis: Prevalence and Risk Factors
Periodontitis represents besides caries the major reason for tooth loss and is vastly prevalent in the population. In Germany, the prevalence of periodontitis according to the case definitions developed by the Centers for Disease Control and Prevention and the American Academy of Periodontology (Eke et al. 2012; Page and Eke 2007) was 71 and 87 % in age cohorts of 35–44 and 65–74 years, respectively; 17 and 42 %, respectively, suffered from severe forms of periodontitis, i.e., advanced loss of the periodontal structures and bone (Holtfreter et al. 2010). Risk factors and risk indicators for periodontal disease may be both nonmodifiable and modifiable; examples of nonmodifiable factors include age, male gender, ethnicity, and genetic factors, while modifiable factors include smoking, alcohol consumption, low educational level, poorly controlled diabetes, obesity and metabolic syndrome, osteoporosis, and stress and its coping mechanisms (Genco and Borgnakke 2013). Page and Kornman (1997) illustrated the interplay between the bacterial attack, the immune response, and the risk factors in the pathogenesis of periodontal disease as shown in Fig. 12.2.
Pathogenesis model of periodontal disease. (Modified from Page and Kornman 1997)
12.1.4 Periodontal Disease Entities
The current classification system of periodontal diseases was established in 1999 at the International Workshop for the Classification of Periodontal Diseases and Conditions (Armitage 1999):
-
1.
Gingival diseases
-
2.
Chronic periodontitis
-
3.
Aggressive periodontitis
-
4.
Periodontitis as a manifestation of systemic diseases
-
5.
Necrotizing periodontal diseases
-
6.
Abscesses of the periodontium
-
7.
Periodontitis associated with endodontic lesions
-
8.
Developmental or acquired deformities and conditions
This chapter deals with osteoimmunological aspects associated with alveolar bone loss occurring in patients with aggressive or chronic periodontitis. Aggressive periodontitis (AgP) is characterized by rapid attachment loss, which is most often inconsistent with the amount of microbial deposits. AgP patients are systemically healthy, i.e., no specific immune compromising condition, but familial aggregation is characteristic. AgP appears clinically as localized and generalized AgP, currently considered to represent two different disease entities. Localized AgP is primarily restricted to first molars and incisors and has a circumpubertal onset, and patients show a high serum antibody response against the infecting agents. In contrast, generalized AgP affects individuals around 30 years of age, but patients may be older; further, generalized AgP patients present a poor serum antibody response against the infecting agents and a pronounced episodic destruction of periodontal attachment (Armitage 1999; Armitage and Cullinan 2010; Tonetti and Mombelli 1999) (Fig. 12.3).
Panoramic radiograph of a 24-year-old man with severe generalized aggressive periodontitis. Yellow arrows indicate the regular height of the alveolar bone in a healthy patient, while the red arrows indicate the bone level of this patient. The light blue arrow is indicating calculus, which is a rare finding in aggressive periodontitis. Severe bone loss is present around the first molar teeth (tooth number 16, 26, and 36; tooth 46 is already missing)
Chronic periodontitis (CP) is the most common form of periodontitis in adults and it is characterized by consistency between the amount of periodontal tissue destruction and the presence of local factors, i.e., large amounts of biofilm. Further, a variable microbial pattern and a slow to moderate rate of progression with possible burst periods of rapid attachment loss are observed. Localized and generalized CP are considered as the same disease, but with different manifestation in terms of percentage of teeth affected, i.e., up to or more than 30 % affected teeth, respectively (Armitage 1999; Armitage and Cullinan 2010; Flemmig 1999) (Fig. 12.4a, b).
Panoramic radiograph (a) and clinical picture (b) of a 52-year-old woman with severe generalized chronic periodontitis. The yellow arrow indicates the regular height of the alveolar bone in a healthy patient, while the red arrows indicate the bone level of this patient. The orange arrows are indicating the lost bone between the roots of the lower molars, i.e., furcation involvement, and the light blue arrow is indicating calculus
In general, the biochemical and histological characteristics, as well as the pathological processes and involved mediators and cells in the lesions, are rather similar between AgP and CP. However, certain, partly genetic, but yet not fully clarified, differences are causing the existing variations in the onset, magnitude, extent, and progression rate of tissue destruction (Kulkarni and Kinane 2014).
12.1.5 Periodontal Disease Progression
In summary, the first defense mechanism against the biofilm is represented by the innate immune system; while at later stages, the adaptive immune system becomes more relevant. After the initial step of biofilm accumulation at the tooth surface, biofilm components are perceived as “danger signals” by cells of the innate immune system; i.e., leukocytes and resident cells (e.g., gingival epithelial cells, periodontal ligament cells) recognize microbial lipopolysaccharide (LPS), DNA, or peptidoglycans via toll-like receptors (TLR). TLR are indispensable for the host to recognize the bacterial attack and their activation induces an intracellular signal pathway, expression of transcription factors, and subsequently release of pro-inflammatory cytokines and chemokines; altogether, an immune response focused on the infecting agents. TLR-2 and TLR-4 are primarily responsible for sensing periodontal pathogens (e.g., A. actinomycetemcomitans or P. gingivalis) (Kikkert et al. 2007). Peptidoglycans and atypical LPS from P. gingivalis activate TLR-2, LPS from gram-negative bacteria in general activate TLR-4, while A. actinomycetemcomitans activates both receptors (Akira and Takeda 2004; Ford et al. 2010). As a response to biofilm accumulation, a massive influx of neutrophils occurs in the tissues and, through the epithelium, in the periodontal sulcus. Neutrophils, except from phagocytosing bacteria, express a variety of pro-inflammatory cytokines, resulting in additional recruitment of various immune cells, including macrophages and T cells, thus propagating inflammation. Among the T cells, mainly CD4+ T cells (T helper cells; Th) are involved in periodontal disease pathogenesis; more specifically the cells of the innate immune system activate Th1 and Th17 cells, which results in a strong pro-inflammatory immune response. Later on Th2 cells promote the establishment of a lesion with predominantly B and plasma cells. Additionally, regulatory T cells (Treg) are activated and exert an anti-inflammatory role.
As already mentioned, the immune response initially causes a local inflammatory reaction, resulting in tissue destruction restricted to the gingiva, i.e., gingivitis. Yet, if the bacterial invasion cannot be controlled in susceptible hosts, the inflammatory reaction proceeds from the gingival margin apically towards the alveolar bone; i.e., the inflammatory response causes detachment of the junctional epithelium and its conversion into an ulcerated pocket epithelium, loss of the connective tissue attachment to the root, and loss of alveolar bone, i.e., periodontitis. An inflammatory process in close proximity to bone regularly supports differentiation and activation of osteoclast precursors, which results in uncoupling of the physiological balanced bone formation and bone resorption, in favor of the latter (Fig. 12.5a, b).
If this, often non-self-resolving, inflammatory process is left untreated, alveolar bone is continuously lost until tooth exfoliation. Hence, the immune response initiated by the bacterial attack is primary responsible for the extent, rate, and severity of host tissue degradation (Bartold et al. 2010; Brook 2003; Ford et al. 2010; Stabholz et al. 2010).
12.1.6 Mediators and Enzymes Involved in the Pathogenesis of Periodontal Disease
12.1.6.1 RANKL/RANK/OPG System
The receptor activator of nuclear factor “kappa-light-chain-enhancer” of the activated B cell ligand (RANKL)/receptor activator of NF-kB (RANK)/osteoprotegerin (OPG) system (RANKL/RANK/OPG system) is regulating the coupling of bone formation and resorption. RANKL, a member of the tumor necrosis factor (TNF) family, induces osteoclastogenesis by binding to its receptor RANK on osteoclast precursors. This step is promoted by macrophage colony-stimulating factor (M-CSF), which is stimulating RANK expression on osteoclast precursors. The activation of RANK by RANKL is essential for osteoclast formation, differentiation, and activity. The soluble counterpart OPG, expressed by osteoblasts, acts as a decoy receptor binding to RANKL and thereby can inhibit the binding of RANKL to RANK (Boyle et al. 2003).
During periodontal disease, elevated levels of RANKL and/or decreased levels of OPG, resulting in a RANKL/OPG ratio that favors bone resorption, are observed (Bartold et al. 2010; Cochran 2008; Crotti et al. 2003). For example, in a preclinical trial on experimental periodontitis in mice, the expression level of RANKL correlated well with the level of various pro-inflammatory cytokines, i.e., interleukin (IL)-1, TNF-α, and interferon (IFN)-γ, during active bone loss (Garlet et al. 2006). Elevated levels of RANKL are also found in active periodontal lesions in patients, i.e., sites showing progressive alveolar bone loss; in contrast, no remarkable change from the physiological RANKL/OPG ratio was found in samples of gingivitis sites, i.e., sites without alveolar bone loss (Menezes et al. 2008). Osteoblasts, dendritic cells, B and T cells, and resident cells (e.g., periodontal ligament cells, gingival fibroblasts) are considered as important sources for the elevated levels of RANKL in periodontal lesions upon pro-inflammatory stimuli and/or TLR activation (Bar-Shavit 2008; Belibasakis et al. 2007; Kawai et al. 2006).
12.1.6.2 Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a family of calcium- and zinc-dependent proteases, which are expressed by immune cells (e.g., neutrophils, macrophages). In healthy periodontal tissues, MMPs control the physiological turnover of extracellular matrix and are regulated by their inhibitors, i.e., tissue inhibitors of metalloproteinases (TIMPs), which are expressed by resident and immune cells (e.g., macrophages, fibroblasts, endothelial cells). During inflammation, presence of pro-inflammatory cytokines deregulates the MMP/TIMP balance, resulting in higher levels of MMPs and/or lower levels of TIMPs. This imbalance leads to increased degradation of periodontal soft tissues (Giannobile 2008; Verstappen and Von den Hoff 2006). Specifically, MMP-8 – being a collagenase – is strongly associated with periodontal disease; progressively increasing levels of MMP-8 are found in sites with increasing severity of disease, i.e., from healthy to gingivitis and to periodontitis. Thus MMP-8 is considered as a strong biomarker for detecting alveolar bone loss (Ebersole et al. 2013; Gursoy et al. 2013; Leppilahti et al. 2014).
12.1.6.3 Pro-inflammatory Interleukins
IL-1 is a strict pro-inflammatory key cytokine expressed by resident (e.g., fibroblasts, epithelial cells) and immune cells (e.g., neutrophils, macrophages). IL-1 stimulates the expression of other pro-inflammatory and chemotactic factors (e.g., IL-6, TNF-α, prostaglandins) and promotes osteoclastogenesis by upregulating RANKL expression. Further, IL-1 enhances matrix degradation by inducing MMPs and impairs the regenerative potential of the tissues by inducing apoptosis of matrix producing cells (Cochran 2008; Garlet et al. 2006; Graves 2008; Graves and Cochran 2003; Wei et al. 2005). Several studies have shown increased levels in gingival crevicular fluid, saliva, and periodontal tissue samples of periodontitis patients (Duarte et al. 2007; Ebersole et al. 2013; Gamonal et al. 2000; Gursoy et al. 2009; Mogi et al. 1999).
IL-2 is a pro-inflammatory cytokine and mainly expressed by Th1 cells. IL-2 affects the differentiation, growth, and activation of T, B, and natural killer cells (Kuziel and Greene 1990). Studies have reported both unaltered and increased levels of IL-2 in chronic periodontitis patients compared to healthy controls (Teles et al. 2009; Tymkiw et al. 2011).
IL-6 is another pro-inflammatory key cytokine, which is expressed by various resident and immune cells (e.g., fibroblasts, neutrophils, macrophages). IL-6 stimulates the expression of other pro-inflammatory factors and promotes – in a similar way as IL-1 – soft and hard tissue degradation by upregulating RANKL and MMP expression and inducing apoptosis of matrix producing cells (Cochran 2008; Garlet et al. 2006; Graves 2008; Graves and Cochran 2003; Wei et al. 2005). However, while IL-1 is a strictly pro-inflammatory cytokine, IL-6 has also a regulatory function; IL-6 was shown to regulate IL-1 and TNF-α by inducing IL-1 receptor antagonist and TNF-α soluble receptor (Irwin and Myrillas 1998). Several studies have shown increased levels of IL-6 in fluid and tissue samples of periodontitis patients (Duarte et al. 2007; Ebersole et al. 2013; Gamonal et al. 2000; Gursoy et al. 2009; Mogi et al. 1999).
IL-8 is a chemokine and secreted by monocytes, macrophages, fibroblasts, keratinocytes, and endothelial cells in the presence of microorganisms and related toxins. Its main function is to attract and activate neutrophils (Bickel 1993). Studies have reported significantly increased levels of IL-8 in periodontally diseased sites (Ertugrul et al. 2013; Gamonal et al. 2000).
IL-12, mainly expressed by immune cells (e.g., dendritic cells, macrophages, neutrophils), is a key mediator of cell-mediated immunity, necessary for the initiation and maintenance of Th1 cell response, including high IFN-γ expression (Park and Scott 2001). The levels of IL-12 in gingival crevicular fluid samples may not always differ significantly between periodontally diseased and healthy patients, but it has been shown that a significant reduction of IL-12 levels occurred after initial treatment in periodontitis patients (Thunell et al. 2010).
IL-17 is a pro-inflammatory cytokine secreted by Th17 cells and by various other cell types (e.g., neutrophils, dendritic cells, periodontal ligament cells). IL-17 increases the levels of MMPs and RANKL and amplifies the pro-inflammatory loop of IL-1−IL-6 and TNF-α (Cardoso et al. 2008; Cheng et al. 2014; Ford et al. 2010; Kotake et al. 1999; Sato et al. 2006). Further, IL-17 plays a role in the defense against pathogens by increasing mobilization and activation of neutrophils (Yu et al. 2007) and by improving the responsiveness of TLR in human gingival epithelial cells (Beklen et al. 2009). Indeed, induced P. gingivalis infection resulted in increased alveolar bone loss in IL-17 receptor knockout mice due to an impaired immune response (Yu et al. 2007). P. gingivalis is considered to promote a Th17 cell response with the corresponding IL-17 expression, and indeed, increased levels of IL-17 have been described in periodontally diseased subjects (Cheng et al. 2014).
IL-18 is a pro-inflammatory cytokine, expressed by immune cells, osteoblasts, and fibroblasts, and upregulates other pro-inflammatory and chemotactic mediators (e.g., IL-1, TNF-α, IL-8). Significantly higher levels of IL-18 have been shown in periodontitis patients compared to healthy controls (Banu et al. 2015; Ozçaka et al. 2011).
IL-23 is expressed by monocytes, macrophages, and dendritic cells and promotes Th17 cell differentiation. Progressively increased levels of IL-23 in the gingival crevicular fluid from healthy to gingivitis and to periodontitis sites have been reported (Gonzales 2015; Himani et al. 2014).
IL-33 is primarily expressed intracellularly, e.g., in monocytes and endothelial and epithelial cells. It is assumed that IL-33 release after cell necrosis represents an alarming signal for cell damage and, hence, causes a pro-inflammatory immune response and cytokine production (Moussion et al. 2008; Nile et al. 2010). In human gingival tissue biopsies, the levels of IL-33 were increased compared to healthy tissue samples, and in a preclinical trial on experimental periodontitis, IL-33 expression was increased in the presence of bacterial infection and mediated bone loss via the RANKL system (Malcolm et al. 2015).
12.1.6.4 Anti-inflammatory Interleukins
IL-4 is expressed by Th2 cells and at the same time also promotes Th2 cell differentiation; further, IL-4 promotes B cell activation, differentiation, and antibody production (Appay et al. 2008; Cronstein 2007). IL-4 exerts its anti-inflammatory and antiresorptive action by decreasing IFN-γ, MMP, and RANKL expression and by elevating IL-10, TIMPs, and OPG levels (Appay et al. 2008; Garlet et al. 2006; Ihn et al. 2002; Jarnicki and Fallon 2003; Saidenberg-Kermanac’h et al. 2004). Indeed, lower IL-4 levels have been recorded in gingival crevicular fluid of periodontally diseased patients compared to those in healthy controls (Pradeep et al. 2008).
IL-10 is an anti-inflammatory cytokine, which is expressed by various cell types (e.g., T and B cells, macrophages, dendritic cells). IL-10 reduces the activity of pro-inflammatory cytokines (e.g., IL-1−IL-17, IFN-γ) (Jovanovic et al. 1998; Naundorf et al. 2009) and upregulates TIMPs and OPG, thereby reducing soft and mineralized periodontal tissue destruction (Garlet et al. 2004, 2006; Zhang and Teng 2006). IL-10 knockout mice presented a remarkably higher susceptibility against P. gingivalis (Sasaki et al. 2004) and a reduced expression of osteoblast and osteocyte markers (Claudino et al. 2010), which indicates that, in addition to its anti-inflammatory and antiresorptive properties, IL-10 appears to also have a direct positive effect on bone formation. In the clinic, patients with a polymorphism reducing IL-10 mRNA transcription showed also reduced TIMP-3 and OPG transcription (Claudino et al. 2008), and therefore IL-10 polymorphism has been suggested as risk factor for chronic periodontitis (Zhong et al. 2012).
IL-13, expressed mainly by Th2 cells, exerts its anti-inflammatory action by downregulating inflammatory cytokine production in monocytes and supports B cell activation and antibody production (Abbas et al. 1996; de Waal Malefyt et al. 1993). During periodontal disease, IL-13 appears to correlate with IL-4 levels but shows in general higher levels (Johnson and Serio 2007).
12.1.6.5 TNF-α
TNF-α is expressed by various resident and immune cells and is, next to IL-1 and IL-6, a pro-inflammatory key cytokine in periodontitis pathogenesis. IL-1, IL-6, and TNF-α seem to somehow complement each other during inflammatory alveolar bone loss, since blocking of all three cytokines is shown to result in a higher inhibition of alveolar bone loss compared to single cytokine blocking (Graves 2008; Zwerina et al. 2004). TNF-α has the potential to induce osteoclastogenesis even in the absence of RANKL and also to diminish bone formation by inhibiting differentiation of osteoblast precursors and proliferation of mature osteoblasts (Bartold et al. 2010; de Vries et al. 2016; Graves et al. 2011; Graves and Cochran 2003; Lacey et al. 2009; Tomomatsu et al. 2009). Progressively increasing gingival crevicular fluid levels of TNF-α from periodontally healthy to gingivitis and to periodontitis patients have been reported (Ertugrul et al. 2013).
12.1.6.6 IFN-γ
IFN-γ is expressed by Th1 cells and natural killer cells and exerts a strong pro-inflammatory action by attracting and enhancing phagocyte activity and by inducing expression of pro-inflammatory cytokines and chemokines. Although it has been shown in vitro that IFN-γ inhibits osteoclastogenesis, due to its strong pro-inflammatory action, i.e., elevation of IL-1, TNF-α, RANKL levels, this direct anti-osteoclastogenic effect is overcome in vivo, resulting altogether in alveolar bone loss (Gao et al. 2007; Garlet et al. 2008; Gemmell and Seymour 1994; Ji et al. 2009). Yet, IFN-γ plays also an important role in the defense mechanism against infections by activating cytotoxic CD8+ T cells and natural killer cells; e.g., it has been shown that IFN-γ knockout mice presented severe defense impairment against A. actinomycetemcomitans (Garlet et al. 2008). In preclinical and clinical trials, increasing levels of IFN-γ correlated well with increasing severity of periodontal lesions (Garlet et al. 2003; Honda et al. 2006; Isaza-Guzmán et al. 2015; Teng et al. 2005).
12.1.6.7 TGF-ß
Transforming growth factor beta (TGF-ß) is part of the TGF-ß superfamily that represents a variety of proteins, including the bone morphogenetic proteins. TGF-ß is secreted by a variety of cells (e.g., Treg, macrophages, neutrophils) and can regulate the immune response by reducing pro-inflammatory cytokine (e.g., IL-1, TNF-α) and tissue-degrading enzyme (e.g., MMPs) expression; additionally, TGF-ß is considered as an important factor for wound healing. High levels of TGF-ß have been found in periodontally inflamed tissues, which might be indicative of the constant wound healing response of the host (Mize et al. 2015; Steinsvoll et al. 1999).
12.1.6.8 Complement System
The complement system consists of more than 40 proteins and can be activated by three different pathways, which converge with cleavage of the complement component 3 (C3) into C3a and C3b. C3a is attracting leukocytes and C3b binds covalently to target surfaces to make the pathogens more susceptible to phagocytosis by leukocytes, i.e., “complement opsonization.” Although the complement system supports the immune system, continuous complement activation can be destructive for the host tissue by indirectly inducing alveolar bone loss (Damgaard et al. 2015). In particular, the membrane attack complex (C5b-9) can lead to activation of phospholipase A2, release of arachidonic acid, and synthesis of prostaglandin E2, which is a very potent osteolytic substance (Klein and Raisz 1970). Further, neutrophils and monocytes express the complement receptor 3, which is strongly activated by P. gingivalis; complement receptor 3 activation increases phagocyte recruitment and pro-inflammatory cytokine expression (e.g., IL-1, IL-6, TNF-α) causing increased alveolar bone loss.
12.1.6.9 Lipid Mediators
Arachidonic acid is released by phospholipase A2 from membrane phospholipids and further processed to either pro- or anti-inflammatory mediators. Pro-inflammatory lipid mediators, i.e., leukotrienes and prostaglandins, induce recruitment of neutrophils (e.g., leukotriene B4) and promote osteoclastogenesis and bone resorption (e.g., prostaglandin E2). Elevated levels of such lipid mediators have been observed in the gingival crevicular fluid of periodontally diseased patients (Offenbacher et al. 1986; Zhong et al. 2007).
The anti-inflammatory lipid mediators, i.e., lipoxins, resolvins, and protectins, are pro-resolving on the inflammation process (Garlet 2010; Graves et al. 2011). Pro-resolution is, in contrast to the passive termination of inflammation, an actively regulated process; e.g., anti-inflammatory lipid mediators were shown to regulate the migration and recruitment of neutrophils and T cells, to attenuate leukotriene B4-dependent pro-inflammatory signals, to reduce the expression of pro-inflammatory cytokines, and to promote T cell apoptosis (Serhan et al. 2008).
12.1.6.10 Nitric Oxide
Nitric oxide (NO), previously named “endothelial-derived relaxing factor,” is synthesized during conversion of L-arginine to L-citrulline by NO-synthases. The endothelial and neuronal NO-synthases are constitutively expressed, whereas the inducible NO-synthases (iNOS) are expressed in neutrophils, macrophages, and/or gingival tissue upon pro-inflammatory cytokine expression and/or in the presence of bacterial products. NO has an important role in vascular regulation, platelet aggregation, regulation of mineralized tissue, and the pathogenesis of inflammatory diseases (Alayan et al. 2006; Daghigh et al. 2002; Matejka et al. 1998; Ugar-Cankal and Ozmeric 2006). The role of NO is most likely dual; on the one side, it is part of the defense mechanism and helps to control the bacterial attack, but on the other side, excessive amounts of NO are toxic for various cells (e.g., fibroblasts, epithelial cells) and NO is suspected to increase MMPs and reduce TIMPs (Brennan et al. 2003; Nguyen et al. 1992; Ugar-Cankal and Ozmeric 2006). Indeed, experimental periodontitis in iNOS knockout mice resulted in an elevated amount of neutrophils in the periodontal tissues and more bone, compared to that in wild-type mice (Alayan et al. 2006; Fukada et al. 2008). Further, elevated levels of iNOS have been observed in periodontally diseased tissue (Lappin et al. 2000; Matejka et al. 1998), while reduced amounts of NO2 −, which is the stable metabolite of NO, have been found in the saliva of periodontally diseased patients (Aurer et al. 2001) (Table 12.1).
12.1.7 Cells Involved in the Pathogenesis of Periodontal Disease
12.1.7.1 Gingival Fibroblasts and Periodontal Ligament Cells
Gingival fibroblasts and periodontal ligament cells constitute the main cellular component of the gingiva and periodontal ligament, respectively, and are responsible for maintenance of tissue integrity. Both cell types can be considered as multipotent cells, possessing an osteogenic potential (Kook et al. 2009; Lee et al. 2009; Mostafa et al. 2011; Rodrigues et al. 2007), and hence may constitute a potential cellular source during bone wound healing. However, this osteogenic potential is diminished in the presence of tissue-degrading pro-inflammatory proteases, i.e., MMPs, during chronic inflammation (Hayami et al. 2008; Joseph et al. 2010).
12.1.7.2 Osteoblasts, Osteoclasts, and Osteocytes
Osteoblasts are derived from mesenchymal cells of the bone marrow, are lining the bone surfaces, and are responsible for bone formation. Osteoclasts are multinucleated monocyte/macrophage lineage cells of hematopoietic origin with the capacity to resorb bone and are found close to resorption lacunae (called Howship’s lacunae). Osteocytes are previous osteoblasts that became entrapped within the bone matrix and reside in lacunae connecting to each other via long cytoplasmic extensions. Osteocytes can regulate the activity of osteoclasts and osteoblasts, resulting in bone loss, through expression of RANKL and sclerostin; specifically, sclerostin expression reduces osteoblast differentiation (Kim et al. 2014, 2015).
Osteoblast and osteoclasts are controlling together bone remodeling and homeostasis, i.e., the lifelong constant coupling of bone formation and resorption (Fuller et al. 1998; Lacey et al. 1998; Quinn et al. 1998; Yasuda et al. 1998). As mentioned earlier, differentiation and activation of osteoclasts are regulated by the RANKL/RANK/OPG system in combination with M-CSF. In periodontitis, osteoclastogenesis is constantly upregulated due to a high RANKL expression by resident and immune cells. Indeed, RANKL levels have been shown to correlate well with the cathepsin K levels; cathepsin K is a protease expressed by osteoclasts and is responsible for degradation of the organic matrix of bone. In addition, LPS from periodontal pathogens seems to impair osteoblastic cell differentiation partially due to TNF-α upregulation (Kadono et al. 1999; Roberts et al. 2008; Tomomatsu et al. 2009; Wang et al. 2010).
12.1.7.3 Neutrophils
Neutrophils (syn. polymorphonuclear leukocytes) differentiate in the bone marrow before entering the blood flow and are the most abundant leukocytes in the blood, accounting for about two thirds of all blood leukocytes. This constant mature state of neutrophils in the blood circulation allows an immediate immune response whenever and wherever necessary; if bacterial invasion occurs, neutrophils can exit the blood flow and migrate into the affected tissue following a chemical gradient, i.e., chemotaxis. In the periodontium, neutrophils enter the tissues constantly from the terminal blood circulation and exit into the gingival crevicular fluid, where they form a “defense wall” against potential invaders.
Neutrophil involvement in the pathogenesis of periodontal disease may be considered as either hyporesponsive or as hyperresponsive. Hyporesponsive neutrophils (e.g., due to defects in chemotaxis, transendothelial migration, or phagocytosis) weaken the first host defense mechanism and the bacterial attack might overwhelm host immunity. In contrast, an intense response of hyperresponsive neutrophils not only degrades the microbial invaders but also causes collateral tissue damage. A hyperresponsive neutrophil response is characterized by excessive release of toxic products, an elevated oxidative burst, and increased secretion of degrading enzymes (e.g., MMPs) (Gustafsson et al. 2006; Nussbaum and Shapira 2011; Ryder 2010; Shaddox et al. 2010).
Further, neutrophils can interfere directly and indirectly with the bone metabolism, being a source of pro-inflammatory cytokines (e.g., IL-1, TNF-α) that further upregulate the immune response and cause an increased degradation of soft and mineralized periodontal tissue. Neutrophils also promote the adaptive immune response via chemotactic effects on Th17 cells, which in turn release high amounts of the pro-inflammatory cytokine IL-17 (Pelletier et al. 2010). In addition, activated neutrophils can express – but not secrete – on their cell surface RANKL; hence, in close proximity to osteoclast precursors, they can directly activate and promote osteoclastogenesis (Bloemen et al. 2010; Chakravarti et al. 2009).
12.1.7.4 Monocytes
Monocytes are myeloid cells of the hematopoietic system, which migrate from the bone marrow with the blood stream in a quite immature state that enables them to further differentiate according to given requirements. For example, monocytes can enter the tissue and differentiate into macrophages, which are typical phagocytosing cells. Macrophages can kill pathogens by producing antimicrobial substances (e.g., myeloperoxidase, reactive oxygen species, reactive nitrogen species) and can ingest, process, and present antigens to T cells. Further, macrophages are contributing to inflammatory tissue damage by secretion of pro-inflammatory cytokines (e.g., IL-1, TNF-α). Another possible differentiation pathway of monocytes – in the presence of M-CSF and RANKL – is into osteoclasts, and thereby they are also contributing to bone resorption (Bar-Shavit 2008; Faust et al. 1999; Massey and Flanagan 1999).
Interestingly, monocytes from periodontally diseased patients may present distinct differences comparing to mononuclear cells from periodontally healthy individuals. Specifically, in vitro studies suggest that a hyper-reactive phenotype may exist in periodontitis patients, which upon stimulation with bacteria or LPS releases increased amounts of pro-inflammatory cytokines and generates more oxygen radicals comparing to monocytes from healthy subjects (Gustafsson et al. 2006). Further, discrepancies in peripheral osteoclastogenesis have been reported, with monocytes from periodontitis patients spontaneously forming osteoclasts without stimulation with M-CSF and/or RANKL. Hence, it has been suggested that the priming of the osteoclast precursors in periodontally diseased patients may already take place – at least partly – in the peripheral blood (Brunetti et al. 2005; Tjoa et al. 2008).
12.1.7.5 Dendritic Cells
Peripheral dendritic cells are professional antigen processing and presenting cells; after antigen incorporation, they prime naive T cells in the lymph nodes (Wolff 1972), and this naive T cell stimulation is promoted in the presence of RANKL (Anderson et al. 1997). In the presence of RANKL and M-CSF, dendritic cells differentiate into mature osteoclasts, while in the absence of RANKL, they differentiate into mature dendritic cells and support the adaptive immunity by processing and presenting antigens. However, once differentiated into mature dendritic cells, they do not posses any longer their osteoclastogenic potential (Liu et al. 2010).
Langerhans cells are dendritic cells located above the basal layer of epithelial cells, e.g., in the skin and oral mucosa. Increased numbers of Langerhans cells have occasionally been observed in the gingiva of periodontitis patients, comparing to what observed in healthy individuals (Ford et al. 2010).
12.1.7.6 Natural Killer Cells
Natural killer cells are a subset of lymphocytes and play an important role in the innate immunity. Natural killer cells express primarily IFN-γ but exhibit additional antimicrobial action by expression of defensins, which are specific antimicrobial peptides. Interestingly, tissue infiltration with activated natural killer cells has been observed in AgP but not CP lesions (Nowak et al. 2013). It has been suggested that this difference is due to the differences among the infecting periodontal pathogens. Specifically, infection with A. actinomycetemcomitans, which is characteristically present in localized AgP, induces via dendritic cells activation of natural killer cells and subsequent IFN-γ expression, while infection with P. gingivalis, which is a key periodontal pathogen in CP, does not (Chalifour et al. 2004; Gonzales 2015; Kikuchi et al. 2004; Nowak et al. 2013).
12.1.7.7 T Cells
T cells are part of the adaptive immunity, derive from the hematopoietic stem cells in the bone marrow, but migrate and mature in the thymus. They can be subdivided based on the expression of the co-receptors CD8 or CD4. CD8 + T cells, also called cytotoxic T cells, control intracellular antigens (e.g., viruses) and may play a role in periodontitis in the presence of coinfection with herpes virus; recognition of herpes virus by the immune system induces a strong CD8+ T cell activation and mobilization (Gonzales 2015; Slots 2010).
CD4 + T cells, also called Th cells, primarily assist other cells of the immune system in their functions; for example, they assist in B cell maturation. CD4+ T cells differentiate further depending on the presence of specific cytokines (Mosmann et al. 1991); while high IL-12 levels favor Th1 cell development, high IL-4 levels promote Th2 cell differentiation. Th1 and Th2 cells can, in turn, be distinguished by differences in their cytokine expression pattern. Th1 cells secrete mainly IFN-γ, IL-2, IL-12, and TNF-α and initiate a cellular and pro-inflammatory immune response characterized by presence of phagocytosing cells (Gonzales 2015), while Th2 cells appear to have a more protective function by expressing IL-4, IL-5, IL-9, IL-10, and IL-13 and promoting B cell activation, differentiation, and antibody production (Appay et al. 2008; Cronstein 2007; Gonzales 2015; Murphy and Reiner 2002). In addition to Th1 and Th2 cells, Th17 and Treg cells have been detected in periodontally diseased tissues (Cardoso et al. 2008; Gaffen and Hajishengallis 2008; Gonzales 2015; Teng 2006b). Th17 cells differentiate in the presence of IL-23 and express IL-6 and mainly IL-17, which – as already described – exerts a strong pro-inflammatory immune response (Cardoso et al. 2008; Cheng et al. 2014; Ford et al. 2010; Kotake et al. 1999; Sato et al. 2006; Yu et al. 2007). In contrast, Treg develops in the presence of TGF-ß and IL-2 and they have a protective role by decreasing periodontal disease progression through expression of IL-10 and TGF-ß (Cardoso et al. 2008; Gonzales 2015).
12.1.7.8 B Cells
B cells belong to the adaptive immunity and they have two main functions: (a) they recognize antigens by using a high-affinity receptor and then process and present them to CD4+ and CD8+ T cells (Gonzales 2015) and (b) they produce and secret antibodies for opsonization of invading pathogens.
In the gingival tissue in periodontitis lesions, B and T cells are the predominant mononuclear cell types, with the number of B cells regularly exceeding that of T cells. B cells exert a dual role in periodontal disease progression; they have a major protective role through opsonization of periodontal pathogens, thus facilitating phagocytosis by neutrophils and macrophages, and through activation of the complement system (Guentsch et al. 2009; Teng 2006a). For example, A. actinomycetemcomitans can only be controlled by neutrophils after opsonization by IgG, while other opsonizing factors (e.g., C3b, LPS-binding proteins, other Ig isotypes) appear not effective against it (Guentsch et al. 2009; Teng 2006a). In a longitudinal human trial, patients with active periodontal lesions presented lower serum levels of IgG against A. actinomycetemcomitans and P. gingivalis compared to patients that were periodontally stable within a regular maintenance program (Rams et al. 2006). However, B cells appear to play also a major role in alveolar bone loss in periodontitis by expression of RANKL after stimulation by periodontal pathogens (Cochran 2008; Han et al. 2009; Han et al. 2006; Kawai et al. 2006); indeed, B cell deletion resulted in remarkably reduced alveolar bone loss (Baker et al. 2009). In addition, autoreactive B cells, that produce antibodies against host tissue components (e.g., collagen, keratin), have also been detected in tissue samples from periodontitis sites (Donati et al. 2009; Koutouzis et al. 2009).
Interestingly, mice severely affected by a combined immunodeficiency resulting in complete deletion of B and T cells experience less bone loss after challenge with P. gingivalis, comparing with non-immunodeficient controls, which indicates that lymphocytes may not be indispensable in infection control, but they play a major role in alveolar bone loss (Baker et al. 1999) (Table 12.2).
12.1.8 Periodontal Treatment
12.1.8.1 Standard Treatment
Cause-related periodontal treatment consists of oral hygiene instructions aiming at the efficient daily removal of the accumulating biofilm from the teeth surfaces and of the mechanical removal of the infectious agents already accumulated on the root surface, i.e., scaling and root planing. The mechanical removal of the biofilm can be combined with the administration of local or systemic use of chemotherapeutic agents (e.g., rinsing solutions based on chlorhexidine, hyaluronan, essential oils; antibiotics). In cases where nonsurgical treatment does not result in disease resolution, periodontal surgery is performed to achieve better access to the root surfaces for efficient mechanical instrumentation. After efficient control of the infection and establishment of inflammation-free tissues, regular maintenance appointments, including mechanical biofilm removal and reinforcement of oral hygiene attitudes, are essential for long-term stability of treatment and for prevention of disease recurrence (Bertl et al. 2015; Deas and Mealey 2010; Zandbergen et al. 2013).
12.1.8.2 “Osteoimmunological Targets” in Periodontal Treatment
Although it is nowadays recognized that the host immune response is the major cause for periodontal tissue destruction, routine periodontal treatment aims primarily to control the bacterial invasion. In periodontitis, unlike in systemic conditions like osteoporosis or rheumatoid arthritis, treatment aiming at interfering with the host’s immune system (e.g., by applying anti-inflammatory or antiresorptive agents) is associated with two major concerns: firstly, any (severe) side effects of systemically administered medications might not justify the possible – most likely small – additional benefit compared to what achieved with standard periodontal treatment alone and secondly any (partial) blocking of the immune system may result in inefficient immune response against the bacterial attack.
In the following, a brief overview of major new therapeutic targets considered in periodontitis treatment is discussed (for summary, see Table 12.3).
12.1.8.2.1 Doxycycline
Doxycycline delivered in subantimicrobial doses (e.g., 20 mg doxycycline twice daily for 3 months) has been shown to downregulate MMP activity and, more recently, also pro-inflammatory cytokine production, i.e., IL-1, IL-17, and TNF-α. A systematic review of the literature and meta-analysis have confirmed that there seems to be a significant improvement of clinical periodontal parameters after nonsurgical periodontal treatment with adjunctive delivery of doxycycline in subantimicrobial doses compared to nonsurgical treatment alone (Castro et al. 2016; Sgolastra et al. 2011).
12.1.8.2.2 Statins
Statins (e.g., simvastatin, atorvastatin) are widely used to control lipid metabolism by reducing serum cholesterol levels. Additionally, they appear to have anti-inflammatory and antiresorptive properties. Clinical trials have reported a significant positive effect of locally or systemically administered statins as adjunct to nonsurgical periodontal treatment, primarily in terms of posttreatment alveolar bone levels but also regarding the clinical periodontal parameters (Fajardo et al. 2010; Pradeep et al. 2012; Pradeep et al. 2013; Pradeep et al. 2015).
12.1.8.2.3 TNF-α Antagonists
TNF-α antagonists are used in the treatment of rheumatoid arthritis. Preclinical trials evaluating the effect of TNF-α antagonists in experimental periodontitis have shown reduced neutrophil infiltration and periodontal inflammation levels (Di Paola et al. 2007; Gonçalves et al. 2014). However, reduced neutrophil infiltration may impair pathogen clearance. For example, increased A. actinomycetemcomitans load and inflammatory marker levels have been detected in a TNF-α knockout mouse model; in particular, knockout mice presented reduced activation, migration, and phagocytosing activity of neutrophils and macrophages in periodontal lesions (Garlet et al. 2007). In a case-control study, however, patients with rheumatoid arthritis taking TNF-α-antagonists presented better periodontal indices and lower gingival crevicular fluid levels of TNF-α, compared to patients taking another medication (Mayer et al. 2009).
12.1.8.2.4 Lipid Mediators
Pro-resolving lipid mediators, i.e., lipoxins, resolvins, and protectins, as adjunct to periodontal therapy, would theoretically reduce the amount of tissue degradation by actively promoting inflammation resolution. Indeed, the extent of tissue breakdown during experimental periodontitis and disease resolution was significantly improved in preclinical trials involving local application of resolvins (Hasturk et al. 2007; Van Dyke et al. 2015).
12.1.8.2.5 OPG
Systemic and local administrations of OPG or OPG analogues in experimental periodontitis models have shown significant alveolar bone loss prevention, due to blocking RANKL binding to RANK and, hence, interfering with the process of osteoclastogenesis (Jin et al. 2007; Lin et al. 2011; Tang et al. 2015; Teng et al. 2000). However, systemic long-term administration of a RANKL inhibitor may entail unwanted systemic side effects on physiologic bone turnover and on the immune system, considering the fact that RANK is not only expressed on osteoclasts and their precursors but also on monocytes/macrophages and dendritic cells (Ferrari-Lacraz and Ferrari 2011; Kong et al. 1999).
12.1.8.2.6 Sclerostin Antibody
In a preclinical trial in rats, administration of a sclerostin antibody significantly improved bone healing in periodontal defects, by blocking the inhibitory effect of sclerostin on osteogenesis (Taut et al. 2013).
12.1.8.2.7 Bisphosphonates
Bisphosphonates reduce osteoclast activity and thereby the amount of bone resorption and are regularly used in the treatment of osteoporosis or tumor-associated osteolysis. Systemic application of bisphosphonates appears not applicable in periodontitis treatment, due to the interference with the physiologic bone turnover, but also because it might cause severe side effects, i.e., bisphosphonate-related osteonecrosis of the jaw; in fact, periodontitis itself appears as risk factor for developing bisphosphonate-related osteonecrosis of the jaw. Yet, it has been demonstrated in preclinical trials of experimental periodontitis models that local administration of bisphosphonates results in reduced alveolar bone destruction and in enhanced alveolar bone regeneration (De Almeida et al. 2015; Furlaneto et al. 2014; Thumbigere-Math et al. 2014).
12.1.8.2.8 Vitamin D and Calcium
Vitamin D plays important role in bone homeostasis and also exerts additional immune regulatory properties; for instance, vitamin D deficiency is associated with reduced calcium levels in the bone and bone volume loss. Indeed, a moderate positive effect of vitamin D and calcium supplement intake has been described in cross-sectional and cohort studies (Garcia et al. 2011; Miley et al. 2009). However, there are currently no randomized controlled clinical trials on vitamin D and calcium supplementation during nonsurgical periodontal treatment.
12.1.8.2.9 Cathepsin K Inhibitors
Cathepsin K inhibitors are considered as promising drugs in osteoporosis treatment. Recently, inhibition of cathepsin K was shown to reduce the extent of the immune response and of alveolar bone loss in experimental periodontitis in mice (Bartold et al. 2010; Hao et al. 2015).
12.1.8.2.10 iNOS Inhibitors
iNOS inhibitors (e.g., mercaptoethyl guanidine, aminoguanidine) are shown in vitro to reduce NO production by human gingival fibroblasts, while, in an experimental periodontitis model, they decreased the level of periodontal inflammation and bone loss (Chang et al. 2014; Daghigh et al. 2002; Di Paola et al. 2004; Lohinai et al. 1998).
12.1.8.2.11 Mitogen-Activated Protein Kinase
Mitogen-activated protein kinases are intracellular molecules involved in signal transduction during inflammation and production of pro-inflammatory cytokines. Administration of mitogen-activated protein kinases inhibitors in an experimental periodontitis model reduced the inflammatory response and thereby also the alveolar bone loss (Kirkwood et al. 2007; Rogers et al. 2007).
12.1.8.2.12 Melatonin
Melatonin, a hormone primary involved in the control of the circadian rhythm, acts also as an antioxidant, immune modulator, and free radical scavenger. It has been demonstrated that the levels of salivary melatonin are decreased in periodontal disease, while systemic melatonin administration during experimental periodontitis reduced the level of pro-inflammatory cytokines and the extent of alveolar bone destruction (Arabacı et al. 2015; Bertl et al. 2013; Kara et al. 2013).
12.1.8.2.13 Angiotensin II Receptor Blockers
Angiotensin II receptor blockers (e.g., azilsartan, olmesartan, telmisartan) exert an anti-inflammatory effect by suppressing TNF-α-induced IL-6 gene promoter activity. In an experimental periodontitis model, angiotensin II receptor blocker administration resulted in reduced tissue degradation, including reduced levels of MMPs and RANKL, increased levels of OPG, reduced levels of IL-1, TNF-α, and increased levels IL-10 cytokines (Araújo et al. 2013a, b, 2014) compared to controls (Table 12.3).
12.1.9 Summary
Periodontal disease is initiated by oral pathogens that provoke a response from the immune system, which exerts a two-sided effect: on one side, it controls the infection and protects the organism from bacterial invasion, but on the other side, it causes collateral tissue destruction within the periodontium. This dual effect is reflected in the often two-sided duties of cells and mediators involved in periodontitis pathogenesis. Interestingly, it appears that the intensity of the immune response is not the main relevant factor for periodontal infection control but is decisive for the extent of periodontal tissue destruction. Thus, the stronger the immune response is, the larger the damage caused in the periodontal tissues (Trombone et al. 2009). In contrast, a deficient immune response also leads to increased tissue damage due to failure in controlling the infection. The increased knowledge on the interactions between cells of the immune system and the resident cells in the periodontal tissues and on the involved enzymes and cytokines offers new exciting targets for the treatment of periodontal disease; yet, evaluation of most of these targets is still on the preclinical level (Fig. 12.6).
Abbreviations
- AgP:
-
Aggressive periodontitis
- C3:
-
Complement component 3
- CP:
-
Chronic periodontitis
- IFN:
-
Interferon
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- iNOS:
-
Inducible NO-synthases
- LPS:
-
Lipopolysaccharide
- M-CSF:
-
Macrophage colony-stimulating factor
- MMP:
-
Matrix metalloproteinases
- NO:
-
Nitric oxide
- OPG:
-
Osteoprotegerin
- RANK:
-
Receptor activator of NF-kB
- RANKL:
-
Receptor activator of nuclear factor “kappa-light-chain-enhancer” of the activated B cell ligand
- TGF:
-
Transforming growth factor
- Th:
-
T helper cells
- TIMP:
-
Tissue inhibitors of metalloproteinase
- TLR:
-
Toll-like receptors
- TNF:
-
Tumor necrosis factor
- Treg:
-
Regulatory T cells
References
Abbas AK, Murphy KM, Sher A (1996) Functional diversity of helper T lymphocytes. Nature 383:787–793
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Alayan J, Ivanovski S, Gemmell E, Ford P, Hamlet S, Farah CS (2006) Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol Immunol 21:360–365
Anderson DM, Maraskovsky E, Billingsley WL et al (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179
Appay V, van Lier RA, Sallusto F, Roederer M (2008) Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73:975–983
Arabacı T, Kermen E, Özkanlar S et al (2015) Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: a biochemical and immunohistochemical study. J Periodontol 86:874–881
Araújo AA, Lopes de Souza G, Souza TO et al (2013a) Olmesartan decreases IL-1β and TNF-α levels; downregulates MMP-2, MMP-9, COX-2, and RANKL; and upregulates OPG in experimental periodontitis. Naunyn Schmiedebergs Arch Pharmacol 386:875–884
Araújo AA, Souza TO, Moura LM et al (2013b) Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J Clin Periodontol 40:1104–1111
Araújo AA, Varela H, Brito GA et al (2014) Azilsartan increases levels of IL-10, down-regulates MMP-2, MMP-9, RANKL/RANK, Cathepsin K and up-regulates OPG in an experimental periodontitis model. PLoS One 9, e96750
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6
Armitage GC, Cullinan MP (2010) Comparison of the clinical features of chronic and aggressive periodontitis. Periodontol 2000 53:12–27
Aurer A, Aleksic J, Ivic-Kardum M, Aurer J, Culo F (2001) Nitric oxide synthesis is decreased in periodontitis. J Clin Periodontol 28:565–568
Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC (1999) CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun 67:2804–2809
Baker PJ, Boutaugh NR, Tiffany M, Roopenian DC (2009) B cell IgD deletion prevents Alveolar bone loss following murine oral infection. Interdiscip Perspect Infect Dis 2009:864359
Banu S, Jabir NR, Mohan R et al (2015) Correlation of Toll-like receptor 4, interleukin-18, transaminases, and uric acid in patients with chronic periodontitis and healthy adults. J Periodontol 86:431–439
Bar-Shavit Z (2008) Taking a toll on the bones: regulation of bone metabolism by innate immune regulators. Autoimmunity 41:195–203
Bartold PM, Cantley MD, Haynes DR (2010) Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000 53:55–69
Beklen A, Sorsa T, Konttinen YT (2009) Toll-like receptors 2 and 5 in human gingival epithelial cells co-operate with T-cell cytokine interleukin-17. Oral Microbiol Immunol 24:38–42
Belibasakis GN, Bostanci N, Hashim A et al (2007) Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by Porphyromonas gingivalis: a putative role of the Arg-gingipains. Microb Pathog 43:46–53
Bertl K, Schoiber A, Haririan H et al (2013) Non-surgical periodontal therapy influences salivary melatonin levels. Clin Oral Investig 17:1219–1225
Bertl K, Bruckmann C, Isberg PE, Klinge B, Gotfredsen K, Stavropoulos A (2015) Hyaluronan in non-surgical and surgical periodontal therapy: a systematic review. J Clin Periodontol 42:236–246
Bickel M (1993) The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol 64:456–460
Bloemen V, Schoenmaker T, de Vries TJ, Everts V (2010) Direct cell-cell contact between periodontal ligament fibroblasts and osteoclast precursors synergistically increases the expression of genes related to osteoclastogenesis. J Cell Physiol 222:565–573
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Bradshaw DJ, Marsh PD, Watson GK, Allison C (1998) Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect Immun 66:4729–4732
Brennan PA, Thomas GJ, Langdon JD (2003) The role of nitric oxide in oral diseases. Arch Oral Biol 48:93–100
Brook I (2003) Microbiology and management of periodontal infections. Gen Dent 51:424–428
Brunetti G, Colucci S, Pignataro P et al (2005) T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J Periodontol 76:1675–1680
Cardoso CR, Garlet GP, Moreira AP, Junior WM, Rossi MA, Silva JS (2008) Characterization of CD4 + CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol 84:311–318
Castro ML, Franco GC, Branco-de-Almeida LS et al (2016) Down-Regulation of protease activated receptor 2, interleukin-17 and other pro-inflammatory genes by subantimicrobial doxycycline dose in a rat periodontitis model. J Periodontol 87:203–210
Chakravarti A, Raquil MA, Tessier P, Poubelle PE (2009) Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood 114:1633–1644
Chalifour A, Jeannin P, Gauchat JF et al (2004) Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104:1778–1783
Chang PC, Chong LY, Tsai SC, Lim LP (2014) Aminoguanidine inhibits the AGE-RAGE axis to modulate the induction of periodontitis but has limited effects on the progression and recovery of experimental periodontitis: a preliminary study. J Periodontol 85:729–739
Cheng WC, Hughes FJ, Taams LS (2014) The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol 41:541–549
Claudino M, Trombone AP, Cardoso CR et al (2008) The broad effects of the functional IL-10 promoter-592 polymorphism: modulation of IL-10, TIMP-3, and OPG expression and their association with periodontal disease outcome. J Leukoc Biol 84:1565–1573
Claudino M, Garlet TP, Cardoso CR et al (2010) Down-regulation of expression of osteoblast and osteocyte markers in periodontal tissues associated with the spontaneous alveolar bone loss of interleukin-10 knockout mice. Eur J Oral Sci 118:19–28
Cochran DL (2008) Inflammation and bone loss in periodontal disease. J Periodontol 79:1569–1576
Cronstein BN (2007) Interleukin-6--a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis 65(Suppl 1):S11–S15
Crotti T, Smith MD, Hirsch R et al (2003) Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res 38:380–387
Daghigh F, Borghaei RC, Thornton RD, Bee JH (2002) Human gingival fibroblasts produce nitric oxide in response to proinflammatory cytokines. J Periodontol 73:392–400
Damgaard C, Holmstrup P, Van Dyke TE, Nielsen CH (2015) The complement system and its role in the pathogenesis of periodontitis: current concepts. J Periodontal Res 50:283–293
De Almeida J, Ervolino E, Bonfietti LH et al (2015) Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. J Periodontol 86:1166–1175
de Vries TJ, Yousovich J, Schoenmaker T, Scheres N, Everts V (2016) Tumor necrosis factor-α antagonist infliximab inhibits osteoclast formation of peripheral blood mononuclear cells but does not affect periodontal ligament fibroblast-mediated osteoclast formation. J Periodontal Res 51:186–195
de Waal Malefyt R, Figdor CG, Huijbens R et al (1993) Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol 151:6370–6381
Deas DE, Mealey BL (2010) Response of chronic and aggressive periodontitis to treatment. Periodontol 2000 53:154–166
Di Paola R, Marzocco S, Mazzon E et al (2004) Effect of aminoguanidine in ligature-induced periodontitis in rats. J Dent Res 83:343–348
Di Paola R, Mazzon E, Muià C et al (2007) Effects of etanercept, a tumour necrosis factor-alpha antagonist, in an experimental model of periodontitis in rats. Br J Pharmacol 150:286–297
Donati M, Liljenberg B, Zitzmann NU, Berglundh T (2009) B-1a cells and plasma cells in periodontitis lesions. J Periodontal Res 44:683–688
Duarte PM, de Oliveira MC, Tambeli CH, Parada CA, Casati MZ, Nociti FH (2007) Overexpression of interleukin-1beta and interleukin-6 may play an important role in periodontal breakdown in type 2 diabetic patients. J Periodontal Res 42:377–381
Ebersole JL, Schuster JL, Stevens J et al (2013) Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J Clin Immunol 33:271–279
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ (2012) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83:1449–1454
Ertugrul AS, Sahin H, Dikilitas A, Alpaslan N, Bozoglan A (2013) Comparison of CCL28, interleukin-8, interleukin-1β and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J Periodontal Res 48:44–51
Fajardo ME, Rocha ML, Sánchez-Marin FJ, Espinosa-Chávez EJ (2010) Effect of atorvastatin on chronic periodontitis: a randomized pilot study. J Clin Periodontol 37:1016–1022
Faust J, Lacey DL, Hunt P et al (1999) Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem 72:67–80
Ferrari-Lacraz S, Ferrari S (2011) Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int 22:435–46
Flemmig TF (1999) Periodontitis. Ann Periodontol 4:32–38
Ford PJ, Gamonal J, Seymour GJ (2010) Immunological differences and similarities between chronic periodontitis and aggressive periodontitis. Periodontol 2000 53:111–123
Foster JS, Kolenbrander PE (2004) Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Appl Environ Microbiol 70:4340–4348
Fukada SY, Silva TA, Saconato IF et al (2008) iNOS-derived nitric oxide modulates infection-stimulated bone loss. J Dent Res 87:1155–1159
Fuller K, Wong B, Fox S, Choi Y, Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188:997–1001
Furlaneto FA, Nunes NL, Oliveira Filho IL et al (2014) Effects of locally administered tiludronic acid on experimental periodontitis in rats. J Periodontol 85:1291–1301
Gaffen SL, Hajishengallis G (2008) A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res 87:817–828
Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A (2000) Levels of interleukin-1 beta, −8, and −10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71:1535–1545
Gao Y, Grassi F, Ryan MR et al (2007) IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest 117:122–132
Garcia MN, Hildebolt CF, Miley DD et al (2011) One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 82:25–32
Garlet GP (2010) Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349–1363
Garlet GP, Martins WJ, Ferreira BR, Milanezi CM, Silva JS (2003) Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res 38:210–217
Garlet GP, Martins WJ, Fonseca BA, Ferreira BR, Silva JS (2004) Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol 31:671–679
Garlet GP, Cardoso CR, Silva TA et al (2006) Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol 21:12–20
Garlet GP, Cardoso CR, Campanelli AP et al (2007) The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immunol 147:128–138
Garlet GP, Cardoso CR, Campanelli AP et al (2008) The essential role of IFN-gamma in the control of lethal Aggregatibacter actinomycetemcomitans infection in mice. Microbes Infect 10:489–496
Gemmell E, Seymour GJ (1994) Cytokines and T cell switching. Crit Rev Oral Biol Med 5:249–279
Genco RJ, Borgnakke WS (2013) Risk factors for periodontal disease. Periodontol 2000 62:59–94
Giannobile WV (2008) Host-response therapeutics for periodontal diseases. J Periodontol 79:1592–1600
Gonçalves DC, Evangelista RC, da Silva RR et al (2014) Infliximab attenuates inflammatory osteolysis in a model of periodontitis in Wistar rats. Exp Biol Med (Maywood) 239:442–453
Gonzales JR (2015) T- and B-cell subsets in periodontitis. Periodontol 2000 69:181–200
Graves D (2008) Cytokines that promote periodontal tissue destruction. J Periodontol 79:1585–1591
Graves DT, Cochran D (2003) The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol 74:391–401
Graves DT, Li J, Cochran DL (2011) Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res 90:143–153
Guentsch A, Puklo M, Preshaw PM et al (2009) Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Periodontal Res 44:368–377
Gursoy UK, Könönen E, Uitto VJ et al (2009) Salivary interleukin-1beta concentration and the presence of multiple pathogens in periodontitis. J Clin Periodontol 36:922–927
Gursoy UK, Könönen E, Huumonen S et al (2013) Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J Clin Periodontol 40:18–25
Gustafsson A, Ito H, Asman B, Bergstrom K (2006) Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J Clin Periodontol 33:126–129
Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35:3–11
Hajishengallis G, Lamont RJ (2012) Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 27:409–419
Hajishengallis G, Liang S, Payne MA et al (2011) Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10:497–506
Hajishengallis G, Darveau RP, Curtis MA (2012) The keystone-pathogen hypothesis. Nat Rev Microbiol 10:717–725
Han X, Kawai T, Eastcott JW, Taubman MA (2006) Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol 176:625–631
Han X, Lin X, Seliger AR, Eastcott J, Kawai T, Taubman MA (2009) Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria. Oral Microbiol Immunol 24:190–196
Hao L, Chen J, Zhu Z et al (2015) Odanacatib, a cathepsin K-Specific inhibitor, inhibits inflammation and bone loss caused by periodontal diseases. J Periodontol 86:972–983
Hasturk H, Kantarci A, Goguet-Surmenian E et al (2007) Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 179:7021–7029
Hayami T, Kapila YL, Kapila S (2008) MMP-1 (collagenase-1) and MMP-13 (collagenase-3) differentially regulate markers of osteoblastic differentiation in osteogenic cells. Matrix Biol 27:682–692
Himani GS, Prabhuji ML, Karthikeyan BV (2014) Gingival crevicular fluid and interleukin-23 concentration in systemically healthy subjects: their relationship in periodontal health and disease. J Periodontal Res 49:237–245
Holt SC, Ebersole JL (2005) Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122
Holtfreter B, Kocher T, Hoffmann T, Desvarieux M, Micheelis W (2010) Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV). J Clin Periodontol 37:211–219
Honda T, Domon H, Okui T, Kajita K, Amanuma R, Yamazaki K (2006) Balance of inflammatory response in stable gingivitis and progressive periodontitis lesions. Clin Exp Immunol 144:35–40
Ihn H, Yamane K, Asano Y, Kubo M, Tamaki K (2002) IL-4 up-regulates the expression of tissue inhibitor of metalloproteinase-2 in dermal fibroblasts via the p38 mitogen-activated protein kinase dependent pathway. J Immunol 168:1895–1902
Irwin CR, Myrillas TT (1998) The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis 4:43–47
Isaza-Guzmán DM, Cardona-Vélez N, Gaviria-Correa DE, Martínez-Pabón MC, Castaño-Granada MC, Tobón-Arroyave SI (2015) Association study between salivary levels of interferon (IFN)-gamma, interleukin (IL)-17, IL-21, and IL-22 with chronic periodontitis. Arch Oral Biol 60:91–99
Jarnicki AG, Fallon PG (2003) T helper type-2 cytokine responses: potential therapeutic targets. Curr Opin Pharmacol 3:449–455
Jenkinson HF, Lamont RJ (2005) Oral microbial communities in sickness and in health. Trends Microbiol 13:589–595
Ji JD, Park-Min KH, Shen Z et al (2009) Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-gamma in human osteoclast precursors. J Immunol 183:7223–7233
Jin Q, Cirelli JA, Park CH et al (2007) RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol 78:1300–1308
Johnson RB, Serio FG (2007) The contribution of interleukin-13 and −15 to the cytokine network within normal and diseased gingiva. J Periodontol 78:691–695
Joseph J, Kapila YL, Hayami T, Kapila S (2010) Disease-associated extracellular matrix suppresses osteoblastic differentiation of human periodontal ligament cells via MMP-1. Calcif Tissue Int 86:154–162
Jovanovic DV, Di Battista JA, Martel-Pelletier J et al (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 160:3513–3521
Kadono H, Kido J, Kataoka M, Yamauchi N, Nagata T (1999) Inhibition of osteoblastic cell differentiation by lipopolysaccharide extract from Porphyromonas gingivalis. Infect Immun 67:2841–2846
Kara A, Akman S, Ozkanlar S et al (2013) Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic Biol Med 55:21–26
Kawai T, Matsuyama T, Hosokawa Y et al (2006) B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169:987–998
Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ (2007) Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol Immunol 22:145–151
Kikuchi T, Hahn CL, Tanaka S, Barbour SE, Schenkein HA, Tew JG (2004) Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gamma interferon responses by natural killer cells. Infect Immun 72:5089–5096
Kim JH, Lee DE, Cha JH, Bak EJ, Yoo YJ (2014) Receptor activator of nuclear factor-kB ligand and sclerostin expression in osteocytes of alveolar bone in rats with ligature-induced periodontitis. J Periodontol 85:e370–e378
Kim JH, Lee DE, Woo GH, Cha JH, Bak EJ, Yoo YJ (2015) Osteocytic sclerostin expression in alveolar bone in rats with diabetes mellitus and ligature-induced periodontitis. J Periodontol 86:1005–1011
Kirkwood KL, Li F, Rogers JE et al (2007) A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther 320:56–63
Klein DC, Raisz LG (1970) Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology 86:1436–1440
Kong YY, Yoshida H, Sarosi I et al (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Kook YA, Lee SK, Son DH et al (2009) Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol Int 33:424–428
Kotake S, Udagawa N, Takahashi N et al (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Koutouzis T, Haber D, Shaddox L, Aukhil I, Wallet SM (2009) Autoreactivity of serum immunoglobulin to periodontal tissue components: a pilot study. J Periodontol 80:625–633
Kulkarni C, Kinane DF (2014) Host response in aggressive periodontitis. Periodontol 2000 65:79–91
Kuziel WA, Greene WC (1990) Interleukin-2 and the IL-2 receptor: new insights into structure and function. J Invest Dermatol 94:27S–32S
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Lacey DC, Simmons PJ, Graves SE, Hamilton JA (2009) Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage 17:735–742
Lappin DF, Kjeldsen M, Sander L, Kinane DF (2000) Inducible nitric oxide synthase expression in periodontitis. J Periodontal Res 35:369–373
Lee HJ, Pi SH, Kim Y et al (2009) Effects of nicotine on antioxidant defense enzymes and RANKL expression in human periodontal ligament cells. J Periodontol 80:1281–1288
Leppilahti JM, Hernández-Ríos PA, Gamonal JA et al (2014) Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J Clin Periodontol 41:348–356
Lin X, Han X, Kawai T, Taubman MA (2011) Antibody to RANKL Ameliorates T cell-mediated Periodontal Bone Resorption. Infect Immun 79:911–7
Listgarten MA (1994) The structure of dental plaque. Periodontol 2000 5:52–65
Liu YC, Lerner UH, Teng YT (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 52:163–206
Loe H, Theilade E, Jensen SB (1965) Experimental gingivitis in man. J Periodontol 36:177–187
Lohinai Z, Benedek P, Feher E et al (1998) Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br J Pharmacol 123:353–360
Malcolm J, Awang RA, Oliver-Bell J et al (2015) IL-33 exacerbates periodontal disease through induction of RANKL. J Dent Res 94:968–975
Massey HM, Flanagan AM (1999) Human osteoclasts derive from CD14-positive monocytes. Br J Haematol 106:167–170
Matejka M, Partyka L, Ulm C, Solar P, Sinzinger H (1998) Nitric oxide synthesis is increased in periodontal disease. J Periodontal Res 33:517–518
Mayer Y, Balbir-Gurman A, Machtei EE (2009) Anti-tumor necrosis factor-alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol 80:1414–1420
Menezes R, Garlet TP, Letra A et al (2008) Differential patterns of receptor activator of nuclear factor kappa B ligand/osteoprotegerin expression in human periapical granulomas: possible association with progressive or stable nature of the lesions. J Endod 34:932–938
Miley DD, Garcia MN, Hildebolt CF et al (2009) Cross-sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol 80:1433–1439
Mize TW, Sundararaj KP, Leite RS, Huang Y (2015) Increased and correlated expression of connective tissue growth factor and transforming growth factor beta 1 in surgically removed periodontal tissues with chronic periodontitis. J Periodontal Res 50:315–319
Mogi M, Otogoto J, Ota N, Inagaki H, Minami M, Kojima K (1999) Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-alpha in gingival crevicular fluid from human periodontal disease. Arch Oral Biol 44:535–539
Mosmann TR, Schumacher JH, Street NF et al (1991) Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev 123:209–229
Mostafa NZ, Uludağ H, Varkey M, Dederich DN, Doschak MR, El-Bialy TH (2011) In vitro osteogenic induction of human gingival fibroblasts for bone regeneration. Open Dent J 5:139–145
Moussion C, Ortega N, Girard JP (2008) The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’. PLoS One 3, e3331
Murphy KM, Reiner SL (2002) The lineage decisions of helper T cells. Nat Rev Immunol 2:933–944
Naundorf S, Schroder M, Hoflich C, Suman N, Volk HD, Grutz G (2009) IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur J Immunol 39:1066–1077
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR (1992) DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A 89:3030–3034
Nile CJ, Barksby E, Jitprasertwong P, Preshaw PM, Taylor JJ (2010) Expression and regulation of interleukin-33 in human monocytes. Immunology 130:172–180
Nowak M, Krämer B, Haupt M et al (2013) Activation of invariant NK T cells in periodontitis lesions. J Immunol 190:2282–2291
Nussbaum G, Shapira L (2011) How has neutrophil research improved our understanding of periodontal pathogenesis. J Clin Periodontol 38(Suppl 11):49–59
Offenbacher S, Odle BM, Van Dyke TE (1986) The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res 21:101–112
Ozçaka O, Nalbantsoy A, Buduneli N (2011) Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res 46:592–598
Page RC, Eke PI (2007) Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78:1387–1399
Page RC, Kornman KS (1997) The pathogenesis of human periodontitis: an introduction. Periodontol 2000 14:9–11
Park AY, Scott P (2001) Il-12: keeping cell-mediated immunity alive. Scand J Immunol 53:529–532
Pelletier M, Maggi L, Micheletti A et al (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115:335–343
Pradeep AR, Roopa Y, Swati PP (2008) Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Periodontal Res 43:712–716
Pradeep AR, Priyanka N, Kalra N, Naik SB, Singh SP, Martande S (2012) Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with Class II furcation defects: a randomized controlled clinical trial. J Periodontol 83:1472–1479
Pradeep AR, Kumari M, Rao NS, Martande SS, Naik SB (2013) Clinical efficacy of subgingivally delivered 1.2% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 84:871–879
Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Guruprasad CN, Kumaraswamy KM (2015) Efficacy of locally delivered 1.2% rosuvastatin gel to non-surgical treatment of patients with chronic periodontitis: a randomized, placebo-controlled clinical trial. J Periodontol 86:738–745
Quinn JM, Elliott J, Gillespie MT, Martin TJ (1998) A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology 139:4424–4427
Rams TE, Listgarten MA, Slots J (2006) Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J Periodontal Res 41:228–234
Ritz HL (1967) Microbial population shifts in developing human dental plaque. Arch Oral Biol 12:1561–1568
Roberts HC, Moseley R, Sloan AJ, Youde SJ, Waddington RJ (2008) Lipopolysaccharide alters decorin and biglycan synthesis in rat alveolar bone osteoblasts: consequences for bone repair during periodontal disease. Eur J Oral Sci 116:207–216
Rodrigues TL, Marchesan JT, Coletta RD et al (2007) Effects of enamel matrix derivative and transforming growth factor-beta1 on human periodontal ligament fibroblasts. J Clin Periodontol 34:514–522
Rogers JE, Li F, Coatney DD et al (2007) A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol 78:1992–1998
Ryder MI (2010) Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000 53:124–137
Saidenberg-Kermanac’h N, Bessis N, Lemeiter D, de Vernejoul MC, Boissier MC, Cohen-Solal M (2004) Interleukin-4 cellular gene therapy and osteoprotegerin decrease inflammation-associated bone resorption in collagen-induced arthritis. J Clin Immunol 24:370–378
Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P (2004) The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res 39:432–441
Sato K, Suematsu A, Okamoto K et al (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682
Schröder HE, Lindhe J (1981) Conditions and pathological features of rapidly destructive, experimental periodontitis in dogs. J Periodontol 51:6–19
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361
Sgolastra F, Petrucci A, Gatto R, Giannoni M, Monaco A (2011) Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis. J Periodontol 82:1570–1581
Shaddox L, Wiedey J, Bimstein E et al (2010) Hyper-responsive phenotype in localized aggressive periodontitis. J Dent Res 89:143–148
Slots J (2010) Human viruses in periodontitis. Periodontol 2000 53:89–110
Stabholz A, Soskolne WA, Shapira L (2010) Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000 53:138–153
Steinsvoll S, Halstensen TS, Schenck K (1999) Extensive expression of TGF-beta1 in chronically-inflamed periodontal tissue. J Clin Periodontol 26:366–373
Tang H, Mattheos N, Yao Y, Jia Y, Ma L, Gong P (2015) In vivo osteoprotegerin gene therapy preventing bone loss induced by periodontitis. J Periodontal Res 50:434–443
Taut AD, Jin Q, Chung JH et al (2013) Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 28:2347–2356
Teles RP, Likhari V, Socransky SS, Haffajee AD (2009) Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res 44:411–417
Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A (2013) Lessons learned and unlearned in periodontal microbiology. Periodontol 2000 62:95–162
Teng YT (2006a) Protective and destructive immunity in the periodontium: Part 1--innate and humoral immunity and the periodontium. J Dent Res 85:198–208
Teng YT (2006b) Protective and destructive immunity in the periodontium: Part 2--T-cell-mediated immunity in the periodontium. J Dent Res 85:209–219
Teng YT, Nguyen H, Gao X et al (2000) Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest 106:R59–R67
Teng YT, Mahamed D, Singh B (2005) Gamma interferon positively modulates Actinobacillus actinomycetemcomitans-specific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun 73:3453–3461
Thumbigere-Math V, Michalowicz BS, Hodges JS et al (2014) Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J Periodontol 85:226–233
Thunell DH, Tymkiw KD, Johnson GK et al (2010) A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res 45:148–152
Tjoa ST, de Vries TJ, Schoenmaker T, Kelder A, Loos BG, Everts V (2008) Formation of osteoclast-like cells from peripheral blood of periodontitis patients occurs without supplementation of macrophage colony-stimulating factor. J Clin Periodontol 35:568–575
Tomomatsu N, Aoki K, Alles N et al (2009) LPS-induced inhibition of osteogenesis is TNF-alpha dependent in a murine tooth extraction model. J Bone Miner Res 24:1770–1781
Tonetti MS, Mombelli A (1999) Early-onset periodontitis. Ann Periodontol 4:39–53
Trombone AP, Ferreira SBJ, Raimundo FM et al (2009) Experimental periodontitis in mice selected for maximal or minimal inflammatory reactions: increased inflammatory immune responsiveness drives increased alveolar bone loss without enhancing the control of periodontal infection. J Periodontal Res 44:443–451
Tymkiw KD, Thunell DH, Johnson GK et al (2011) Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol 38:219–228
Ugar-Cankal D, Ozmeric N (2006) A multifaceted molecule, nitric oxide in oral and periodontal diseases. Clin Chim Acta 366:90–100
Van Dyke TE, Hasturk H, Kantarci A et al (2015) Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res 94:148–156
Verstappen J, Von den Hoff JW (2006) Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. J Dent Res 85:1074–1084
Wang YH, Jiang J, Zhu Q et al (2010) Porphyromonas gingivalis lipids inhibit osteoblastic differentiation and function. Infect Immun 78:3726–3735
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL (2005) IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115:282–290
Wolff K (1972) The langerhans cell. Curr Probl Dermatol 4:79–145
Yasuda H, Shima N, Nakagawa N et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Yu JJ, Ruddy MJ, Wong GC et al (2007) An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109:3794–3802
Zandbergen D, Slot DE, Cobb CM, Van der Weijden FA (2013) The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: a systematic review. J Periodontol 84:332–351
Zhang X, Teng YT (2006) Interleukin-10 inhibits gram-negative-microbe-specific human receptor activator of NF-kappaB ligand-positive CD4 + −Th1-cell-associated alveolar bone loss in vivo. Infect Immun 74:4927–4931
Zhong Y, Slade GD, Beck JD, Offenbacher S (2007) Gingival crevicular fluid interleukin-1beta, prostaglandin E2 and periodontal status in a community population. J Clin Periodontol 34:285–293
Zhong Q, Ding C, Wang M, Sun Y, Xu Y (2012) Interleukin-10 gene polymorphisms and chronic/aggressive periodontitis susceptibility: a meta-analysis based on 14 case–control studies. Cytokine 60:47–54
Zwerina J, Hayer S, Tohidast-Akrad M et al (2004) Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum 50:277–290
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bertl, K., Pietschmann, P., Stavropoulos, A. (2016). Osteoimmunological Aspects of Periodontal Diseases. In: Pietschmann, P. (eds) Principles of Osteoimmunology. Springer, Cham. https://doi.org/10.1007/978-3-319-34238-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-34238-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-34236-8
Online ISBN: 978-3-319-34238-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)