Abstract
Mast cells (MCs) are resident inflammatory cells positioned in the proximity of vascular basal lamina in the cerebral cortex, meninges, and certain deep cerebral structures. MCs are most commonly regarded as key effectors in the pathogenesis of allergic diseases and anaphylaxis, but have been linked to a number of chronic diseases with an autoimmune component in their pathophysiology. MCs produce effects by rapid release or de novo synthesis of various cytokines such as TNF-α, potent proteases such as chymase and tryptase, chemokines, vasoactive substances such as histamine and eicosanoids, and regulators of hemostasis such as heparin and tissue plasminogen activator. They also produce and release matrix metalloproteases. This chapter will review recent experimental research suggesting that MCs and their nominal responses increasing the permeability of the vascular wall play a significant, deleterious role following acute cerebral ischemia. Inhibition of MC effects by pharmacological agents or gene manipulation has been demonstrated to alleviate postischemic blood–brain barrier (BBB) failure, expansive edema, inflammation, neuroinjury, and neurologic dysfunction in models of experimental cerebral ischemia. MCs should be regarded as a potent ischemia-responding cell type that can interact within the neurovascular unit via a multitude of mediators and signalosomes. MCs are a promising novel pharmacologic target in the quest to limit early inflammation and vasculopathic deteriorations within the acute phase of cerebral ischemia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebral ischemia
- Blood–brain barrier damage
- Brain edema
- Mast cell
- Meninges
- Neurovascular unit
- Inflammation
- Neutrophil
- Stroke
- Tumor necrosis factor-α
1 Introduction
Mast cells (MCs) are bone marrow derived, tissue-homing mononuclear white blood cells (leukocytes) that undergo differentiation upon entry into target organs. Committed bone marrow mast cell progenitors are released into the circulation from where they subsequently migrate into peripheral tissues, undergo maturation, and become terminally differentiated by the influence of cytokines within the surrounding milieu [1]. The progression of these cells to fully mature MCs is dependent on KIT activation which occurs as a consequence of stem cell factor (SCF)-induced KIT dimerization and autophosphorylation [1]. Central to this process is stem cell factor (SCF), the ligand for the c-kit tyrosine kinase receptor expressed on the surface of mast cells. KIT activation significantly regulates many aspects of MC differentiation, proliferation, activation, and survival [1, 2]. Mutant mice lacking either c-kit or SCF are MC deficient. MCs characteristically express a high-affinity receptor (FcεRI) for the Fc region of IgE, the least-abundant member of the antibodies. This receptor is of high affinity so that binding of IgE molecules is in essence irreversible.
MCs reside within or in the proximity to tissues that form a barrier to diverse noxious environmental challenges, i.e., in epithelium, mucosa, and the perivascular space. They are most commonly regarded as key effectors in the pathogenesis of allergic diseases and anaphylaxis [2]. However, the meticulous study of MC biology has established that they can generate or release various cytokines such as TNFα, potent proteases such as chymase and tryptase, vasoactive substances such as histamine and eicosanoids, and regulators of hemostasis such as heparin and tPA [3]. They also produce and release numerous interstitial matrix-degrading enzymes such as matrix metalloproteases (e.g., MMP-9). This armamentarium of mediators equips MCs to take part in a wide array of biological processes. This chapter describes recent evidence strongly supporting a role for MCs in the pathophysiology of cerebral ischemia.
2 Mast Cells in Health and Disease
MCs have gained a notorious reputation as mediators of anaphylaxis and allergic, atopic disorders. The spectrum of mast cell mediators, however, indicate a key role in diverse pathophysiological processes, such as chronic inflammatory processes, wound healing, angiogenesis, fibrosis, and tumors. Recent research has also demonstrated an important role in cardiac diseases, cardiac failure, cardiomyopathy, coronary syndromes, and atherosclerosis. Terminally differentiated, tissue-resident MCs are long lived [4], which coheres with a role in modulating local immunopathogenetic effects in chronic vascular disease. At least in the human the longevity of MCs is dependent upon the continued presence of SCF [1]. Clearly, MCs perform a critical role in protecting the host from a variety of environmental hazards [4, 5], but in the long-lived human, the cellular functions of MCs become involved in many disorders with a strong autoimmune component. The role of MCs has been established in the pathophysiology of atopic dermatitis, psoriasis, asthma, and interstitial cystitis, and is highly probable in multiple sclerosis, coronary artery disease, irritable bowel disorder, and arthritis. The sphere of potential diseases where MCs and their mediators may play a role is, however, extensive and we must refer to other comprehensive reviews for more detailed scrutiny [6, 7].
3 Mechanisms of Action as an Inflammatory Cell
MCs are most efficiently activated via the classical IgE-mediated pathway but they can also be activated by a host of substances such as lipopolysaccharide, cytokines, hormones, immunoglobulins, neuropeptides (such as substance P), endothelin I, and activated complement components [8]. Functional activation of MCs leads to rapid degranulation of their mediators, many of which are preformed and stored in secretory granules. Piecemeal degranulation is an alternative form of mediator discharge permitting the selective secretion of certain mediators (and not the whole content) of the secretory granules. The preformed mediators include tryptase, histamine, serotonin (5-hydroxytryptamine), serine proteases, proteoglycans and cytokines such as tumor necrosis factor α (TNFα) and neutrophil chemokines CXCL1 and CXCL2 [9].
MC activation also leads to de novo synthesis of chemokines, cytokines, and eicosanoids that requires time for synthesis. For example, arachidonic acid metabolites (prostaglandins and leukotrienes), platelet activating factor (PAF), and several chemokines (e.g., CXCL1 and CXCL2) and cytokines (Il-1β and IL-6) can be synthesized de novo. The balance of engaging inhibitory and activatory cell-surface receptors on MCs determines whether the cell becomes active on encountering a challenge. Once activated, MC’s response is further regulated by the balance of both positive and negative intracellular molecular events that extend well afar from the conventional role of kinases and phosphatases [10, 11]. MCs are equipped with particular antigen presenting capacity and phagocytosis of microbes and antigens [2, 12], although their main mode of immunologic function is to act as local effector cells and attract other immune cells to the site of inflammation.
4 Cerebrocranial Mast Cells
The presence and activity of MCs within the cerebrum, already recognized by Nobel-prize winning immunologist Paul Ehrlich in the late nineteenth century, has received little attention up until now. MCs are not easily visible in standard histopathological stainings such as hematoxylin-eosin. They can be clearly detected with a handful of specialized staining procedures, many of which utilize visualization of the abundant heparin sulfate contained in the cytoplasmic MC granules. These can be demonstrated by metachromatic dyes like toluidine blue (Fig. 1a), azures A and B, thionin and methylene blue, which induce purple or violet staining of the granules. Toluidine blue and Giemsa are the most routinely used stainings. Egg white avidin attaches to heparin as well and has been used in a fluorophore-conjugated staining regimen. Tissue MCs can also be detected by enzyme cytochemistry by demonstration of Naphtol AS-D chloroacetate esterase activity (Leder staining) (Fig. 1b), by immunocytochemistry against tryptase, and by visualization of histamine fluorescence after o-phthaldialdehyde exposure [13].
Mast cells in cerebral and meningeal tissues. (a) Toluidine blue staining of a rat brain section demonstrating a densely packed granule-containing MC in association to a small blood vessel. A few solitary granules are also visible surrounding the outer cell membrane. (b) Demonstration of a rat brain MC with naphtol AS-D chloroacetate esterase (Leder) staining. A typical position close to a cortical penetrating arteriole can be observed. (c) Toluidine blue staining of mouse dura, showing the closely set localisation of MC in meningeal tissues
Using these techniques, the presence of MCs has long been observed in substantial numbers in various craniocerebral structures. In the mammalian brain, MCs are found abundantly in the meninges, choroid plexus, hypothalamus, thalamus, olfactory bulb, and the midbrain [14–17]. MCs are also observed in the cerebral cortex [14, 15, 18], characteristically at the branching points of cortical penetrating arterioles (Fig. 1b). A typical position for MCs is the Virchow–Robin perivascular space. They are situated in the brain parenchymal side of basal lamina, nesting between glial processes [19]. MCs are very abundant also in the dural meninges (Fig. 1c). From these locations, MCs can influence not only the blood vessels but interact also with neurons, glial cells, microglia, and extracellular matrix components. Intramuscular injection of compound 48/80 in several species has triggered striking Evans blue extravasation in MC-rich brain areas but not in MC-devoid regions equipped with fenestrated capillaries [20]. A tremendous challenge for research is that the type of MCs is to a large extent species-dependent, and the cell population fluctuates dynamically during development as well as in the course of behavioral and physiological events, and various kinds of stress.
The role of craniocerebral MCs remains elusive, but can be reflected upon an evolutionary perspective. MCs might have transformed from a cell which was directly involved in the compartmentalization and killing of pathogens into a cell type which orchestrates complex reactions leading to segregation and clearing of invading microbes. From a simple effector cell, the MC has become a coordinating cell which operates not only through innate mechanisms but also with the contribution of adaptive immune mechanisms [21]. It is of fundamental importance for the pathogenic mechanisms within the CNS, that of the resident cells in the brain, only MCs are able to acutely respond by releasing massive amounts of preformed mediators (reviewed in [22, 23]). The impact of MC activation is not uniformly proinflammatory, but can also attenuate the response of circulating leukocytes as in the mouse hippocampal brain trauma model [24].
In the brain, MCs have been ascribed a wide spectrum of physiological functions serving not only host response and innate immunity but also endocrine regulation [20]. MCs have been traditionally held as a first line of host defense against pathogens, allergens, toxins, and tissue injury, but with evolutionary accumulation of various membrane-bound receptors and diverse granule-contained mediators, MCs have engaged in quite diverse regulating functions also in many normal physiological and homeostatic functions along with the evolution of CNS.
5 Diversity of MCs
MCs of different organotypic origins show a substantial heterogeneity regarding their granule content and certain outer membrane epitopes. The organotypic difference has been used to classify MCs, which traditionally have been divided into mucosal or connective tissue type serosal (peritoneal) types. One key diversity is that of the main protease content: in humans, MCs can be distinguished based upon whether they contain tryptase (MCT) or tryptase and chymase (MCTC) [25]. Another example of diversity is the lack of c-kit receptor for SCF reported in some cerebral MCs [26]. Interestingly, blood transfusion results in MCs of donor origin settling in tissues of WT mice including the brain, and this relocation is restricted to regions bearing host MCs, but transfusion in KitW-sh/W-sh MC-deficient mice strain results in MCs in the pinna of the ear, not in the brain [27]. Another brain tissue-specific MC characteristic is the low expression of the IgE-binding, high-affinity receptor FcεRI. The molecular and anatomical heterogeneity of the brain MCs has recently been reviewed in detail [20].
However, experimental studies have indicated that, in keeping with the perivascular position of MCs in the brain, the released vasoactive mediators furnish them with a capacity to regulate the permeability of BBB [28, 29].
6 MCs in the Priming of Inflammatory Cell Response
MCs are best known for their potent effector functions in allergic and hypersensitivity disorders. These phlogistic responses have long been known to be biphasic, the immediate phase occurring within minutes, and the late phase within several hours. The former is caused by the release of MC granule contents (histamine, TNFα, chymase, tryptase, heparin, cathepsin-G, and assorted chemokines). The second phase is characterized by de novo synthesis of MC mediators, such as leukotrienes, cytokines, prostaglandins, and additional chemotactic factors. However, MCs recently have been demonstrated to be involved in a vast array of considerably more complex immune functions that go well beyond allergies and contain the development of autoimmune disorders and peripheral tolerance, as well as the initiation and maintenance of adaptive and innate host responses [30].
In the development of local inflammatory tissue response, MCs have been implicated in recruiting neutrophils that are frequently the first immune cells to enter an inflamed or infected tissue site [31, 32]. In a mouse model of lipopolysaccharide (LPS)-induced peritonitis, MC granules containing preformed chemokines CXCL1 and CXCL2 were shown to be released by MCs within 15 min of in vivo stimulation, constituting an ideal mechanism to stimulate local neutrophil entry from the circulation at an early stage following the inflammatory signal [33]. Earlier work has emphasized the role of MC-granule dependent TNFα in mediating the initial phase of neutrophil recruitment following immune-complex mediated peritonitis in mice [34]. In addition, MCs, like macrophages, also were demonstrated to newly synthesize CXCL1 and CXCL2, making detectable amounts within 1 h of LPS treatment. Neutrophil extravasation was diminished in mice devoid of MCs [33].
Another facet of the local inflammatory response is the increase of vasopermeability and tissue edema. A well-established view is that several MC mediators, especially the vasoactive amines histamine, serotonin, and vascular endothelial growth factor A (VEGF-A), increase capillary leakage and can rapidly trigger tissue edema [35], but numerous other MC mediators such as PAF and leukotrienes participate in this response. Histamine receptor antagonists are therapeutically used to treat edema formation and allergic reactions associated with aberrant MC activity e.g., in pulmonary asthma, which can be also treated by the classic MC-stabilizing medicament, sodium cromoglycate. MCs can also trigger vascular permeability by paracrine mechanisms such as heparin-initiated bradykinin formation [36].
Although sparsely scattered in tissues, MCs are strategically positioned in close proximity to blood vessels and thus in a position to directly influence circulating leukocytes [33]. The same principle seems to be active in mounting local inflammation also in the various inflammatory diseases of the brain [6]. It is noteworthy that the described physiological and pathophysiological roles of MCs are likely to be strongly species-specific, because the immunological key characteristic such as the palette of granule constituents, membrane receptors, and activation modes have significant variability across different animal species.
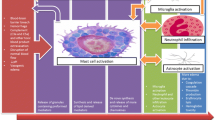
7 Mast Cells as a Member of the Neurovascular Unit
The co-orchestrated roles of endothelial cells, neurons, astrocytes, pericytes, and extracellular matrix in the pathobiology of cerebral blood vessels have been encompassed under the term “neurovascular unit” (NVU). We and others have viewed MCs in the context of the NVU responding to cerebral ischemia [23, 37]. In the micromilieu of the brain parenchyma resident MCs lie in close proximity to other cell types of the NVU (Fig. 2). In addition to their role as initiators of acute inflammatory and vasoactive events, functional, unstressed MCs are certain to interact with these surrounding cell types, with bidirectional effects. Modes of interaction are likely to include direct cellular connections and released mediators, but interestingly, may also include cell signaling via release of exosomes, small membrane vesicles packed with RNA, capable of altering function of surrounding cell types [38]. For now, most research on cerebral MCs has focused on the pathophysiology of different neurological disorders, and more detailed investigation of MC interaction with other NVU cell types has been scarce, mostly relying on in vitro cell culture methods. Therefore the participation of MCs in the various physiological functions of the NVU is still to be characterized.
8 Evidence for a Role of MCs in Cerebral Ischemia
8.1 Mast Cell Activation in Experimental Models of Cerebral Ischemia
Initial observations of MC activation in experimental focal cerebral ischemia were made 15 years ago in our laboratory [39, 40]. In these experiments, degranulated cerebral MCs were observed in perivascular locations often associated with adjacent perivascular edema, based on which we hypothesized MCs to be involved in the pathophysiology of cerebral ischemia. Later on, MC activation and subsequent mediator release were reported in an in vitro model of oxygen-glucose deprivation [41]. In a separate study we further showed a dose-dependent activation of MCs (as measured by histamine release) in vitro as a reaction to tissue plasminogen activator (tPA), used for treatment of focal cerebral ischemia [42]. MC activation has also been described in neonatal rat stroke models. Biran et al. showed that ischemia induced histamine accumulation in the infarct core at 6–12 h after ischemia induction, whereas it was located in the penumbra at 24–48 h [43]. This study also showed that histamine accumulated in neuronal cells before they degenerated, which was accompanied by an increase in the MC count at 12 and 48 h. Activation of MCs after experimental hypoxic-ischemia injury in the neonate brain has later been replicated by additional studies [44].
8.2 The Effects of MCs in Experimental Focal Ischemic Brain Damage
Investigation on the role of MCs in focal cerebral ischemia began with experiments using a transient middle cerebral artery occlusion (MCAO) model in rats. Applying pharmacological activation and inhibition of MCs, we found MCs to play a central part in the development of space occupying brain edema, BBB disruption, and neutrophil infiltration 3 h after transient ischemia and reperfusion [18]. For example, MC stabilization with intraventricularly applied cromoglycate led to a 39 % reduction in hemispheric expansion, 51 % reduction in BBB leakage, and a 37 % reduction in postischemic neutrophil infiltration. Involvement of MCs was subsequently studied using the same model together with intravenous tPA, simulating thrombolytic treatment [42]. MC stabilization showed reduction not only of edema, BBB disruption, and neutrophil infiltration, but also of perilous hemorrhage formation, neurological deterioration, and mortality. In both of these proof-of-concept studies, experiments were repeated using gene-manipulated MC-devoid rats lacking the c-kit ligand necessary for stem cell-dependent MC differentiation, demonstrating an even stronger protective effect with MC deficiency [18, 42].
More recently, our initial experimental findings in rats were replicated by two groups using a mouse MCAO model of transient ischemia. Studying later timepoints at 3 days and 2 weeks, Arac et al. showed significant reductions in brain edema, granulocyte infiltration, and infarct size in two different MC-deficient mouse strains [45]. Importantly, the study demonstrated the central participation of the meningeal MC population mediated via interleukin (IL)-6 and, to a lesser extent, chemokine ligand 7. Another study with pharmacological stabilization of MCs and genetically MC-deficient mice supported the involvement MCs in the pathophysiology of ischemia-mediated edema and inflammation, and suggested a role for endoglin, endothelin-1, and MMP-9, but not for TNFα [46]. Interestingly, this study also reported an increase of MC count by 50 % in the infarcted hemisphere 4 h after transient MCAO. Together, these observations provide solid evidence to support direct involvement of MCs in the pathophysiology of focal cerebral ischemia.
8.3 MCs and Neuroprotection
The initial in vivo stroke model experiments were not designed to study late-stage neuroprotection, and did not notice any effect regarding the lesion volume in association with MC stabilization or genotypic MC deficiency [18]. However, another study with longer follow-up of 24-h revealed a clear effect on functional recovery both after pharmacological stabilization of mast cells and in rats with mast cell deficiency [42], although no significant differences in infarct sizes were seen. More recent studies have also showed a reduction of infarct volume after MC stabilization in adult wild-type mice [45, 46]. Again, additional supporting evidence of MC involvement in tissue injury comes from neonate models. One set of experiments showed MC involvement in hypoxic-ischemic brain damage in the immature rat [47] and later observations showed that MC stabilization translated into reduced neuronal loss and brain atrophy [44]. The authors suggested the possibility of MC-derived IL-9 to be involved in the detrimental effect, which was supported by others [48]. Immunohistochemical co-localization studies of histamine and microtubule-associated protein 2 revealed accumulation in neuronal cells prior to their degeneration, and increased MC counts in the corresponding regions [43]. Histamine immunoreactivity was detected in MCs at 2, 6, and 12 h after ischemia, but disappeared at 24 h along with a concomitant observation of MC degranulation. Another study showed an effect of cyclosporin A in protecting against mild ischemic injury in neonatal rat brain [49]. The observed effect in reduction of histamine release from MCs is, however, IgE-dependent, and may not be of major importance in this setting. These data support a role for MCs in experimental ischemia-induced neuronal death, at least in neonatal cerebral ischemia.
9 Mast Cells and BBB in Ischemia–Reperfusion Injury
As the neurovascular unit responds to the sudden insult of ischemia and subsequent reperfusion, MCs are ideally located to initiate and aggravate known pathways of BBB disruption [23]. As discussed earlier, the activation of MCs is known to occur in a biphasic manner [3], starting with acute release of potent preformed granule contents that quickly spread to interact with the abluminal side of endothelial cells, the surrounding basal lamina, and other cell types of the neurovascular unit (Fig. 3). The second, later phase of MC activation is characterized by de novo production and release of mediators to support and prolong the initiated inflammatory response [3].
Very early on hypoxia, acidosis, formation of reactive oxygen species, and changes in blood flow act to disrupt cellular homeostasis of the neurovascular unit [37]. These reactions, together with intravascular blood coagulation, complement activation, and activation of the sympathetic nervous system, are likely initiators of MC activation next to parenchymal microvessels and within meningeal tissues [23].
The most imminent MC effects are amplified through the adjacent endothelium, which is the initial site of BBB leakage and failure early after reperfusion [50]. Histamine, an abundant and highly soluble MC mediator, acts through endothelial histamine receptors to activate calcium influx and convert the cells into a proinflammatory state [51]. The carefully characterized effects of histamine include increased endothelial permeability [51, 52], activation of endothelial nitric oxide synthase (eNOS) [53], and acute release of Weibel–Palade bodies (WPBs) [54], the main storage site for von Willebrand Factor (VWF) and P-selectin that act to support acute leukocyte infiltration [55, 56]. The MC protease tryptase may further activate endothelium through cleavage of the proteinase activated receptor 2 (PAR-2), with similar effects to histamine [57]. A third MC mediator, Cathepsin G, has also been shown to induce endothelial permeability and influx of calcium into endothelial cells [58, 59].
As reperfusion injury advances, a storm of proteolytic enzymes is activated within the structures of the cerebral microvasculature [60, 61]. Ultrastructural evidence suggests that an early disruption of basal lamina [62], endothelial tight junctions [63], and other cellular connections ensues [64], and may locally progress to cause structural failure of the vascular wall, leading to edema and eventually hemorrhage [65]. Experimental evidence indicates that the wide armamentarium of MC mediators may have a central role in enhancing this proteolytic cascade [23].
The family of matrix metalloproteinase (MMP) enzymes, especially the gelatinases MMP-2 and -9, are thought to be a central proteolytic pathway, minutely characterized in experimental stroke models [61, 66–68]. For example, in human stroke patients plasma levels of MMP-9 are correlated with the incidence of significant hemorrhagic transformation [69, 70]. Using a rat MCAO stroke model, we found that both genetic MC deficiency and pharmacological MC stabilization with intracerebroventricular cromoglycate were able to significantly reduce the percentage of microvessels with high gelatinase activity in the ischemic hemisphere as early as 3 h after reperfusion (−64 % and −36 %, respectively) [71]. This finding is likely a sum of several MC-mediated effects on the MMP-cascade.
MCs have been shown to release both MMP-2 and -9 [72], which fits with the gelatinolytic activity we observed in the granules of activated cerebral MCs [71]. The MC protease chymase is capable of activating proMMP-1, proMMP-2, and proMMP-9 [72–74] and degrades tissue inhibitor of metalloproteinases (TIMP) -1, an important endogenous MMP-inhibitor [75]. MC tryptase has also been shown to activate proMMP-2 and proMMP-3 [76, 77]. Further on, in vitro studies have shown that histamine induces MMP-2 production in endothelial cells [78] and MMP-9 production in astrocytes [79]. MC proteases can also degrade components of the basal lamina directly: chymase is capable of degrading fibronectin [80] and cathepsin G, found in a subset of MCs, is able to degrade fibronectin and laminin [81, 82].
Progression of postischemic BBB disruption is accompanied by unrestrained granulocyte infiltration, beginning hours after reperfusion [83], which acts to further drive inflammation, increase proteolysis and barrier permeability [84], and disrupt microvascular flow [85]. MCs seem to have a central role in activating leukocyte recruitment, as data from three individual laboratories show that MC inhibition significantly reduces both early and late granulocyte infiltration after transient MCAO (3–6 h and 3 days postreperfusion, respectively) [18, 42, 45, 46].
MCs secrete a wide range of mediators that can augment granulocyte infiltration. In addition to the endothelial dependent effects of histamine and tryptase described above, MCs are capable of releasing preformed TNFα, which further increases endothelial permeability, endothelial adhesion molecule expression, and neutrophil infiltration [86–88]. Moreover, chymase is thought to have direct chemotactic effects on neutrophils [89]. As MC activation endures, de novo production of mediators continues to support infiltration of granulocytes. IL-1 is capable of increasing both endothelial barrier permeability and neutrophil infiltration [90, 91]. Further, in a recent report, Arac et al. demonstrated that IL-6 is central for MC-dependent neutrophil infiltration in a later phase, 3 days postreperfusion. In these experiments reconstitution of MC-deficient mice with wild-type MCs returned typical neutrophil infiltration and brain swelling, while reconstitution with IL-6-deficient MCs did not [45].
To sum up, the effects of MCs in experimental models of ischemic stroke are well in line with the known effects of the wide armamentarium of MC mediators. However, the individual contribution of these mediators is still unknown, and will require further experimental work, preferably by reconstituting MC-deficient mice with MCs deficient for the studied mediator [45]. Although meningeal MCs appear to be central at later timepoints after reperfusion (3 days and 2 weeks) [45], the relative contribution of different MC populations in the ultra-acute and early phases (0–48 h) of ischemic stroke is still unknown.
10 Mast Cells, Blood Coagulation, and Fibrinolysis
In addition to their potent vasoactive, proteolytic, and chemotactic effects, MCs are known as a profibrinolytic, anticoagulant, and antithrombotic cell type, with several effects on thrombotic pathways [23, 92]. As reperfusion injury advances to break down structures of the vascular wall, the unphysiologically strong activation of MCs may partake in initiating dangerous intraparenchymal hemorrhage, one of the most feared complications of acute stroke treatment. Supporting this hypothesis, in experiments using a rat stroke model with 90 min of transient MCAO combined with intravenous tPA, both genetic and pharmacological MC inhibition led to almost total abrogation of intraparenchymal hemorrhage [42]. Again, a spectrum of MC mediators have effects on hemostasis.
Heparin is the central anticoagulant mediator released from MCs, a negatively charged glycosaminoglycan which catalyzes antithrombin III-mediated inactivation of coagulation factors, most importantly activated factor X and thrombin [93]. Heparin can inhibit binding of platelets onto collagen IV [94], revealed upon disruption of the vascular wall. Further, heparin also releases tissue factor pathway inhibitor (TFPI) from the surface of endothelial cells, a mediator which can inhibit arterial thrombosis [95]. Lastly, heparin has recently been shown to activate the plasma contact system, inducing rapid generation of bradykinin without activation of blood coagulation [36], which may act to further increase endothelial permeability and leukocyte infiltration.
MCs also have more direct effects on fibrinogen and fibrin. MC tryptase has been shown to degrade fibrinogen, preventing normal fibrin formation [96, 97]. Tryptase also activates pro-urokinase [98], an important plasminogen activator, initiating plasmin-mediated breakdown of fibrin. Moreover, MCs have been shown to directly secrete tissue plasminogen activator (tPA), another central plasminogen activator, without accompanying secretion of plasminogen activator inhibitors, like plasminogen activator inhibitor-1 (PAI-1) [99]. Importantly, a wide collection of experimental evidence has shown that plasminogen activators, especially tPA, have important effects on proteolysis and inflammation at the BBB, in addition to direct pro-excitotoxic effects [100]. Of note, a positive feedback loop may exist between fibrinolysis and further MC activation, as certain fibrinolytic breakdown products of fibrinogen have been shown to activate MCs [101].
The endothelial effects of MC mediators contribute an additional pathway for modification of hemostasis. In patients with anaphylaxis, extremely strong MC activation and subsequent release of endothelial WPBs have been shown to induce a rapid increase in circulating levels of both vWF and tPA, and induce systemic plasminogen activation [102]. The significance of this pathway during localized MC activation is still to be uncovered, but may have both fibrinolytic and proaggregatory effects.
In the setting of acute inflammation, the physiological purpose of these described anticoagulant, fibrinolytic, and antithrombotic MC effects may be in regulating thrombosis activated by inflammatory pathways, to ensure adequate blood flow to the inflamed tissue area, and counteract the inhibitory effects of fibrin formation on leukocyte recruitment [103]. More generally, in the resting state, MCs have been suggested to protect the brain microvasculature against thrombotic challenges [104]. In line with these hypotheses, several products of the coagulation cascade have been shown to activate MCs, including bradykinin, thrombin, and activated factor X [105–107].
11 Conclusions
To conclude, recent experimental research suggests that MCs and their nominal responses increasing the permeability of the vascular wall play a significant, deleterious role following acute cerebral ischemia. MCs should be regarded as a potent inflammatory cell type that can interact via a multitude of mediators and signalosomes with its neighboring cells within the NVU, and may also have more distant effects within the CNS. MCs are a unique resident inflammatory cell type, settled in the proximity of the vascular wall already at the outset of ischemia, capable of quickly degranulating, leading to degradation of the basal membrane, BBB damage, brain edema, and hemorrhage.
Future goals of MC research include examination of whether MC mediators are released early after cerebral ischemia in man. In view of the significant species differences in the immunological characteristics and mediator contents of MCs, more evidence is needed on the magnitude of MC-dependent chemokine release, neutrophil targeting, and other secondary effects on postischemic tissue integrity. The potential involvement of the meningeal MC population is an attractive area of future research, as these MCs may be more readily influenced with pharmacological means due to their localization outside the BBB. MCs are candidate cells to become novel pharmacologic targets at the NVU to limit ischemic and hemorrhagic brain damage associated with reperfusion therapies.
Abbreviations
- BBB:
-
blood–brain barrier
- eNOS:
-
endothelial nitric oxide synthase
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- MC:
-
mast cell
- MMP:
-
matrix metalloproteinase
- MCAO:
-
middle cerebral artery occlusion
- NVU:
-
neurovascular unit
- PAF:
-
platelet-activating factor
- PAI-1:
-
plasminogen activator inhibitor-1
- PAR:
-
proteinase activated receptor 2
- SCF:
-
stem cell factor
- TIMP:
-
tissue inhibitor of metalloproteinases
- tPA:
-
tissue plasminogen activator
- TNFα:
-
tumor necrosis factor-α
- WPBs:
-
Weibel–Palade bodies
- VWF:
-
von Willebrand factor
References
Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2–12.
Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40(7):1843–51.
Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118(20):5383–93.
Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–75.
Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121(10):4180–91.
Theoharides TC, Alysandratos K-D, Angelidou A, Delivanis D-A, Sismanopoulos N, Zhang B, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822(1):21–33.
Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115(24):4981–90.
Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–52.
Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–94.
Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117(6):1214–25. quiz1226.
Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228(1):149–69.
Hofmann AM, Abraham SN. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr Opin Immunol. 2009;21(6):679–86.
Edvinsson L, Cervoś-Navarro J, Larsson LI, Owman C, Rönnberg AL. Regional distribution of mast cells containing histamine, dopamine, or 5-hydroxytryptamine in the mammalian brain. Neurology. 1977;27(9):878–83.
Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46(9):607–17.
Silver R, Silverman AJ, Vitković L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19(1):25–31.
Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left–right asymmetry. Brain Res. 1984;323(2):209–17.
Dropp JJ. Mast cells in mammalian brain. Acta Anat. 1976;94(1):1–21.
Strbian D, Karjalainen-Lindsberg M-L, Tatlisumak T, Lindsberg PJ. Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab. 2006;26(5):605–12.
Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20(1):401–8.
Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36(9):513–21.
Crivellato E, Ribatti D. The mast cell: an evolutionary perspective. Biol Rev Camb Philos Soc. 2010;85(2):347–60.
Strbian D, Kovanen PT, Karjalainen-Lindsberg M-L, Tatlisumak T, Lindsberg PJ. An emerging role of mast cells in cerebral ischemia and hemorrhage. Ann Med. 2009;41(6):438–50.
Lindsberg PJ, Strbian D, Karjalainen-Lindsberg M-L. Mast cells as early responders in the regulation of acute blood–brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30(4):689–702.
Hendrix S, Kramer P, Pehl D, Warnke K, Boato F, Nelissen S, et al. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. FASEB J. 2013;27(3):920–9.
Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80.
Shanas U, Bhasin R, Sutherland AK, Silverman AJ, Silver R. Brain mast cells lack the c-kit receptor: immunocytochemical evidence. J Neuroimmunol. 1998;90(2):207–11.
Nautiyal KM, Liu C, Dong X, Silver R. Blood-borne donor mast cell precursors migrate to mast cell-rich brain regions in the adult mouse. J Neuroimmunol. 2011;240–241:142–6.
Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood–brain barrier. J Neurobiol. 1996;31(4):393–403.
Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, et al. Acute stress increases permeability of the blood–brain-barrier through activation of brain mast cells. Brain Res. 2001;888(1):117–27.
Metz M, Maurer M. Mast cells—key effector cells in immune responses. Trends Immunol. 2007;28(5):234–41.
Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–70.
Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–82.
De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–7.
Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258(5090):1957–9.
Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–19.
Oschatz C, Maas C, Lecher B, Jansen T, Björkqvist J, Tradler T, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34(2):258–68.
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ. Mast cells in ischemic rat brain. Conference Proceeding. San Diego: Society for Neuroscience; 2001. Abstract No: 330.11
Strbian D, Tatlisumak T, Karjalainen-Lindsberg ML, Lindsberg PJ. Mast cells regulate ischemic brain edema. Conference Proceeding. J Cereb Blood Flow Metab. 2003;23(Suppl 1):166
Hu W, Shen Y, Fu Q, Dai H, Tu H, Wei E, et al. Effect of oxygen-glucose deprivation on degranulation and histamine release of mast cells. Cell Tissue Res. 2005;322(3):437–41.
Strbian D, Karjalainen-Lindsberg M-L, Kovanen PT, Tatlisumak T, Lindsberg PJ. Mast cell stabilization reduces hemorrhage formation and mortality after administration of thrombolytics in experimental ischemic stroke. Circulation. 2007;116(4):411–8.
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Héron A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008;18(1):1–9.
Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40(9):3107–12.
Arac A, Grimbaldeston MA, Nepomuceno ARB, Olayiwola O, Pereira MP, Nishiyama Y, et al. Evidence that meningeal mast cells can worsen stroke pathology in mice. Am J Pathol. 2014;184(9):2493–504.
McKittrick CM, Lawrence CE, Carswell HVO. Mast cells promote blood–brain barrier breakdown and neutrophil infiltration in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35(4):638–47.
Jin Y, Silverman A-J, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;29(4–5):373–84.
Patkai J, Mesples B, Dommergues MA, Fromont G, Thornton EM, Renauld JC, et al. Deleterious effects of IL-9-activated mast cells and neuroprotection by antihistamine drugs in the developing mouse brain. Pediatr Res. 2001;50(2):222–30.
Leger P-L, De Paulis D, Branco S, Bonnin P, Couture-Lepetit E, Baud O, et al. Evaluation of cyclosporine A in a stroke model in the immature rat brain. Exp Neurol. 2011;230(1):58–66.
Kuntz M, Mysiorek C, Pétrault O, Pétrault M, Uzbekov R, Bordet R, et al. Stroke-induced brain parenchymal injury drives blood–brain barrier early leakage kinetics: a combined in vivo/in vitro study. J Cereb Blood Flow Metab. 2014;34(1):95–107.
Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39(4–5):173–85.
Winter MC, Shasby SS, Ries DR, Shasby DM. Histamine selectively interrupts VE-cadherin adhesion independently of capacitive calcium entry. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L816–23.
Li H, Burkhardt C, Heinrich U-R, Brausch I, Xia N, Förstermann U. Histamine upregulates gene expression of endothelial nitric oxide synthase in human vascular endothelial cells. Circulation. 2003;107(18):2348–54.
van Mourik JA, Romani de Wit T, Voorberg J. Biogenesis and exocytosis of Weibel-Palade bodies. Histochem Cell Biol. 2002;117(2):113–22.
Jones DA, Abbassi O, McIntire LV, McEver RP, Smith CW. P-selectin mediates neutrophil rolling on histamine-stimulated endothelial cells. Biophys J. 1993;65(4):1560–9.
Pendu R, Terraube V, Christophe OD, Gahmberg CG, de Groot PG, Lenting PJ, et al. P-selectin glycoprotein ligand 1 and beta2-integrins cooperate in the adhesion of leukocytes to von Willebrand factor. Blood. 2006;108(12):3746–52.
Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol Cell Physiol. 2005;289(6):C1485–91.
Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145(8):2652–61.
Peterson MW, Gruenhaupt D, Shasby DM. Neutrophil cathepsin G increases calcium flux and inositol polyphosphate production in cultured endothelial cells. J Immunol. 1989;143(2):609–16.
Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35(4):998–1004.
Yang Y, Rosenberg GA. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323–8.
Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26(11):2120–6.
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709.
Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28(4):858–65.
Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16(6):1373–8.
Amantea D, Corasaniti MT, Mercuri NB, Bernardi G, Bagetta G. Brain regional and cellular localization of gelatinase activity in rat that have undergone transient middle cerebral artery occlusion. Neuroscience. 2008;152(1):8–17.
Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–8.
Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–91.
Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34(1):40–6.
Rosell A, Ortega-Aznar A, Alvarez-Sabín J, Fernández-Cadenas I, Ribó M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–406.
Mattila OS, Strbian D, Saksi J, Pikkarainen TO, Rantanen V, Tatlisumak T, et al. Cerebral mast cells mediate blood–brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke. 2011;42(12):3600–5.
Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999;162(9):5528–35.
Tchougounova E, Lundequist A, Fajardo I, Winberg J-O, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280(10):9291–6.
Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269(27):18134–40.
Frank BT, Rossall JC, Caughey GH, Fang KC. Mast cell tissue inhibitor of metalloproteinase-1 is cleaved and inactivated extracellularly by alpha-chymase. J Immunol. 2001;166(4):2783–92.
Lohi J, Harvima I, Keski-Oja J. Pericellular substrates of human mast cell tryptase: 72,000 dalton gelatinase and fibronectin. J Cell Biochem. 1992;50(4):337–49.
Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84(5):1657–62.
Doyle JL, Haas TL. Differential role of beta-catenin in VEGF and histamine-induced MMP-2 production in microvascular endothelial cells. J Cell Biochem. 2009;107(2):272–83.
Patel A, Vasanthan V, Fu W, Fahlman RP, MacTavish D, Jhamandas JH. Histamine induces the production of matrix metalloproteinase-9 in human astrocytic cultures via H1-receptor subtype. Brain Struct Funct. 2015:1–16
Okumura K, Takai S, Muramatsu M, Katayama S, Sakaguchi M, Kishi K, et al. Human chymase degrades human fibronectin. Clin Chim Acta. 2004;347(1–2):223–5.
Vartio T, Seppä H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981;256(1):471–7.
Heck LW, Blackburn WD, Irwin MH, Abrahamson DR. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol. 1990;136(6):1267–74.
Lindsberg PJ, Carpén O, Paetau A, Karjalainen-Lindsberg M-L, Kaste M. Endothelial ICAM-1 expression associated with inflammatory cell response in human ischemic stroke. Circulation. 1996;94(5):939–45.
Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39(4):1121–6.
del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22(10):1276–83.
Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28(6):1233–44.
Gotsch U, Jäger U, Dominis M, Vestweber D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes Commun. 1994;2(1):7–14.
Wong D, Prameya R, Dorovini-Zis K. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J Neuroimmunol. 2007;184(1–2):136–48.
Tani K, Ogushi F, Kido H, Kawano T, Kunori Y, Kamimura T, et al. Chymase is a potent chemoattractant for human monocytes and neutrophils. J Leukoc Biol. 2000;67(4):585–9.
Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26(4):676–80. discussion 681.
Hara H, Fink K, Endres M, Friedlander RM, Gagliardini V, Yuan J, et al. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17(4):370–5.
Valent P. New aspects in thrombosis research: possible role of mast cells as profibrinolytic and antithrombotic cells. Thromb Haemost. 2002;87(5):786–90.
Hirsh J. Heparin. N Engl J Med. 1991;324(22):1565–74.
Lassila R, Lindstedt K, Kovanen PT. Native macromolecular heparin proteoglycans exocytosed from stimulated rat serosal mast cells strongly inhibit platelet–collagen interactions. Arterioscler Thromb Vasc Biol. 1997;17(12):3578–87.
White TA, Johnson T, Zarzhevsky N, Tom C, Delacroix S, Holroyd EW, et al. Endothelial-derived tissue factor pathway inhibitor regulates arterial thrombosis but is not required for development or hemostasis. Blood. 2010;116(10):1787–94.
Schwartz LB, Bradford TR, Littman BH, Wintroub BU. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985;135(4):2762–7.
Thomas VA, Wheeless CJ, Stack MS, Johnson DA. Human mast cell tryptase fibrinogenolysis: kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry. 1998;37(8):2291–8.
Stack MS, Johnson DA. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase). J Biol Chem. 1994;269(13):9416–9.
Sillaber C. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J Immunol. 1999;162(2):1032–41.
Vivien D, Gauberti M, Montagne A, Defer G, Touzé E. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J Cereb Blood Flow Metab. 2011;31(11):2119–34.
Basheer M, Schwalb H, Nesher M, Gilon D, Shefler I, Mekori YA, et al. Mast cell activation by fibrinogen-related homologous c-terminal peptides (haptides) modulates systemic blood pressure. J Allergy Clin Immunol. 2010;126(5):1041–8.
van der Linden PW, Hack CE, Struyvenberg A, Roem D, Brouwer MC, de Boer JP, et al. Controlled insect-sting challenge in 55 patients: correlation between activation of plasminogen and the development of anaphylactic shock. Blood. 1993;82(6):1740–8.
Kaplan ZS, Zarpellon A, Alwis I, Yuan Y, McFadyen J, Ghasemzadeh M, et al. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors GPIb and PAR4. Nat Commun. 2015;6:7835.
Kitamura Y, Taguchi T, Yokoyama M, Inoue M, Yamatodani A, Asano H, et al. Higher susceptibility of mast-cell-deficient W/WV mutant mice to brain thromboembolism and mortality caused by intravenous injection of India ink. Am J Pathol. 1986;122(3):469.
Bueb JL, Mousli M, Landry Y, Regoli D. Structure–activity studies of bradykinin analogues on rat mast cell histamine release. Peptides. 1993;14(4):685–9.
Razin E, Marx G. Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J Immunol. 1984;133(6):3282–5.
Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, et al. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997;99(10):2446–51.
Acknowledgments
Academic stroke research in Helsinki is supported by financial resources from governmental and nonprofit foundations, the Helsinki University Central Hospital governmental subsidiary funds for clinical research (DS, PJL), the Finnish Medical Foundation (DS), the Sigrid Jusélius Foundation (PJL), the Maire Taponen Foundation (PJL), and the Paavo Nurmi Foundation (PJL).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lindsberg, P.J., Mattila, O.S., Strbian, D. (2016). Mast Cell as an Early Responder in Ischemic Brain Injury. In: Chen, J., Zhang, J., Hu, X. (eds) Non-Neuronal Mechanisms of Brain Damage and Repair After Stroke. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-32337-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-32337-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32335-0
Online ISBN: 978-3-319-32337-4
eBook Packages: MedicineMedicine (R0)