Abstract
Inflammation is a hallmark in the pathogenesis of acute stroke and contributes substantially to secondary neuronal degeneration. Circulating leukocytes—particularly pro-inflammatory T cells—invading the ischemic brain contribute to post-stroke neuroinflammation and exacerbation of stroke outcome. However, a subpopulation of T cells, regulatory T cells (Treg), are potent immunosuppressant cells involved in resolution of tissue inflammation and have a key role in inflammatory diseases. Recent studies have identified a prominent role of Foxp3+ Treg cells in modulating the inflammatory response to acute brain ischemia and thereby contributing to stroke outcome. Most studies observed a neuroprotective function of Treg cells by inhibiting an overshooting immunological “collateral damage” after stroke and first Treg-targeted therapeutic strategies have been tested. This chapter provides a comprehensive overview of the current knowledge on Treg cells in experimental and clinical stroke.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Post-stroke neuroinflammation has come into the focus of current preclinical stroke research [1]. Among the pathophysiological mechanisms of microglial activation, brain leukocyte invasion and secretion of pro-inflammatory factors, lymphocytes have been uncovered as the key leukocyte subpopulation determining the neuroinflammatory outcome. Several studies have shown that pro-inflammatory lymphocytes such as TH1, TH17, and γδ-T cells worsen stroke outcome and that blocking their brain invasion is neuroprotective [2–5]. Contrary to pro-inflammatory lymphocytes, regulatory T cells have been characterized in primary inflammatory diseases as disease-limiting protective cells [6]. Finding this key role of regulatory T cells in other T cell-driven pathologies has initiated productive research efforts on the role of regulatory T cells also in ischemic brain injury over the past years. Due to the complex function of regulatory cells in immune homeostasis and disease as well as partially divergent findings using different stroke models, uncertainty has emerged about the pathophysiological function of regulatory lymphocytes in stroke.
2 Regulatory T Cells in Post-Stroke Neuroinflammation
The immune system has evolved several regulatory mechanisms to inhibit an overshooting immune reaction to tissue damage including cell depletion, anergy, and unresponsiveness to self-antigens. The presence of regulatory T cells (Treg) suppressing actively (self-reactive) immunity is one of the key mechanisms of preserving immune homeostasis and limiting inflammatory collateral damage [7]. For example, depletion of CD25+ CD4+ Treg cells naturally arising in the immune system induces autoimmune diseases and reconstitution of this cell population prevents disease development [8]. Lack of Treg has been shown to be a primary cause of autoimmune diseases in humans [9]. In addition to sustaining self-tolerance, Treg are also intricate in suppressive control of a broad spectrum of immune responses including those against autologous tumor cells [10], allergens [11] and organ transplantation [12]. Although Treg might be simplistically defined as immunosuppressive T cells, several phenotypically and functionally distinct Treg subpopulations have been defined, such as induced Treg populations, Tr1, TH3, and various others [13]. Yet, the best investigated population of regulatory lymphocytes are CD4+ CD25+ Foxp3+ naturally occurring regulatory T cells, which are physiologically produced in the thymus as a mature and functional cell population [14]. While it is still unknown at which specific site Treg act as immunomodulators after stroke, their kinetics and magnitude of brain infiltration have been characterized in several studies using models of experimental middle cerebral artery occlusion (MCAO). One of the first studies systematically analyzing brain leukocyte invasion after transient brain ischemia (filament-induced MCAO resulting in large hemispheric lesions) by flow cytometry was performed by Gelderblom and colleagues [15]. In this study only a very low number of CD25+ Foxp3+ Treg cells at a frequency of less than 5 % of all CD4+ T cells was observed within the first week after transient ischemia. In contrast, using a permanent MCA occlusion model we detected substantial T cell and Treg counts in the ischemic hemisphere (Fig. 1) with Foxp3+ Treg cells constituting approx. 20 % of all CD4+ cells [16]. Correspondingly, it was previously shown that distal, permanent occlusion induces a significantly stronger neuroinflammatory reaction with manifold higher T cell counts in the ischemic hemisphere than in proximal, transient occlusion models [17]. Also in contrast to the initial study by Gelderblom et al., a more recent work by Stubbe et al. has investigated cerebral Treg counts also at later stages after large hemispheric lesions and detected only negligible Foxp3+ cells in ischemic hemispheres early after MCAO but substantial brain Treg invasion at 14days and 30days after MCAO. Additionally, the percentage of Treg cells within the Thelper cell population was increased in brains after stroke compared to peripheral lymphatic organs [18].
Apart from the evidence of variable Treg cell invasion depending of the used stroke model, it is feasible to assume that Treg cells might execute their function on target cells in the peripheral immune system. The majority of studies has detected the effector function of Treg cells in depletion or therapeutic paradigms (see below) already within the first days after brain injury before a considerable amount of Treg have even invaded the brain. Likewise, delayed deletion of Treg cells by antibodies or in inducible Foxp3-KO mice at 3days after MCAO was not effective in altering stroke outcome [18, 19]. Hence, although the definite site of action for Treg after stroke is still unsolved, currently available data suggests that Treg fulfill their immunomodulatory function within the first 3days after stroke and most likely outside the CNS. Potential peripheral target sites of Treg cells might include suppression of peripheral effector T cell activation, inhibition of autoantigen-specific clonal expansion or priming of transendothelial effector T cell-migration via currently unknown mechanisms.
3 Treg Depletion in Experimental Stroke
Depletion of the regulatory T cell population has been used as an experimental paradigm to investigate the functional role of Treg in brain ischemia in several publications (Table 1). Methodologically, two different approaches have been used for Treg depletion, antibody-mediated cell lysis using CD25-specific antibodies or the use of transgenic mice with a diphtheria toxin receptor (DTR) transgene under the control of the Foxp3 promotor for inducible Treg depletion. Using Treg-depletion paradigms about half of the experiments performed revealed an increased infarct volume while the other half did not detect any effect on stroke outcome and one study even observed a reduction of infarct size in Treg-deficient mice (Table 1). This discrepancy of Treg depletion on stroke outcome led to an unsolved debate on their role and denomination of Treg as a “double-edged sword” in acute brain injury [20–22]. This discrepancy could not be attributed to the used depletion paradigm, since genetic deletion models can be found on both sides of the “efficacy spectrum”. Notably, the three publications using inducible Foxp3-KO mice used in each case a different mouse strain: Ren et al. [23] used the original Foxp3DTR mouse [24], Liesz et al. [19] the Foxp3.LuciDTR.4 mouse line [25], and Kleinschnitz et al. [26] the DEREG mouse [24]. All three of these DTR-transgene-mediated Foxp3-deletion models are using different transgene constructs, are variable in Treg-depletion efficacy and the used DT treatment protocol. Nevertheless, a systematic bias by the used depletion paradigm did not become evident for the authors. In contrast, it is apparent that the utilized stroke models, and more precisely the resulting volume of the ischemic lesion, do indeed predict a deterioration of outcome versus no effect or improved lesion size after Treg depletion (Table 1). In this line, both studies from our group have detected an increase of stroke volume after Treg depletion only in small, permanent ischemia lesion models but not after extensive infarction induced by transient MCAO [16, 19]. A recent study by Xie et al. has also detected an increase of infarct volume after antibody-mediated Treg depletion in a rat model of moderate brain ischemia with rapamycin pretreatment [27]. In contrast, all studies investigating effects of Treg depletion without detection of an effect on stroke outcome [16, 18, 19, 23, 28] or even an increase of lesion volume [26] were performed in transient mechanical occlusion models with extensive brain lesions.
The immunological effects of Treg depletion on the neuroinflammatory outcome after stroke have unfortunately only been investigated in a small fraction of the studies (Table 2). All three studies detecting an exacerbation of stroke outcome after Treg depletion also measured an associated increase in neuroinflammatory biomarkers [16, 19, 27]. The most robust findings were an increase in leukocyte brain invasion, particularly of lymphocyte subpopulations, and an increase in pro-inflammatory cytokine secretion such as TNF-α, IL-1β, IL-12, and IFN-γ. Among the studies using models of extensive brain injury with no effect of Treg depletion on stroke outcome, only one study [18] has investigated neuroimmunological readouts. Correspondingly to the missing effect on lesion volume, also inflammatory markers were not altered by the antibody-mediated Treg depletion in this study.
4 Treg-Therapies for Ischemic Stroke
Despite the obvious and still unresolved discrepancies arising from studies investigating effects of Treg depletion, a rapidly increasing number of reports have analyzed different strategies to increase the Treg number and/or function for experimental stroke therapy (Table 3). Out of 15 independent experiments reported in 13 studies, 13 have detected an improvement of stroke outcome while two have demonstrated an increase in infarct volume when using two independent Treg-targeted therapies. This discrepancy could not be attributed to the used therapeutic paradigm since both studies showing an exacerbation of lesion volume (adoptive Treg transfer and CD28SA) have been respectively tested in three or more other experiments that have been showing an improved outcome (Table 3). Again, it seems that the lesion size induced by the used stroke model might be a predictor of the neuroprotective effect of Tregs: out of the 13 experiments detecting a benefit of Treg-therapy, only two have reported infarct volumes in the control group of more than 40 % of the hemisphere, while the other studies deployed models inducing small to moderate-sized lesions. Yet, particularly two studies [29, 30] using both the CD28SA treatment in transient ischemia models with similar lesion size have reported contradicting results, indicating the presence of other confounding factors independent of lesion volume. We have performed in a recent review a Meta-analysis in order to assess an approximation of the efficacy of Treg-targeted therapeutic approaches in experimental stroke models [31]. For this, all studies having investigated interventions with the aim to modulate Treg (defined at least by CD25 or Foxp3 expressing CD4+ T cells) number and/or efficacy in models of experimental brain ischemia were included in the analysis. The overall effect size estimation revealed an odds ratio favoring Treg-targeted interventions [31]. However, a large heterogeneity between the included studies regarding study design, experimental model and species (rat and mouse) has to be taken into account. Therefore, the number of studies investigating Treg interventions has to be increased to more robustly estimate a realistic effect size. Also the publication of negative results has to be encouraged to avoid a publication bias in such an emerging research field as Treg-targeted cell therapies for acute brain injuries.
5 Mechanisms of Treg Function in Post-Stroke Neuroinflammation
Basic immunological research on Treg function has identified several mechanisms by which Treg cells suppress an immune reaction [32]. These can be mainly divided into mechanisms acting on lymphocyte activation and mechanisms inhibiting antigen-presenting cells of the innate immune system. While most of these mechanisms can be recognized using in vitro model systems, the contribution of individual mechanisms such as different anti-inflammatory cytokines or cell–cell-contact dependent suppression is highly diverse in between in vivo disease models. The most prominent mechanisms of Treg function in vivo are the secretion of anti-inflammatory cytokines (IL-10, TGF-β), expression of immunosuppressant molecules (CTLA-4, CD39, PD, consumption of vital cytokines (IL-1, IL-2) and the secretion of cytolytic molecules (granzymes, perforin) [6].
In the model of experimental brain ischemia, several studies have demonstrated that IL-10 is a critical neuroprotective cytokine regulating post-stroke neuroinflammation [33–36]. The main sources of cerebral IL-10 are regulatory lymphocytes (T and B cells) and microglia/monocytes. The key role of IL-10 as a primary effector mechanism of Treg cells in tissue injury [37] was verified in some of the experimental stroke studies [16, 19, 38]. Accordingly, strategies increasing lymphocyte-derived IL-10 production [19, 29, 35, 39] or therapeutic IL-10 administration [33, 36, 40] have been shown to be beneficial for stroke outcome. In addition, alternative Treg mechanisms have been identified also in brain ischemia models such as the expression of TGF-β and IL-35 [27] as well as a role of Treg cells in the PD-1/PD-L1 pathway. Pro-inflammatory lymphocytes and brain-resident microglia/macrophages have been characterized as the target cells for the immunosuppressive effect of Treg cells after brain injury. Studies that have identified a beneficial effect of Treg detected an inhibition of T cell brain invasion [16, 28], suppression of effector T cell proliferation [19], reduced production of lymphocytic and monocytic cerebral cytokines [27, 28, 41], suppression of microglia/monocyte activation [16] or a priming towards a M2-like microglial phenotype [27]. In contrast, studies detecting an exacerbation of stroke burden by Treg-targeted therapies [26, 30] have found an association of the increased lesion volume with amplified cerebral immune cell accumulation [30], however, it is assumable that the deleterious effects observed by Treg in these two studies might be independent of an immunological function but rather attributable to an impact on secondary microthrombosis [26].
6 Circulating Treg Cells After Stroke
Acute brain ischemia induces profound alterations of the peripheral immune reaction, encompassing peripheral immune activation in the acute phase after brain injury [42] and an immunosuppressive syndrome in the later phase [43]. In addition to the effect of Treg in modulating central neuroinflammation, their functional role has also been detected in alterations of the peripheral immune system after stroke. A consistent finding in the subacute phase after extensive experimental stroke is that cellular immunosuppression and splenic atrophy is accompanied by a relative expansion of Treg cells in spleen and blood [44, 45]. Interestingly, a recent report suggested that the adoptive transfer of Treg cells reduced on one side the systemic inflammatory reaction in the acute phase after stroke and on the other side also ameliorated the extent of immunosuppression (i.e., lymphopenia) in the later subacute phase [46]. This finding of a dualistic role of Treg in systemic immunity comparing the acute and subacute phases after stroke is well in line with the concept that the initial overactivation of the peripheral immune system might result in the later immunosuppression syndrome [47]. Thereby, the potent homeostatic function of Treg might suppress the initial systemic inflammation, attenuating the subsequent immune disturbances such as lymphopenia due to activation-induced lymphopenia cell death and exhaustion of antigen-presenting cells.
7 Regulatory T Cells in Stroke Patients
Only very few studies have investigated regulatory T cells in stroke patients. Understandably, these studies have focused on peripheral immune effects after stroke by investigating blood samples of patients with different stroke entities. The first study specifically analyzing Treg function after stroke by Hug et al. has found that Treg function is preserved in the context of post-stroke immunosuppression in contrast to the dysfunction of effector cell populations such as circulating monocytes or helper T cells [48]. In contrast, a second study has found reduced suppressive capacities of post-stroke Treg in female but not male stroke patients, proposing sex-specific effects on post-stroke peripheral immunity [49]. This difference between the studies could be explained by differing patient characteristics in terms of comorbidities and stroke severity. While the latter study has detected a robust increase of Treg cell counts after stroke in accordance with experimental studies, another report has shown the opposite. Li et al. reported a significant reduction of peripheral Treg in stroke patients [50]. In contrast to the previous studies investigating post-stroke effects on peripheral Treg function, a report by Wigren et al., analyzed the association of Treg counts in a cohort of 700 participants of the Malmö Diet and Cancer study with the prospective incidence of stroke. While low Treg counts at baseline were associated with an increased risk of myocardial infarction, this association was not present for stroke [51]. Overall, clinical data is supporting the experimental finding of substantial peripheral immunomodulation after stroke, which is also affecting the Treg population, yet, specific changes might depend on stroke entity, severity, and patient characteristics.
8 Potential Causes for Differential Effects of Treg Function in Post-Stroke Neuroinflammation
As previously discussed, considerable discrepancy on the role of Treg in stroke can be observed in the literature. Based on our current knowledge of mechanisms involved in post-stroke secondary neuroinflammation as well as the impact of acute brain injuries on the peripheral immune system [1, 43, 52], the most probable reasons for this phenomenon shall be discussed in the following paragraph.
8.1 Thromboinflammation and Secondary Microthrombosis
The commonly used transient MCA occlusion models by intraluminal filaments which were also used in the two studies detecting a deleterious role of Treg in stroke [26, 30] can be described as a transient mechanical vascular occlusion model (TMVO) with prompt revascularization. One prominent and distinct feature of this model is the occurrence of a delayed neuronal damage due to microthrombosis [53]. Notably, the occurrence of microthrombosis is not common to human stroke and animal models of permanent or gradual reperfusion [54] and might rather represent a subpopulation of stroke patients receiving endovascular reperfusion therapy with immediate recanalization of a proximal artery. In experimental models, the presence of such microthrombosis might be determined by the extent of endothelial damage induced by the filament insertion, speed of filament retraction, and occlusion time. Finally, these at first-sight minor methodological differences might have a major pathophysiological impact by the presence or absence of secondary thrombosis [54, 55].
8.2 The Impact of Lesion Volume on Stroke-Immunology
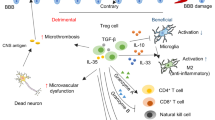
As noted above, it seems that lesion size might be a confounder independent of reperfusion. It is well acknowledged that the phenomenon of post-stroke peripheral immunosuppression occurs in stroke patients and animal stroke models only after extensive brain tissue injury [43, 45, 52, 56]. While mice and men with substantial brain lesions develop substantial lymphopenia and alterations of the monocyte population, small ischemic injuries induce only a minor immunomodulation but no immunosuppressive syndrome. While the impact of peripheral immunosuppression after major stroke on secondary neuroinflammation was to our knowledge not systematically investigated, it is conceivable that peripheral immune alterations might affect also central neuroinflammation. Indeed, a recent previous study performing a face-to-face comparison of the extent of cerebral leukocyte invasion, microglial activation, and cytokine secretion in three common models of brain ischemia of differing lesion size detected crucial difference in between models with manifold stronger inflammatory reaction in small permanent ischemia models than in extensive hemispheric lesions after transient MCA occlusion [17]. Therefore, it is plausible that regulatory T cells have an inferior role in stroke models with only minor bystander inflammation compared to the critical role of Treg cells in lesion models with an overshooting immune reaction (Fig. 2).
Differential role of regulatory T cells in experimental stroke models. The schematic diagram illustrates the discrepancy of the observed role of Treg in the brain (upper panels) versus peripheral immune system (lower panels) in models of permanent ischemia with small- to moderate-sized lesions or after extensive brain lesions in TMVO models. Permanent occlusion of the distal middle cerebral artery (MCA; left panels) induces a strong neuroinflammatory reaction, but preserves peripheral immune homeostasis with only minor immunomodulation. In this context, Treg have a primary role in inhibiting an overshooting inflammatory reaction mediated by pro-inflammatory leukocytes. It is assumed that this immunosuppressive function of Treg takes place in the periphery even before brain invasion. In contrast, TMVO models with extensive brain injuries (right panels) have less pronounced neuroinflammation, but induce an immunosuppressive phenotype of the peripheral immune system. Here, Treg have only a minor function in suppressing the neuroinflammatory response, and might even have a non-immunologic function in the manifestation of secondary microthrombosis and thromboinflammation. The principal role of Treg in TMVO models seems rather to be in ameliorating immune disturbances by inhibiting initial immunologic activation or overactivation and later immunosuppression, thereby preserving homeostatic systemic immune function. IL-10, interleukin 10; TGFβ, tumor growth factor β (Reproduced from Liesz et al., Stroke, 2015)
8.3 Milieu-Dependent Treg Function
Moreover, the above stated methodological difference including the presence of microthrombosis/thromboinflammation, induction of peripheral immune alterations, and the extent of neuroinflammation might have a direct impact on the functional properties of natural Treg cells. It is known from other disease paradigms that Treg function in vivo might be particularly dependent of the immunological milieu. For example in cancer research this phenomenon—very much alike to stroke-immunology—has been termed as the “Janus-faced function of Treg” [57, 58]. In addition, the currently predominant perception of neuroinflammation after acute brain injury and particularly stroke research as too much of a bad thing should be revisited. Several elegant reports by the groups of Michal Schwartz and Jonathan Kipnis have established the concept of “protective autoimmunity” (see Schwartz and Raposo [59] for a recent review of the concept). This concept ascribes secondary inflammation as a generally physiological and protective mechanisms in which too much of immune activation as well as immunosuppression might be deleterious. Accordingly, in certain situations of acute brain lesions and neurodegeneration models—determined by the time-dependent inflammatory milieu of the brain during disease progression—Treg depletion as well as its augmentation might negatively affect the outcome [60–62]. Therefore, the very specific properties of the used stroke model and targeted mechanism of Treg function have to be carefully investigated before considering Treg in a potentially oversimplified model as good or bad immune cells after stroke.
References
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808.
Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15(8):946–50.
Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120(18):3793–802. Epub Nov 1 2012. English.
Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134(Pt 3):704–20.
Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69(1):119–29.
Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500.
Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–8.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. Epub 1995/08/01. eng.
Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–64. Epub 2005/01/22. eng.
Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16(2):98–105. Epub 2005/12/28. eng.
Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116(5):949–59; quiz 60. Epub 2005/11/09. eng.
Battaglia M, Roncarolo MG. Induction of transplantation tolerance via regulatory T cells. Inflamm Allergy Drug Targets. 2006;5(3):157–65. Epub 2006/08/22. eng.
Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354(11):1166–76. Epub 2006/03/17. eng.
Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73.
Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–57.
Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–9.
Zhou W, Liesz A, Bauer H, Sommer C, Lahrmann B, Valous N, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol. 2013;23(1):34–44. Epub 2012/07/11. eng.
Stubbe T, Ebner F, Richter D, Randolf Engel O, Klehmet J, Royl G, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2012;33(1):37–47.
Liesz A, Zhou W, Na SY, Hammerling GJ, Garbi N, Karcher S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. 2013;33(44):17350–62. Epub 2013/11/01. eng.
Kleinschnitz C, Wiendl H. Con: regulatory T cells are protective in ischemic stroke. Stroke. 2013;44(8):e87–8. Epub 2013/07/04. eng.
Hu X, Li P, Chen J. Pro: regulatory T cells are protective in ischemic stroke. Stroke. 2013;44(8):e85–6. Epub 2013/07/04. eng.
Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009;158(3):1174–83. Epub 2008/07/16. eng.
Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+ FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26(1):87–90. PMCID: 3070853. Epub 2010/11/18. eng.
Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. PMCID: 2118432. Epub 2007/01/04. eng.
Suffner J, Hochweller K, Kuhnle MC, Li X, Kroczek RA, Garbi N, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3.LuciDTR mice. J Immunol. 2010;184(4):1810–20. Epub 2010/01/20. eng.
Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121(4):679–91. Epub 2012/11/20. eng.
Xie L, Sun F, Wang J, Mao X, Xie L, Yang SH, et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol. 2014;192(12):6009–19. PMCID: 4128178. Epub 2014/05/16. eng.
Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74(3):458–71. PMCID: 3748165. Epub 2013/05/16. eng.
Na SY, Mracsko E, Liesz A, Hunig T, Veltkamp R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke. 2015;46(1):212–20. Epub 2014/11/08. Eng.
Schuhmann MK, Kraft P, Stoll G, Lorenz K, Meuth SG, Wiendl H, et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cereb Blood Flow Metab. 2015;35(1):6–10. Epub 2014/10/16. Eng.
Liesz A, Hu X, Kleinschnitz C, Offner H. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke. 2015;46(5):1422–30.
Shevach EM. Review mechanisms of Foxp3 + T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45.
Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251(3):189–92.
Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, et al. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12(7):2265–72.
Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab Brain Dis. 2013;28(3):375–86. PMCID: 3737266. Epub 2013/05/04. eng.
Liesz A, Bauer A, Hoheisel JD, Veltkamp R. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci Lett. 2014;579:18–23. Epub 2014/07/16. eng.
O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114(10):1372–8.
Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J Immunol. 2003;171(12):6549–55.
Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233(1-2):125–32.
Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21(5):427–49.
Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E-selectin promotes the survival of newly generated neuroblasts via regulatory T-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009;29(3):606–20.
Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26(5):654–65. Epub 2005/08/27. eng.
Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–86.
Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176(11):6523–31. Epub 2006/05/20. eng.
Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, et al. The spectrum of systemic immune alterations after murine focal ischemia: immunodepression versus immunomodulation. Stroke. 2009;40(8):2849–58. Epub 2009/05/16. eng.
Li P, Mao L, Zhou G, Leak RK, Sun BL, Chen J, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44(12):3509–15. PMCID: 3895539. Epub 2013/10/05. eng.
Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–98.
Hug A, Liesz A, Muerle B, Zhou W, Ehrenheim J, Lorenz A, et al. Reduced efficacy of circulating costimulatory cells after focal cerebral ischemia. Stroke. 2011;42(12):3580–6.
Yan J, Read SJ, Henderson RD, Hull R, O’Sullivan JD, McCombe PA, et al. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol. 2012;243(1–2):89–94.
Li Q, Wang Y, Yu F, Wang YM, Zhang C, Hu C, et al. Peripheral Th17/Treg imbalance in patients with atherosclerotic cerebral infarction. Int J Clin Exp Pathol. 2013;6(6):1015–27. PMCID: 3657353.
Wigren M, Bjorkbacka H, Andersson L, Ljungcrantz I, Fredrikson GN, Persson M, et al. Low levels of circulating CD4+ FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32(8):2000–4.
Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8(7):401–10.
Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–6. PMCID: 3390813. Epub 2012/01/12. eng.
Gauberti M, Martinez de Lizarrondo S, Orset C, Vivien D. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J Thromb Haemost. 2014;12(3):409–14. Epub 2013/12/21. eng.
Gauberti M, Vivien D. Letter by Gauberti and Vivien regarding article, “amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice”. Stroke. 2015;46(2):e50–1.
Liesz A, Ruger H, Purrucker J, Zorn M, Dalpke A, Mohlenbruch M, et al. Stress mediators and immune dysfunction in patients with acute cerebrovascular diseases. PLoS One. 2013;8(9):e74839. PMCID: 3777986. Epub 2013/09/27. eng.
Levings MK, Allan S, d’Hennezel E, Piccirillo CA. Functional dynamics of naturally occurring regulatory T cells in health and autoimmunity. Adv Immunol. 2006;92:119–55. Epub 2006/12/06. eng.
Danese S, Rutella S. The Janus face of CD4+ CD25+ regulatory T cells in cancer and autoimmunity. Curr Med Chem. 2007;14(6):649–66. Epub 2007/03/10. eng.
Schwartz M, Raposo C. Protective autoimmunity: a unifying model for the immune network involved in CNS repair. Neuroscientist. 2014;20(4):343–58. Epub 2014/01/08. Eng.
Walsh JT, Zheng J, Smirnov I, Lorenz U, Tung K, Kipnis J. Regulatory T cells in central nervous system injury: a double-edged sword. J Immunol. 2014;193(10):5013–22. Epub 2014/10/17. eng.
Kipnis J, Schwartz M. Controlled autoimmunity in CNS maintenance and repair: naturally occurring CD4+ CD25+ regulatory T-Cells at the crossroads of health and disease. Neuromol Med. 2005;7(3):197–206.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+ CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99(24):15620–5.
Stubbe T, Ebner F, Richter D, Engel O, Klehmet J, Royl G, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab. 2013;33(1):37–47. PMCID: 3597367. Epub 2012/09/13. eng.
Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, et al. Mucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci U S A. 2003;100(25):15107–12. PMCID: 299916. Epub 2003/12/03. eng.
Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39(5):1575–82.
Li P, Mao L, Liu X, Gan Y, Zheng J, Thomson AW, et al. Essential role of program death 1-ligand 1 in regulatory T-cell-afforded protection against blood–brain barrier damage after stroke. Stroke. 2014;45(3):857–64. PMCID: 3939692. Epub 2014/02/06. eng.
Brea D, Agulla J, Rodriguez-Yanez M, Barral D, Ramos-Cabrer P, Campos F, et al. Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J Cell Mol Med. 2014;18(8):1571–9. Epub 2014/06/04. eng.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Liesz, A. (2016). Regulatory T Cells in Ischemic Brain Injury. In: Chen, J., Zhang, J., Hu, X. (eds) Non-Neuronal Mechanisms of Brain Damage and Repair After Stroke. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-32337-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-32337-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32335-0
Online ISBN: 978-3-319-32337-4
eBook Packages: MedicineMedicine (R0)