Abstract
The fact that cellular development and fate is very dependent, not just of the biochemical signals of the environment and of their own genetic background, but also of the mechanical properties and mechanical stimuli acting within the extra cellular matrix, has promoted the birth of a new field of science and technology at the interface of biology and engineering, that of mechanobiology. Such novel field focuses on the way that physical forces, stresses and strains, and changes in cell or tissue mechanics contribute to tissue development, to the success of physiological interactions and even to the appearance of disease. A major challenge in the field is linked to understanding the complex mechanisms by which cells sense and respond to mechanical signals: the mechanotransduction properties of cells. Although completely understanding how cells respond to mechanical stimuli and learning about their mechano-sensitive properties and about the mechanical forces they can develop is a complex task, the use of biomedical microdevices with controlled microstructures and microtextures can be a useful strategy for the progressive comprehension of single and collective cell behavior. This chapter provides some introductory examples of microsystems developed for generating knowledge for the field of mechanobiology. The cases of study include cell culture platforms for obtaining different types of cell aggregations, biomedical microsystems for studying the impact of surface texture on cell behavior and some final textured surfaces with details reaching nanometric details.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction to Mechanobiology and Its Connections with Tribology
A key element involved in tissue engineering processes is the extra cellular matrix or scaffold which serves as substrate or framework for cell growth, aggregation and tissue development (Langer and Vacanti 1993). These scaffolds must be porous so as to allow cell migration during the colonization process as well as the transport of nutrients and waste to and from cells, but they have to be also resistant enough to withstand possible mechanical demands, especially if final scaffold (or device) implantation is desired.
Additionally, as cells are able to feel their microenvironment and substrate texture upon which they lie by changing their morphology, cytoskeleton configuration, and intra- and extracellular signaling, increasing efforts are continuously being focused on advanced design and manufacturing technologies, so as to generate and modify the structures and surfaces of biomaterials. Aspects such as porosity, pore size, and surface microtexture promote cell adherence, migration and proliferation within the scaffold, for subsequent differentiation into relevant cell types. Thus, tissue progenitor cells and the scaffold plays a fundamental role in most tissue engineering strategies as its properties can deeply influence the global success of new tissue formation and the controlled fabrication of the scaffold structures is becoming increasingly important for novel approaches within regenerative medicine (Thomas et al. 2010; Chen et al. 2010; Buxboim and Discher 2010).
The fact that cellular development and fate is very dependent, not just of the biochemical signals of the environment and of their own genetic background, but also of the mechanical properties and mechanical stimuli acting within the extra cellular matrix, has promoted the birth of a new field of science and technology at the interface of biology and engineering, that of mechanobiology.
Mechanobiology focuses on the way that physical forces, stresses and strains, and changes in cell or tissue mechanics contribute to cell and tissue development, to the success of physiological interactions and even to the appearance of disease. A major challenge in the field is linked to understanding the complex mechanisms by which cells sense and respond to mechanical signals: the mechanotransduction properties of cells (Jacobs et al. 2012).
Cells and tissues can be therefore considered “smart materials and structures” capable of sensing and actuating as a consequence of external stimuli. The possibility of controlling their response by means of adequate stimuli opens new horizons, even beyond mechanobiology, for the development of cell-based sensors and actuators, as discussed in Chap. 21.
Although completely understanding how cells respond to mechanical stimuli and learning about their mechano-sensitive properties and about the mechanical forces they can develop is a complex task, the use of biomedical microdevices with controlled microstructures and microtextures can be a useful strategy for the progressive comprehension of single and collective cell behavior.
For instance, devices with micropillars have been found useful for measuring cell forces during movement, implants with different microtextures have led to different biological responses and osseointegration rates and microsystems with textured channels have shown the impact of roughness on cell dynamics.
In consequence, mechanotransduction is profoundly connected with the field of tribology, the science aimed at studying contact phenomena, including adhesion, lubrication and wear. Fundamental aspects for an adequate tribological performance of materials and components, such as surface roughness, material hardness, material elasticity, among others, also affect cell behavior and should be taken into account as parameters of influence for mechanobiology, as well as for the design of microsystems aimed at studying cell-material interactions, cell dynamics and cell development into relevant tissues. Micro- and nano-manufacturing resources and surface functionalization techniques also affect the tribological properties of materials and structures and play a key role in the development of microdevices for studying, understanding and modeling cell behavior and fate.
The following sections provide some examples of microsystems developed for generating knowledge for the field of mechanobiology.
2 Microstructured and Microtextured Systems for Studying Cell Mechanobiology
As previously mentioned, devices with micropillars have been found useful for measuring cell forces during movements (Trichet et al. 2012). In addition, devices with micropillars can be also submitted to resonance for applying controlled stimuli upon the cells attached to them. These micro-pillar structures can be manufactured resorting to several procedures, including subtractive ones, such as the use of UV-photo-lithography and chemical etching, and additive ones, such as the employment of high precision stereolithography, among other options (see Chap. 8 for further information on micro-fabrication of biomedical microdevices).
To show the potential of these micro-structured devices, Fig. 13.1 includes the finite-element model and the simulation results of a single cell interacting in different ways with a micro-pillar platform used as culture substrate for studying cell mechanobiology. These FEM-assisted simulations show the deformations of the cell during its expansion and during its movement and the related micro-pillar deformations. The lower images compare the displacement and stress solutions for the same loading case and boundary conditions (the cell crawling). The designs, models and simulations are performed using NX-8.5 (Siemens PLM Solutions).
Finite-element model and simulation results of cellular interactions with a micro-pillar platform for studying cell mechanobiology. Simulations show the deformations of a cell during expansion and movement and the related micro-pillar deformations. Lower images compare the displacement and stress solutions for the same loading case and boundary conditions (cell crawling). The deformation of pillars helps to assess the forces performed by the cells. Simulations performed with the help of NX-8.5 (Siemens PLM Solutions)
The most relevant aspect of these types of studies is that the displacements and deformations of pillars help to assess the forces performed by the cells (Sun and Fu 2013) during their development, growth, movement, interaction with the surrounding materials, gene expression, reproduction, evolution towards relevant tissues and death. Using high-precision imaging systems and related software resources, the deformations and stresses can be monitored in real time and relevant information regarding cell behavior can be obtained.
3 Microtopographies and Their Impact on Cell Behavior
The use of microtopographies is not only useful from a mechanobiological point of view, for obtaining useful information on the stresses and strains that cells can develop and on the mechanical aspects of cell-material interactions, but also for helping cells behave in a desired way, for promoting interesting cell-cell interactions and even for controlling the final tissues formed. Several studies have focused on the importance of surface topography and microtexture for promoting positive effects in all kinds of biomedical devices (De la Guerra et al. 2012), from implantable prosthesis to extra cellular matrixes and scaffolds for cell growth and tissue engineering. These textures have a significant influence in osseointegration of prosthesis, cell proliferation and tissue growth; given that those cells and tissues seem to be more “comfortable” and spread more quickly when faced with biodevices with similar surface properties. In addition the use of biomimetic surfaces can help to introduce numerous desirable phenomena in machine, mechanical and structural elements, thus improving contact between parts, reducing wear or even obtaining self-cleaning objects (Barthlott and Neinhuis 1997; Groenendijk 2007).
Consequently, topography also plays a determinant role in material selection in engineering design, especially in the field of micro and nanosystem development for biomedical engineering, where the effects of topography on the incorporation of advanced properties are even more significant. More specifically, in the field of tissue engineering and regenerative medicine, surface topography plays a determinant role in several strategies, both in vitro and in vivo, as it influences the attachment of cells to substrates for in vitro studies and to extra cellular matrices and implants aimed at tissue repair. It also helps control cellular dynamics and serves to orient cellular proliferation, aggregation and differentiation and has an impact on final tissue formation (Francesco et al. 2010; Kumar et al. 2012). As already introduced, cells are able to feel the microenvironment and substrate texture on which they develop, by changing their morphology, cytoskeleton configuration, intra- and extracellular signaling, and gene expression, therefore increasing efforts are continuously being focused on advanced design and manufacturing technologies capable of generating and modifying the structures and surface topographies of biomaterials (Thomas et al. 2010; Chen et al. 2010; Buxboim and Discher 2010).
However, the process of introducing desired roughness on the surfaces of man-made objects is still mainly linked to carrying out machining operations, laser processing or chemical attacks. In all these cases, post-processing operations can be difficult to control and it would be very positive to directly impose special topographies from the design stage. Fortunately, as detailed in Chap. 8, advances in computer-aided design and in high-precision additive manufacturing technologies, based on layer-by-layer deposition or construction, are opening new horizons for controlling the topographies of surfaces. They are being used from the design stage and can be applied in a manner that is very direct, rapid and simple. This is enabling the prototyping of multi-scale designs and hierarchical structures.
Even though conventional computer-aided design packages are only capable of handling Euclidean geometries and mainly rely on simple operations (sketch based operations, extrusions, pads, holes, circular grooves, etc.) for obtaining “soft” solids and surfaces, recent approaches relying on the use of matrix-based programming have already proved to be useful for designing rough surfaces and textured objects adequately described by fractal geometries (Mandelbrot 1982; Falconer 2003).
In parallel, the continued progress in additive manufacturing technologies, especially during the last decade, has increased the range of materials capable of being additively processed and greatly promoted their precision, even down to nanometric features. This has implications in the development of advanced materials and metamaterials, many of which benefit from multi-scale approaches (Bückmann et al. 2012; Röhrig et al. 2012).
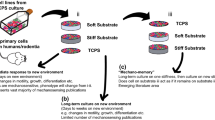
Some development examples have been already provided in Chap. 8. In this section we provide additional cases of study, linked to the complete development of cell culture platforms, with controlled surface topography. They are aimed at obtaining different types of cell aggregations with potential for the development of different types of tissues.
The cell culture platforms with soft and wavy surfaces shown in Fig. 13.2 are designed with the help of conventional CAD software (Solid Edge v.18), while those with fractal features are obtained by the processes previously described in Chaps. 6 and 8. Prototyping is accomplished in two steps by means of additive photopolymerization combined with a final diamond-like carbon coating obtained by physical-vapour deposition, in order to promote biocompatibility. Additional details can be found elsewhere (Díaz Lantada et al. 2012).
In vitro assessment is carried out using h-MSCs (human mesenchymal stem cells) expanded, seeded upon the cell culture platforms and visualized in a fluorescence inverted microscope (Olympus IX81) coupled to a CCD camera, following previously described processes (Javed et al. 2000; Romero-Prado et al. 2006). Results, summarized in Fig. 13.2, show the clear impact of surface topography on cell behavior and aggregation. The more planar substrate leads to a more expanded growth, while substrates with micro-bumps lead to aligned cell aggregations, which may be of interest for mimicking 3D cell dynamics. Larger topographies promote isolated compact growth, in the valleys of the microstructure, forming spherical aggregations, with potential application for promoting differentiation into osteocytes for bone repair. Regarding the impact of the DLC coating, it is important to highlight that it dramatically promotes the cell-scaffold interaction, helping to resolve the typical problems of photopolymeric resins with acrylic-based chemistry for in vitro trials and medical applications.
Next sections further explore the topic, providing also an example of a microsystem for studying the impact of surface topography on cell motility and detailing current capabilities of high-precision additive manufacturing for the development of biomedical microdevices capable of mechanically interacting at a cellular and even molecular level (Fig. 13.3).
4 Case Study: Microsystem for Studying the Impact of Microtextures on Cell Behavior
The proposed microsystem of present section includes two microchambers connected by several microchannels to guide cell movement, each with a different texture defined from the design stage at its bottom. The cell motility experiment should begin adding cells (with or without growth factors or nutrients) to one of the chambers and, eventually, adding growth factors, nutrients, chemicals, pathogens… to the other one, so as to promote or prevent cell movement from one chamber to another (details: Díaz Lantada et al. 2015).
The design presented here is obtained by means of design and manufacturing procedures detailed in Chaps. 6, 8–10. It is adapted to scales well suited to interacting at a cellular level and to enabling the cell culture processes without resorting to ultra-high precision manipulators.
In short, the overall structure, which mainly comprises the different walls of the two pools and the six microchannels, has been designed using conventional 3D computer-aided design methods. The CAD files can be converted into .stl (standard tessellation language) format, currently the most common file type used in 3D additive manufacturing. Different technologies, including digital light processing, conventional laser stereolithography, selective laser sintering, direct laser writing or melting and fused deposition modeling, allow .stl file as information input. The specific method chosen depends on the desired material and precision.
In our case we use an EnvisionTEC digital light processing machine with acrylic resin for the master prototypes. The 3D design can also be converted into a black-white mask for 2D½ manufacture of the overall structure using lithographic approaches typical of the electronic industry. Subsequently, to incorporate the desired microtextures (capable of interacting at a cellular level) into the channels, additional design operations rely on the generation of fractal-based geometries via matrix-based approaches.
In such matrix-based designs the geometries are stored in the form of [X, Y, Z(x, y)] matrices, where X and Y are column vectors with the x and y components of the working grid, and Z(x, y) is a column vector whose components are the height values for each (x, y) couple (spherical and cylindrical coordinates can be used for the cases of spherical and cylindrical meshes). Then, fractal features can be introduced to incorporate controlled random textures into the initially regular meshes (z0), as previously detailed in Chap. 6. We use a fractional Brownian fractal surface model for defining the microtextures and control them by changing the value of the typical parameter “alfa” from one channel to another.
The microtextures obtained following this strategy are more similar to the surfaces of biological materials than artificial textures based on pillars, struts, wood-pile structures or similar designs based on Euclidean geometry. An additional planar control channel is included for purposes of comparison.
The channels are 300 μm wide and around 7 mm long and the pools have a volume of 4 × 2 × 0.6 mm3. The intermediate walls between channels are 300 μm wide and 200 μm high, which in some cases promotes cell escape from some channels, but otherwise proves to be an adequate design. Surface roughness goes from 130 μm in the “softest” fractal channel to 250 μm in the roughest fractal channel. The planar channel can be just used as control if desired.
The master models are manufactured using digital light processing, a high precision rapid prototyping technology working on an additive approach that layer by layer projects on a photopolymer, images corresponding to the slices of the three-dimensional objects being built. For that purpose a Perfactory SXGA machine (EnvisionTec GmbH) is used, together with the R11 EnvisionTec acrylate based photo-resin. Figure 13.4 includes the master prototypes (red-orange resin) directly obtained from the three-dimensional geometries stored in the .stl computer-aided design files. As the acrylic resin used for obtaining the master models is not apt for cell culture processes, replication technologies are used for producing the final micro injected parts in poly(methyl methacrylate) (PMMA).
Using the master model, a mold insert for is manufactured. In short, the process includes gluing the masters to a thick copper substrate and evaporation for coating the master and substrate with layers of 7 nm chromium and 50 nm gold. This is followed by: (i) immersion in a galvanic bath for nickel electroplating until a thickness of 6 mm is reached, (ii) separation from the substrate and (iii) cutting and rinsing steps with ethyl acetate and acetone. The process leads to a stiff homogeneous metal block which can withstand the forces applied in the injection molding process. Additional details can be found elsewhere (Díaz Lantada et al. 2014, 2015).
The first action before starting the injection molding trials is to adjust the electroplated nickel mold inserts to a standard mold. Replication is accomplished on a Ferromatik Elektra 50S injection molding machine, which is equipped with the necessary features, such as tool evacuation and vario-thermal-temperization. This procedure allows for the replication of very fine structures with outstanding surface qualities. Figure 13.4 shows a replicated PMMA sample. Micro injection molding stands out for the degree of precision attainable and for the possibility of manufacturing large series of replicas for systematic trials. In our case, 200 copies of the PMMA microsystem were obtained and at least 10 of them were used to adjust the cell-culture processes.
The repeatability is outstanding and the final parts are compact, but without some of the typical injection molding problems, such as the presence of pores or wrapping, in spite of the precise dimensions of interest. The replicas obtained have several advantages when compared with the original masters. They are made of bioinert polymers typically used in the medical industry (polycarbonate and poly(methyl methacrylate)), and are therefore adequate for in vitro trials. They are also transparent, which constitutes an enormous help for cell culture processes and related fluorescent microscopy tasks, while it is easier to manipulate them thanks to a supporting structure.
In vitro assessment is carried out using h-MSCs (human mesenchymal stem cells) expanded, seeded upon the cell culture platforms and visualized in a fluorescence inverted microscope (Olympus IX81) coupled to a CCD camera, following previously described processes (Javed et al. 2000; Romero-Prado et al. 2006). Results are summarized in Figs. 13.5 and 13.6, which respectively show the impact of channel microtexture on cell adhesion and on cell motility.
According to the presented results, an increase on surface roughness promotes cell adhesion, with an almost linear tendency. This result is in accordance with the adherent nature of mesenchymal stem cells; an increase in the channel roughness allows them to extend the number of their focal adhesion points with the surface, thereby helping them maintain their energy balance by themselves.
Regarding cell motility, our interpretation is that the presence of an artificial surface roughness may promote opposing effects on the cells. On the one hand, the mere presence of obstacles and artificial textures may be sensed by the cells as something that must be avoided to maintain their energy metabolism. On the other hand, the increase in the surface to volume ratio and adhesion with the roughness and the fractal dimension may provide additional anchorage options for cell pseudopodia and help them crawl. At lower roughnesses the first effect seems to be prevalent, while at higher roughnesses the second effect stands out.
Taking into account that the cells have been cultured for 24 h in the motility study and that they have not reached the final pool, the proposed microsystem may also be useful for quantifying not only the impact of surface topography on cell adhesion, motility and behavior, but also for obtaining an estimation of cell velocity on different surfaces. In the microsystem, hMSCs reach a velocity value of 6000 μm/day, helping to show the positive impact of incorporating design-defined fractal microtextures to promote cell motility and eventual tissue growth.
In our opinion, the use of design-defined microtextured channels provides a more three-dimensional approach, in accordance with recent results, highlighting that 1D cell culture is more similar to 3D conditions than more conventional 2D cell culture on planar surfaces (Doyle et al. 2009). According to Doyle and colleagues, cell migration in both 1D and 3D is rapid, uniaxial and independent of extra cellular matrix ligand density, in contrast to 2D cultures, which brings out the need for alternative solutions aimed at a more adequate reproduction of the 3D environment (Doyle et al. 2009). We truly believe that our approach of providing 1D cell culture along channels, with inner three-dimensional features, can help with further studies linked to comparing 1D, 2D and 3D cell culture, as well as to take into account non-integer values, using a fractal-based definition of dimension. The noteworthy values of cell velocity obtained help to verify the potential of these kinds of design combinations.
The presented culture microsystem is aimed at the promotion of a real 3D cell culture environment and the microchannels may help cells to crawl in the adequate direction for a systematic cost- and time-efficient study. In fact, recent studies have put forward that 1D cell culture resembles the real behavior of cells in three-dimensional environments, than conventional 2D cell culture on dishes.
Combining manufacturing technologies in order to obtain similar microsystems to the one proposed here, but covering a wider range of surface topographies, from hundreds of nm to hundreds of μm, will be useful for increasing our knowledge of cell dynamics and response to surface topography. We expect to achieve this in forthcoming studies in collaboration with all colleagues that may find this approach of interest or, at least, of help as a complement to their current methods. Fractal-based design approaches, together with additive manufacture and replication techniques, may well indeed be useful for controlling such textures from the design stage, as detailed in the forthcoming section.
5 Case Study: Microtextured Platforms for Studying and Controlling Cell Behavior and Fate
Towards more precise studies, capable of addressing the mechanical behaviour of biological structures at sub-cellular and molecular levels, microsystems with nanometric features are required. Conventional additive manufacturing resources, commonly know as 3D printers, do not provide the required precision and novel high-precision processes are needed. Currently, two-photon polymerization is the most precise additive manufacturing technology and enables details down to nanometric scale. When combined with advanced computer-aided design and with additional processes for surface modification (see Chaps. 6, 8 and 9), very remarkable results can be obtained, regarding the development of biomedical microsystems for interacting at cellular and even molecular level.
The rough surfaces of Fig. 13.7 are designed using a fractional Brownian fractal surface model evaluated upon a working grid, in which points are separated 1 μm, as detailed in Chap. 6 and in previous microsystems with controlled roughnesses. The use of additional “mesh to solid” converters leads to final solid files, as those shown in Fig. 13.8, which can be used as normal CAD parts for further design, simulation and computer-aided manufacturing tasks.
The process can be adapted to the surfaces of any computer-aided designed implant and multi-scale designs are possible, normally using more conventional Euclidean surfaces for micrometric–millimetric features and the addition of the fractal term for the 100 nm–10 μm range. Hence, biomimetic approaches are promoted.
Even though converting the generated surfaces into solid .stl files is almost direct with CAD resources, subsequent geometry slicing (a typical operation of the software used for controlling layer by layer manufacturing machines) leads to very slow and expensive manufacturing processes. In our case, for speeding up the manufacturing process, the surfaces are obtained upon supporting pillars, as shown in the prototypes from Fig. 13.9. With this pillar-based design, a fractal surface of 40 × 40 μm2 can be manufactured in just 30 min, increasing production speed in more than an order of magnitude, when compared with the initial solid model, and reducing material and laser power consumption. The prototypes of the nano-textured surfaces are manufactured using the Photonic Professional System from NanoScribe GmbH (www.nanoscribe.de), the first commercial direct laser writing system.
Textured surfaces with nanometric details obtained by direct laser writing. Roughness can be controlled from the design stage and details are even smaller than single eukaryotic cells. Process developed in collaboration between Universidad Politécnica de Madrid and the Karlsruhe Institute of Technology, with support from the KNMF—Karlsruhe Nano-Micro Facility (http://www.knmf.kit.edu/) (additional details: Hengsbach and Díaz Lantada 2014)
In this system, the structures are not written layer-by-layer, but following three-dimensional paths connected from the beginning to the end of the writing process, so additional programming for converting the original CAD files into writable structures is usually needed. In any case, the attainable degree of precision is outstanding and opens new horizons in the field of biomimetic development of biomedical microsystems.
Multi-scale approaches are also possible, by combining different prototyping technologies, a more conventional 3D printer for the larger details and 2PP for the tiniest features (Hengsbach and Díaz Lantada 2014).
6 Main Conclusions and Future Research
Although completely understanding how cells respond to mechanical stimuli and learning about their mechano-sensitive properties and about the mechanical forces they can develop is a complex task, the use of biomedical microdevices with controlled microstructures and microtextures can be a useful strategy for the progressive comprehension of single and collective cell behavior.
This chapter has tried to provide some introductory examples of microsystems developed for generating knowledge for the field of mechanobiology. The cases of study detailed include cell culture platforms for obtaining different types of cell aggregations, biomedical microsystems for studying the impact of surface texture on cell behavior and some final textured surfaces with details reaching nanometric details.
As for the future, the use of the described procedures and technologies for the control of surface topography at micro- and nano-metric levels and its application to larger biomedical microdevices, with more complex geometries for studying cell-material and cell-cell interactions and for in vitro reproducing physiological phenomena is just a matter of time.
References
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8
Bückmann T, Stenger N, Kadic M, Kaschke J, Frölich A, Kennerknecht T, Eberl C, Thiel M, Wegener M (2012) Tailored 3D mechanical metamaterials made by dip-in direct-laser-writing optical lithography. Adv Mater 24:2710–2714
Buxboim A, Discher DE (2010) Stem cells feel the difference. Nat Methods 7(9):695
Chen WL, Likhitpanichkul M, Ho A, Simmons CA (2010) Integration of statistical modeling and high-content microscopy to systematically investigate cell-substrate interactions. Biomaterials 31:2489
De la Guerra Ochoa E, Del Sordo Carrancio D, Echávarri Otero J, Chacón Tanarro E, Díaz Lantada A, Lafont Morgado P (2012) The influence of textured surfaces on the lubrication of artificial joint prostheses. In: Biodevices 2012—international conference on biomedical electronics and devices. IEEE Engineering in Medicine and Biology Society
Díaz Lantada A, Endrino JL, Sánchez Vaquero V, Mosquera AA, Lafont Morgado P, García-Ruíz JP (2012) Tissue engineering using novel rapid prototyped diamond-like carbon coated scaffolds. Plasma Process Polym Online (4 Oct 2011). doi:10.1002/ppap.201100094; 9(1):98–107, 2012
Díaz Lantada A, Piotter V, Plewa K, Barié N, Guttmann M, Wissmann M (2014) Towards mass production of microtextured microdevices: Linking rapid prototyping with micro injection molding. Int J Adv Manuf Technol. doi:10.1007/s00170-014-6333-2
Díaz Lantada A, Alarcon Iniesta H, García-Ruíz JP (2015) Multi-channelled polymeric microsystem for studying the impact of surface topography on cell adhesion and motility. 7(11):2371–2388
Doyle AD, Wang FW, Matsumoto K, Yamada K (2009) One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 184(4):481–490
Falconer K (2003) Fractal geometry: mathematical foundations and applications. Wiley
Francesco G, Tirinato L, Battista E, Causa F, Liberale C, Di Fabrizio EM, Decuzzi P (2010) Cells preferentially grow on rough substrates. Biomaterials 31(28):7205–7212
Groenendijk M (2007) Self cleaning Lotus leaf imitated in plastic by using a femtosecond laser. University of Twente. www.physorg.com
Hengsbach S, Díaz Lantada A (2014) Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed Microdevices 16(4):617–627
Jacobs CR, Huang H, Kwon RY (2012) Introduction to cell mechanics and mechanobiology. Garland Science
Javed A, Guo B, Hiebert S, Choi JY, Green J, Zhao SC, Osborne MA, Stifani S, Stein JL, Lian JB, van Wijnen AJ, Stein GS (2000) Groucho/TLE/R-esp proteins associate with the nuclear matrix and repress RUNX (CBF(alpha)/AML/PEBP2(alpha)) dependent activation of tissue-specific gene transcription. J Cell Sci 113(12):2221
Kumar G, Waters MS, Farooque TM, Young MF, Simon CG Jr (2012) Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials 33(16):4022–4030
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920
Mandelbrot B (1982) The fractal geometry of nature. W.H. Freeman, San Francisco
Röhrig M, Thiel M, Worgull M, Hölscher H (2012) Hierarchical structures: 3D direct laser writing of nano-microstructured hierarchical gecko-mimicking surface. Small 8(19):3009–3015
Romero-Prado M, Blázquez C, Rodríguez-Navas C, Muñoz J, Guerrero I, Delgado-Baeza E, García-Ruíz JP (2006) Functional characterization of human mesenchymal stem cells that maintain osteochondral fates. J Cell Biochem 98:1457
Sun Y, Fu J (2013) Mechanobiology: a new frontier for human pluripotent stem cells. Integr Biol 5(3):450–457
Thomas WE, Discher DE, Shastri VP (2010) Mechanical regulation of cells by materials and tissues. MRS Bull 35:578
Trichet L, Le Digabel J, Hawkins RJ, Vedula SK, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B (2012) Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. PNAS 109(18):6933–6938
Acknowledgements
We gratefully acknowledge the support of the Karlsruhe Nano Micro Facility (KNMF, http://www.knmf.kit.edu/) a Helmholtz research infrastructure at the Karlsruhe Institute of Technology (KIT). Proposal KNMF-2013-010001542 (muFractal: Microsystem for studying the influence of fractal dimension on cell behaviour), linked to the rapid manufacture of microtextured microsystems, and proposal KNMF-2012-009001145 (Replic-AS: Replication of advanced scaffolds with biomimetic fractal features), linked to replicating the presented multi-channelled microsystem with fractal channels, and the co-authors and their teams that made them possible are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Díaz Lantada, A., de Blas Romero, A., García Ruíz, J.P., Alarcón Iniesta, H., Hengsbach, S., Piotter, V. (2016). Microstructured Devices for Studying Cell Adhesion, Dynamics and Overall Mechanobiology. In: Díaz Lantada, A. (eds) Microsystems for Enhanced Control of Cell Behavior. Studies in Mechanobiology, Tissue Engineering and Biomaterials, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-319-29328-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-29328-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29326-4
Online ISBN: 978-3-319-29328-8
eBook Packages: EngineeringEngineering (R0)