Abstract
Mitochondrial dysfunction is increasingly recognised as a cause of neurodegeneration in both primary mitochondrial diseases and common neurodegenerative diseases, including Parkinson’s, Alzheimer’s and Huntington’s diseases and amyotrophic lateral sclerosis (ALS).
In this chapter, we will focus on the molecular basis of mitochondrial dysfunction and the mechanisms bridging it to neurodegeneration. In the first part, we will summarise some basic concepts of mitochondrial biology. We will then cover paediatric diseases, including different types of encephalopathies, Leigh disease, leukoencephalopathies and a variety of syndromes with peculiar features. Finally, we will cover diseases in adults, including MNGIE disease, Friedreich ataxia and the disorders associated with alterations in mitochondrial dynamics and quality control. These include axonal Charcot–Marie–Tooth neuropathy, hereditary spastic paraplegia, and autosomal dominant optic atrophy, as well as the inherited and idiopathic forms of common neurodegenerative diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mitochondria
- Mitochondrial disease
- Leigh disease
- Leukoencephalopathy
- Encephalocardiomyopathy
- CoQ10 deficiency
- Mitochondrial DNA depletion syndrome
- Microphthalmia with linear skin defects
- Neurodegeneration with iron accumulation in the brain
- Friedreich ataxia
- Mitochondrial neurogastrointestinal encephalomyopathy
- Hereditary spastic paraplegia
- Charcot–Marie–Tooth peripheral neuropathy
- Dominant optic atrophy
- Parkinson’s disease
- Alzheimer’s disease
- Amyotrophic lateral sclerosis
1 Introduction
Mitochondria are essential organelles present in almost all eukaryotic organisms with key roles in a number of processes such as heat generation, calcium, iron and sulphur homeostasis, haem biosynthesis, cell quality control and apoptosis, etc. [1].
Since mitochondria are under the double genetic control of mitochondrial DNA (mtDNA) and nuclear DNA (nDNA), mutations in either genome can thus cause mitochondrial disease, by a number of different mechanisms. Defects of mtDNA are maternally inherited. Mutations can affect all mtDNA copies (homoplasmy) and in this case are often non-pathogenic variants or are present only in some copies of mtDNA (heteroplasmy). In this case, the percentage of mutated mtDNA is correlated to the severity of the disorder, and there is a threshold necessary for the expression of the biochemical defect and clinical phenotype. Mutations in nDNA are inherited as Mendelian traits, and therefore mitochondrial disorders may be transmitted as autosomal recessive (such as Leigh disease due to mutations in SURF1), dominant (for instance, progressive external ophthalmoplegia (PEO) due to mutations in ANT1) or X-linked (for instance, microphthalmia with linear skin lesions due to mutations in COX7B) traits. Tissue and organ function critically depends on adequate ATP production, especially in cells and tissues with high-energy demand, such as neurons and muscle fibres. This explains why primary disorders of mitochondrial bioenergetics usually cause neurodegeneration, heart abnormalities, and/or muscle weakness, leading to neuro(cardio)muscular disease in children and adults. Mitochondrial disorders are a group of highly heterogeneous conditions affecting humans at any age [2]. Individually rare, when taken as a whole, mitochondrial disorders are among the most frequent genetic diseases in humans, with an estimated prevalence of about 1 in 5,000 individuals in the European population, although healthy carriers are much more common [3].
In a large proportion of mitochondrial disease cases, it is still not possible to reach a molecular genetic diagnosis, so that more than 50 % of adult patients, and an even greater percentage of paediatric cases, remain genetically undefined. This gap between biochemical readout and genetic characterisation reflects the complexity and intricacy of the homeostatic pathways related to mitochondrial bioenergetics. In these cases, the diagnosis is thus based solely on biochemical and/or morphological findings, in skeletal muscle or, more rarely, in cultured fibroblasts, limiting the possibility of genetic counselling. Whilst the analysis of mtDNA is a relatively standardised procedure in most specialised centres, new technologies based on next-generation sequencing offer the realistic possibility of rapidly filling the gap between clinical/biochemical characterisation and genetic diagnosis in these diseases.
This chapter will focus on the repertoire of nuclear genes related to mitochondrial neurodegeneration in children and adults.
2 Diseases in Children
In mitochondrial disorders of infancy and childhood, clinical and genetic findings differ from those found in adults: in general, the phenotypes are much more severe, typically involving the brain, either in isolation or as part of a multisystem condition, and mutations in nDNA are more frequent than in adulthood.
The syndromes described in this chapter are related to mutations in nuclear genes, whose protein products are imported into the mitochondria. However, it should be noted that similar clinical phenotypes can also be observed in mtDNA mutations, as described in Chap. 3.
2.1 Leigh Disease
Leigh disease (LD) is the most frequent mitochondrial encephalopathy in infancy and childhood. LD is a neuropathological–neuroradiological entity characterised by focal bilateral lesions in one or more areas of the deep grey matter, including the basal ganglia, thalamus, brainstem, cerebellum and rostral spinal cord. The lesions are characterised by symmetrical areas of demyelination, gliosis, necrosis with spongiotic vacuolisation and capillary proliferation.
Neurological symptoms are related to the areas affected. Clinical signs at onset usually consist of hypotonia and psychomotor regression, followed by dystonia, ataxia, involuntary movements, seizures, spasticity, evidence of brainstem dysfunction as dysphagia and respiratory problems. Neurological signs are often accompanied by general symptoms, such as failure to thrive and recurrent vomiting. The peripheral nervous system is also frequently affected with axonal and demyelinating polyneuropathy. Lactic acidosis is a virtually constant finding.
MRI neuroimaging, an essential tool for diagnosis, typically shows high T2 signal in the involved areas, most frequently in the caudate nucleus and putamen, but also in the subthalamic nuclei, periaqueductal grey matter, tegmentum of the midbrain and pons, red nuclei and dentate nuclei (Fig. 4.1a, b).
In spite of the homogeneous clinical and neuroradiological presentation, at least 15 genes have been associated to LD. The most frequent biochemical findings are isolated defects of complex I, complex IV or complex V. Mutations have been identified in both nuclear and mitochondrial genes, encoding not only single respiratory chain (RC) subunits but also assembly factors of the RC complexes or enzymes catalysing the synthesis of haem moieties or cofactors (Table 4.1). Pyruvate dehydrogenase complex (PDHC) deficiency is also related to LD with a high number of mutations in PDHA1, an X-linked gene encoding for the alpha subunit of the first catalytic enzyme of the complex [4]. Sporadic de novo mutations and affected females due to skewed X inactivation are not uncommon. However, PDHC deficiency can also account for other mitochondrial encephalomyopathies, with early-onset and neuroradiological findings different from those of typical LD (see below). SURF1 is the most common nuclear gene involved in LD [5]. This gene encodes a 30 kDa protein involved in the formation of complex IV which explains the isolated, severe, generalised complex IV deficiency characterising the biochemical profile of this condition.
Although the precise mechanistic role of SURF1 is still poorly understood, it is thought to stabilise the S2–S3 assembly intermediate subcomplexes during COX assembly [6, 7]. The majority of the mutations (deletions, insertions, frameshift and nonsense) lead to a truncated protein, usually correlated to a severe phenotype, whereas patients carrying missense mutations may present with a milder phenotype characterised by later onset and longer survival [8–10].
SURF1 mutant patients often present some peculiar signs, such as open wide “staring” eyes with abnormal eye movements due to ocular ataxia, and hypertrichosis; the presence of these signs may orientate towards the correct diagnosis “at first glance”.
Occasional patients may have normal MRI or different MRI patterns, such as severe cerebellar atrophy or diffuse white matter involvement [11, 12].
2.2 Ethylmalonic Encephalopathy
A particular form of Leigh-like disease is ethylmalonic encephalopathy (EE). EE is an example of mitochondrial disorder caused by genetically determined poisoning of the respiratory chain [13]. The ETHE1 gene encodes the mitochondrial sulphur dioxygenase, an enzyme that takes part in the aerobic energetic exploitation of, and detoxification from, hydrogen sulphide, which is converted into sulphite and eventually fully oxidised to harmless sulphate. Mutations in ETHE1 lead to accumulation of sulphide, a toxic compound that inhibits the activity of enzymes such as COX and short-chain acyl-CoA dehydrogenase, acts as a potent vasodilator and damages the colonic mucosa and the endothelia of small vessels. EE is characterised by an early onset with microangiopathy, chronic diarrhoea and neurological signs such as microcephaly, hypotonia, psychomotor delay, seizures and pyramidal signs. High excretion of ethylmalonic acid in urine and thiosulphate in plasma is a pathognomonic biomarker of the disease. Severe deficiency of complex IV is detected in the muscle biopsy [14].
2.3 Leukoencephalopathy
In about 10–20 % of infantile mitochondrial diseases, diffuse white matter involvement has been reported as a predominant neuroimaging feature, with little or no involvement of other structures [15].
The white matter damage is associated with early impairment of mitochondrial energy production that is crucial for myelination and maintenance of compact myelin.
Leukoencephalopathy may be related to different mitochondrial syndromes and molecular/biochemical phenotypes (Table 4.2): (i) late onset syndromes such as mitochondrial neurogastrointestinal encephalomyopathy (MNGIE); (ii) isolated or combined OXPHOS deficiencies; (iii) multiple mitochondrial dysfunction syndromes (MMDS), due to mutations in genes involved in iron–sulphur (Fe–S) cluster biosynthesis and associated to defects of complexes I, II and III and PDHC [16]; (iv) mitochondrial disorders due to mutations in mtDNA translation, particularly defects of aminoacyl-tRNA synthetases, again associated with multiple defects in complex I-III-IV-V [17]; and (v) a still poorly defined miscellaneous group of conditions (e.g. mutations in APOPT1 or FBXL4).
In MNGIE, white matter alterations are caused by an alteration of the blood–brain barrier and their clinical implications are unclear.
Early-onset OXPHOS deficiencies associated with leukoencephalopathy are most commonly characterised by isolated complex I or II defects or by MMDS. Two major clinical presentations are reported: (i) psychomotor delay since the first months of life, failure to thrive, growth impairment and a rapidly progressive course resulting in severe spastic quadriparesis and cognitive impairment and (ii) acute onset after a free period with focal motor signs, sometimes seizures, and slowly progressive course, motor impairment being usually more severe than cognitive impairment.
The MRI pattern (Fig. 4.2a, b) discloses diffuse involvement of supratentorial deep white matter, often associated with large cystic lesions within the affected white matter and progressive vacuolisation. In an increasing number of cases, involvement of brainstem or spinal cord white matter has been reported to form a specific pattern, e.g. leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL) or leukoencephalopathy with thalamus and brainstem involvement and high lactate (LTBL) [18, 19]. LBSL was first reported as a peculiar leukoencephalopathy and later recognised as a mitochondrial disease, due to mutations in DARS2, the gene encoding the mitochondrial aspartyl-tRNA synthetase [20, 21]. The MRI pattern shows signal abnormalities in the centrum semiovale, but sparing the U fibres, posterior arm of the internal capsule, splenium of the corpus callosum, the cerebellar peduncles, the intraparenchymal tract of the fifth cranial nerve, down to the pyramids, and the dorsal columns and corticospinal tracts of the rostral spinal cord, LBSL was initially described as a relatively mild disorder, characterised by juvenile onset of slowly progressive ataxia, spasticity and dorsal column dysfunction; an increasing number of infantile onset cases have later been reported, characterised by more rapid neurological deterioration and – in the most severe cases – early death [19]. LTBL is an early-onset leukoencephalopathy hallmarked by unique MRI features involving the centrum semiovale but consistently sparing the periventricular rim; the corpus callosum, basal ganglia, thalamus, midbrain, pons, medulla oblongata and cerebellar white matter are also consistently affected [18]. A milder phenotype was reported, with biphasic improvement of the MRI and clinical stabilisation. The responsible gene, identified by exome next-generation sequencing, is EARS2 encoding the mitochondrial glutamyl-tRNA synthetase [18]. An autosomal recessive spastic ataxia frequently with leukoencephalopathy, thin corpus callosum and cerebellar atrophy has been associated with mutation in MARS2 [22].
In patients with neonatal onset, particularly those affected by PDHC deficiency or EF-Tu gene mutation, leukoencephalopathy is frequently combined with brain structural malformations, e.g. polymicrogyria [23, 24].
Other inherited forms of mitochondrial leukodystrophy must be considered in the differential diagnosis of non-mitochondrial entities, such as vanishing white matter disease, Alexander’s disease, Canavan’s disease and megalencephalic leukoencephalopathy with subcortical cysts. Brain proton spectroscopy (H+-MRSI) may be useful in these cases, as lactate concentration is usually high in brain lesions associated with mitochondrial disease [25]. However, a lactate peak may be found also in the active phase of other inherited leukodystrophies as well as in acute ischaemic or inflammatory lesions; likewise, its absence cannot rule out the diagnosis of a mitochondrial aetiology. A specific, albeit exceptionally rare, MRSI finding is a peak of succinate [26], hallmarking complex II deficiency, particularly SDHAF1 mutations [27].
2.4 Encephalocardiomyopathy
Combined encephalocardiomyopathy is a severe, usually fatal, early-onset mitochondrial condition.
Subjects may present signs of the disease in utero, including intrauterine growth retardation, polyhydramnios and malformations. Patients are often critically ill at birth, with severe heart failure and lactic acidosis. Clinical findings include hypertrophic – rarely dilating – cardiomyopathy, severe hypotonia, failure to thrive and respiratory distress. Microcephaly, facial dysmorphism and liver failure are occasionally present. The clinical course may be fulminant in the neonatal period. Patients who survive usually develop neurological impairment with psychomotor delay, ataxia, pyramidal or extrapyramidal signs, cognitive stagnation and abnormal ocular movements, often associated with an MRI pattern of Leigh disease or leukoencephalopathy.
The most frequent biochemical abnormalities in encephalocardiomyopathies are isolated defects of complex I, complex IV or complex V [15]. Complex I deficiency was reported in patients with encephalocardiomyopathy, carrying mutations in genes encoding structural subunits (i.e. NDUFS2, NDUFV2, NDUFA11) or complex I assembly factors (NDUFAF4, ACAD9); defects of complex IV are often associated to mutations in SCO2 or COX15, which are factors involved in the incorporation of copper or in the biosynthesis of the haem-a moiety, respectively, whereas defects of complex V are usually related to mutations in TMEM70, encoding a putative complex V assembly factor.
More recently other genotypes have been reported causing mitochondrial encephalomyopathies, with or no specific biochemical defects, for instance, MTO1 and GTPBP3 [28], encoding two partner proteins involved in the post-transcriptional maturation of mt-tRNAs, and mitochondrial ribosomal proteins MRP22 and MRPL44 [29].
2.5 CoQ10 Deficiency: Related Diseases
Coenzyme Q10 (CoQ10), or ubiquinone, is a lipoidal quinone that shuttles electrons to complex III. Different syndromes associated with CoQ10 deficiency in the brain/muscle have been described [15], including encephalomyopathy with seizures, ataxia or mental retardation; multisystem infantile encephalopathy, cardiomyopathy and renal failure; ataxia and cerebellar atrophy; Leigh syndrome with growth retardation; and an isolated myopathy. Several cases have been reported with recurrent myoglobinuria and with ragged-red fibres/lipid storage in the muscle.
Mutations in the CoQ10 biosynthetic genes COQ2, COQ4, COQ9, PDSS1 and PDSS2 were reported in patients with severe infantile mitochondrial syndromes and tissue CoQ10 deficiency [30]. Mutations in CABC1 (also known as ADCK3) usually cause spinocerebellar ataxia type 9. ADCK3 is the human ortholog of yeast CoQ8, whose specific function in CoQ10 biosynthesis is still unknown. Another form of ataxia with partial CoQ10 deficiency is due to mutations in APTX (aprataxin), causing ataxia-oculomotor apraxia syndrome, which suggest that CoQ10-associated ataxias are genetically heterogeneous and may be secondary [15].
2.6 Miscellaneous
The use of targeted sequencing of gene panels or exome sequencing is expanding the genetic characterisation of highly heterogeneous mitochondrial syndromes. Definite conclusions about genotype–phenotype correlation are difficult to draw in these cases, due to the exiguity of the cohort of patients so far reported in the literature. Here are a few examples.
Mutations in AIFM1, encoding a mitochondrial apoptosis-inducing factor (AIF), have been reported in early-onset, severe encephalopathy and axonal sensory–motor neuropathy [31], encephalomyopathy with moderate clinical severity, slow progressive course and cerebellar ataxia [32] and Charcot–Marie–Tooth X4 [33].
Mutations in TTC19 – encoding a subunit of mitochondrial respiratory chain complex III – have been reported in few patients with heterogeneous phenotypes ranging from early-onset neurodegenerative disorders [34–37] to adult forms with psychiatric manifestations and cerebellar ataxia [38, 39].
An early-onset encephalomyopathy with progressive cerebral atrophy and involvement of the white matter, deep grey nuclei and brainstem structures has been associated with mutations in FBXL4, encoding a mitochondrial F-box domain-containing protein [40].
And a few individuals with isolated COX deficiency and slowly progressive, early-onset encephalomyopathy of variable severity had loss of function mutations in APOPT1, a gene encoding a protein of the inner mitochondrial compartment, the function of which is at present unknown [41].
2.7 Mitochondrial Disorders Due to Defects of Nuclear–Mitochondrial Intergenomic Signalling: mtDNA Depletion Syndromes (MDS)
Mitochondrial DNA depletion syndromes (MDS) result from a defect in nuclear-encoded factors involved in mtDNA maintenance. A spectrum of disorders of increasing severity is associated to instability of mtDNA. The most striking examples of this are the diseases associated with mutations in POLG1, ranging from relatively mild phenotypes associated to the presence of multiple deletions to very severe ones associated to profound mtDNA depletion.
Profound reduction of the mtDNA copy number is the molecular hallmark of MDS [42]. MDS differ from other respiratory chain disorders, as mtDNA depletion may manifest only in specific organs and so the manifestations vary from tissue-specific mtDNA depletion to widespread multisystemic disorders. MtDNA depletion causes a combined respiratory chain deficiency of the complex I, III, IV and V. Biochemical analysis of MRC enzyme activities may be normal in the muscle if the muscle is not the affected tissue. Nine genes have been so far implicated in MDS (Table 4.3).
2.7.1 Hepatocerebral Forms: MDS3, MDS4A, MDS6 and MDS7
Hepatocerebral MDS are probably the most common variant of MDS. Mutations have been found in four genes.
2.7.1.1 MDS4A, Mutation in POLG1
Mutations in POLG1 are the most frequent cause of hepatocerebral MDS.
POLG1 encodes pol gamma A, the catalytic subunit of polymerase gamma, which is the only known mtDNA polymerase and is essential for mtDNA replication [43]. More than a hundred mutations have been identified in POLG1, associated with a wide clinical spectrum raging from MDS4A [44], also known as Alpers-Huttenlocher syndrome (AHS), to juvenile-onset spinocerebellar ataxia and epilepsy (SCAE) syndrome, its variant sensory ataxia neuropathy, dysarthria, and ophthalmoparesis and dominant or recessive adult-onset progressive external ophthalmoplegia (PEO). AHS is the most severe phenotype among hepatocerebral MDS. It was described by Bernard Alpers in 1931, long before being recognised as a mitochondrial disease and associated with mutations in POLG1 [44, 45]. AHS is hallmarked by diffuse progressive degeneration of the grey matter associated with a variable degree of liver involvement ranging from increased levels of hepatic enzymes in plasma to severe liver failure. The onset is in infancy or early childhood, although juvenile and adult cases have been reported [46, 47]. Principal clinical findings are psychomotor regression, refractory seizures and liver failure. Status epilepticus is common and poorly responsive to usual antiepileptic drugs; valproate should be avoided as it may precipitate hepatic failure [48]. In most patients with early onset, the course is rapidly progressive leading to death usually before 3 years of age [15, 49]. The MRI shows severe and progressive cortical and subcortical atrophy and often involvement of deep grey structures, particularly the thalami (Fig. 4.3 a, b). In the liver, mtDNA content can be decreased over 90 %.
2.7.1.2 MDS3, Mutation in DGUOK
Mutations in DGUOK are a frequent cause of infantile hepatocerebral MDS with over 80 reported patients and over 40 different mutations [50]. The mitochondrial deoxyguanosine kinase (dGK) mediates the phosphorylation of purine deoxyribonucleosides into the corresponding nucleotides. Most of the mutations found in DGUOK gene are nonsense changes, although missense mutations have also been found. MDS3 manifests at birth or during the first months of life, and liver involvement seems to be the most prominent feature. The patients present at onset with hepatic cytolysis, cholestasis, jaundice and hypoglycaemia. Encephalopathy may be mild and neurological symptoms appear usually during the course of disease: the patients develop hypotonia, nystagmus and developmental delay. Null mutations tend to cause hepatocerebral disease, whilst missense mutations can cause pure hepatopathy. Patients with hepatocerebral forms usually suffer mortality due to liver failure around the age of 2 years; patients with isolated hepatopathy have better prognosis and survive longer. Liver transplantation is indicated for patients with isolated hepatopathy. mtDNA deletion in the liver is severe (<10 % residual mtDNA amount) [51].
2.7.1.3 MDS6, Mutation in MPV17
MPV17 mutations have been described in MDS6 and in Navajo neurohepatopathy which has a high prevalence in Navajo populations caused by the missense mutation W50Q with a founder effect. MPV7 encodes for a protein that probably has multiple functions that are as yet still poorly understood; evidence obtained in yeast suggests its role in the structural preservation of the inner mitochondrial membrane and in the control of mtDNA maintenance and stability [52]. MDS6 may have different clinical presentations: an early phenotype with variable age of onset ranging from the first months of life till 5 years characterised by recurrent episodes of hypoglycaemia and severe progressive liver dysfunction and a later-onset form with moderate hepatopathy and progressive sensory-motor axonal neuropathy. All forms are also associated with variable degrees of demyelination both in central and peripheral nervous systems (see “leukoencephalopathy” previously in the text). The patients with early-onset forms usually go on to develop neurological symptoms as ataxia, hypotonia, dystonia and psychomotor regression. Liver transplant may be efficacious in the acute phase of liver dysfunction but will not prevent patients from developing neurological progressive syndrome. Marked mtDNA depletion in the liver, ranging from 5 % to 15 %, and multiple defects of mtDNA-related respiratory chain complexes are present in liver biopsy, whilst in the skeletal muscle, the amount of mtDNA may be only slightly reduced.
2.7.1.4 MDS7, Mutation in C10ORF2
C10ORF2 encodes for mtDNA helicase Twinkle protein that has a functional interaction with POLG and is necessary for mtDNA replication. Different phenotypes are related to mutations in C10ORF2 including autosomal dominant PEO, infantile-onset spinocerebellar ataxia (IOSCA) and hepatocerebral phenotype (MDS7). Neonatal or infantile onset of MDS7 is very rare, and clinical manifestations are those of severe hepatocerebral forms reminiscent of Alpers disease [53]. Later in infancy and adolescent, patients develop ataxia, sensory neuropathy, athetosis, sensorineural deafness and epilepsy. MRI shows atrophy of the cerebellum, brainstem and spinal cord. Lactate is elevated in plasma and CSF. More recently a novel homozygous mutation in C10ORF2 has been described associated with hepatocerebral forms and renal tubulopathy, suggesting an expanding phenotype in “hepato-cerebro-renal” [54].
2.7.2 Encephalomyopathic Forms: MDS5, MDS8A and MDS9
2.7.2.1 MDS5 and MDS9, Mutation in SUCLA2 and SUCLG1
SUCLG1 and SUCLA2 encode, respectively, the alpha- and beta-subunits of succinyl-CoA synthetase, a mitochondrial matrix enzyme that catalyses the reversible conversion of substrates succinyl-CoA and GDP/ADP to succinate and GTP/ATP in the tricarboxylic acid pathway. So far about 34 patients with SUCLA2 mutations have been reported with a high prevalence in the Faroe Islands due to a founder effect [55]. At onset patients have psychomotor delay with irritability and hypotonia, and then as the disease progress, they develop sensorineural deafness, severe spastic dystonic quadriparesis, athetoid or choreiform movements, feeding difficulty, failure to thrive, growth retardation and respiratory insufficiency. MRI shows cerebral, cerebellar and medulla oblongata atrophy and bilateral basal ganglia involvement [56]. Almost all the patients have increased urinary excretion of methylmalonic acid that should be considered as a useful marker although recently two cases without methylmalonic aciduria have been reported [57, 58]. SUCLG1 is rarer and presents with a similar, but usually worse phenotype and more severe mtDNA depletion in the muscle [59].
2.7.2.2 MDS8A, Mutation in RRM2B
RRM2B gene encodes the small subunit of p53-inducible ribonucleotide reductase, essential for the conversion of ribonucleoside diphosphates to deoxyribonucleoside diphosphates, which is crucial for mtDNA synthesis. Mutations in RRM2B have been reported in association to MNGIE-like phenotype or with a severe infantile encephalomyopathy with renal tubulopathy and mtDNA depletion or autosomal dominant progressive external ophthalmoplegia (PEO) with multiple mtDNA deletions. The infantile phenotype is characterised by early-onset hypotonia, failure to thrive, psychomotor delay, myopathy, central hypomyelination, sensorineural deafness and tubulopathy. The disease progresses leading to death in early months and lactic acidosis is a constant feature [60, 61].
2.8 Infantile Mitochondrial Neurological Diseases Not Related to MRC Dysfunction
2.8.1 Microphthalmia with Linear Skin Defects
The microphthalmia with linear skin defects (MLS) syndrome is an X-linked neurocutaneous disorder manifesting exclusively in females, suggesting embryonic lethality in hemizygous males. Affected individuals typically show uni- or bilateral microphthalmia and/or anophthalmia and linear skin lesions, usually associated with ocular anomalies, such as microcornea, coloboma, anterior chamber defects, optic nerve hypoplasia, etc. Developmental delay, abnormalities of the central nervous system, short stature and cardiac defects have also been observed.
Three mitochondrial genes have been found implicated in the pathogenesis of this disease [62]: HCCS, encoding the mitochondrial holocytochrome c-type synthase; COX7B, encoding a poorly characterised structural subunit of cytochrome c oxidase; and NDUFB11, encoding a poorly characterised supernumerary subunit of CI.
2.8.2 Neurodegeneration with Brain Iron Accumulation
Neurodegeneration with brain iron accumulation (NBIA) is a family of degenerative extrapyramidal monogenic disorders characterised by focal accumulation of iron in the brain, usually in the basal ganglia [63]. They are characterised by early or late onset, with the main symptoms associated to problems in movement, spasticity and cognitive impairment. NBIA is a general definition gathering a number of diseases due to mutations in genes, some of which are present in mitochondrial isoforms, including: pantothenate kinase-associated neurodegeneration, PKAN, due to mutations in PANK2; phospholipase 2, group VI-associated neurodegeneration (PLAN), due to mutations in PLA2G6; mitochondrial membrane protein-associated neurodegeneration (MPAN), due to mutations in C19ORF12, a gene of unknown function; fatty acid hydroxylase-associated neurodegeneration (FAHN), due to mutations in FAH2; and COASY protein-associated neurodegeneration (CoPAN), due to mutations in the CoA synthetase gene COASY.
A common feature of PKAN and PLAN is the fragmentation of the mitochondrial network with alteration of cristae morphology, probably linked to impaired synthesis (PanK2) or the remodelling (iPLA2β) of membrane lipids, suggesting a role for lipid metabolism alterations in the pathogenesis of the disease [64].
3 Diseases in Adults
3.1 Friedreich Ataxia
Friedreich ataxia (FRDA) is an autosomal recessive disorder caused by pathological GAA trinucleotide repeat expansions in the first intron of the FXN gene [65]. The encoded protein, frataxin, is a mitochondrial matrix, 21 kDa polypeptide involved in the formation of iron–sulphur clusters, which are critical components of the mitochondrial respiratory chain complexes and of many redox enzymes not only in mitochondria, but also in the cytoplasm and nucleus. FXN mutations impair OXPHOS and result in abnormal accumulation of intra-mitochondrial iron, which eventually reaches toxic levels. In addition, since frataxin has antioxidant properties, cellular defences against ROS are impaired in FRDA, further exacerbating neuronal damage. The onset of the disease is in the second decade of life, with progressive gait ataxia, loss of deep tendon reflexes, dysarthria, distal limb weakness, pes cavus, scoliosis and arrhythmias secondary to hypertrophic cardiomyopathy. In addition, optic nerve dysfunction is often present, although FXN mutations probably cause RGC loss via other disease mechanisms compared with OPA1 and the primary mtDNA LHON mutations [66].
3.2 MNGIE
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an autosomal recessive disease caused by loss-of-function mutations in TYMP, encoding thymidine phosphorylase (TP), a key enzyme in the degradation of pyrimidine nucleosides, thymidine (dThd) and deoxyuridine (dUrd) [67]. In MNGIE patients, TP deficiency leads to systemic accumulation of dThd and dUrd. This overload results in imbalanced mitochondrial deoxynucleotides, which impairs replication of mitochondrial DNA (mtDNA), and accumulation of point mutations and multiple deletions, resulting in mitochondrial dysfunction. The clinical phenotype is multisystemic, the skeletal and smooth muscle and peripheral nerves being exquisitely affected. As a result, polyneuropathy, myopathy with CPEO and severe impairment of intestinal peristalsis lead to progressive cachexia and neuromuscular impairment. As already mentioned, a functional leukoencephalopathy of uncertain significance is a virtually consistent MRI finding.
3.3 Diseases of Mitochondrial Dynamics and Quality Control
Mitochondria are tightly regulated by a complex quality control system, acting at either the organellar or protein level (see Chaps. 11 and 12). Mitochondria are highly dynamic organelles, undergoing continuous fusion and fission events [68] (see Chap. 7). In general, mitochondria undergoing fusion are protected from degradation, whereas fragmented organelles are prone to disposal [69]. Dynamin-related GTPases on the OM (mitofusins, MFN1 and MFN2) and IM (OPA1) control the fusion process, whereas fission is regulated by the cytosolic soluble dynamin-related protein 1 (DRP1). DRP1 interacts with docking adaptors (FIS1, MFF and MiD49/51), which form spiral filaments that constrict around mitochondria determining fission. This process is important not only for the distribution of mitochondria during cell division, but also for the elimination of dysfunctional mitochondria, as fragmented organelles are targeted for autophagic degradation, a process called mitophagy (see Chap. 11). Mitophagy is controlled by the PTEN-induced putative kinase 1 (PINK1) and by parkin [70]. PINK1 targets to mitochondria, but under normal conditions, it is rapidly degraded by a number of mitochondrial intermembrane and matrix peptidases [71], whereas parkin is a cytosolic component of E3 ubiquitin-protein ligase that ubiquitinates proteins targeted for degradation. When mitochondria depolarise, PINK1 is prevented from degradation and accumulates on the outer mitochondrial membrane, where it phosphorylates MFN2, which acts as a receptor for parkin. Parkin is then brought in close proximity to and phosphorylated by PINK1, initiating the ubiquitination of downstream targets (discussed in detail in Chap. 11).
A second line of defence operates within mitochondria and consists of chaperones and proteases, which promote folding of newly imported pre-proteins, protect mitochondrial proteins against stress and degrade irreversibly damaged polypeptides [72]. Once a mitochondrial precursor protein has been imported into the mitochondria, it is processed by the mitochondrial processing peptidase (MPP), which removes the mitochondrial signalling peptide that then gets degraded by the presequence peptidase (PreP). The mature protein is subsequently folded by the chaperones of the Hsp60 and Hsp70 families. Misfolded proteins are eventually degraded by the Lon protease.
In the IM, protein quality control is monitored by two AAA proteolytic complexes: the intermembrane space-ATPase Associated with various cellular Activities (i-AAA) protease and the matrix-ATPase Associated with various cellular Activities (m-AAA) protease. The i-AAA protease is a homo-oligomeric machine composed of a single protein subunit (YME1L). Conversely, the m-AAA protease is composed by either homo-oligomeric or hetero-oligomeric complexes composed of paraplegin (encoded by SPG7) and AFG3L2 [72].
Notably, the two quality control systems are somehow interconnected. In fact, the machinery controlling the dynamic behaviour of the mitochondrial network through balanced fusion and fission is controlled by the activity of the mitochondrial proteases, which, in turn, thus control the fission–fusion processes. An example is OPA1, a master regulator of mitochondrial fusion, whose processing is controlled by YME1L and by another IM metallopeptidase (OMA1) [73]. This regulation has a profound impact on cell survival. In fact, fusion is a pro-survival mechanism protecting against apoptosis and neurodegeneration, whilst fission occurs early during mitophagy and apoptosis.
3.3.1 Hereditary Spastic Paraplegia
Pathogenic mutations in the SPG7 lead to an autosomal recessive form of hereditary spastic paraplegia (HSP), a progressive disorder characterised by weakness, spasticity (muscle rigidity) and loss of the vibratory sense of the lower limbs. HSP is caused by the selective retrograde degeneration of the longest motor and sensory axons of the central nervous system, the corticospinal tracts and the fasciculus gracilis.
Mutations in AFG3L2 have been associated with an autosomal dominant form of spinocerebellar ataxia, SCA28 [74]. SCA28 patients are affected by a slowly progressive gait and limb incoordination of juvenile onset, dysarthria, hyperreflexia at lower limbs, nystagmus, ptosis and ophthalmoplegia. Accumulation of multiple mtDNA deletions has been described in patients with mutations in both AFGL3L2 and SPG7, encoding paraplegin, the molecular partner of AFGL3L2 in the AAA+ complex [75, 76].
A homozygous mutation in AFG3L2 in a highly conserved tyrosine at the beginning of the peptidase domain to a cysteine has been associated with an early-onset syndrome characterised by severe spastic paraplegia, ataxia, ptosis, oculomotor apraxia, dystonic movements and stimulus-induced myoclonus [77]. Strikingly, this phenotype combines manifestations of both HSP and SCA28 along with clinical features of other mitochondrial diseases (such as ptosis, ophthalmoplegia and myoclonic epilepsy). Defective mitochondrial translation has been documented in a Afg3l2 –/– mouse model of the disease, suggesting a critical role of this process in the pathogenesis of the disease [78].
3.3.2 Charcot–Marie–Tooth Peripheral Neuropathy
Charcot–Marie–Tooth (CMT) disease is characterised by progressive degeneration of the peripheral nerves [79]. Patients develop distal muscle weakness and sensory loss, but depending on the actual causative gene, there can be quite marked variations in age of onset and rate of clinical progression. Mutations in MFN2 have been found in families with CMT2A, an autosomal dominant form of CMT [80]. The MFN2 gene consists of 19 exons and codes for a 757 amino acid, dynamin-related GTPase protein, which is anchored within the outer mitochondrial membrane. Although severe peripheral neuropathy with prominent proprioceptive loss is the main clinical sign, some patients show subacute visual failure and optic atrophy [79]. In other cases, a multisystem encephalomyopathy associated with accumulation of multiple deletions.
3.3.3 Dominant Optic Atrophy
Dominant optic atrophy (DOA) is the most common inherited optic nerve disorder. Although the onset may be in childhood, symptoms progress very slowly and poor vision becomes manifest in adulthood. Neuronal loss is typically limited to retinal ganglion cells (RGC), particularly those forming the papillo-macular bundle, leading to progressive optic nerve degeneration and visual failure. In most of the cases, DOA is due to heterozygous mutations in OPA1, encoding a dynamin-related GTPase localised in the inner mitochondrial membrane, although several other loci have been found but not characterised except OPA3, whose role and localisation are however still debated. OPA1 is by far the best characterised DOA gene [79]. It is expressed ubiquitously but particularly enriched within the RGC layer and photoreceptors. Accordingly, OPA1 mutations can affect other CNS populations, peripheral nerves and skeletal muscle (the so-called DOA+ phenotypes).
The majority of OPA1 mutations result in premature termination codons, leading to truncated mRNA species, which are rapidly degraded via nonsense-mediated mRNA decay. Haploinsufficiency, therefore, is thus a major disease mechanism in DOA. The spectrum of OPA1-linked phenotypes now encompasses a wide range of prominent neuromuscular features such as ataxia, myopathy, peripheral neuropathy and classical chronic progressive external ophthalmoplegia (CPEO), with unusually high frequency of late-onset Parkinsonism [81].
4 Secondary Mitochondrial Involvement in Neurodegeneration
Mitochondrial impairment has been proven to be involved in the pathogenesis of a number of classical neurodegenerative disorders including Parkinson’s disease and Parkinsonisms. In other neurodegenerative conditions, such as Huntington’s disease, Alzheimer’s disease and amyotrophic lateral sclerosis, a role of mitochondrial dysfunction has been hypothesised, but more work is needed to test this mechanistically.
4.1 Parkinson’s Disease
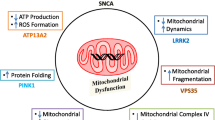
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases in the ageing population. It is characterised by the clinical triad of rigidity, bradykinesia and tremor, due to the loss of dopaminergic neurons in the substantia nigra with the presence of typical intracytoplasmic ubiquitin- and α-synuclein-positive inclusions, dubbed Lewy bodies. The link between mitochondrial dysfunction and PD stems from several observations, including those that neurotoxins which affect respiratory chain complex I induce specific death of dopaminergic neurons. Multiple mtDNA deletions accumulate in dopaminergic neurons, and a number of causative genes in familial forms of PD have been found in juvenile-onset familial Parkinson’s disease [82]. These include PINK1, Parkin and DJ1, encoding a mitochondrial and cytosolic chaperone; and LRRK2, encoding a protein primarily localised to the cytoplasm and membranes, including the mitochondrial membrane, where it has multiple functions such as kinase, GTPase and scaffolding protein [83].
4.2 Huntington’s Disease
HD is a devastating neurodegenerative disease resulting from an expansion of CAG triplet repeats in the HTT gene. This causes a corresponding expansion of the polyglutamine tract in the N-terminal region of the huntingtin protein. This polyQ expansion eventually leads to the accumulation of intracellular HTT aggregates [84]. Neurodegeneration occurs in the striatum during early pathogenesis, but broad areas of the brain are also affected during later stages of disease progression. Changes in mitochondrial dynamics have been found in HD patients and animal models of the disease, whose mitochondria are excessively fragmented and show decreased motility and respiration. This effect could be mediated by Drp1, a potential target of mutant HTT.
4.3 Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia, affecting over 24 million people worldwide [85]. The onset is usually between 60 and 70 years of age. AD is clinically characterised by memory loss and impairment of other cognitive including language, orientation, constructional abilities, abstract thinking, ability to solve problems, etc. The two main neuropathological hallmarks currently used to diagnose AD are the accumulation of intracellular neurofibrillary tangles, consisting mainly of hyperphosphorylated forms of the microtubule-associated protein tau, and the deposition of extracellular neuritic plaques mainly composed of β-amyloid (Aβ), a small peptide derived from processing of the amyloid-β precursor protein (APP; gene APP). The vast majority of cases of AD are sporadic, although in about 1 % of patients the disease is inherited (usually as an autosomal-dominant trait) as a result of mutations in one of three genes: APP, PSEN1 (presenilin-1; PS1) and PSEN2 (presenilin-2; PS2). PSEN1 and PSEN2 are components of the γ-secretase complex, which cleaves the C-terminal membrane domain of APP to produce Aβ peptides. Clinically, familial AD (FAD) is similar to the sporadic form (SAD), but has an earlier age of onset and is more aggressive [86].
The pathogenesis of the disease is highly debated, but two main hypotheses are nowadays the most accepted [85]. The first model is the “amyloid cascade” hypothesis, which proposes that the disease is due to the accumulation of Aβ1–42 plaques, which are toxic to the cells. The resulting cellular stress triggers tau hyperphosphorylation via upregulation of calcium-sensitive kinases, leading to tangle formation.
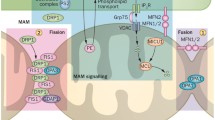
The alternative is the mitochondrial cascade hypothesis, which stresses the role of age-dependent mitochondrial dysfunction and increased ROS production in Aβ deposition and AD pathogenesis. Mitochondrial dysfunction is among the earliest observed pathogenic alterations, detected well before the accumulation of neuritic plaques. A growing number of studies are emerging which report impaired mitochondrial functions including electron transfer, ATP synthesis, mitochondrial transcription, translation and protein synthesis, upregulation of voltage-dependent anion channel (VDAC) and increased production of reactive oxygen species (ROS) in AD patients and in AD transgenic mouse models. The Aβ accumulation in mitochondria from postmortem AD brains and cellular and transgenic mice models has been well documented, providing evidence that Aβ is physically localised within or on the surface of mitochondria, where it has been reported to interact with a number of factors, such as Drp1, Aβ-binding alcohol dehydrogenase (ABAD), cyclophilin D (CypD), cytochrome c oxidase, VDAC and hPreP. However, the connection between mitochondrial dysfunction and the progression of AD is not fully understood, especially whether mitochondrial dysfunction triggers Aβ accumulation, or it is the latter that cause mitochondrial impairment. In addition, it is still unclear how Aβ enters mitochondria [87]. Notably, it has been recently observed that the presenilins and the γ-secretase activity are predominantly located in the mitochondria-associated ER membranes (MAMs), a dynamic and highly specialised subdomain of the ER facing to and connected with the mitochondrial outer membrane, playing essential roles in lipid synthesis and transport between the two organelles, fatty acids, glucose and cholesterol metabolism, Ca2+ homeostasis and apoptosis [88].
4.4 Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is the most prevalent, adult-onset, motor neuron disease, characterised by the degeneration of upper and lower motor neurons, progressive muscle weakness and atrophy, leading to muscle paralysis.
Approximately, 90 % of patients develop ALS with unknown aetiology, classified as sporadic ALS (sALS), whereas the remaining 10 % are familial ALS cases (fALS) due to genetic defects [89]. An increasing number of genes are being linked to ALS. Superoxide dismutase 1 (SOD1) was the first gene to be discovered as responsible for fALS [90]. SOD1 mutations account for 20 % of fALS dominant cases, whereas they are rare in idiopathic cases. In the last decade, a number of mutations have been identified in several DNA-/RNA-binding proteins (e.g. TDP-43 and FUS). Other fALS genes, such as VCP and SQSTM1, are related to proteostatic pathways and cytoskeletal and axonal transport, such as PFN1 and DCTN1. Recently, the largest proportion of fALS cases (40 %) has been linked to intronic hexanucleotide repeat expansions in C9ORF72, a gene with still unknown function.
The proposed pathogenic mechanisms include oxidative stress, toxic activity of misfolded and aggregated proteins, endoplasmic reticulum stress, mitochondrial dysfunction and axonal disorganisation, including organelle transport defects [89].
5 Conclusion
The finding of numerous mitochondrial genes involved in mitochondrial neurodegeneration has highlighted the central role of mitochondria in neurodegenerative processes. The elucidation of the pathways in which these genes are involved will shed new light not only on the specific disease, but likely on the processes of idiopathic neurodegenerative diseases.
References
Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Ann Rev Genet. 2005;39:359–407.
Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004;127(Pt 10):2153–72.
Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83(2):254–60.
Matthews PM, Marchington DR, Squier M, Land J, Brown RM, Brown GK. Molecular genetic characterization of an X-linked form of Leigh’s syndrome. Ann Neurol. 1993;33(6):652–5.
Finsterer J. Leigh and Leigh-like syndrome in children and adults. Pediatr Neurol. 2008;39(4):223–35.
Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet. 2001;106(1):46–52.
Zeviani M, Corona P, Nijtmans L, Tiranti V. Nuclear gene defects in mitochondrial disorders. Ital J Neurol Sci. 1999;20(6):401–8.
Lee IC, El-Hattab AW, Wang J, Li FY, Weng SW, Craigen WJ, et al. SURF1-associated Leigh syndrome: a case series and novel mutations. Hum Mutat. 2012;33(8):1192–200.
Piekutowska-Abramczuk D, Magner M, Popowska E, Pronicki M, Karczmarewicz E, Sykut-Cegielska J, et al. SURF1 missense mutations promote a mild Leigh phenotype. Clin Genet. 2009;76(2):195–204.
Tiranti V, Jaksch M, Hofmann S, Galimberti C, Hoertnagel K, Lulli L, et al. Loss-of-function mutations of SURF-1 are specifically associated with Leigh syndrome with cytochrome c oxidase deficiency. Ann Neurol. 1999;46(2):161–6.
Rahman S, Brown RM, Chong WK, Wilson CJ, Brown GK. A SURF1 gene mutation presenting as isolated leukodystrophy. Ann Neurol. 2001;49(6):797–800.
Salviati L, Freehauf C, Sacconi S, DiMauro S, Thoma J, Tsai AC. Novel SURF1 mutation in a child with subacute encephalopathy and without the radiological features of Leigh Syndrome. Am J Med Genet Part A. 2004;128A(2):195–8.
Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15(2):200–5.
Tiranti V, D’Adamo P, Briem E, Ferrari G, Mineri R, Lamantea E, et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am J Hum Genet. 2004;74(2):239–52.
Uziel G, Ghezzi D, Zeviani M. Infantile mitochondrial encephalopathy. Semin Fetal Neonat Med. 2011;16(4):205–15.
Invernizzi F, Ardissone A, Lamantea E, Garavaglia B, Zeviani M, Farina L, et al. Cavitating leukoencephalopathy with multiple mitochondrial dysfunction syndrome and NFU1 mutations. Front Genet. 2014;5:412.
Diodato D, Ghezzi D, Tiranti V. The mitochondrial aminoacyl tRNA synthetases: genes and syndromes. Int J Cell Biol. 2014;2014:787956.
Steenweg ME, Ghezzi D, Haack T, Abbink TE, Martinelli D, van Berkel CG, et al. Leukoencephalopathy with thalamus and brainstem involvement and high lactate ‘LTBL’ caused by EARS2 mutations. Brain. 2012;135(Pt 5):1387–94.
van Berge L, Hamilton EM, Linnankivi T, Uziel G, Steenweg ME, Isohanni P, et al. Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain. 2014;137(Pt 4):1019–29.
Scheper GC, van der Klok T, van Andel RJ, van Berkel CG, Sissler M, Smet J, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007;39(4):534–9.
van der Knaap MS, van der Voorn P, Barkhof F, Van Coster R, Krageloh-Mann I, Feigenbaum A, et al. A new leukoencephalopathy with brainstem and spinal cord involvement and high lactate. Ann Neurol. 2003;53(2):252–8.
Bayat V, Thiffault I, Jaiswal M, Tetreault M, Donti T, Sasarman F, et al. Mutations in the mitochondrial methionyl-tRNA synthetase cause a neurodegenerative phenotype in flies and a recessive ataxia (ARSAL) in humans. PLoS Biol. 2012;10(3):e1001288.
Moroni I, Bugiani M, Bizzi A, Castelli G, Lamantea E, Uziel G. Cerebral white matter involvement in children with mitochondrial encephalopathies. Neuropediatrics. 2002;33(2):79–85.
Valente L, Tiranti V, Marsano RM, Malfatti E, Fernandez-Vizarra E, Donnini C, et al. Infantile encephalopathy and defective mitochondrial DNA translation in patients with mutations of mitochondrial elongation factors EFG1 and EFTu. Am J Hum Genet. 2007;80(1):44–58.
Sofou K, Steneryd K, Wiklund LM, Tulinius M, Darin N. MRI of the brain in childhood-onset mitochondrial disorders with central nervous system involvement. Mitochondrion. 2013;13(4):364–71.
Brockmann K, Bjornstad A, Dechent P, Korenke CG, Smeitink J, Trijbels JM, et al. Succinate in dystrophic white matter: a proton magnetic resonance spectroscopy finding characteristic for complex II deficiency. Ann Neurol. 2002;52(1):38–46.
Ghezzi D, Goffrini P, Uziel G, Horvath R, Klopstock T, Lochmuller H, et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat Genet. 2009;41(6):654–6.
Kopajtich R, Nicholls TJ, Rorbach J, Metodiev MD, Freisinger P, Mandel H, et al. Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy. Am J Hum Genet. 2014;95(6):708–20.
Baertling F, Haack TB, Rodenburg RJ, Schaper J, Seibt A, Strom TM, et al. MRPS22 mutation causes fatal neonatal lactic acidosis with brain and heart abnormalities. Neurogenetics. 2015;16(3):237–40.
Brea-Calvo G, Haack TB, Karall D, Ohtake A, Invernizzi F, Carrozzo R, et al. COQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am J Hum Genet. 2015;96(2):309–17.
Ghezzi D, Sevrioukova I, Invernizzi F, Lamperti C, Mora M, D’Adamo P, et al. Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor. Am J Hum Genet. 2010;86(4):639–49.
Ardissone A, Piscosquito G, Legati A, Langella T, Lamantea E, Garavaglia B, et al. A slowly progressive mitochondrial encephalomyopathy widens the spectrum of AIFM1 disorders. Neurology. 2015;84(21):2193–5.
Rinaldi C, Grunseich C, Sevrioukova IF, Schindler A, Horkayne-Szakaly I, Lamperti C, et al. Cowchock syndrome is associated with a mutation in apoptosis-inducing factor. Am J Hum Genet. 2012;91(6):1095–102.
Ardissone A, Granata T, Legati A, Diodato D, Melchionda L, Lamantea E, et al. Mitochondrial complex III deficiency caused by TTC19 defects: report of a novel mutation and review of literature. JIMD Rep. 2015;22:115–20.
Atwal PS. Mutations in the complex III assembly factor tetratricopeptide 19 gene TTC19 are a rare cause of Leigh syndrome. JIMD Rep. 2014;14:43–5.
Ghezzi D, Arzuffi P, Zordan M, Da Re C, Lamperti C, Benna C, et al. Mutations in TTC19 cause mitochondrial complex III deficiency and neurological impairment in humans and flies. Nat Genet. 2011;43(3):259–63.
Mordaunt DA, Jolley A, Balasubramaniam S, Thorburn DR, Mountford HS, Compton AG, et al. Phenotypic variation of TTC19-deficient mitochondrial complex III deficiency: a case report and literature review. Am J Med Genet Part A. 2015;167(6):1330–6.
Morino H, Miyamoto R, Ohnishi S, Maruyama H, Kawakami H. Exome sequencing reveals a novel TTC19 mutation in an autosomal recessive spinocerebellar ataxia patient. BMC Neurol. 2014;14:5.
Nogueira C, Barros J, Sa MJ, Azevedo L, Taipa R, Torraco A, et al. Novel TTC19 mutation in a family with severe psychiatric manifestations and complex III deficiency. Neurogenetics. 2013;14(2):153–60.
Gai X, Ghezzi D, Johnson MA, Biagosch CA, Shamseldin HE, Haack TB, et al. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. Am J Hum Genet. 2013;93(3):482–95.
Melchionda L, Haack TB, Hardy S, Abbink TE, Fernandez-Vizarra E, Lamantea E, et al. Mutations in APOPT1, encoding a mitochondrial protein, cause cavitating leukoencephalopathy with cytochrome c oxidase deficiency. Am J Hum Genet. 2014;95(3):315–25.
Moraes CT, Shanske S, Tritschler HJ, Aprille JR, Andreetta F, Bonilla E, et al. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991;48(3):492–501.
Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23(12):2423–9.
Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol. 2004;55(5):706–12.
Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Markusic D, Karpinski NC, et al. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers’ syndrome. Ann Neurol. 1999;45(1):54–8.
Uusimaa J, Hinttala R, Rantala H, Paivarinta M, Herva R, Roytta M, et al. Homozygous W748S mutation in the POLG1 gene in patients with juvenile-onset alpers syndrome and status epilepticus. Epilepsia. 2008;49(6):1038–45.
Wiltshire E, Davidzon G, DiMauro S, Akman HO, Sadleir L, Haas L, et al. Juvenile Alpers disease. Arch Neurol. 2008;65(1):121–4.
Horvath R, Hudson G, Ferrari G, Futterer N, Ahola S, Lamantea E, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain. 2006;129(Pt 7):1674–84.
Milone M, Massie R. Polymerase gamma 1 mutations: clinical correlations. Neurologist. 2010;16(2):84–91.
Poulton J, Hirano M, Spinazzola A, Arenas Hernandez M, Jardel C, Lombes A, et al. Collated mutations in mitochondrial DNA (mtDNA) depletion syndrome (excluding the mitochondrial gamma polymerase, POLG1). Biochim Biophys Acta. 2009;1792(12):1109–12.
Suomalainen A, Isohanni P. Mitochondrial DNA depletion syndromes – many genes, common mechanisms. Neuromuscul Disord. 2010;20(7):429–37.
Dallabona C, Marsano RM, Arzuffi P, Ghezzi D, Mancini P, Zeviani M, et al. Sym1, the yeast ortholog of the MPV17 human disease protein, is a stress-induced bioenergetic and morphogenetic mitochondrial modulator. Hum Mol Genet. 2010;19(6):1098–107.
Hakonen AH, Davidzon G, Salemi R, Bindoff LA, Van Goethem G, Dimauro S, et al. Abundance of the POLG disease mutations in Europe, Australia, New Zealand, and the United States explained by single ancient European founders. Eur J Hum Genet. 2007;15(7):779–83.
Prasad C, Melancon SB, Rupar CA, Prasad AN, Nunez LD, Rosenblatt DS, et al. Exome sequencing reveals a homozygous mutation in TWINKLE as the cause of multisystemic failure including renal tubulopathy in three siblings. Mol Genet Metab. 2013;108(3):190–4.
Ostergaard E, Hansen FJ, Sorensen N, Duno M, Vissing J, Larsen PL, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130(Pt 3):853–61.
Carrozzo R, Dionisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di Giandomenico S, et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain. 2007;130(Pt 3):862–74.
Jaberi E, Chitsazian F, Ali Shahidi G, Rohani M, Sina F, Safari I, et al. The novel mutation p.Asp251Asn in the beta-subunit of succinate-CoA ligase causes encephalomyopathy and elevated succinylcarnitine. J Hum Genet. 2013;58(8):526–30.
Lamperti C, Fang M, Invernizzi F, Liu X, Wang H, Zhang Q, et al. A novel homozygous mutation in SUCLA2 gene identified by exome sequencing. Mol Genet Metab. 2012;107(3):403–8.
Rouzier C, Le Guedard-Mereuze S, Fragaki K, Serre V, Miro J, Tuffery-Giraud S, et al. The severity of phenotype linked to SUCLG1 mutations could be correlated with residual amount of SUCLG1 protein. J Med Genet. 2010;47(10):670–6.
Acham-Roschitz B, Plecko B, Lindbichler F, Bittner R, Mache CJ, Sperl W, et al. A novel mutation of the RRM2B gene in an infant with early fatal encephalomyopathy, central hypomyelination, and tubulopathy. Mol Genet Metab. 2009;98(3):300–4.
Bornstein B, Area E, Flanigan KM, Ganesh J, Jayakar P, Swoboda KJ, et al. Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene. Neuromuscul Disord. 2008;18(6):453–9.
van Rahden VA, Fernandez-Vizarra E, Alawi M, Brand K, Fellmann F, Horn D, et al. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am J Hum Genet. 2015;96(4):640–50.
Gregory A, Hayflick SJ. Genetics of neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2011;11(3):254–61.
Brunetti D, Dusi S, Morbin M, Uggetti A, Moda F, D’Amato I, et al. Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model. Hum Mol Genet. 2012;21(24):5294–305.
Isaya G. Mitochondrial iron-sulfur cluster dysfunction in neurodegenerative disease. Front Pharmacol. 2014;5:29.
Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies – disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30(2):81–114.
Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011;134(Pt 11):3326–32.
Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–46.
Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14.
Durcan TM, Fon EA. The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29(10):989–99.
Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, et al. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep. 2012;13(4):378–85.
Rugarli EI, Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31(6):1336–49.
Anand R, Langer T, Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta. 2013;1833(1):195–204.
Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, et al. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010;42(4):313–21.
Gorman GS, Pfeffer G, Griffin H, Blakely EL, Kurzawa-Akanbi M, Gabriel J, et al. Clonal expansion of secondary mitochondrial DNA deletions associated with spinocerebellar ataxia type 28. JAMA Neurol. 2015;72(1):106–11.
Pfeffer G, Gorman GS, Griffin H, Kurzawa-Akanbi M, Blakely EL, Wilson I, et al. Mutations in the SPG7 gene cause chronic progressive external ophthalmoplegia through disordered mitochondrial DNA maintenance. Brain. 2014;137(Pt 5):1323–36.
Pierson TM, Adams D, Bonn F, Martinelli P, Cherukuri PF, Teer JK, et al. Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet. 2011;7(10):e1002325.
Almajan ER, Richter R, Paeger L, Martinelli P, Barth E, Decker T, et al. AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival. J Clin Invest. 2012;122(11):4048–58.
Burte F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11(1):11–24.
Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36(5):449–51.
Carelli V, Musumeci O, Caporali L, Zanna C, La Morgia C, Del Dotto V, et al. Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann Neurol. 2015;78(1):21–38.
Haelterman NA, Yoon WH, Sandoval H, Jaiswal M, Shulman JM, Bellen HJ. A mitocentric view of Parkinson’s disease. Ann Rev Neurosci. 2014;37:137–59.
Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–73.
Itoh K, Nakamura K, Iijima M, Sesaki H. Mitochondrial dynamics in neurodegeneration. Trend Cell Biol. 2013;23(2):64–71.
Pinho CM, Teixeira PF, Glaser E. Mitochondrial import and degradation of amyloid-beta peptide. Biochim Biophys Acta. 2014;1837(7):1069–74.
Jayadev S, Leverenz JB, Steinbart E, Stahl J, Klunk W, Yu CE, et al. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain. 2010;133(Pt 4):1143–54.
Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol Cell Neurosci. 2013;55:26–36.
de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29(16):2715–23.
Palomo GM, Manfredi G. Exploring new pathways of neurodegeneration in ALS: the role of mitochondria quality control. Brain Res. 1607;2015:36–46.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- Ataxia
-
Lack of voluntary coordination of muscle movements that includes gait abnormality
- Athetosis
-
Slow, involuntary, convoluted, movements of the fingers, hands, toes and feet
- Coloboma
-
A hole in one of the structures of the eye (e.g. iris, retina, choroid or optic disc)
- Demyelinating polyneuropathy
-
Neurological disorder characterised by progressive weakness and impaired sensory function in the legs and arms due to the damage to the myelin sheath
- Dysarthria
-
Speech disorder due impaired coordination of movements in the muscles used for speech production
- Dysphagia
-
Difficulty in swallowing
- Dystonia
-
A state of abnormal muscle tone resulting in muscular spasm and abnormal posture
- Hyperreflexia
-
Abnormal reaction of the involuntary (autonomic) nervous system to stimulation
- Hypertrichosis
-
Abnormal amount of hair growing over the body
- Hypotonia
-
Low resistance to stretch of muscles
- Leukoencephalopathy
-
Disease affecting the brain white matter
- Megalencephalic leukoencephalopathy
-
Progressive condition that affects brain development and function, characterised by an enlarged brain
- Microangiopathy
-
Disease affecting small vessels
- Myoclonus
-
Brief, involuntary twitching of a muscle or a group of muscles
- Myoglobinuria
-
Presence of myoglobin in the urine
- Nystagmus
-
Repetitive, uncontrolled movements of the eyes
- Ophthalmoplegia
-
Slowly progressive paralysis of the extraocular muscles
- Pes cavus
-
High arch of the foot
- Polyhydramnios
-
Excessive accumulation of amniotic fluid
- Pyramidal signs
-
Symptoms related to the descending tract originated from pyramidal cells of motor cortex
- Quadriparesis
-
Weakness of the four limbs
- Seizures
-
Symptoms due to abnormal excessive or synchronous neuronal activity in the brain
- Spasticity
-
Abnormal muscle contraction characterised by a combination of paralysis, increased tendon reflex activity and hypertonia
- Supratentorial
-
The brain area located above the tentorium cerebelli, the membranous roof over the cerebellum
- T1, T2, T2 FLAIR
-
Neuroradiological terms that refer to the relaxation times that protons need to revert back to their resting states after the initial pulse at a certain radiofrequency
Rights and permissions
Copyright information
© 2016 Springer International Publishing
About this chapter
Cite this chapter
Viscomi, C., Ardissone, A., Zeviani, M. (2016). Mitochondrial Genes and Neurodegenerative Disease. In: Reeve, A., Simcox, E., Duchen, M., Turnbull, D. (eds) Mitochondrial Dysfunction in Neurodegenerative Disorders. Springer, Cham. https://doi.org/10.1007/978-3-319-28637-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-28637-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28635-8
Online ISBN: 978-3-319-28637-2
eBook Packages: MedicineMedicine (R0)