Abstract
MS patients commonly experience symptoms related to dysregulated gastrointestinal function, and these problems contribute to significant impairment in quality of life. Oropharyngeal dysphagia and anorectal dysfunction have traditionally garnered the most attention due to their more obvious impacts on daily functions. For example, convergent evidence suggests a prevalence of oropharyngeal dysphagia in 25–40 % of all MS patients. Similarly, anorectal dysfunction is quite common with ~40 % of MS patients reporting constipation and ~25 % reporting frequent fecal incontinence. In addition, many MS patients experience mixed forms of anorectal dysfunction with both constipation and fecal incontinence. There are a diverse range of potential pathophysiological mechanisms that contribute to these problems, including general impairments in skeletal motor function that are typically experienced by MS patients. However, recent research has revealed that gastrointestinal symptoms in the MS population are not limited to oropharyngeal dysphagia and anorectal dysfunction, but include dyspepsia and abdominal pain. The latter associations may reveal a broader impact of MS disease beyond impairments in skeletal motor function to include disruptions in the central neural regulation of autonomic and/or sensory processing. Despite the significant impact of gastrointestinal dysfunction on MS patient quality of life, there remains a paucity of published literature on therapeutic options for these disorders in this patient population. Thus, there is a compelling need to develop effective treatment options that should translate into improved patients’ quality of life. Collaborative work between neurologists and gastroenterologists will have the best chance to advance the field and to optimize the care of MS patients suffering from symptoms related to impaired gastrointestinal function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple sclerosis
- Gastroenterological disorders
- Dysphagia
- Anorectal dysfunction
- Fecal incontinence
- Constipation
- Dyspepsia
- Autonomic nervous system
Introduction
The complex and integrative functions of the gastrointestinal (GI) system require a fine coordination of skeletal muscle movements, sensory feedback, and autonomic nerve activity, all of which are influenced by the central nervous system. Thus, it is not surprising that impairments in GI system function are frequently experienced by patients with central nervous system disorders such as multiple sclerosis (MS). However, MS does not have a uniform presentation nor is it a static disorder. The specific location and extent of neuroinflammation and neuronal dysfunction varies greatly between patients and even within an individual patient over time. Therefore, the various impacts of MS disease on gastrointestinal function are inherently variable between patients and even within single individuals over time. Yet, despite this heterogeneity in the potential physiological impacts of MS disease, there are some general patterns of GI-related symptoms that many MS patients experience during the course of their illness. The aim of this chapter is to review what is currently known about the nature and prevalence of some of the more common gastrointestinal symptoms observed in MS disease. The discussion will also focus on contributing pathophysiological mechanisms and the unique management challenges posed by several of these problems. While the focus of our discussion is on MS, many of the problems described and their treatment approaches are relevant for other diseases of the central and/or peripheral nervous system that also impact GI function in patients with impaired sensory, motor, and/or cognitive function.
Prevalence of Gastrointestinal Symptoms in Multiple Sclerosis
The classical evaluation and treatment of patients with multiple sclerosis placed an emphasis on skeletal muscle function and the impact of the disease on mobility. Yet, impairments of skeletal muscle function not only affect mobility, but can compromise swallowing (deglutition), urination, and defecation. The normal processes of ingestion and elimination require highly coordinated patterns of voluntary skeletal muscle activity as well as reflexive activity that are collectively integrated within the spinal cord, brainstem, and higher-order neural systems. Thus, deglutition and elimination processes can be quite sensitive to even subtle disruption in such neural regulation. Because impairment of deglutition and elimination are common in multiple sclerosis patients [22] and contribute to poor quality of life, these symptoms have become recognized as important markers of MS disease. For at least the past 25 years, impairments in swallowing, urination, and defecation have been incorporated into widely used patient assessment instruments, such as the expanded disability status scale (EDSS) [26].
How Common Are Problems with Deglutition in Multiple Sclerosis Patients?
The specific answer reflects the methodology used to assess oropharyngeal dysphagia, the predominant form of dysphagia experienced in this population. These study methods range from standardized symptom questionnaires to more direct, objective methods such as fiberoptic visualization endoscopic evaluations of swallowing (FEES), electrophysiological study of swallowing (EPSS), or dynamic fluoroscopic methods such as videofluoroscopic swallowing study (VFSS) using contrast radiography. Distinguishing the methodology of assessment is important because patients may experience symptoms of dysphagia with or without objective abnormalities in the motor domain [53]. For example, subtle sensory abnormalities of input from the mouth, tongue, and posterior pharynx may lead to changes in the perceived timing of bolus movement during swallowing that could drive symptom reporting, even if the overall motor pattern is largely intact as assessed by the currently accepted standard methods. Dysphagia prevalence may also vary in different MS disease subgroups, such as those with primary progressive disease versus relapsing remitting disease [9], or in patients with differing MS disease severity [12]. In this context, it is not surprising that the published literature shows a range of prevalence estimates for dysphagia in large groups of MS patients with heterogeneous mixes of disease subtypes and severity. A review of 11 studies [1, 2, 4, 5, 9, 11, 12, 21, 27, 39, 49] that used either symptom questionnaires and/or objective visualization measures to assess dysphagia shows that the prevalence of swallowing disorders among patients with multiple sclerosis is likely between 25 and 40 % (Fig. 17.1a). As shown in Fig. 17.1a, the variability in prevalence estimates is high for small studies, but converges to a smaller range in studies with larger numbers of study participants. Although the reported data may be skewed due to tertiary referral center bias or failure to stratify for disease subtypes or MS symptom severity, it nonetheless demonstrates the potential importance of this often underappreciated problem.
What Is the Prevalence of Anorectal Dysfunction in Multiple Sclerosis Patients?
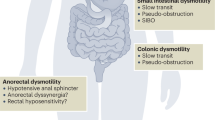
Anorectal dysfunction is a common complaint among patients with MS. Patients with MS commonly have a variety of impairments in the strength and recruitment of pelvic floor muscles that are critical for coordinating the timing and efficacy of defecation, as well as for maintaining fecal continence. Furthermore, impaired colonic motility may result from either a direct impact of MS disease, or as a side effect of medications used to alleviate other MS-related symptoms, and contribute to problems with defecation. Finally, the initiation of defecation requires effective straining, which can also be impaired as the illness advances to include ineffective recruitment of abdominal wall muscles needed to increase intraabdominal pressure. Collectively, anorectal dysfunction or bowel dysfunction are general clinical terms that incorporate symptoms of constipation or fecal incontinence. Constipation and fecal incontinence can exist in isolation or occur together. As with the studies of dysphagia in MS patients, there is a range of published prevalence estimates for constipation and fecal incontinence in this population. This is again likely due to differences in study methodology, as different studies use varying definitions of constipation and/or fecal incontinence. Most prevalence studies have used general consensus criteria and a combination of patient self-assessments and validated scoring systems. Some additional studies incorporate physiological markers (such as whole gut transit time assessed with Sitz markers) in combination with reported symptoms. We recently examined the published literature on the prevalence of anorectal dysfunction in the multiple sclerosis population [35]. As shown in Fig. 17.1b, our review reported a range of prevalence estimates for constipation across 17 studies (cited in [35]). However, studies with larger populations generally converged with estimates close to 40 %, which thus represents the best estimate for the true prevalence of constipation in MS patients and is consistent with the clinical experience of the authors. The prevalence of fecal incontinence in MS patients is lower than constipation. As seen in Fig. 17.1c, our same review reported prevalence estimates of fecal incontinence in MS patients from 19 studies (cited in [35]), most of which were smaller studies involving fewer than 50 patients. Therefore, there was significant variability in the estimate of symptom prevalence of fecal incontinence in this population. In studies with greater than 100 MS patients, the range of estimates was still wide-ranging, from 3.4 to 51 % [35]. It is generally accepted that the typical prevalence of fecal incontinence in the general population is on the order of ~10 % [7]. Despite the described shortcomings of published studies, the aggregate data clearly indicate a higher prevalence with ~25 % of MS patients experiencing fecal incontinence. Finally, the prevalence of the mixed form of anorectal dysfunction (i.e., patients experience both constipation and fecal incontinence) is less clear, with a wide range of estimates from between 6 and 52 % of MS patients (Fig. 17.1d) [35]. Although mixed forms of anorectal dysfunction are likely less common than the isolated occurrence of constipation or incontinence, this population of MS patients constitutes an important subgroup that poses significant therapeutic challenge for clinicians.
The impact of multiple sclerosis on gastrointestinal function may not be limited to impairments in skeletal muscle coordination, and historically, this possibility has received less attention. Depending upon the specific location of MS lesions and the severity of neuroinflammation, there could be a range of potential disruptions to the central neural circuits that govern sensation and autonomic regulation. Such central neural circuit disruption could directly generate gastrointestinal symptoms beyond the aforementioned difficulties with deglutition and defecation. However, the range of GI symptoms typically experienced by MS patients has not been well established. To address this gap in knowledge, we recently conducted a large, comprehensive survey to assess the extent and prevalence of GI symptoms in MS patients [27]. In this study, the validated Rome III questionnaire was used to assess the prevalence of GI symptoms in a single center cohort of 218 patients with MS disease. Our analysis showed that the majority of patients (66 %) experienced at least one chronic GI symptom. Not surprisingly, we reconfirmed the presence of dysphagia (21 %), constipation (37 %), and fecal incontinence (15 %) in our cohort, with prevalence estimates that generally correspond to other studies (see Fig. 17.1). However, we also discovered a fairly high prevalence of dyspepsia (28 %) and abdominal pain (14 %), symptoms which are not traditionally regarded as being associated with MS (Fig. 17.2). Dyspepsia incorporates symptoms of early satiety, postprandial fullness, and epigastric discomfort and points toward dysfunction in the sensory and motor function of the stomach. The neural mechanisms that integrate these functions are distinct from those that drive impaired skeletal muscle coordination, and therefore MS patients may have dyspepsia but no dysphagia or anorectal dysfunction. Further evidence for disruption in the normal motility or sensory function of the GI tract in MS patients is the significant number of patients that reported bloating, belching, and nausea (Fig. 17.2). Thus, patients with MS may suffer from a variety of GI symptoms, and these symptoms are not constrained to mechanisms dependent upon impaired control of skeletal muscle function, but rather may involve more global dysfunction of sensory and autonomic regulation.
Prevalence of gastrointestinal symptoms in a single center cohort of 218 MS patients as assessed by the Rome III questionnaire (Modified from Levinthal et al. [27])
Pathophysiological Mechanisms and Treatments for MS-Associated Dysphagia
Swallowing involves the successful manipulation and propulsion of ingested material from the oral cavity into the stomach while simultaneously preventing material from entering the proximal airway. Although swallowing can be triggered volitionally, many aspects of the process rely on highly coordinated patterns of activity among dozens of striated muscles within the oropharynx and proximal esophagus. This aspect of swallowing (deglutition) is referred to as the oropharyngeal phase, and disruption in this phase defines oropharyngeal dysphagia. Swallowing also requires the coordination of skeletal and smooth muscle activity within the tubular esophagus and sphincteric structures. This latter aspect of swallowing is referred to as the esophageal phase, and disruption in this phase defines esophageal dysphagia. The precise interplay of muscle movements that support normal swallowing requires intact sensory, motor, and autonomic nerves, with appropriate reflexive integration within the central nervous system. Hence, the symptom of dysphagia can result from any disturbance within these varied sensorimotor and autonomic systems. MS patients are particularly vulnerable to developing dysphagia given the likelihood that the disease causes neural dysfunction within one or more of the distributed CNS sites required to coordinate optimal swallowing. However, normal esophageal peristalsis that propels an ingested bolus along the smooth muscle portion of the esophagus is largely preprogrammed by the enteric nervous system. As enteric neurons are not directly affected by a central demyelinating process, most MS patients experience oropharyngeal dysphagia rather than esophageal dysphagia [39, 48]. Although many clinical studies have demonstrated an association of severe oropharyngeal dysphagia with more severely progressed MS-related disability [9, 12, 39], even patients with mild or moderate MS disease severity can experience some degree of oropharyngeal phase impairments in swallowing [39].
MS-related dysphagia remains an important and active area of research because the clinical consequences of untreated dysphagia can be severe. Beyond its potential impact on nutrition, oropharyngeal dysphagia carries an inherently high risk of aspiration pneumonia if left unaddressed. Indeed, some MS patients with severe oropharyngeal dysphagia ultimately require long-term restrictions on oral intake to minimize the likelihood of aspiration. While behavioral modifications, food choices, and manipulations of food consistency potentially mitigate this risk, there are few effective therapies to improve the true underlying problem. Prolonged oral restriction may require the placement of a percutaneous gastrostomy (PEG) tube. While PEG tube placement may ensure adequate enteral alimentation, it can significantly interfere with patient quality of life. We recently reviewed patterns of hospitalization and treatments in MS patients. We found that slightly less than 1 % of all MS-related hospital admissions were associated with the placement of a gastrostomy tube, which was presumably performed due to patient difficulties with oral intake in the context of oropharyngeal dysphagia [36]. Unfortunately, the rate of gastrostomy tube placement remained stable between 2001 and 2010, despite the advent and widespread adoption of disease-modifying agent use over this time period [36]. Thus, the cumulative clinical impact of oropharyngeal dysphagia on important clinical outcomes (gastrostomy tube placement, aspiration pneumonia, etc.) and associated impairments in MS patient quality of life continues to increase.
Ongoing research continues to define the precise locations of CNS sites that are required to support optimal swallowing. Several brainstem nuclei are known to contain the motor neurons that directly influence the muscles of the tongue, epiglottis, and pharynx. Thus, it is not surprising that MS patients with documented brainstem lesions suffer disproportionately from oropharyngeal dysphagia [2, 9]. However, disruption of either sensory inputs and/or descending commands from higher brain sites to these brainstem nuclei can also lead to oropharyngeal dysphagia. For example, impaired integration in the brainstem as assessed by delayed or absent gag reflex is associated with dysphagia in MS patients [53]. Similar mechanisms may link the presence of an impaired gag reflex with impairments in the protective cough reflex, and thus MS patients with brainstem lesions may be at especially high risk to develop “silent” aspiration. At the cerebral cortical level, multiple sites have been implicated in the regulation of tongue and pharyngeal muscle contractions that support the act of swallowing [19, 24, 38]. These cortical sites include regions of the lateral frontal operculum [38] as well as more rostromedial regions of the lateral hemispheres that span premotor areas and the anterior motor cortex [19]. Most people have an asymmetric, bilateral representation of the pharyngeal muscles, with one of these representations dominating pharyngeal control [19, 20]. Disruption in neural activity within the dominant pharyngeal cortical control region is sufficient to induce oropharyngeal dysphagia, even in normal individuals [24]. Interestingly, the cortical systems that regulate swallowing are capable of undergoing significant neuroplastic changes. For example, strokes involving the dominant motor cortical representation of the pharynx can lead to dysphagia, and dysphagia recovery is associated with an increase in the areal distribution and increase in the cortical excitability within the non-lesioned, previously non-dominant hemispheric representation [20]. Indeed, repetitive transcranial magnetic stimulation (rTMS) directed to the contralesional pharyngeal motor cortex is capable of increasing cortical excitability within the region and is associated with improvements in both poststroke dysphagia symptoms and objective measures of swallowing function [37]. This important clinical observation suggests that rTMS therapy to the contralesional pharyngeal motor cortex in MS patients may be developed as a viable therapy to reduce MS-related oropharyngeal dysphagia that is not clearly related to brainstem disease.
The published literature that details specific, objective measures of swallowing function in MS patients is quite sparse (Table 17.1). These methods include bedside swallowing evaluations, fiberoptic visualization endoscopic evaluation of swallowing (FEES), videofluoroscopic swallowing studies (VFSS), and electrophysiological assessments [2, 3, 9, 49, 53]. Despite being fairly small clinical studies with heterogeneous groups of MS patients, there are several common findings. These studies demonstrate a range of tongue, laryngeal, epiglottic, soft palate, and pharyngeal muscle impairments, even in MS patients without symptomatic oropharyngeal dysphagia. Interestingly, some of these muscles demonstrate spastic changes rather than weakness. For example, a subset of MS patients experience dysphagia associated with impaired relaxation of the cricopharyngeal muscle, which is a major contributor to the upper esophageal sphincter (UES). This latter observation may be particularly relevant for oropharyngeal dysphagia therapy, as such abnormalities can be objectively determined with appropriate diagnostic studies to identify a subset of patients with a high likelihood to respond to therapies directed at the cricopharyngeal muscle.
There are no current systemic pharmacological treatments for MS-related oropharyngeal dysphagia. Typically, therapeutic interventions in patients with oropharyngeal dysphagia of any cause include functional swallowing therapies that include altering the diet to accommodate the degree of dysfunction (i.e., thickening liquids), methods to compensate or adapt to the dysfunction (i.e., tucking the chin while swallowing to passively direct material to the esophagus and close off the upper airway; Mendelsohn maneuver with external lift to the cricoid during swallowing to reduce laryngeal movement), and exercises to attempt to maintain residual function. Only two therapeutic trials have been conducted in MS patients with oropharyngeal dysphagia. The first study investigated the efficacy of cricopharyngeal-directed botulinum toxin A injection in 14 MS patients with a documented hypertonic upper esophageal sphincter [44]. The results of this small study showed at least moderate improvement in dysphagia in all 14 patients, but the effects were relatively short-lived with a return to baseline dysfunction at 6 months following the injections. As indicated above, cricopharyngeal botulinum toxin injection would not be predicted to benefit MS patients with predominantly oral dysphagia or oropharyngeal dysphagia not associated with UES hyperactivity. A second study by the same research group investigated the ability of direct electrical pharyngeal muscle stimulation to improve oropharyngeal dysphagia in 20 symptomatic MS patients [43]. A catheter fitted with bipolar platinum ring electrodes was placed transnasally with the electrode tips located 3 cm above the UES. Patients then underwent either five consecutive daily sessions with 10 min of stimulation (75 % of pain threshold) or sham stimulation. Interestingly, the treatment group experienced a significant improvement in VFSS measures which were durable for at least 4 weeks after the final stimulation session. The mechanisms that mediate the reported treatment effect are not clear. Nevertheless, pharyngeal muscle electrical stimulation may be a promising intervention that should be further explored for the treatment of oropharyngeal dysphagia in MS patients.
Pathophysiological Mechanisms and Treatment for MS-Associated Anorectal Dysfunction
Normal defecation patterns require both conscious and unconscious sensory and motor processing within the CNS to support the perception of rectal filling, the ability to retain stool in the rectum without anal leakage, and the volitional elimination of stool without difficulty. The disruption in any of these processes leads to anorectal dysfunction, a term that encapsulates the symptoms of constipation and/or fecal incontinence. Anorectal dysfunction is a source of significant impairment in quality of life in affected MS patients and is a source of substantial caregiver burden [35]. MS patients may experience anorectal dysfunction because even subtle impairments in sensation from the anorectum and/or the timing and efficacy of abdominal, pelvic floor, and anal muscle contractions are sufficient to generate symptoms. For example, impaired sensation of rectal filling may predispose to fecal incontinence (due to lack of perceived urge) or even fecal impaction. Altered central neural control may result in spasticity of pelvic floor muscles that can impair the ability to evacuate rectal contents easily. Additionally, it is possible that MS disease itself could directly influence colonic transit and contractility due to disruption in normal patterns of CNS influences over the autonomic regulation of the colon. Because these various sensory, motor, and autonomic functions are supported by neurons in disparate regions of the brain, brainstem, and spinal cord, there is an increased likelihood that any one MS lesion could impact some aspect of anorectal function. However, clinical features of many MS patients potentially confound the attribution of anorectal dysfunction as a direct result of an MS-related CNS lesion. For example, medications that are frequently used to alleviate other MS-related symptoms, such as urinary incontinence and hyperactive bladder, pain, muscle spasticity, or mood disorders, can lead to constipation as a side effect. Physical activity is independently linked with colonic motility through unclear mechanisms. Thus, MS patients with impaired mobility may suffer from constipation via reduced colonic transit time, and fecal incontinence may increase in frequency simply because such patients do not have time to reach the commode. Lastly, a subset of female MS patients could develop anorectal dysfunction due to the effects of prior obstetric trauma.
The extrinsic innervation of the pelvic structures involved in defecation arise from the pudendal nerves (motor function to the pelvic floor muscles and external anal canal; sensation from the genitalia, perineum, and anus), the pelvic nerves (parasympathetic innervation of the colon, rectum, and internal anal sphincter), and the hypogastric nerves (sympathetic innervation of the distal-most colon, rectum, and internal anal sphincter). These nerves are most immediately influenced by the activity in neurons contained within autonomic and motor nuclei of the lumbosacral spinal cord. A similar pattern of spinal and autonomic innervation also supports urinary function. Thus, MS patients with low spinal involvement are likely to experience anorectal dysfunction, often in conjunction with difficulties with urination [31, 41]. Ongoing research continues to help define the precise locations of supraspinal CNS neurons that are required to support optimal anorectal function. Multiple higher-order centers in the midbrain and brainstem have been shown to exert an influence over both bladder and anorectal function, including Barrington’s nucleus, the periaqueductal gray, and the parabrachial nucleus, among others [13]. Ultimately, the cerebral cortex integrates social cues and context with sensory input to both consciously and unconsciously influence pelvic floor motor programs that are required to retain and/or eliminate stool. These cerebral cortical sites are widely distributed across bilateral regions located both in the medial wall, in particular, the supplementary motor cortex and mid-cingulate cortex [25, 45], and within lateral regions that include the medial primary motor cortex [50], insula, lateral operculum, and posterior parietal cortex [45]. Because of the disparate locations of the cerebral cortical areas that are involved in pelvic floor function, it would follow that periventricular and internal capsular white matter disease associated with MS could easily interfere with tracts carrying descending commands to the pelvic floor. To date, there have been no reports investigating this possibility, although modern brain-imaging techniques such as diffusion tensor tractography would be well suited to do so. For example, similar methods have already been used to reveal abnormalities in white matter tracts that are associated with symptoms of fatigue, disrupted emotional regulation, and impaired cognition in MS patients [6, 33].
The published literature that details specific, objective measures of anorectal function in MS patients is limited. The few clinical studies that have investigated specific mechanisms that may contribute to anorectal dysfunction in MS patients have collectively employed a variety of colonic, rectal, and anal pressure-sensing devices, sensory testing, radiographic studies, and electrophysiological testing, as well as assessments of colonic motility. However, these studies have been conducted in relatively small numbers of mostly symptomatic patients (Table 17.2). Yet, what is apparent is that while no single mechanism can fully account for the experience of anorectal dysfunction in these MS patients, abnormalities are common. Importantly, many of these studies cannot determine the sufficiency or necessity of any given physiological biomarker because MS patients without anorectal dysfunction were typically excluded from analysis.
Nevertheless, several results were frequently observed in those studies that focused on MS patients with constipation and/or fecal incontinence. For example, most studies have demonstrated decreased resting anal tone and decreased volitional squeeze pressures, particularly in those patients with fecal incontinence. Sensory deficits were less prevalent, with only two studies demonstrating decreased subjective anal or rectal perception [34, 52]. One small study in advanced MS patients showed that such decreased sensation may occur at the brain level, rather than at the primary afferent or spinal level [18]. Defecographic methods demonstrated impaired puborectalis relaxation as a contributing factor to constipation [10, 15, 51]. Measures of colonic transit or colonic pressure-volume relationships showed delayed colonic transit and evidence for decreased colonic compliance. However, decreased colonic transit may be confounded by the presence of impaired evacuation. Finally, electrophysiological techniques reliably demonstrated impaired central motor latencies along with intact peripheral nerve-motor endplate latencies, consistent with an impact of CNS disease, rather than peripheral nerve injury.
There are few treatment options for MS patients with anorectal dysfunction, and clinical studies in this population are limited to only a few small, mostly uncontrolled trials (Table 17.3). These include studies using behavioral interventions and biofeedback in order to alter sphincteric function that showed some clinical benefit [30, 42, 54]. Unfortunately, these benefits were primarily restricted to those with mild disease. This observation is not surprising, as biofeedback requires intact central motor control systems that are likely to be disrupted in advanced MS patients with more severe forms of anorectal dysfunction. One study investigated various techniques of abdominal massage to improve constipation in MS patients and demonstrated a small effect size in the treatment group [29]. However, the intervention appears to be time consuming and may be difficult for MS patients with advanced disease to accomplish independently due to difficulties in positioning, dexterity, and strength. Two small studies using transanal irrigation in MS patients with anorectal dysfunction showed similar results with improvement in ~40–53 % of patients [14, 40].
A practical approach to the treatment of anorectal dysfunction must consider the dominant symptom, level of MS disability (particularly in regard to mobility and dexterity), and patient and caregiver preferences. For milder problems, some general approaches are likely to be effective. For constipation in those with retained mobility and intact sensation, changes in fiber intake or flexibly dosed osmotically active agents (e.g., polyethylene glycol, magnesium citrate, etc.) should suffice. For those with primarily fecal incontinence, bulking agents or antimotility agents (e.g., loperamide) could be quite helpful to firm stool consistency. The rationale for the latter recommendation is that looser stool consistency is the factor most highly associated with fecal incontinence in the general population [8]. These two general approaches may work for many MS patients with anorectal dysfunction, but are unlikely to be effective for those with severe mobility impairment and/or the presence of both constipation and fecal incontinence. For example, patients with severe mobility impairment or sensory deficits may easily develop fecal incontinence with standard therapies for constipation, or fecal impaction with standard therapies for fecal incontinence. In these circumstances, timed evacuations using scheduled administration of enemas or laxating rectal suppositories could be effective. Alternatively, for those with more severe fecal incontinence at baseline, the combination of timed evacuation with suppositories could be coupled with the cautious use of antidiarrheals between evacuations. The Consortium for Multiple Sclerosis Centers (CMSC) sponsored a meeting in the fall of 2011 to develop a practical treatment approach based upon expert experience to treat both mild and severe forms of anorectal dysfunction in MS patients [32]. These treatment guidelines incorporate many of the practical approaches mentioned above and were devised using input from both authors. However, the efficacy of these guidelines has remained untested.
Unaddressed Needs and Future Directions
As is clear from more recent symptom surveys, gastrointestinal dysfunction remains a very common contributor to impaired quality of life in many MS patients. Oropharyngeal dysphagia and anorectal dysfunction have traditionally garnered the most attention, perhaps because of their more obvious, daily impact on eating and defecation. However, treatment options and efficacy for these problems remain limited, and more research is needed to optimize the care of MS patients with gastrointestinal dysfunction. In a fragmented healthcare delivery system, the reciprocal relationship between many neurological disorders with gastrointestinal symptoms constitutes an important clinical challenge. Treatment choices must not only target the underlying gastrointestinal abnormality, but also consider the often significant neurological deficits that may influence treatment results and feasibility. While neurological illnesses such as MS directly affect many other body systems as outlined above, understanding this interdependence also requires healthcare providers to understand treatments and their side effects, as many of the medical interventions for neurological illness inherently alter gut function. Several other gastroenterological symptoms are just beginning to be recognized in MS patients. More research is needed to quantify the impact of specific symptoms on disease-related quality of life, as this will help prioritize future clinical studies and the development of treatment options. For example, dyspepsia is surprisingly common in MS patients and is associated with significant impairment in quality of life. However, the mechanisms that drive this symptom are not clear. Future research should work to uncover contributing mechanisms that drive dyspeptic symptoms in MS patients, as well as evaluate the efficacy of treatments. Collaborative work between neurologists and gastroenterologists will have the best chance to advance the field and optimize the care of MS patients that suffer from impaired gastrointestinal function.
References
Abraham S, Scheinberg LC, Smith CR, LaRocca NG. Neurologic impairment and disability status in outpatients with multiple sclerosis reporting dysphagia symptomatology. J Neurol Rehab. 1997;11:7–13.
Abraham SS, Yun PT. Laryngopharyngeal dysmotility in multiple sclerosis. Dysphagia. 2002;16:69–74.
Alfonsi E, Bergamaschi R, Cosentino G, Ponzio M, Montomoli C, Restivo DA, Brighina F, Ravaglia S, Prunetti P, Bertino G, Benazzo M, Fontana D, Moglia A. Electrophysiological patterns of oropharyngeal swallowing in multiple sclerosis. Clin Neurophysiol. 2013;124(8):1638–45.
Bergamaschi R, Crivelli P, Rezzani C, Patti F, Solaro C, Rossi P, Restivo D, Maimone D, Romani A, Bastianello S, Tavazzi E, D’Amico E, Montomoli C, Cosi V. The DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. J Neurol Sci. 2008;269(1–2):49–53.
Bergamaschi R, Rezzani C, Minguzzi S, Amato MP, Patti F, Marrosu MG, Bonavita S, Grasso MG, Ghezzi A, Rottoli M, Gasperini C, Restivo D, Maimone D, Rossi P, Stromillo ML, Montomoli C, Solaro C, DYMUS Group. Validation of the DYMUS questionnaire for the assessment of dysphagia in multiple sclerosis. Funct Neurol. 2009;24(3):159–62.
Bester M, Lazar M, Petracca M, Babb JS, Herbert J, Grossman RI, Inglese M. Tract-specific white matter correlates of fatigue and cognitive impairment in benign multiple sclerosis. J Neurol Sci. 2013;330(1–2):61–6.
Bharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, Mott L, Rogers RG, Zinsmeister AR, Whitehead WE, Rao SS, Hamilton FA. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110(1):127–36.
Bharucha AE, Zinsmeister AR, Schleck CD, Melton 3rd LJ. Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139(5):1559–66.
Calcagno P, Ruoppolo G, Grasso MG, De Vincentiis M, Paolucci S. Dysphagia in multiple sclerosis–prevalence and prognostic factors. Acta Neurol Scand. 2002;105:40–3.
Chia YW, Gill KP, Jameson JS, Forti AD, Henry MM, Swash M, Shorvon PJ. Paradoxical puborectalis contraction is a feature of constipation in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;60(1):31–5.
Danesh-Sani SA, Rahimdoost A, Soltani M, Ghiyasi M, Haghdoost N, Sabzali-Zanjankhah S. Clinical assessment of orofacial manifestations in 500 patients with multiple sclerosis. J Oral Maxillofac Surg. 2013;71:290–4.
De Pauw A, Dejaeger E, D’hooghe B, Carton H. Dysphagia in multiple sclerosis. Clin Neurol Neurosurg. 2002;104(4):345–51.
Drake MJ, Fowler CJ, Griffiths D, Mayer E, Paton JF, Birder L. Neural control of the lower urinary and gastrointestinal tracts: supraspinal CNS mechanisms. Neurourol Urodyn. 2010;29(1):119–27.
Faaborg PM, Christensen P, Kvitsau B, Buntzen S, Laurberg S, Krogh K. Long-term outcome and safety of transanal colonic irrigation for neurogenic bowel dysfunction. Spinal Cord. 2009;47(7):545–9.
Gill KP, Chia YW, Henry MM, Shorvon PJ. Defecography in multiple sclerosis patients with severe constipation. Radiology. 1994;191(2):553–6.
Glick ME, Meshkinpour H, Haldeman S, Bhatia NN, Bradley WE. Colonic dysfunction in multiple sclerosis. Gastroenterology. 1982;83(5):1002–7.
Guinet A, Jousse M, Damphousse M, Hubeaux K, Le Breton F, Sheikh Ismael S, Amarenco G. Modulation of the rectoanal inhibitory reflex (RAIR): qualitative and quantitative evaluation in multiple sclerosis. Int J Colorectal Dis. 2011;26(4):507–13.
Haldeman S, Glick M, Bhatia NN, Bradley WE, Johnson B. Colonometry, cystometry, and evoked potentials in multiple sclerosis. Arch Neurol. 1982;39:698–701.
Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2(11):1217–24.
Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicholson DA, Tallis RC, Thompson DG. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–12.
Herrera W, Zeligman BE, Gruber J, Jones M, Monica C, Pautler R, Wriston R, Cain M, Prescott T, Cobble N, Burks JS. Dysphagia in multiple sclerosis: clinical and videofluoroscopic correlations. J Neurol Rehabil. 1990;4:1–8.
Hinds JP, Eidelman BH, Wald A. Prevalence of bowel dysfunction in multiple sclerosis. A population survey. Gastroenterology. 1990;98:1538–42.
Jameson JS, Rogers J, Chia YW, Misiewicz JJ, Henry MM, Swash M. Pelvic floor function in multiple sclerosis. Gut. 1994;35:388–90. (PMID: 8150353).
Jayasekeran V, Singh S, Tyrrell P, Michou E, Jefferson S, Mistry S, Gamble E, Rothwell J, Thompson D, Hamdy S. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138(5):1737–46.
Kuhtz-Buschbeck JP, van der Horst C, Wolff S, Filippow N, Nabavi A, Jansen O, Braun PM. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions--an fMRI study. Neuroimage. 2007;35(2):449–57.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis. Neurology. 1983;33:1444–52.
Levinthal DJ, Rahman A, Nusrat S, O’Leary M, Heyman R, Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int. 2013;2013:319201.
Mathers SE, Ingram DA, Swash M. Electrophysiology of motor pathways for sphincter control in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1990;53(11):955–60.
McClurg D, Hagen S, Hawkins S, Lowe-Strong A. Abdominal massage for the alleviation of constipation symptoms in people with multiple sclerosis: a randomized controlled feasibility study. Mult Scler. 2011;17(2):223–33.
Munteis E, Andreu M, Martinez-Rodriguez J, Ois A, Bory F, Roquer J. Manometric correlations of anorectal dysfunction and biofeedback outcome in patients with multiple sclerosis. Mult Scler. 2008;14(2):237–42.
Munteis E, Andreu M, Téllez MJ, Mon D, Ois A, Roquer J. Anorectal dysfunction in multiple sclerosis. Mult Scler. 2006;12(2):215–8.
Namey M. Elimination dysfunction in multiple sclerosis. Int J MS Care. 2012;14(S1):1–26.
Nigro S, Passamonti L, Riccelli R, Toschi N, Rocca F, Valentino P, Nisticò R, Fera F, Quattrone A. Structural ‘connectomic’ alterations in the limbic system of multiple sclerosis patients with major depression. Mult Scler. 2015;21(8):1003–12.
Nordenbo AM, Andersen JR, Andersen JT. Disturbances of ano-rectal function in multiple sclerosis. J Neurol. 1996;243(6):445–51.
Nusrat S, Gulick E, Levinthal D, Bielefeldt K. Anorectal dysfunction in multiple sclerosis: a systematic review. ISRN Neurol. 2012; http://dx.doi.org/10.5402/2012/376023. Article ID 376023.
Nusrat S, Levinthal D, Bielefeldt K. Hospitalization rates and discharge status in multiple sclerosis. Mult Scler Int. 2013;2013:436929.
Park JW, Oh JC, Lee JW, Yeo JS, Ryu KH. The effect of 5Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013;25(4):324–e250.
Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(4):389–443.
Poorjavad M, Derakhshandeh F, Etemadifar M, Soleymani B, Minagar A, Maghzi AH. Oropharyngeal dysphagia in multiple sclerosis. Mult Scler. 2010;16:362–5.
Preziosi G, Gosling J, Raeburn A, Storrie J, Panicker J, Emmanuel A. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis Colon Rectum. 2012;55(10):1066–73.
Preziosi G, Raptis DA, Raeburn A, Thiruppathy K, Panicker J, Emmanuel A. Gut dysfunction in patients with multiple sclerosis and the role of spinal cord involvement in the disease. Eur J Gastroenterol Hepatol. 2013;25(9):1044–50.
Preziosi G, Raptis DA, Storrie J, Raeburn A, Fowler CJ, Emmanuel A. Bowel biofeedback treatment in patients with multiple sclerosis and bowel symptoms. Dis Colon Rectum. 2011;54(9):1114–21.
Restivo DA, Casabona A, Centonze D, Marchese-Ragona R, Maimone D, Pavone A. Pharyngeal electrical stimulation for dysphagia associated with multiple sclerosis: a pilot study. Brain Stimul. 2013;6(3):418–23.
Restivo DA, Marchese-Ragona R, Patti F, Solaro C, Maimone D, Zappalá G, Pavone A. Botulinum toxin improves dysphagia associated with multiple sclerosis. Eur J Neurol. 2011;18(3):486–90.
Schrum A, Wolff S, van der Horst C, Kuhtz-Buschbeck JP. Motor cortical representation of the pelvic floor muscles. J Urol. 2011;186(1):185–90.
Sørensen M, Lorentzen M, Petersen J, Christiansen J. Anorectal dysfunction in patients with urologic disturbance due to multiple sclerosis. Dis Colon Rectum. 1991;34(2):136–9.
Swash M, Snooks SJ, Chalmers DH. Parity as a factor in incontinence in multiple sclerosis. Arch Neurol. 1987;44(5):504–8.
Tassorelli C, Bergamaschi R, Buscone S, Bartolo M, Furnari A, Crivelli P, Alfonsi E, Alberici E, Bertino G, Sandrini G, Nappi G. Dysphagia in multiple sclerosis: from pathogenesis to diagnosis. Neurol Sci. 2008;29:S360–3.
Thomas FJ, Wiles CM. Dysphagia and nutritional status in multiple sclerosis. J Neurol. 1999;246:677–82.
Turnbull GK, Hamdy S, Aziz Q, Singh KD, Thompson DG. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117:32–9.
Waldron DJ, Horgan PG, Patel FR, Maguire R, Given HF. Multiple sclerosis: assessment of colonic and anorectal function in the presence of faecal incontinence. Int J Colorectal Dis. 1993;8(4):220–4.
Weber J, Grise P, Roquebert M, Hellot MF, Mihout B, Samson M, Beuret-Blanquart F, Pasquis P, Denis P. Radiopaque markers transit and anorectal manometry in 16 patients with multiple sclerosis and urinary bladder dysfunction. Dis Colon Rectum. 1987;30(2):95–100.
Wiesner W, Wetzel SG, Kappos L, Hoshi MM, Witte U, Radue EW, Steinbrich W. Swallowing abnormalities in multiple sclerosis: correlation between videofluoroscopy and subjective symptoms. Eur Radiol. 2002;12(4):789–92.
Wiesel PH, Norton C, Roy AJ, Storrie JB, Bowers J, Kamm MA. Gut focused behavioural treatment (biofeedback) for constipation and faecal incontinence in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(2):240–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Levinthal, D.J., Bielefeldt, K. (2016). The Impact of Multiple Sclerosis on Gastrointestinal System Function. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)