Abstract
Portable XRF (pXRF) instruments can be used in the field for measurement of soil elemental concentrations. The pXRF measurements in the field, however, are affected by several factors including soil texture, moisture and sample heterogeneity. In this chapter, we investigated the effect of moisture on the Fe concentration of pXRF laboratory and field measurements. In the laboratory study, soil samples were analysed that were wetted to different moisture contents. In the field study, pXRF measurements were made from topsoil samples and the data were compared to samples that were air-dried and ground to pass a 2-mm sieve. Soil moisture mainly dampens the X-ray intensity resulting in lower Fe concentrations, and this effect is more pronounced for clayey soils (higher Fe content) as compared to sandy soils (lower Fe content). In addition, the field moisture content is highly related to clay and Fe content. Thus, the response of XRF intensity to soil moisture content depends on soil texture and mineralogy. We could not find a general correction factor for soil moisture and X-ray intensity but the relationship between air-dried and field-moist Fe concentration is highly linear which allows to correct for moisture content by establishing an empirical correction function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Handheld portable X-ray fluorescence (pXRF) devices offer great opportunity to enrich the in situ description of soil profiles by rapidly measuring the elemental concentration of unprocessed soil materials and are therefore a highly valuable tool in digital soil morphometrics and pedology studies (Hartemink and Minasny 2014).
XRF devices operate on the principle of energy dispersive spectrometry whereby the amount of emitted fluorescence photons is directly measured by an X-ray detector that simultaneously analyses their energy levels. The number of element-specific characteristic X-rays produced (or intensity) determines the quantity of a given element. Measurements by portable XRF measurements, however, can be affected by soil particle size, moisture, the presence of coarse organic residues, soil structure, smearing, stoniness, mottles and redoximorphic features. For example, a soil sample ground to finer particle sizes can show higher elemental concentrations when compared to the same soil sample unground or of coarser particle sizes (Markowicz 2008). This effect is more pronounced for elements with small atomic numbers such as K, V, Cr, Mn and Ca (Laiho and Perämäki 2005). There are two factors that affect the measurement of pXRF in the field, i.e. sample heterogeneity and soil moisture (Ge et al. 2005; Horta et al. 2015).
In the laboratory, soil samples are usually ground to pass certain size fractions (<2 mm) to homogenise the sample and eliminate coarse fragments. When the soil particle size decreases, the XRF intensity is increased because of smaller incident angles (Maruyama et al. 2008). Generally, sieving the soil to <2 mm is recommended because it results in less variation (Laiho and Perämäki 2005). In the field, the soil particle sizes can be variable, and pXRF measurements from the field can be different from laboratory measurements.
Soil moisture affects XRF measurements in two ways (Ge et al. 2005). First, the presence of water particles enhances the absorption of X-rays which reduces the intensity of the X-ray signal. Secondly, the presence of water particles can cause primary X-rays to scatter which results in the increase of the X-ray intensity. Therefore, the elemental concentration in wet soil is generally lower than in dry soil, and this can lead to a lower precision, poor detection limit and overall lower accuracy. Kalnicky and Singhvi (2001) and Laiho and Perämäki (2005) recommended scanning soil samples with gravimetric moisture content less than 20 %. Most of these studies used samples from a single source with limited variability (e.g. Parsons et al. 2013 and Bastos et al. 2012). They have not considered different soil textures and soil water potentials. The 20 % moisture is rather arbitrarily defined, as 20 % gravimetric water content in a clayey and a sandy soil is different in terms of its water potential.
The aim of this study was to investigate the influence of soil moisture on the pXRF spectrum, in particular Fe concentration and to explore the potential for quantifying and removing this effect and to increase the accuracy of field pXRF measurements.
2 Materials and Methods
We conducted two studies to investigate the effect of soil moisture on the measurement of elemental concentrations, particularly Fe. The XRF spectra were collected in SOILMODE using an Olympus Delta Premium Handheld pXRF Analyzer which operates at three beam configurations of 50, 40 and 15 kV, respectively (Olympus InnovX-Systems, USA, 2010).
2.1 Laboratory-Based Study
Eleven soil samples with texture contents ranging from loam to medium clay were collected from the Hunter Valley, NSW, Australia. The samples were air-dried and ground to pass a 2-mm sieve. The air-dried samples were evenly wetted until they reached a sticky consistency (~30 % wetness), and subsequently scanned with a pXRF at different moisture conditions after air-drying the samples for 1 day in a controlled laboratory environment. Wetness (%) was determined gravimetrically by measuring the amount of water in the soil by drying in the oven at 105 °C for 24 h. Maximum peak heights as well as the total peak area around 6.2 and 6.6 keV for Fe were used to investigate the response of the XRF spectrum to different moisture conditions.
2.2 Field Study
This study used 120 topsoil samples (0–7.5 cm depth) from Nowley Farm in the Liverpool Plains, NSW, Australia. The samples were collected using a stratified random sampling design. The samples were scanned with pXRF under field-moist conditions. The samples were then dried in the oven at 40 °C for 24 h, ground to pass a 2-mm sieve and rescanned under these air-dried conditions. All scans were replicated thrice.
3 Results and Discussion
3.1 Laboratory Study
Figure 5.1 shows the intensity of the Fe peak at 6.40 keV at different moisture contents for a sample from the Bt horizon of a Dermosol. The intensity decreases with increasing moisture content. Ge et al. (2005) proposed a model for the correction of soil moisture on XRF measurement based on the principle that the reduction in X-ray intensity is proportional to the increment of water content of the sample:
where dI x is the reduction in the X-ray intensity, I x is the X-ray intensity, dw is the increment of gravimetric water content in the sample, and \(\mu_{\text{w}}\) is the attenuation coefficient of moisture. Integrating this equation from w = 0, gives:
where I 0 is the intensity at w = 0. This implies that based on Beer–Lambert’s law, the intensity will attenuate exponentially with increasing moisture content. Ge et al. (2005) estimated w from the intensity of scattered radiation.
The laboratory study using different soils showed that the response of the intensity (or Fe-peak height) decreases with moisture content but that the decrease is not uniform (Fig. 5.2). Although the three soil samples can all be classified as clay-rich Bt horizons (35–55 % clay content), and belong to the same soil order (Dermosol), and also show the existence of an exponential relationship between wetness and intensity, the attenuation factor is different for the three soil samples. The correction factor as proposed by Ge et al. (2005) can therefore not be uniformly applied.
3.2 Field Study
In the field study, we collected a range of topsoil samples with soil textures ranging from sand to clay. Figure 5.3 shows the relationship between Fe-peak height for soil samples collected under field and air-dried conditions. The Fe content (as estimated by the maximum Fe-peak height) was underestimated for field-moist soil as compared to air-dry soils. The relationship of Fe content between air-dried and field-moist conditions is linear. Some field-moist samples showed higher Fe concentrations compared to air-dry samples possibly due to the scattering effects. Hürkamp et al. (2009) also found a solid linear relationship for Pb content between field measurement and laboratory measurement (air-dried and ground to pass a 2-mm sieve). Tjallingii et al. (2007) who worked on marine sediments found that heavier elements (such as K, Ca, Ti and Fe) are relatively unaffected by soil moisture, but that lighter elements (such as Al and Si) are more sensitive to moisture changes. In this study, we have not included any comparisons for heavy or light elements yet.
Clay-rich soils (>40 % clay) showed higher concentrations of Fe compared to sandy soils (>60 % sand). There is a tendency for the relative difference of the max Fe peaks (or Fe content) to be larger (between the field-moist and air-dry soil samples) in Fe-rich soils (Fig. 5.4). The general relationship proposed by Ge et al. (2005) \(w\sim \mu_{w} \log \left( {I_{0} /I_{w} } \right)\) is not observed which suggests that the attenuation coefficient is soil-dependent.
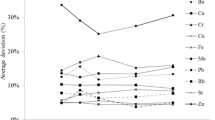
As we measured three replicates for each soil sample, the standard deviation and coefficient of variation of measurements can be calculated. Table 5.1 shows the mean and standard deviation of measurements along with the average standard deviation and coefficient of variation (CV) of the measurements. The concentration of Fe is lower under field conditions as compared to air-dry conditions (Fig. 5.3). However, the standard deviation and CV of the measurements are relatively similar between field-moist and air-dry soil samples. This suggests that measuring the soil under field conditions does not introduce much more variation when compared to a more uniform dried and sieved sample. This differs from the findings of Laiho and Perämäki (2005). We hypothesised that our samples are more variable, and thus, the field variation is larger than the variation in measurement.
We mapped the Fe concentration using the 240 observations collected for the study area (2083 ha) of Nowley Farm using ordinary kriging. As shown in Fig. 5.5, the spatial pattern of Fe content between field-moist and air-dried conditions is similar. Since the relationship of Fe content between field and air-dried conditions is linear, we can correct the observed offset using an empirical relationship. We selected 10 samples from the whole range of Fe concentrations and fitted a linear relationship:
This relationship is applied to the field-moist map, which results in an air-dried corrected map (Fig. 5.5c) that is identical to the map produced using samples scanned in air-dried condition. Thus in practice, we can derive such correction functions from field measured data by selecting a subsample (e.g. 10 samples) covering the whole elemental concentration. These subsamples were air-dried and ground and remeasured under laboratory conditions, and thus, a correction function can be derived.
4 Conclusions
The response of XRF intensity to soil moisture content is affected by mineralogy and clay content. The correction factor proposed by Ge et al. (2005) does not apply for soil with varying clay content and mineralogy. The effect of moisture dampens the X-ray intensity resulting in lower elemental concentrations. The effect of soil moisture on Fe content is more pronounced for clayey soils than sandy soils. Furthermore, the relationship between air-dried and field-moist Fe concentrations is linear. As the coefficient of variation between air-dry and field-moist measurements is similar, we can measure elemental concentrations directly in the field. We propose a pragmatic way to correct for the moisture effect during field pXRF measurement by taking representative samples (from high to low elemental concentration), and subsequently preparing and measuring those samples under air-dried condition. From this, a linear correction factor can be developed to correct for field-moist measurements.
References
Bastos RO, Melquiades FL, Biasi GEV (2012) Correction for the effect of soil moisture on in situ XRF analysis using low-energy background. X-Ray Spectrom 41(5):304–307
Ge L, Lai W, Lin Y (2005) Influence of and correction for moisture in rocks, soils and sediments on in situ XRF analysis. X-Ray Spectrom 34:28–34. doi:10.1002/xrs.782
Hartemink AE, Minasny B (2014) Towards digital soil morphometrics. Geoderma 230–231:305–317
Horta A, Malone B, Stockmann U, Minasny B, Bishop TF, McBratney B, Pallasser R, Pozza L (2015) Potential of integrated field spectroscopy and spatial analysis for enhanced assessment of soil contamination: a prospective review. Geoderma 241–242:180–209
Hürkamp K, Raab T, Völkel J (2009) Two and three-dimensional quantification of lead contamination in alluvial soils of a historic mining area using field portable X-ray fluorescence (FPXRF) analysis. Geomorphology 110(1):28–36
Kalnicky DJ, Singhvi R (2001) Field portable XRF analysis of environmental samples. J Hazard Mater 83:93–122
Laiho JVP, Perämäki P (2005) Evaluation of portable X-ray fluorescence (PXRF) sample preparation methods. Special Paper of the Geological Survey of Finland 73–82
Markowicz AA (2008) Quantification and correction procedures. In: Potts PJ, West M (eds) Portable X-ray fluorescence spectrometry: capabilities for in situ analysis. The Royal Society of Chemistry, Cambridge, pp 13–38
Maruyama Y, Ogawa K, Okada T, Kato M (2008) Laboratory experiments of particle size effect in X-ray fluorescence and implications to remote X-ray spectrometry of lunar regolith surface. Earth Planet Space 60:293–297
Parsons C, Grabulosa EM, Pili E, Floor GH, Roman-Ross G, Charlet L (2013) Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: considerations for sample preparation and measurement conditions. J Hazard Mater 262:1213–1222
Tjallingii R, Röhl U, Kölling M, Bickert T (2007) Influence of the water content on X‐ray fluorescence core‐scanning measurements in soft marine sediments. Geochem Geophys Geosyst 8(2)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stockmann, U., Jang, H.J., Minasny, B., McBratney, A.B. (2016). The Effect of Soil Moisture and Texture on Fe Concentration Using Portable X-Ray Fluorescence Spectrometers. In: Hartemink, A., Minasny, B. (eds) Digital Soil Morphometrics. Progress in Soil Science. Springer, Cham. https://doi.org/10.1007/978-3-319-28295-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-28295-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28294-7
Online ISBN: 978-3-319-28295-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)