Abstract
The presence of cervical lymph node metastases remains one of the most important prognostic factors for various solid tumours of the head and neck, including melanoma, squamous cell carcinoma and Merkel cell carcinoma. In patients with clinically evident neck involvement, the regional lymphatics clearly require directed treatment, and this may involve therapeutic neck dissection or radiotherapy. However, the decision whether or not to electively treat patients with clinically uninvolved cervical lymphatics is usually less clear-cut. On the one hand, elective neck dissection simultaneously allows for accurate pathologic neck staging and definitive surgical management of patients found to harbour occult metastatic disease. On the other hand, the majority of patients with clinically negative necks do not harbour occult disease and would therefore be overtreated by an elective neck dissection. The significant morbidity associated with neck dissection means that this is a real concern, and efforts to minimise the extent of surgical intervention while maintaining oncologic safety are ongoing.
The radical en bloc cervical lymph node dissections introduced at the start of the twentieth century have largely been surpassed by more focused surgical procedures, including the modified radical neck dissection (MRND) and, more recently, selective neck dissection (SND). The operative morbidity of MRND and SND procedures compares favourably with more extensive dissections, though it remains significant. Sentinel lymph node biopsy (SLNB) represents an extension of this principle; by super-selecting the small subset of lymph nodes most likely to harbour disease, the extent of surgical intervention can be further minimised without adversely affecting diagnostic accuracy. The sentinel node concept states that tumour spread occurs in a stepwise progression from the primary tumour to the first-echelon lymph nodes, before progression to the remainder of the lymphatic basin.
These first-echelon lymph nodes, known as the sentinel nodes, can be harvested, examined for the presence of tumour and used to predict the disease status of the entire basin. In the head and neck region, considerable variability exists in the patterns of lymphatic drainage from each primary tumour site, and the exact location of the sentinel nodes therefore varies between patients. In order to accurately locate the SLNs, a number of techniques may be employed. Preoperatively, radiolabelled tracer is injected in a peritumoral fashion, travelling via the lymphatics to the first-echelon nodes where it may be detected by gamma camera during lymphoscintigraphy (LSG). A handheld gamma probe is utilised intraoperatively to afford more precise radiolocalisation, and some surgeons choose also to inject peritumoral blue dye, easing visual identification of the lymphatics. These comprise the sentinel lymph node biopsy technique, which has been applied to a variety of solid tumours including breast cancer, malignant melanoma and penile cancer.
This chapter describes SLNB as it relates to the management of solid tumours in the head and neck region, particularly malignant melanoma, squamous cell carcinoma and Merkel cell carcinoma. A brief history of the development of the technique and its reported accuracy are presented, and the advantages and disadvantages of this relatively new application are discussed. Finally, this chapter will explore the possible roles that SLNB may play in the future management of head and neck cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sentinel node biopsy
- Head and neck cancer
- Neck dissection
- Melanoma
- Squamous cell carcinoma
- Merkel cell carcinoma
15.1 Introduction
Head and neck cancers comprise a diverse group of tumours arising from the epidermis, with significant differences in tumour biology, disease characteristics and prognosis. The three most common types of head and neck cancer are malignant melanoma (MM), arising from melanocytes; squamous cell carcinoma (SCC), arising from keratinocytes; and Merkel cell carcinoma (MCC), a rare aggressive skin tumour arising from neuroendocrine cells.
Despite their differences in many regards, these cancer types share one important characteristic: their prognosis is heavily dependent on the presence or absence of lymph node metastases. Patients with malignant melanoma and nodal involvement demonstrate less than 50 % 5-year survival [1], and similar figures have been reported for patients with SCC [2]. In Merkel cell carcinoma, the presence of nodal disease has been shown to be the most important prognostic indicator by multivariate analysis [3], with a further study demonstrating a drop from 40 months to 13 months median survival with nodal involvement [4].
Virchow [5] was the first to postulate that lymph nodes act as a barrier to particulate matter, and in particular cancer cells. The contention that cancer progression followed a sequential route from the primary site to the regional lymphatics before distant metastasis laid the way for development of regional surgical treatments for a variety of cancers: first, Halsted’s radical mastectomy for breast cancer [6] and, in the case of the head and neck, the radical neck dissection as described by Crile [7].
15.2 Anatomy of the Cervical Lymph Node Basin
The lymphatic anatomy of the head and neck is complex, comprising approximately 250–350 lymph nodes and demonstrating great variability in the patterns of lymph flow observed [8]. The cervical lymph nodes may be divided into superficial and deep chains. The superficial chain lies between the skin and the superficial fascia of the face and scalp, following the anatomy of the major veins, and eventually drains into the deep chain. The deep chain lies along the course of the internal jugular vein under the sternocleidomastoid muscle, draining inferiorly from the base of the skull to the brachiocephalic junction, where the lymph is returned to the venous system. The most popular system of classification for cervical lymphatic anatomy was developed at the Memorial Sloan Kettering Cancer Center [9] and forms the basis for describing the various types of neck dissection in current usage [10]. In this system, the cervical lymph nodes are divided into levels I through VI. The anatomy and classification system are illustrated in Fig. 15.1.
15.3 Neck Dissection
The introduction of the radical neck dissection (RND) in 1906 [7] represented an important step for both staging and treatment of patients with head and neck cancer. However, the morbidity associated with such an extensive dissection was considerable. Complications included shoulder stiffness, pain, muscle atrophy, facial swelling and cosmetic defects, while the mortality rate following bilateral RND was reported as high as 10 % [11]. A number of “modified radical” neck dissections were developed as a means of minimising associated morbidity, being designated MRND I–III depending on the structures preserved (accessory nerve, sternocleidomastoid and/or internal jugular vein) [12]. Studies demonstrating the oncologic safety of the MRND led to its adoption as the standard of care, and the radical neck dissection fell out of favour [13].
The goal of reducing morbidity continues to push the development of more conservative surgical management techniques, however, and this is particularly true for patients with clinically uninvolved necks. Improved understanding of the lymphatic anatomy of the head and neck has facilitated the development of more selective lymphadenectomies, concentrating on the groups of lymph nodes most likely to be involved [14–16]. These selective neck dissections (SND) require less extensive dissection, leaving more of the normal lymphatic anatomy intact, and have been shown to cause less morbidity when compared with MRND [17]. The various types of neck dissection are outlined in Table 15.1.
Despite these recent advances, neck dissection remains an invasive procedure with appreciable morbidity [18], and, while its use in clinically node-positive patients is well established, elective neck dissection for patients with clinically negative (cN0) necks remains controversial. Traditionally considered the gold standard, END provides tissue for accurate pathologic staging while also treating the neck by removing lymph nodes at risk for involvement [19]. However, the majority of cN0 patients do not in fact harbour occult nodal metastases and may be unnecessarily subjected to the morbidity associated with the procedure.
As a result, selection of patients who would benefit most from neck dissection becomes increasingly important. Clinical staging of the cervical lymph nodes is unreliable, with poor reported sensitivities for both palpation and clinical imaging, and it is generally accepted that an occult nodal metastasis rate of 20–30 % persists despite meticulous clinical staging [20–22]. For SCC, elective neck dissection is currently recommended for patients with a greater than 20 % risk of occult nodal metastases based on primary tumour characteristics such as site and T-stage [23]. The role of END for cN0 head and neck melanoma patients is unclear, with no consistent survival benefit demonstrated [24]. It has been suggested that END may be most beneficial for patients with primary tumours between 1.5 mm and 3.99 mm in thickness [25].
15.4 Sentinel Node Biopsy
Sentinel node biopsy represents a means of super-selecting the group of lymph nodes most at risk for disease involvement, allowing histopathologic staging of the neck while minimising the extent of surgical intervention for patients without nodal involvement. The sentinel node concept is based on the assumption that spread from the primary tumour occurs to a single node (or group of nodes) before progressing to the remaining nodal basin and systemic metastasis (Fig. 15.2). Identification of these sentinel nodes allows for selective biopsy and pathologic evaluation of the nodes most likely to represent the disease status of the remaining nodal basin [26]. The results of sentinel node biopsy (SNB) can then be used to guide further management, with SNB-positive patients going on to receive definitive (therapeutic) neck dissection and/or parotidectomy, while SNB-negative patients may be followed clinically. These SNB-negative patients may therefore avoid some of the morbidity associated with neck dissection [27].
The potential advantages of sentinel node biopsy over neck dissection are manyfold, including its minimally invasive nature, a lower per-patient cost compared with comprehensive neck dissection [28, 29] and a drastic reduction in the number of lymph nodes submitted for pathologic evaluation. In turn, this allows a more in-depth search for micrometastatic deposits utilising techniques such as step-serial sectioning and immunohistochemistry [30, 31]. However, SNB can be a technically challenging technique with a steep learning curve [26, 32], and as such, investigators wishing to begin using the technique for SCC are recommended to do so within the context of SNB-assisted END [33]. As with any biopsy technique, there exists the potential for sampling error, and the reported false-negative rate ranges from 0 to 10.5 % in most studies for both SCC and melanoma [33–39]. Finally, the usefulness of SNB is currently restricted to cN0 patients, since distortion of the normal lymphatic anatomy by extensive tumour infiltration may lead to unexpected drainage patterns and increase the likelihood of false-negative results [40].
15.5 Development of the Sentinel Node Concept
The first description of a “sentinel” lymph node dates back to 1960 with a total parotidectomy reported by Gould et al., during which frozen section examination of a single facial lymph node was used to guide the decision for neck dissection [41]. Subsequently, Cabanas et al. reported direct drainage from the penis to the lymph nodes associated with the superficial epigastric vein in a series of 46 patients with penile SCC and described 90 % survival for sentinel node-negative patients [42]. Similarly, Weissbach and Boedefeld suggested a limited retroperitoneal lymph node dissection in patients with testicular cancer, in order to detect lymphatic involvement while minimising operative intervention [43]. Holmes et al. introduced the use of colloidal gold injections to demonstrate the actual patterns of lymph drainage for ambiguous areas such as the midline [44] and followed this in 1992 with the description of intraoperative vital dye injection, providing a means of visually tracing dye-stained lymphatics to the first-echelon nodes [26]. In 1993, Alex and Krag described the intraoperative use of a handheld gamma probe, easing the detection of the sentinel nodes and improving identification rates [45]. Since these early studies, SNB has gone on to become increasingly important as a staging tool for patients with early-stage melanoma [46], and work is underway to fully elucidate its utility in SCC management [33, 47]. The role played by SNB in the management of these and other head and neck cancers will be described later in this chapter.
15.6 Technique of Sentinel Node Biopsy
In general, sentinel node biopsy is comprised of three parts: preoperative lymphoscintigraphy, intraoperative identification and harvest and pathologic evaluation of sentinel nodes. These components will be described in detail in this section, with reference to the minor differences in protocol for each of the major head and neck cancer types.
15.6.1 Preoperative Lymphoscintigraphy
The lymphatic anatomy of the head and neck is complex and variable, with discordance between predicted and actual lymphatic drainage in up to 67 % of patients [8]. Aberrant drainage patterns can lead to inaccurate placement of the initial access incision and may contribute to failure of sentinel node identification [15]. The goal of preoperative lymphoscintigraphy is to demonstrate the location of sentinel nodes prior to incision. This begins with injection of a radiolabelled colloid solution at the site of the primary tumour. The radiocolloid may then track along the same afferent lymphatics draining the tumour, accumulating in the first-echelon lymph nodes where the resultant radioactivity may be detected by gamma camera. Lymphoscintigraphy may be carried out up to 24 h before surgery, or on the day of surgery, and this should be coordinated between the nuclear medicine physician and the surgeon.
The technique of radiocolloid injection varies according to the type of cancer being studied. For melanoma and other cutaneous tumours, multiple intradermal injections should be employed to completely encircle the tumour or site of previous excision biopsy. There has been considerable debate regarding the accuracy of lymphoscintigraphy, and SNB in general, in cases where wide local excision (WLE) has previously been carried out. While it is strongly preferred that SNB be performed prior to excision, there is some evidence to suggest that previous WLE is not an absolute contraindication [48]. For intraoral lesions, the majority of which are SCC, multiple mucosal/submucosal injections should be performed around the periphery of the tumour or scar margin, and deeper injections may be employed according to the depth of the lesion [49]. Ideally, the operating surgeon should be present for the injections to ensure consistency with injection of blue dye if used. The volume injected varies according to the location and size of the lesion and ranges from two to four aliquots. A mouthwash should be employed following intraoral injections, to prevent sumping or swallowing of radiotracer.
The ideal radiotracer should emit only gamma rays, be cleared rapidly from the injection site, have a uniform particle size and persist in the lymph nodes until imaging can be performed [50, 51]. A variety of technetium99m (99Tcm)-labelled colloids are available, including 99Tcm human serum albumin, 99Tcm colloidal albumin, 99Tcm antimony sulphur colloid and 99Tcm sulphur colloid, although regional licensing issues may restrict the available choices. In Europe and parts of the USA, AlburesTM and NanocollTM (Nycomed Amersham, Buckinghamshire, UK) are the most commonly available colloidal albumin preparations. The larger particle size of AlburesTM (500 nm) limits its use to primary tumour sites with high lymphatic density, such as the anterior tongue or floor of the mouth, while the 50 nm particle size of NanocollTM allows its use in other sites [33, 51]. For regions where human albumin-based colloids have not been approved, sulphur colloid preparations are available in both unfiltered (300–340 nm) and filtered (<200 nm) forms [52]. There is little consensus on the optimum activity for injection, which varies from 15 to 120 MBq between studies with higher doses or repeat injections being employed for the 2-day protocol [53–55]. However, it has been suggested that much lower doses (0.37–2.2 MBq) may be used in the setting of head and neck melanoma [56].
Planar lymphoscintigraphic imaging may be static or dynamic or a combination of the two. The addition of dynamic imaging for melanoma patients improves the detection of “in-transit” nodes, which are reported to occur in 5–8 % of the population and should also be considered sentinel nodes [57, 58]. To date, there have been no reports of in-transit nodes in patients with SCC. There is currently no evidence favouring either technique in these patients, and the exact timing of static image acquisition varies between centres. Images should be obtained in two planes: anterior and lateral or lateral oblique. A gamma camera fitted with a low-energy, high-resolution (LEHR) collimator is used to image the patient, whose silhouette can be delineated by a flood source of 57Co or 99mTc placed behind the patient or by tracing his/her outline with a 57Co-labelled marker pen. At this point, it may be helpful to mark the skin overlying visualised sentinel nodes with indelible marker pen [33, 49, 51]. However, this practice has not been universally accepted due to concerns that the change in positioning between lymphoscintigraphy and surgery may misguide the placement of initial access incision [59].
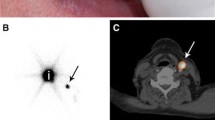
Recent studies have reported potential improvements in preoperative sentinel node identification through the use of single-photon emission computed tomography (SPECT/CT) imaging [60, 61]. This hybrid anatomical/functional imaging modality affords better topographical orientation and separation of SLNs from adjacent structures, compared with planar lymphoscintigraphy alone, allowing the surgeon to see 3-dimensional images of the nodes. It may also provide more consistency in identification of sentinel nodes, as evidenced by Uren et al. [62] who found that different nodes may be identified on lymphoscintigraphy performed on the same site a day apart. However, this problem may also be found in SPECT, and no studies have shown that ~SPECT performed on different days always identifies the same nodes. This problem may be addressed intraoperatively with freehand SPECT, but studies are required to investigate this.
In the melanoma literature, it appears that SPECT/CT can lead to more accurate incision placement and improvements in SLN detection rates [61, 63–65], and freehand SPECT has shown encouraging results for intraoperative imaging [66]. For SCC there have been promising reports regarding the use of SPECT/CT [67]; however, these have yet to be consistently reproduced [68].
Bluemel et al. showed that freehand SPECT can accurately predict SLN status intraoperatively in oral/oropharyngeal SCC including for floor of the mouth tumours where it may reduce the shine-through effect. One limitation of freehand SPECT is the need for repeated scans due to artefacts.
15.6.2 Surgical Technique
Within 24 h of lymphoscintigraphy, patients may undergo the operative portion of SNB. Although SNB of cervical lymph nodes under local anaesthesia has been reported [69], most surgeons prefer to employ general anaesthesia for this technique. The patient is prepared and draped as for a standard excision and neck dissection. Preoperative lymphoscintigraphy images should be available for reference in the operating suite, in electronic or hard-copy form, and these may be used to guide the placement of the initial access incision. If skin markings have been placed in the nuclear medicine suite, underlying radioactivity levels should be verified using a handheld gamma probe prior to making the incision. The orientation of the incision should be such that it may be easily excised in the event of a future neck dissection.
If injection of vital (blue) dye is desired, this may be carried out prior to preparing and draping. Injections should be undertaken by the same operator as the radiotracer injection in order to ensure consistency, and the pattern and depth of injection should mirror that of the radiotracer. The brand of dye used varies according to geographical region, with Patent Blue V Dye (Laboratoire Guerbet, Aulnay-Sous-Bois, France) available in Europe and LymphazurinTM (Tyco Healthcare Group LP, Norwalk, CT, USA) in the USA. The technique of blue dye injection, introduced by Morton et al., provides a means of visually identifying the small lymphatic vessels intraoperatively, allowing them to be traced to the first-echelon nodes [26]. However, the success rate for identification of SLNs by blue dye injection is less than that for radiolocalisation by gamma probe, and the technique has a steeper learning curve [70]. In a study of 55 patients with head and neck melanoma, Wells et al. reported a 67 % identification rate by blue dye mapping and 95 % utilising a combined approach [38].
While most blue dye-stained SLNs are also found to be radioactive or “hot”, a small minority of SLNs are “cold”, and proponents of blue dye injection report facilitation of intraoperative identification [33, 49, 71]. The major perceived disadvantages to blue dye are related to persistent cutaneous staining and masking of true surgical margins; however, rare cases of anaphylactic reactions have also been reported [72]. As a result, the use of blue dye is considered optional, though many authors employ a combined approach.
Guided by the preoperative lymphoscintigraphy images, skin markings (if present) and the handheld gamma probe, a small skin incision (2–4 cm) is made and limited skin flaps elevated. Dissection is carried through the superficial fascia and is guided by the handheld gamma probe. If blue-stained lymphatics are visualised, these may be followed to the draining lymph node(s); if no staining is present (or dye was not used), the dissection may be guided solely by the gamma probe, which is fitted with a 14 mm diameter straight collimated probe. The angle of the probe may be gradually altered while watching or listening for a change in the counts per second (cps). In cases where the primary tumour site lies in close proximity to the regional lymph nodes, a particular problem for floor of the mouth SCCs, radioactive “shine through” from the primary tumour site may mask the true position of the sentinel node. In these patients, the use of malleable lead plates between the injection site and the nodal basin may address this issue [26, 45, 49, 51]. All radioactive and/or blue-stained nodes are clipped and excised, and radioactivity is confirmed ex vivo. Following excision, the remaining basin is examined with the gamma probe, and no further SLNs are considered present when the residual count rate is less than 10 % that of the “hottest” excised SLN [73]. This strategy is somewhat arbitrary, as there are no specific guidelines on the optimum strategy for SLN identification. The 10 % rule has been reported to lead to unnecessary removal of non-SLNs [74], and some authors advocate for removal of the “hottest two plus blue rule” which potentially reduces operative time and number of nodes removed without increasing false-negative rates in melanoma [75]. In SCC it has been shown that removing the hottest three nodes would be sufficient to ensure accurate results [76, 77]. Patients undergoing SNB-assisted END may then proceed to completion neck dissection.
15.7 Further Developments in Lymph Node Identification
Several studies have reported initial successes with near-infrared imaging using indocyanine green dye. Benefits of this include good tissue penetration, direct real-time transcutaneous intraoperative visual feedback of draining lymph channels and excellent safety profile. It has poor results when used alone particularly as transcutaneous feedback is unreliable, but it has excellent results when combined with radioisotopes [78–80]. However, due to the small particle size and speed of travel through lymphatics, non-sentinel lymph nodes may be inappropriately identified and removed, and the technique’s usefulness has yet to be clarified by large randomised studies [79]. More studies are required to further evaluate the role of indocyanine in SNB.
15.8 Pathologic Evaluation of Sentinel Nodes
Detection of metastatic disease in sentinel nodes by pathologic examination is intrinsic to the success of the procedure and offers a number of advantages over traditional elective neck dissection. Principally, the absolute number of lymph nodes examined is far fewer during SNB, allowing the pathologist to perform a more thorough search for micrometastatic deposits.
15.9 Metastases, Micrometastases and Isolated Tumour Cells
Previously, the degree of tumour burden identified in lymph nodes was classified as metastases, micrometastases or isolated tumour cells (defined as tumour size <0.2 mm, single cells or small clusters, with no stromal reaction or contact with the vessel wall). Isolated tumour cells did not previously upstage the neck to node positive. However, according to the most recent American Joint Committee of Cancer (AJCC) guidelines, a single isolated cell detected by IHC defines positive SLN involvement [81]. The recent AJCC guidelines (shown in Table 15.2) state that nodal metastases can be confirmed using either H&E or IHC to identify at least one melanocyte-specific marker.
In order to compare results across studies, uniform reporting standards for pathologic staging are critical. For each of the head and neck cancer types, the sequence of pathologic examination is broadly similar and involves gross examination, bivalving of the lymph node, sectioning at predefined intervals and staining with a variety of histopathologic techniques. However, there are a number of minor differences in protocol according to the type of tumour being studied, and exact sectioning/staining protocols vary between centres. In some cases, additional techniques such as real-time polymerase chain reaction (RT-PCR) may also be employed; these differences are briefly outlined below [82, 83].
15.10 Melanoma
The addition of immunohistochemical techniques to standard H&E examination has been shown to increase melanoma detection rates by at least 10 % [84], and a number of sectioning/staining protocols have been described in an effort to maximise detection rates while minimising unnecessary workload. Some authors have advocated examination of only the central portion of the lymph node, based on the suggestion by Cochran et al. that the vast majority of micrometastases occur centrally [85], while other suggested protocols have included sectioning of the entire node into 1 mm slices [86], or examination of one half of the SLN using a combination of histology and immunohistochemistry, and the other half using RT-PCR with a variety of probes [87].
RT-PCR detection of occult metastatic deposits is an attractive technique, potentially reducing the cost and labour associated with SLN evaluation. However, disadvantages include its destructive nature, inability to distinguish benign and malignant cells and positivity rates of up to 70 % in some studies [88]. False positives may be due to capsular or trabecular naevus cells, nerves or macrophages. In a recent report by Cook et al., utilising an extended stepwise study of bivalved nodes with immunohistochemistry, the discrepancy between detection rates using histology/IHC and RT-PCR was found to be only 3–5 %. Several studies have since shown that RT-PCR can upstage up to 30 % of patients who were initially found to have negative SLNs on H&E or IHC staining [89, 90]. Nevertheless, the exact role of RT-PCR remains to be fully elucidated, and the authors therefore recommend the routine use of their extended histology/IHC protocol, which sections deeper into the periphery of the node, until further data become available [82].
The protocol currently recommended by the EORTC is illustrated in Fig. 15.3. Briefly, the sequence involves bivalving the formalin-fixed SLN, embedding in paraffin and sectioning at 50 μm intervals to a total depth of 250 μm. Several sections are taken at each interval and are alternately stained with H&E, S100 and/or HMB45 for IHC. Sections found positive by IHC are compared with adjacent H&E-stained sections in order to confirm the presence of viable tumour cells. The use of this extended sectioning protocol results in thorough evaluation of the central 700–800 μm of each SLN and is thought to represent the best balance between sensitivity, cost-effectiveness and pathologist workload [82]. The EORTC protocol outlined above involves more extensive processing when compared to techniques used in trials such as MSLT-1 suggesting that the false-negative rate reported in such trials could potentially be lowered by more thorough histopathologic processing.
False positive may arise as a result of the inability to distinguish between malignant melanoma cells and benign intranodal naevi cells. HMB45 is often used to distinguish but can still be present in a significant number of nodal naevi [91]. Lee et al. [92] showed that benign naevi cells retain high levels of nuclear staining for the epigenetic hallmark 5-hydroxymethylcytosine which has the potential to accurately distinguish benign and malignant cells. This was confirmed in their study with all 18 malignant cases showing complete loss of staining and all 10 benign naevus cases retaining staining. Chen et al. [93] also found the nuclear biomarker SOX2 to have potential to differentiate benign and malignant cells.
15.11 Squamous Cell Carcinoma
For SCC, there remains considerable debate regarding the optimal method for sectioning SLNs. Current recommendations were formulated during the Second International Conference on Sentinel Node Biopsy in Mucosal Head and Neck Cancer in 2003 and are included in the recent joint guideline published by the European Association of Nuclear Medicine (EANM) and European Sentinel Node Trial (SENT) committee [54, 83].
SLNs less than 2 mm in longest dimension are processed whole, while those measuring 2–5 mm should be bivalved and both halves processed en face. Nodes greater than 5 mm are cut into 2 mm slices, and each slice is processed en face. A section from each slice is stained with H&E, and positive nodes/slices result in upstaging of the patient. Step-serial sectioning (SSS) at finer intervals of 150 μm (six sections per interval) should be carried out for SLNs found negative after initial sectioning, and these are H&E stained and examined as before. Finally, SLNs that remain negative are subjected to immunohistochemical (IHC) staining with pancytokeratin antibody (AE1/AE3 or MNF116). The combination of SSS and IHC has previously been shown to detect an additional 10 % of occult/micrometastatic deposits compared with H&E alone [33]. If no disease is found following H&E and IHC staining, the lymph node is considered free of tumour. For SLNs with positive IHC staining, the positive section must be compared with the immediately adjacent serial section in order to avoid false positives due to non-viable tumour cells, artefacts and/or inclusion of other cell types [54].
The use of intraoperative frozen section analysis of SLNs offers the potential advantage of avoiding a second anaesthetic for SNB-positive patients, but has traditionally been avoided due to concerns regarding freezing artefacts and loss of tissue. However, several recent studies have shown promising results with only 10–17 % of SNB-positive patients requiring a second procedure [35, 94, 95]. The technique has not yet gained universal acceptance, and others have questioned the sensitivity of frozen section when compared with standard practices [34] for identification of micrometastases and isolated tumour cells [96, 97].
Novel techniques such as imprint cytology [98] and intraoperative real-time genetic evaluation [99] currently remain under investigation. In particular, one-step nucleic acid amplification has the potential to allow fast intraoperative detection of lymph node metastases, reducing the need for a second procedure. Ferris et al. [100] showed excellent reproducibility and 94.2 % accuracy in 103 lymph nodes with their tumour-associated calcium signal transducer 1 and pemphigus vulgaris antigen assay. Although in the early stages, this technique has exciting potential.
15.12 Merkel Cell Carcinoma
Pathologic evaluation of the sentinel nodes in MCC is similar to that for melanoma, though no standardised protocol has yet been adopted. The differences lie mainly in the type of step-serial sectioning, which varies from 2–3 mm slices [101] to 1 mm slices with multiple 200 μm sections per slice [102], and the use of anti-CK-20 staining (Dako Corp, Carpinteria, Calif.) in place of S100/HMB-45 for immunohistochemistry. CK-20 is well established as the most sensitive and specific marker currently available for the detection of MCC [103].
15.13 The Role of SNB in Current Practice
15.13.1 Melanoma
Following the initial reports of SNB for cutaneous melanoma using blue dye only, technical difficulties and the significant learning curve associated with the procedure led to variable technical success rates ranging from 60 to 80 % [46]. Subsequently, the introduction of radiolabelled tracer injection, preoperative lymphoscintigraphy and intraoperative gamma probe guidance led to significant improvements in identification rates to greater than 90 %, and the use of both blue dye and radiotracers quickly gained acceptance [36, 59, 104]. Since then, the technique of SNB has been demonstrated to accurately predict the disease status of the remaining nodal basin in a number of landmark studies of cutaneous melanoma (all sites) [48, 105, 106].
The presence of metastases within SLNs has been demonstrated to be the most accurate predictor of outcome in melanoma patients without clinical lymph node involvement [107], and its benefits as a prognostic tool are universally accepted. As a result, SNB is widely regarded as the gold standard for staging the lymphatic basins of intermediate-thickness melanoma (1–4 mm) patients without clinical evidence of nodal involvement [46]. The recently published results of the MSLT-1 trial confirmed that SNB is the most effective staging tool for primary melanoma [108]. Current guidelines recommend sentinel lymph node staging in all primary melanomas greater than 1 mm Breslow thickness; however, there remains debate as to the benefits of SLNB in patients with thick and thin melanomas, and this will be discussed further.
The greatest area of controversy surrounding SNB in melanoma is whether there is a survival benefit for therapeutic lymphadenectomy in the clinical-negative SNB-positive patient group. A small but significant survival benefit was reported in an early report, based on subgroup analysis [109]. However, the final results after 10 years of follow-up of the landmark MSLT-1 trial published in 2014 have provided more reliable evidence as to the benefits of SNB in melanoma [108].
The MSLT-1 trial randomised patients with melanoma greater than 1.2 mm Breslow thickness to either wide local excision (WLE) of primary tumours plus SNB and lymphadenectomy if positive or WLE plus observation and lymphadenectomy if nodal disease developed. The study found no significant melanoma-specific survival advantage for patients having SNB compared to those undergoing observation and therapeutic lymphadenectomy in intermediate-thickness melanoma (1.2–3.5 mm). However, some authors have argued that the trial was underpowered and that the data showed a trend towards a likely melanoma-specific survival benefit as well as demonstrating a significantly improved 10-year disease-free survival in both intermediate and thick melanomas [110].
Furthermore, the trial did show a statistically significant improvement in melanoma-specific survival for sentinel node-positive patients with intermediate-thickness tumours who underwent immediate lymphadenectomy after SNB. The overall 10-year melanoma specific survival was 62.1 % in this group compared to 41.5 % in the observation arm. This survival benefit was also shown to remain significant even when false-negative cases were included in the analysis, confirming the accuracy of the findings. This group of SNB-positive patients constitutes approximately 20 % of the overall population, a significant proportion of patients.
However, critics argue that this benefit is only conferred upon 20 % of the population despite the morbidity of the procedure affecting the entire SNB cohort and that the overall population did not show a survival advantage even when thick tumours, which are most likely to affect survival, were excluded from the analysis [111, 112]. They argued that the trial was always unlikely to show survival advantage from SNB, given that the majority of patients will not develop nodal disease and therefore will gain no benefit from further staging after primary resection [113]. Authors also highlight the limitations of the study design with possible ascertainment bias and small population size limiting the value of the conclusions [114].
However, others have argued that the clinically and statistically significant survival benefit of early treatment to patients with positive nodes, even if they only constitute 20 % of the patient population, is a justification for SNB [115]. They argue this is particularly true as MSLT-1 also showed that morbidity from lymphadenectomy was significantly reduced when done early after positive SNB compared to when performed later after nodal recurrence, with the benefits most marked in lymphoedema [114]. Also, early intervention guided by SNB reduces the extent of nodal involvement at surgery by half [115]. Furthermore, similar melanoma-specific survival has been demonstrated in other large studies [116]. The MSLT-1 trial also showed an improvement in recurrence-free survival in both intermediate and thick tumours. However, some authors have argued that this result was inevitable given the flaw in the study design in that the observation arm had an intact nodal basin and therefore had a much higher chance of nodal recurrence than those with previously treated nodal basins [112, 117]. Thomas also argued that a proportion of SNB-positive patients were false positive and therefore influenced the final results [117]. He argued that because the cumulative incidence of nodal recurrence was not the same even after 10-year follow-up, then some cases must be false positive. However, in response Thompson and colleagues point out that the difference in nodal recurrence is beginning to converge after 10 years and that differences are not statistically different and therefore are unlikely to skew the results [115].
Given the continuing controversies surrounding the results of MSLT-1, it can only be stated that the results offer guidance rather than definitive proof of the survival benefits of SNB in melanoma.
The issue of whether SNB should be offered to patients with thick melanomas also remains controversial. Current NCCN and ASCO guidelines recommend SNB for all patients with tumours greater than 1 mm Breslow thickness due to its value as prognostic tool [118, 119]. However, MSLT-1 showed that SNB offered no benefit to melanoma-specific and distant recurrence survival compared to observation for patients with thick tumours. Moreover, the survival benefits seen in SNB-positive patients with intermediate-thickness tumours are not found in patients with thick tumours. In fact overall melanoma-specific survival was worse in thick tumours compared to the observation arm, most likely because the false-negative patients did particularly poorly. This has led some authors to argue that SNB in patients with thick tumours is unnecessary given the high risk of metastases and lack of survival benefit of early intervention given that the only benefit SNB provides is prognostic information [114]. However, MSLT-1 did find improved recurrence-free survival in thick tumours undergoing SNB compared to observation as well as a short time to recurrence in patients under observation suggesting that offering early intervention with SNB may be justifiable [114].
The benefit of SNB in patients with thin tumours is also controversial particularly given that a large proportion of melanoma patients have tumours <1 mm thick [120]. Guidelines recommend that patients with tumours 0.76–1 mm should be considered if there are other high risk factors such as high mitotic rate, but there is little proof that the procedure provides any benefit [119]. Van der Ploeg et al. found no survival difference for patients undergoing SNB versus observation with thin tumours [121]. Bartlett et al. showed that patients with thin tumours and no other significant histopathologic features have an extremely low nodal positivity rate of 0.7 %, but this increases to 3.7 % in patients with mitoses or high Clark level [122]. The NCCN guidelines state that when offering patients SNB for tumours 0.76–1 mm, patients must be informed of the limited evidence to suggest benefit and low rate of positivity [118].
An argument for the use of SNB in melanoma is that early staging may allow early enrollment into adjuvant trials and mutation testing for targeted therapies. There have recently been developments in the drug agents available to treat patients with advanced melanoma. In particular the BRAF signalling molecule inhibitors vemurafenib and dabrafenib have shown some survival improvement in melanoma patients who have positive BRAF V600 mutations [123, 124]. Early staging with sentinel node biopsy would allow patients to be BRAF tested early and allow them to receive drug therapy or start adjuvant trials early. In addition many trials stipulate that patients must have been staged via sentinel biopsy before being considered for enrolment. Currently, the benefits of current adjuvant therapies are limited, with many patients only showing partial response or developing resistance to treatment. As these adjuvant treatment options improve, there may be stronger indications for SNB to direct early treatment, particularly in thin and thick melanomas, and in the long term, this may potentially show an improvement in survival.
There is some debate as to whether the results of MSLT-1 are applicable to head and neck tumours due to the complex anatomy and often close proximity of lesions to first-echelon nodes [125, 126]. This means that there is less consensus amongst surgeons as to the benefit of SNB in head and neck tumours, as illustrated by SEER database analysis which reports only 60 % patients of SNB-positive patients undergoing lymphadenectomy in head and neck melanoma [127].
In the head and neck, the prognostic significance of sentinel node status is less clear, with SLN-negative patients demonstrating a 5-year disease-free survival rate of only 55 % in one report. In their review of the existing head and neck melanoma literature, the authors noted false-negative rates in excess of 10 % in 12 of 21 studies and suggested that this high false-negative rate may contribute to the poor survival they observed in their series [127]. Similar results were described in the large Sunbelt Melanoma Trial, where false-negative rates were 12 % for the head and neck, compared with 2–3 % for other sites [37]. However, this view has been challenged by Civantos et al., who contended that surgeons with a subspecialty focus on the head and neck may achieve negative predictive values comparable to the 98.2 % for cutaneous malignancies and 92 % for oral cancer described in their series of 106 patients with head and neck malignancy [91]. Furthermore, a large single centre study showed that SNB status was the best prognostic indicator in HNM and that its results are comparable to those of other nodal basins for false positivity [128]. Several other studies have also illustrated that SNB in HNM is an accurate and safe staging technique [129]. Concluding their review, Tanis et al. stated that there is currently no conclusive survival advantage for either elective lymph node dissection or SNB in patients with intermediate-thickness melanoma of the head and neck; however, the benefits of SNB may potentially justify its use in this patient population. These benefits include early prognostic information for patient and physician, reduced tumour load due to earlier lymphadenectomy and the possibility of a survival advantage based on subgroup analysis [127].
A variety of micromorphometrical parameters of SN tumour deposits have been used in an attempt to determine the likelihood of further disease in the remaining nodal basin, such as tumour penetrative depth from the central plane, location within the node and size. The potential applications for these measurements would include guidance of the decision to proceed with formal lymphadenectomy and prediction of survival.
For example, the knowledge that only 10–30 % of patients with positive SLNs are found to have additional positive “non-SLN” nodes following lymphadenectomy has led some authors to suggest that formal lymphadenectomy may not be required in patients with SLN deposits <0.1 mm in size [130]. However, despite several studies suggesting that patients with low volume disease may be able to avoid lymphadenectomy in the head and neck [131–133], these results have not been universally reproduced in other studies, and as a result the prognostic significance of tumour burden in the sentinel nodes has not yet been fully elucidated. In the meantime, it is recommended that all patients with detectable disease in the sentinel nodes be treated as SN positive and offered formal lymphadenectomy [46, 87].
15.14 Future Application of SNB for Melanoma of the Head and Neck
For melanoma, SNB is well established as a staging tool for patients with intermediate-thickness primary tumours and for selected patients in other groups. The main questions now focus on the optimal management of SNB-positive patients, and these questions are still unanswered as we await the results of the MSLT-2 trial. The MSLT-2 trial is a prospective randomised controlled trial, comparing the outcomes of completion lymphadenectomy and observation alone for SNB-positive patients. The study aims to address whether completion lymph node dissection is always required or whether sentinel node-positive patients can be safely observed. MSLT-1 showed that benefits of SNB and lymphadenectomy are combined, and current guidelines recommend completion lymphadenectomy for all positive cases. A study by Kachare et al. showed that melanoma-specific survival was improved in patients undergoing immediate lymphadenectomy after positive SNB compared to delayed, although this did not reach statistical significance due to small population size [116]. Conversely, Wong et al. showed no difference in melanoma-specific survival between immediate lymphadenectomy and observation in SNB-positive patients in a study of 298 patients. Similarly Gyorki et al. found no difference in the head and neck although this was a small study [125, 134]. In addition to the main question, the differences in technical success and false-negative rates for SNB in the head and neck compared with other sites suggest that the results of large-scale prospective RCTs reporting all-sites melanoma data may not be immediately applicable to the head and neck population. Therefore, similar prospective trials tailored specifically to this patient group are required before definitive conclusions regarding optimal management can be reached.
15.15 Oral/Oropharyngeal Squamous Cell Carcinoma
In patients with oral/oropharyngeal SCC, the current gold standard staging procedure for the clinically node negative neck is elective neck dissection (END). However, this can lead to overtreatment in up to 80 % of cases with associated morbidity, as only 20 % cases have occult metastases. Therefore, SNB has been extensively investigated as a staging procedure for these patients. The vast majority of the tumours studied to date are located in the oral cavity or accessible oropharynx, and, while some reports do exist of SNB for other locations such as the hypopharynx and larynx [135–137], the status of the technique should remain “investigational” in these sites until further data becomes available. Furthermore, the use of SNB may be limited in patients with larger tumours which may be difficult to completely surround with tracer injections and which may ultimately require a neck dissection for tumour access or reconstruction purposes [51].
Early validation studies demonstrated that SNB may be safely and successfully applied to patients with T1 or T2 disease and clinically negative necks in oral/oropharyngeal tumours [33, 54]. These studies demonstrated a false-negative rate of approximately 5 %, comparable to rates with melanoma, leading some centres to adopt SNB as the sole staging tool for patients with early OSCC with only those SNB positive undergoing completion lymphadenectomy [33, 35].
The applications for SNB in early OSCC include staging of the ipsilateral cN0 neck, staging bilateral cN0 necks for tumours with ambiguous drainage (i.e. midline) and staging the contralateral cN0 neck for a midline tumour with an ipsilateral cN+ neck. Other applications, including the use of SNB for patients with recurrent primary tumours or following prior treatment to the neck, remain under investigation.
In a large prospective study, the European multicentre trial included patients from six centres and demonstrated a 93 % SN identification rate and 91 % sensitivity in cT1/T2 N0 OSCC at 5-year follow-up. The authors concluded that SNB is a safe staging tool in early OSCC but advised caution in floor-of-mouth tumours due to lower identification rates and sensitivity likely because of technically challenging access to these tumours and close proximity to the first-echelon lymph nodes [33]. Stoeckli et al. [35] reported a 98 % identification rate and 94 % negative predictive value in the largest single centre study at the time of publication.
Since these early studies, several authors have reported promising results with regard to SNB for OSCC. A meta-analysis by Thompson et al. [136] showed a sensitivity and NPV of 94 and 96 % illustrating the technique is both accurate and of value in providing prognostic information and allowing selection of patients who would benefit from further neck dissection. The authors also concluded that patients with negative SNB can avoid further neck dissection without compromising recurrence, a finding also shown in the study by Yuen et al. [138]. Another study also found that patients who were sentinel node negative had improved survival rates compared to those undergoing observation illustrating the prognostic value of SNB [139]. The accuracy and prognostic value of SNB in OSCC have also been validated by several other studies [140, 141]. The ACOSOG trial [142] also found a 96 % NPV in a study that included floor-of-mouth tumours; however, they did find a higher false-negative rate in FOM similar to Alkureishi et al., highlighting the caution required in these patients [143]. The ACOSOG trial also found that increased surgical experience significantly improved the NPV suggesting that centres and surgeons specialising in this procedure are more likely to demonstrate benefit from it. Broglie et al. found that SNB is not only accurate in assessing nodal status but also in identifying unexpected drainage patterns as 12 % of their study population showed aberrant drainage pathways which would have led to under- or overtreatment by traditional methods [77]. SNB can be particularly useful in identifying unexpected drainage patterns and tailoring dissection in previously treated necks which are more likely to have aberrant drainage pathways [144]. In addition to the accuracy of SNB as a staging procedure, some authors have suggested that SNB is both cheaper [145] and associated with better quality of life outcomes compared to immediate END, due to reduced surgical morbidity [146, 147].
However, despite its benefits as a staging procedure and prognostic tool, several trials have failed to demonstrate any survival benefit of SNB versus END [147, 148] and early intervention versus observation [138, 149]. Therefore, the exact role of SNB in patients with head and neck SCC has yet to be fully elucidated, and END remains the gold standard in most centres.
The European Sentinel Node Trial is a large prospective multicentre study incorporating data from the previous two European trials. In a report of their preliminary results, the authors reported that 52 % of additional nodes found on completion lymphadenectomy after positive SNB were located in the same level as the original positive sentinel node and only 4 % were located outside the two adjacent neck levels. Therefore, they concluded that it may be reasonable to limit therapeutic lymphadenectomies following positive SNB to three levels—one above and one below the positive SLN—potentially further reducing the morbidity associated with treatment of the neck. Follow-up results of this trial have yet to be published, and no further studies have ratified their conclusions.
There is controversy over which neck levels require dissection in oral SCC. Some authors have argued that oral SCCs show predictable drainage patterns to ipsilateral levels I–III, and these should be targeted [148]. However, dissection of level IV nodes may also be required due to the potential of skip metastases to this level without involvement of levels I–III [143, 150–152]. Broglie et al. [77] demonstrated that the majority of OSCCs show predictable drainage to levels I–III; however, 12 % showed unexpected drainage patterns. There is no current consensus on the most appropriate level of dissection required; however, less radical dissection is desirable due to increased morbidity and reduced quality of life with more radical surgery [150, 153].
15.16 Cutaneous SCC of the Head and Neck
For patients with cutaneous SCC, the rate of nodal metastasis is much lower, ranging from 0.3 to 16 % [154, 155]. As a result, SNB has not been well studied in this patient group. As part of a larger series of multiple tumour types, Civantos et al. undertook SNB in a series of 10 patients with “high-risk” cutaneous SCC and detected occult nodal disease in only one patient [156]. Since this study, a review of the literature found that the false-negative rate is approximately 4.76 % similar to that of other regions suggesting that SNB is accurate for cutaneous SCC [157]. Furthermore, a study by Takahashi et al. showed that SNB-positive patients had a worse survival rate compared with SNB-negative patients, suggesting SNB may be used as a prognostic indicator [158]. Several small studies have suggested that tumour thickness is the most reliable predictor of nodal positivity, with tumours less than 2 mm extremely unlikely to be positive and those greater than 6 mm having approximately a 16 % positivity rate [159, 160]. However, there remains a severe lack of evidence as to the value of SNB in cutaneous SCC, and larger prospective studies are required to determine the most appropriate management.
15.17 Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is a rare, highly aggressive neuroendocrine tumour arising from the Merkel mechanoreceptor of the skin. It is associated with the Merkel cell polyomavirus [161] and has an overall 5-year survival of 30–64 %, with a high incidence of local recurrence, regional lymph node involvement and distant metastasis [162, 163].
In part due to the rarity of this tumour, there is no consensus on the current standard of care for management. Excision of the primary tumour may require wide margins for elective local control [164] or the addition of adjuvant radiotherapy if smaller margins are used [165]. In some series, radiotherapy alone has been shown to achieve similar local control rates to primary excision [166]. Elective treatment of the lymph nodes should be strongly considered due to a clinically N0 neck being a poor indicator of nodal metastases with a high occult metastatic rate [167] and reported nodal recurrence rates of up to 76 % of stage I MCC patients in some series [107]. Prophylactic lymph node dissection appears to improve regional control, but does not lead to improved survival [168]. As a result, there is some disagreement regarding the utility of prophylactic node dissection in this population [102, 169].
Similarly, the utility of SNB in patients with early-stage MCC is a topic of considerable debate. It is extremely difficult to predict metastatic risk in MCC with no accurate histopathologic risk factors identified, meaning there is no clear consensus as to who the procedure would benefit. Furthermore, even with negative risk factors and small primary tumours, the risk of metastatic disease is high [170–172]. The lack of consensus is particularly notable in head and neck MCC, as highlighted by analysis of the SEER database which found that only 8.6 % patients undergo SNB, significantly less than at other sites [173].
Advocates of the technique contend that SNB can help identify patients with occult nodal disease, demonstrate aberrant drainage patterns and may prevent unnecessary neck dissection, parotidectomy and/or irradiation [101, 102]. In a review of the literature by Mehrany et al. [174], the authors found that SNB-positive patients were 18.9 times more likely to have nodal recurrence than SNB-negative patients, although the follow-up was only 7 months. Schmalbach et al. [101] also highlighted only one case of false positivity in a study of 10 cases with 34-month follow-up. These two studies suggest that SNB is both accurate and of prognostic value in MCC.
In a meta-analysis by Sadeghi et al., the authors demonstrated that positive sentinel node status is a strong predictor of poor survival and recurrence [175]. They argued that SNB gives a survival benefit versus nodal observation due to early diagnosis of metastases, early surgical intervention and commencement of adjuvant therapies. However, all the studies analysed had low numbers, short follow-up and heterogeneous methodologies which reduces the robustness of their conclusions. A large study of 403 cases by Shibayama et al. with a positive SNB rate of 31.8 % demonstrated that positive SNB was a predictor of distant recurrence, highlighting the possible prognostic benefits of SNB [176]. However, the study had a high false-negative rate of 12.9 % illustrating the unpredictability of MCC and the caution of interpreting conclusions with regard to SNB. A large study by Paulson et al. [177] found that patients with negative SNB had improved outcomes compared to those undergoing a watch and wait policy. In the most recent large study published on the subject, Kachare et al. analysed SEER database data and found that SNB does improve survival in patients with MCC [178]. However, their conclusions have been questioned by some authors due to possible biases in their methodology. In particular, the fact that patients who underwent SLNB were more frequently given radiotherapy could be partly responsible for the improved outcomes [179]. Several other methodology biases were also highlighted including the large number of patients excluded and the fact SNB was more likely offered to younger and fitter patients. This may be of particular importance given that older age has been shown to be significantly associated with SLNB positivity [173].
Despite the positive findings in some studies, several authors have questioned the benefit of SNB in MCC. Warner et al. [180] found that SLN status is not an accurate predictor of locoregional recurrence, and the authors instead advocate the use of local and regional radiotherapy to obtain disease control. This is an argument also advocated by Shibayama et al. who suggest that the high rate of false positivity in their study (12.9 %) justifies the use of adjuvant radiotherapy in SNB-negative patients [176]. Other studies have shown false-negative rates of up to 33 % [179, 181–183] leading many authors to question the validity of SNB as a prognostic tool. In the largest single centre study conducted to date, Fields et al. [184] did not find SLN status to be a predictor of recurrence or survival in MCC. Frisch et al. [173] also concluded that SLN status did not predict survival in 173 patients studied. Given the lack of evidence for any benefit, and the high rates of metastases in high-risk MCC, some authors advocate that SNB is unlikely to be beneficial in high-risk cases, particularly as radiotherapy and chemotherapy provide relatively good outcomes [179]. Some authors also argue that alternative staging modalities such as FDG-PET may be a more accurate and less invasive method and further studies are warranted to determine their suitability [185]. However, Shnayder concluded that, in this patient population with very high rates of occult micrometastatic lymph node involvement, the true utility of SNB may be in ensuring that all at-risk nodes are adequately addressed, even in cases of “aberrant” drainage. Furthermore, SNB may allow for accurate staging in patients who are reluctant to undergo formal lymphadenectomy [102].
As with melanoma and SCC, the true prognostic significance of submicroscopic lymph node metastases, which are reported to occur in up to 100 % of MCC patients, remains unclear [186]. Further study will be required to clarify the exact role of SNB in this population.
In the USA, the National Comprehensive Cancer Network (NCCN) currently recommends SNB for all patients presenting with previously untreated, localised stage I disease [118].
15.18 Complications of Sentinel Node Biopsy
The steep learning curve, technical difficulty and minimally invasive approach of SNB may potentially lead to a higher risk of complications compared with formal lymphadenectomy, principally damage to the facial or spinal accessory nerve. In addition, the requirement for a completion lymphadenectomy in SLN-positive patients represents a second procedure in an inflamed, recently operated surgical field, theoretically contributing to the risk of iatrogenic injury [91]. However, in experienced hands the incidence of complications following SNB is reported to be as low as 1 % [37, 187], and several large studies have shown that the effect on morbidity and quality of life is significantly higher in those undergoing lymphadenectomy versus SNB.
For nodes located in the parotid gland, high rates of facial nerve paresis in selected studies have led some authors to recommend superficial parotidectomy over biopsy alone. However, others have shown that SNB can be safely and accurately performed in the parotid gland with continuous nerve monitoring and careful dissection [188–190].
15.19 Summary
Sentinel node biopsy represents a useful tool for staging the clinically negative lymphatic basins in patients with selected head and neck malignancies. For patients with melanoma, SNB is widely accepted as the gold standard staging tool for patients with intermediate-thickness tumours. It has also been shown to give a survival benefit to patients with sentinel node-positive disease who then undergo immediate lymphadenectomy. However, questions remain with regard to the overall survival benefit of SNB, the optimal management of SNB-positive patients, its usefulness in thin and thick tumours and the prognostic significance of very small tumour deposits. For the management of patients with early OSCC, SNB has not yet gained universal acceptance as a sole staging tool despite encouraging results, and further studies are required to clarify its role. Finally, the prognostic value of SNB for Merkel cell carcinoma has been questioned, and its utility may ultimately be limited to improvements in staging.
References
Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–48.
Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (n0): implications and outcome. Otolaryngol Head Neck Surg. 1996;114(1):65–70.
Poulsen M, Rischin D, Walpole E, Harvey J, Mackintosh J, Ainslie J, Hamilton C, Keller J, Tripcony L. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a trans-Tasman radiation oncology group study-trog 96:07. J Clin Oncol. 2003;21(23):4371–6.
Morrison WH, Peters LJ, Silva EG, Wendt CD, Ang KK, Goepfert H. The essential role of radiation therapy in securing locoregional control of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1990;19(3):583–91.
Virchow R. Die krankhanften Geschwülste (3rd lesson, November 22, 1862). Berlin: A. Hirschwald; 1963.
Halsted WS. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20(5):497–555.
Crile G. Excision of cancer of the head and neck with special reference to the plan of dissection based on one hundred and thirty-two operations. JAMA. 1987;258(22):3286–93.
Norman J, Cruse CW, Espinosa C, Cox C, Berman C, Clark R, Saba H, Wells K, Reintgen D. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991;162(5):432–7.
Shah JP, Strong E, Spiro RH, Vikram B. Surgical grand rounds neck dissection: current status and future possibilities. Clin Bull. 1981;11(1):25–33.
Robbins KT, Clayman G, Levine PA, Medina J, Sessions R, Shaha A, Som P, Wolf GT. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128(7):751–8.
Razack MS, Baffi R, Sako K. Bilateral radical neck dissection. Cancer. 1981;47(1):197–9.
Suarez O. El problema de las metastasis linfaticas y alejadas del cancer de laringe e hipofaringe. Rev Otorrinolaringol. 1963;23:83–99.
Bocca E, Pignataro O, Oldini C, Cappa C. Functional neck dissection: an evaluation and review of 843 cases. Laryngoscope. 1984;94(7):942–5.
Fisch UP, Sigel ME. Cervical lymphatic system as visualized by lymphography. Ann Otol Rhinol Laryngol. 1964;73:870–82.
Werner JA, Dunne A, Myers JN. Functional anatomy of the lymphatic drainage system of the upper aerodigestive tract and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25(4):322–32.
Shah JP, Andersen PE. The impact of patterns of nodal metastasis on modifications of neck dissection. Ann Surg Oncol. 1994;1(6):521–32.
Chepeha DB, Hoff PT, Taylor RJ, Bradford CR, Teknos T, Esclamado RM. Selective neck dissection for the treatment of neck metastasis from squamous cell carcinoma of the head and neck. Laryngoscope. 2002;112(3):434–8.
Sobol S, Jensen C, Sawyer W, Costiloe P, Thong N. Objective comparison of physical dysfunction after neck dissection. Am J Surg. 1985;150(4):503–9.
Pitman KT, Johnson JT, Myers EN. Effectiveness of selective neck dissection for management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1997;123(9):917–22.
Alkureishi LWT, Ross GL, MacDonald DG, Shoaib T, Gray HW, Robertson AG, Soutar DS. Sentinel node in head and neck cancer: use of size criterion to upstage the N0 neck in head and neck squamous cell carcinoma. Head Neck. 2007;29(2):95–103.
O'Brien CJ, Traynor SJ, McNeil E, McMahon JD, Chaplin JM. The use of clinical criteria alone in the management of the clinically negative neck among patients with squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2000;126(3):360–5.
Rossi CR, Scagnet B, Vecchiato A, Mocellin S, Pilati P, Foletto M, Zavagno G, Casara D, Montesco MC, Tregnaghi A, Rubaltelli L, Lise M. Sentinel node biopsy and ultrasound scanning in cutaneous melanoma: clinical and technical considerations. Eur J Cancer. 2000;36(7):895–900.
Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, Ho CM. Clinicopathological analysis of elective neck dissection for n0 neck of early oral tongue carcinoma. Am J Surg. 1999;177(1):90–2.
Fisher SR. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: analysis of 1444 patients from 1970 to 1998. Laryngoscope. 2002;112(1):99–110.
Medina JE. Malignant melanoma of the head and neck. Otolaryngol Clin North Am. 1993;26(1):73–85.
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–9.
Wrightson WR, Wong SL, Edwards MJ, Chao C, Reintgen DS, Ross MI, Dirk Noyes R, Viar V, Cerrito PB, McMasters KM. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–80.
Kosuda S, Kusano S, Kohno N, Ohno Y, Tanabe T, Kitahara S, Tamai S. Feasibility and cost-electiveness of sentinel lymph node radiolocalization in stage N0 head and neck cancer. Arch Otolaryngol Head Neck Surg. 2003;129(10):1105–9.
Brobeil A, Cruse CW, Messina JL, Glass LF, Haddad FF, Berman CG, Marshburn J, Reintgen DS. Cost analysis of sentinel lymph node biopsy as an alternative to elective lymph node dissection in patients with malignant melanoma. Surg Oncol Clin N Am. 1999;8(3):435–45. viii.
van den Brekel MW, Stel HV, van der Valk P, van der Waal I, Meyer CJ, Snow GB. Micrometastases from squamous cell carcinoma in neck dissection specimens. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology. Head Neck Surg. 1992;249(6):349–53.
Gershenwald JE, Colome MI, Lee JE, Mansfield PF, Tseng C, Lee JJ, Balch CM, Ross MI. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol. 1998;16(6):2253–60.
van der Veen H, Hoekstra OS, Paul MA, Cuesta MA, Meijer S. Gamma probe-guided sentinel node biopsy to select patients with melanoma for lymphadenectomy. Br J Surg. 1994;81(12):1769–70.
Ross GL, Soutar DS, MacDonald DG, Shoaib T, Camilleri IG, Roberton AG, Sorensen JA, Thomsen JB, Grupe P, Alvarez JA, Barbier L, Santamaria J, Poli T, Massarelli O, Sesenna E, Kovacs AF, Grunwald F, Barzan L, Sulfaro S, Alberti F. Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol. 2004;11(7):690–6.
Civantos FJ, Moffat FL, Goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope. 2006;112(3 Pt 2 Suppl 109):1–15.
Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope. 2007;117(9):1539–51.
Bostick P, Essner R, Sarantou T, Kelley M, Glass E, Foshag L, Stern S, Morton D. Intraoperative lymphatic mapping for early-stage melanoma of the head and neck. Am J Surg. 1997;174(5):536–9.
Chao C, Wong SL, Edwards MJ, Ross MI, Reintgen DS, Noyes RD, Stadelmann WK, Lentsch E, McMasters KM. Sentinel lymph node biopsy for head and neck melanomas. Ann Surg Oncol. 2003;10(1):21–6.
Wells KE, Rapaport DP, Cruse CW, Payne W, Albertini J, Berman C, Lyman GH, Reintgen DS. Sentinel lymph node biopsy in melanoma of the head and neck. Plast Reconstr Surg. 1997;100(3):591–4.
Jansen L, Koops HS, Nieweg OE, Doting MH, Kapteijn BA, Balm AJ, Vermey A, Plukker JT, Hoefnagel CA, Piers DA, Kroon BB. Sentinel node biopsy for melanoma in the head and neck region. Head Neck. 2000;22(1):27–33.
Dunne AA, Kulkens C, Ramaswamy A, Folz BJ, Brandt D, Lippert BM, Behr T, Moll R, Werner JA. Value of sentinel lymphonodectomy in head and neck cancer patients without evidence of lymphogenic metastatic disease. Auris Nasus Larynx. 2001;28(4):339–44.
Gould EA, Winship T, Philbin PH, Kerr HH. Observations on a “sentinel node” in cancer of the parotid. Cancer. 1960;13:77–8.
Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39(2):456–66.
Weissbach L, Boedefeld EA. Localization of solitary and multiple metastases in stage II non-seminomatous testis tumor as basis for a modified staging lymph node dissection in stage I. J Urol. 1987;138(1):77–82.
Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186(4):481–90.
Alex JC, Krag DN. Gamma-probe guided localization of lymph nodes. Surg Oncol. 1993;2(3):137–43.
Balch CM, Morton DL, Gershenwald JE, McMasters KM, Nieweg OE, Powell B, Ross MI, Sondak VK, Thompson JF. Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60(5):872–5.
Civantos FJ, Zitsch R, Bared A, Amin A. Sentinel node biopsy for squamous cell carcinoma of the head and neck. J Surg Oncol. 2008;97(8):683–90.
Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, Glass EC, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Wang H. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–11. discussion 311–3.
Shoaib T, Soutar DS, Prosser JE, Dunaway DJ, Gray HW, McCurrach GM, Bessent RG, Robertson AG, Oliver R, MacDonald DG. A suggested method for sentinel node biopsy in squamous cell carcinoma of the head and neck. Head Neck. 1999;21(8):728–33.
Cody HS. Sentinel lymph node mapping in breast cancer. Breast Cancer. 1999;6(1):13–22.
Ross GL, Shoaib T, Soutar DS, MacDonald DG, Camilleri IG, Bessent RG, Gray HW. The first international conference on sentinel node biopsy in mucosal head and neck cancer and adoption of a multicenter trial protocol. Ann Surg Oncol. 2002;9(4):406–10.
Tafra L, Chua AN, Ng PC, Aycock D, Swanson M, Lannin D. Filtered versus unfiltered technetium sulfur colloid in lymphatic mapping: a significant variable in a pig model. Ann Surg Oncol. 1999;6(1):83–7.
De Cicco C, Trifirò G, Calabrese L, Bruschini R, Ferrari ME, Travaini LL, Fiorenza M, Viale G, Chiesa F, Paganelli G. Lymphatic mapping to tailor selective lymphadenectomy in cn0 tongue carcinoma: beyond the sentinel node concept. Eur J Nucl Med Mol Imaging. 2006;33(8):900–5.
Stoeckli SJ, Pfaltz M, Ross GL, Steinert HC, MacDonald DG, Wittekind C, Soutar DS. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann Surg Oncol. 2005;12(11):919–24.
Gershenwald JE, Thompson W, Mansfield PF, Lee JE, Colome MI, Tseng CH, Lee JJ, Balch CM, Reintgen DS, Ross MI. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976–83.
Rasgon BM. Use of low-dose technetium Tc 99m sulfur colloid to locate sentinel lymph nodes in melanoma of the head and neck: preliminary study. Laryngoscope. 2001;111(8):1366–72.
Uren RF, Howman-Giles R, Thompson JF, McCarthy WH, Quinn MJ, Roberts JM, Shaw HM. Interval nodes: the forgotten sentinel nodes in patients with melanoma. Arch Surg. 2000;135(10):1168–72.
Vidal-Sicart S, Pons F, Piulachs J, Castel T, Palou J, Herranz R. Mid-arm sentinel lymph nodes showing surprising drainage from a malignant melanoma in the forearm. Clin Nucl Med. 1998;23(5):273–4.
Alex JC. The application of sentinel node radiolocalization to solid tumors of the head and neck: a 10-year experience. Laryngoscope. 2004;114(1):2–19.
Bilde A, Von Buchwald C, Mortensen J, Marving J, Therkildsen MH, Kirkegaard J, Charabi B, Specht L. The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Otolaryngol. 2006;126(10):1096–103.
Even-Sapir E, Lerman H, Lievshitz G, Khaff A, Fliss DM, Schwartz A, Gur E, Skornick Y, Schneebaum S. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003;44(9):1413–20.
Uren RF, Howman-Giles R, Chung DK, et al. The reproducibility in routine clinical practice of sentinel lymph node identification by pre-operative lymphoscintigraphy in patients with cutaneous melanoma. Ann Surg Oncol. 2007;14:899–905.
van der Ploeg IMC, Valdés Olmos RA, Kroon BBR, van den Brekel MW, Vogel WV, Hoefnagel CA, Nieweg OE. The yield of SPECT/CT for anatomical lymphatic mapping in patients with melanoma. Ann Surg Oncol. 2009;16(6):1537–42.
Manca G, Rubello D, Romanini A, Boni G, Chiacchio S, Tredici M, et al. Sentinel lymph node mapping in melanoma: the issue of false-negative findings. Clin Nucl Med. 2014;39:e346–54.
Zender C, Guo T, Weng C, Faulhaber P, Rezaee R. Utility of SPECT/CT for periparotid sentinel lymph node mapping in the surgical management of head and neck melanoma. Am J Otolaryngol. 2014;35(1):12–8.
Bluemel C, Herrmann K, Muller-Richter U, Lapa C, Higuchi T, Wild V, et al. Freehand SPECT-guided sentinel lymph node biopsy in early oral squamous cell carcinoma. Head Neck. 2014;36(11):E112–6.
Sabate-Llobera A, Benitez-Segura A, Mari A, Arranz C, Bajen MT, Maymo-Garrdio S, et al. Lymphoscintigraphy in oral squamous cell carcinoma sentinel node biopsy and its role in the surgical planning. Clin Nucl Med. 2014;39(2):e142–5.
Sidler SK, Stoeckli SJ, Haerle SK, Hany TF. Is there an additional value of SPECT/CT over lymphoscintigraphy for sentinel node mapping in oral/oropharyngeal squamous cell carcinoma? Ann Surg Oncol. 2009;16(11):3118–24.
Koljonen V, Suominen S. Sentinel node biopsy in local anaesthesia in patients with head and neck Merkel cell carcinoma. Eur J Plast Surg. 2007;5(30):205–10.
Habib FA, Lodish ME, Mittal VK, Young SC. Sentinel lymph node dissection for primary cutaneous melanoma: a community hospital’s initial experience. Am Surg. 2000;66(3):291–5.
Shoaib T, Soutar D, MacDonald DG, Camilleri IG, Dunaway DJ, Gray HW, McCurrach GM, Bessent RG, MacLeod TI, Robertson AG. The accuracy of head and neck carcinoma sentinel lymph node biopsy in the clinically n0 neck. Cancer. 2001;91(11):2077–83.
Leong SP, Donegan E, Heffernon W, Dean S, Katz JA. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7(5):361–6.
McMasters KM, Noyes RD, Reintgen DS, Goydos JS, Beitsch PD, Davidson BS, Sussman JJ, Gershenwald JE, Ross MI. Lessons learned from the sunbelt melanoma trial. J Surg Oncol. 2004;86(4):212–23.
Coit DG. The “true” sentinel lymph node: in search of an operational definition of a biological phenomenon. Ann Surg Oncol. 2001;8:187–9.
Murphy AD, Britten A, Powell B. Hot or not? The 10% rule in sentinel lymph node biopsy for malignant melanoma revisited. J Plast Reconstr Aesthet Surg. 2014;67:316–9.
Atula T, Shoaib T, Ross GL, Gray HW, Soutar DS. How many sentinel nodes should be harvested in oral squamous cell carcinoma? Eur Arch Otorhinolaryngol. 2008;265 Suppl 1:S19–23.
Broglie MA, Haerle SK, Huber GF, Haile SR, Stoeckli SJ. Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: impact on survival. Head Neck. 2013;35(5):660–6.
Conway WC, Faries MB, Nicholl MB, et al. Age-related lymphatic dysfunction in melanoma patients. Ann Surg Oncol. 2009;16:1548–52.
Cloyd JM, Wapnir IL, Read BM, Swetter S, Greco RS. Indocyanine green and fluorescence lymphangiography for sentinel lymph node identification in cutaneous melanoma. J Surg Oncol. 2014;110(7):888–92.
Korn JM, Tellez-Diaz A, Bartz-Kurycki M, Gastman B. Indocyanine green SPY elite-assisted sentinel lymph node biopsy in cutaneous melanoma. Plast Reconstr Surg. 2014;133(4):914–22.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. Chapter 6. Melanoma of the skin. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010.
Cook MG, Green MA, Anderson B, Eggermont AMM, Ruiter DJ, Spatz A, Kissin MW, Powell M. The development of optimal pathological assessment of sentinel lymph nodes for melanoma. J Pathol. 2003;200(3):314–9.
Alkureishi LWT, Alvarez JA, Ballinger J, Bilde A, Britten AJ, Calabrese L, Chiesa C, Chiti A, de Bree R, Gray HW, Hunter K, Kovacs AF, Lassmann M, Leemans CR, Mamelle G, McGurk M, Mortensen J, Poli T, Shoaib T, Sloan P, Sorensen JA, Stoeckli SJ, Thomsen JB, Trifiro G, Werner J, Ross G, Burak Z. Joint practice guidelines for radionuclide lymphoscintigraphy for sentinel node localisation in oral/oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2009;36(11):1915–36.
Messina JL, Glass LF, Cruse CW, Berman C, Ku NK, Reintgen DS. Pathologic examination of the sentinel lymph node in malignant melanoma. Am J Surg Pathol. 1999;23(6):686–90.
Cochran AJ. Surgical pathology remains pivotal in the evaluation of ‘sentinel’ lymph nodes. Am J Surg Pathol. 1999;23(10):1169–72.
Starz H, Balda BR, Krämer KU, Büchels H, Wang H. A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer. 2001;91(11):2110–21.
Scolyer RA, Murali R, McCarthy SW, Thompson JF. Pathologic examination of sentinel lymph nodes from melanoma patients. Semin Diagn Pathol. 2008;25(2):100–11.
Bieligk SC, Ghossein R, Bhattacharya S, Coit DG. Detection of tyrosinase mRNA by reverse transcription-polymerase chain reaction in melanoma sentinel nodes. Ann Surg Oncol. 1999;6(3):232–40.
Romanini A, Manca G, Pellegrino D, et al. Molecular staging of the sentinel lymph node in melanoma patients: correlation with clinical outcome. Ann Oncol. 2005;16:1832–40.
Mocellin S, Hoon DS, Pilati P, et al. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta-analysis of prognosis. J Clin Oncol. 2007;25:1588–95.
Holt JB, Sangueza OP, Levine EA, et al. Nodal melanocytic nevi in sentinel lymph nodes. Correlation with melanoma-associated cutaneous nevi. Am J Clin Pathol. 2004;121:58–63.
Lee JJ, Granter SR, Laga AC, Saavedra AP, Zhan Q, Guo W, et al. 5-Hydroxymethylcytosine exppression in metastatic melanoma versus nodal nevus in sentinel lymph node biopsies. Mod Pathol. 2015;28(2):218–29.
Chen PL, Chen WS, Li J, et al. Diagnostic utility of neural stem and progenitor cell markers nestin and SOX2 in distinguishing nodal melanocytic nevi from metastatic melanomas. Mod Pathol. 2013;26:44–53.
Terada A, Hasegawa Y, Yatabe Y, Hyodo I, Ogawa T, Hanai N, Ikeda A, Nagashima Y, Masui T, Hirakawa H, Nakashima H. Intraoperative diagnosis of cancer metastasis in sentinel lymph node of oral cancer patients. Oral Oncol. 2008;44(9):838–43.
Tschopp L, Nuyens M, Stauffer E, Krause T, Zbären P. The value of frozen section analysis of the sentinel lymph node in clinically n0 squamous cell carcinoma of the oral cavity and oropharynx. Otolaryngol Head Neck Surg. 2005;132(1):99–102.
Vorburger MS, Broglie MA, Soltermann A, Haerle SK, Haile SR, Huber GF, Stoeckli SJ. Validity of frozen section in sentinel lymph node biopsy for the staging in oral and oropharyngeal squamous cell carcinoma. J Surg Oncol. 2012;106:816–9.
Trivedi NP, Ravindran HK, Sundram S, et al. Pathologic evaluation of sentinel lymph nodes in oral squamous cell carcinoma. Head Neck. 2010;32:1437–43.
Asthana S, Suryanarayana Deo SV, Shukla NK, Jain P, Anand M, Kumar R. Intraoperative neck staging using sentinel node biopsy and imprint cytology in oral cancer. Head Neck. 2003;25(5):368–72.
Hamakawa H, Onishi A, Sumida T, Terakado N, Hino S, Nakashiro KI, Shintani S. Intraoperative real-time genetic diagnosis for sentinel node navigation surgery. Int J Oral Maxillofac Surg. 2004;33(7):670–5.
Ferris RL, Xi L, Seethala RR, et al. Intraoperative qRT-PCR for detection of lymph node metastasis in head and neck cancer. Clin Cancer Res. 2011;17:1858–66.
Schmalbach CE, Lowe L, Teknos TN, Johnson TM, Bradford CR. Reliability of sentinel lymph node biopsy for regional staging of head and neck Merkel cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131(7):610–4.
Shnayder Y, Weed DT, Arnold DJ, Gomez-Fernandez C, Bared A, Goodwin WJ, Civantos FJ. Management of the neck in Merkel cell carcinoma of the head and neck: University of Miami experience. Head Neck. 2008;30(12):1559–65.
Sian SU, Wagner JD, Sood R, Park HM, Havlik R, Coleman JJ. Lymphoscintigraphy with sentinel lymph node biopsy in cutaneous Merkel cell carcinoma. Ann Plast Surg. 1999;42(6):679–82.
Albertini JJ, Cruse CW, Rapaport D, Wells K, Ross M, DeConti R, Berman CG, Jared K, Messina J, Lyman G, Glass F, Fenske N, Reintgen DS. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg. 1996;223(2):217–24.
Wagner JD, Corbett L, Park HM, Davidson D, Coleman JJ, Havlik RJ, Hayes JT. Sentinel lymph node biopsy for melanoma: experience with 234 consecutive procedures. Plast Reconstr Surg. 2000;105(6):1956–66.
Yee VSK, Thompson JF, McKinnon JG, Scolyer RA, Li LL, McCarthy WH, O’Brien CJ, Quinn MJ, Saw RPM, Shannon KF, Stretch JR, Uren RF. Outcome in 846 cutaneous melanoma patients from a single center after a negative sentinel node biopsy. Ann Surg Oncol. 2005;12(6):429–39.
Amersi F, Morton DL. The role of sentinel lymph node biopsy in the management of melanoma. Adv Surg. 2007;41:241–56.
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609.
Hammond R, Rosenbaum P, Guerry D, Gimotty PA, Yoon F. Sentinel lymph node biopsy (SLNB) improves survival among SEER patients with melanoma. J Clin Oncol. 2008;26:Abstr 9005.
Thompson JF, Faries MB, Cochran AJ. Sentinel lymph node biopsy for melanoma: a plea to let the data be heard. Ann Surg Oncol. 2014;21(11):3362–4.
Coit D. Sentinel lymph node biopsy for melanoma: a plea to let the data speak. Ann Surg Oncol. 2014;21(11):3359–61.
Yang JC, Sherry RM, Rosenberg SA. Why is sentinel lymph node biopsy ‘standard of care’ for melanoma? Nat Rev Clin Oncol. 2014;11:245–6.
Durham AB, Wong SL. Sentinel lymph node biopsy in melanoma: final results of MSLT-1. Future Oncol. 2014;10(7):1121–3.
Sondak VK, Zager JS. MSLT-1 – putting sentinel lymph node biopsy into context. Nat Rev Clin Oncol. 2014;11:246–8.
Thompson JF, Cochran AJ, Faries MB. Sentinel-node biopsy in melanoma. N Engl J Med. 2014;370(22):2149–50.
Kachare SD, Brinkley J, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. The influence of sentinel lymph node biopsy on survival for intermediate-thickness melanoma. Ann Surg Oncol. 2014;21(11):3377–85.
Thomas JM. Sentinel-node biopsy in melanoma. N Engl J Med. 2014;370(22):2148.
Coit DG, Thompson JA, Andtbacka R, Anker CJ, Bichakjian CK, Carson 3rd WE, et al. Melanoma, version 4.2014. J Natl Compr Canc Netw. 2014;12(5):621–9.
Wong SL, Balch CM, Hurley P, Agarwala SS, Akhurst TJ, Cochran A, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. Ann Surg Oncol. 2012;19(11):3313–24.
Han D, Zager JS, Shyr Y, Chen H, Berry LD, Ivengar S, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31(35):4387–93.
Van der Ploeg AP, Haydu LE, Spillane AJ, Quinn MJ, Saw RP, Shannon KF, et al. Outcome following sentinel node biopsy plus wide local excision versus wide local excision only for primary cutaneous melanoma: analysis of 5840 patients treated at a single institution. Ann Surg. 2014;260(1):149–57.
Bartlett EK, Phyllis AG, Sinnamon AJ, Wachtel H, Roses RE, Schuchter L. Clark level risk stratifies patients with mitogenic thin melanomas for sentinel lymph node biopsy. Ann Surg Oncol. 2014;21:643–9.
McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, Garbe C, Testori A, Maio M, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–32.
Hauschild A, Grob J-J, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller Jr WH, Kaempgen E. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–65.
Gyorki DE, Boyle JO, Ganly I, Morris L, Shaha AR, Singh B, et al. Incidence and location of positive nonsentinel lymph nodes in head and neck melanoma. Eur J Surg Oncol. 2014;40(3):305–10.
Smith VA, Cunningham JE, Lentsch EJ. Completion node dissection in patients with sentinel node-positive melanoma of the head and neck. Otolaryngol Head Neck Surg. 2012;146:591–9.
Tanis PJ, Nieweg OE, van den Brekel MW, Balm AJM. Dilemma of clinically node-negative head and neck melanoma: outcome of “watch and wait” policy, elective lymph node dissection, and sentinel node biopsy-a systematic review. Head Neck. 2008;30(3):380–9.
Erman AB, Collar RM, Griffith KA, et al. Sentinel lymph node biopsy is accurate and prognostic in head and neck melanoma. Cancer. 2012;118:1040–7.
de Rosa N, Lyman GH, Silbermins D, et al. Sentinel node biopsy for head and neck melanoma: a systematic review. Arch Otolaryngol Head Neck Surg. 2011;145:375–82.
van Akkooi ACJ, de Wilt JHW, Verhoef C, Schmitz P, van Geel AN, Eggermont AMM, Kliffen M. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17(10):1578–85.
van der Ploeg AP, van Akkooi AC, Rutkowski P, Nowecki ZI, Michej W, Mitra A, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol. 2011;29(16):2206–14.
Murali R, Desilva C, Thompson JF, Scolyer RA. Non-Sentinel Node Risk Score (N-SNORE): a scoring system for accurately stratifying risk of non-sentinel node positivity in patients with cutaneous melanoma with positive sentinel lymph nodes. J Clin Oncol. 2010;28:4441–9.
Gershenwald JE, Andtbacka RH, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26:4296–303.
Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13(6):809–16.
Flach GB, Bloemena E, van Schie A, Hoekstra OS, van Weert S, Leemans CR, de Bree R. Sentinel node identification in laryngeal cancer: feasible in primary cancer with previously untreated neck. Oral Oncol. 2013;49:165–8.
Thompson CF, St John MA, Lawson G, Grogan T, Elashoff D, Mendelsohn AH. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol. 2013;270(7):2115–22.
Werner JA, Dünne AA, Ramaswamy A, Dalchow C, Behr T, Moll R, Folz BJ, Davis RK. The sentinel node concept in head and neck cancer: solution for the controversies in the n0 neck? Head Neck. 2004;26(7):603–11.
Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009;31(6):765–72.
Matsuzuka T, Suzuki M, Saijo S, Matsui T, Nomoto Y, Ikeda M, et al. Usefulness of sentinel node navigation surgery in the management of early tongue cancer. Auris Nasus Larynx. 2014;41(5):475–8.
Chaturvedi P, Datta S, Arya S, Rangarajan V, Kane SV, Nair D et al. Prospective study of ultrasound-guided fine-needle aspiration cytology and sentinel node biopsy in the staging of clinically negative T1 and T2 oral cancer. Head Neck. 2015;37(10):1504–8.
Govers TM, Hannink G, Merkx MA, Takes RP, Rovers MM. Sentinel node biopsy for squamous cell carcinoma of the oral cavity and oropharynx: a diagnostic meta-analysis. Oral Oncol. 2013;49(8):726–32.
Civantos FJ, Zitsch RP, Schuller DE, Agrawal A, Smith RB, Nason R, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28(8):1395–400. ACOSOG multi-institutional trial demonstrating accuracy of SLNB in a prospective cohort of early stage oral cancer patients.
Alkureishi LW, Ross GL, Shoaib T, Soutar DS, Robertson AG, Thompson R. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol. 2010;17(9):2459–64.
Flach GB, Broglie MA, van Schie A, Bloemena E, Leemans CR, de Bree R, Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma in the previously treated neck. Oral Oncol. 2012;48(1):85–9.
O’Connor R, Pezier T, Schilling C, McGurk M. The relative cost of sentinel lymph node biopsy in early oral cancer. J Craniomaxillofac Surg. 2013;41(8):721–7.
Schiefke F, Akdemir M, Weber A, Akdemir D, Singer S, Frerich B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck. 2009;31(4):503–12.
Alvarez J, Bidaguren A, McGurk M, Diaz-Basterra G, Brunso J, Andikoetxea B, et al. Sentinel node biopsy in relation to survival in floor of the mouth carcinoma. Int J Oral Maxillofac Surg. 2014;43(3):269–73.
Fan SF, Zeng ZY, Peng HW, Guo ZM, Wang SL, Zhang Q. Sentinel lymph node biopsy versus elective neck dissection in patients with cT1-2N0 oral tongue squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(2):186–90.
Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 2011;9:CD006205.
Hernando J, Villarreal P, Alvarez-Marcos F, Gallego L, García-Consuegra L, Junquera L Comparison of related complications: sentinel node biopsy versus elective neck dissection. Int J Oral Maxillofac Surg. 2014;43(11):1307–12.
Genden EM, Ferlito A, Silver CE, et al. Contemporary management of cancer of the oral cavity. Eur Arch Otorhinolaryngol. 2010;267:1001–17.
Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14–9.
Kuntz AL, Weymuller Jr EA. Impact of neck dissection on quality of life. Laryngoscope. 1999;109:1334–8.
Kraus DH, Carew DF, Harrison LB. Regional lymph node metastasis from cutaneous squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124(5):582–7.
Dinehart SM, Pollack SV. Metastases from squamous cell carcinoma of the skin and lip. An analysis of twenty-seven cases. J Am Acad Dermatol. 1989;21(2 Pt 1):241–8.
Civantos FJ, Zitsch R, Bared A. Sentinel node biopsy in oral squamous cell carcinoma. J Surg Oncol. 2007;96(4):330–6.
Ahmed MM, Moore BA, Schmalbach CE. Utility of head and neck cutaneous squamous cell carcinoma sentinel node biopsy: a systematic review. Otolaryngol Head Neck Surg. 2014;150(2):180–7.
Takahashi A, Imafuku S, Nakayama J, Nakaura J, Ito K, Shibayama Y, et al. Sentinel node biopsy for high-risk cutaneous squamous cell carcinoma. Eur J Surg Oncol. 2014;40(10):1256–62.
Fukushima S, Masuguchi S, Igata T, Harada M, Aoi J, Miyashita A, et al. Evaluation of sentinel node biopsy for cutaneous squamous cell carcinoma. J Dermatol. 2014;41(6):539–41.
Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, Breuninger H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713–20.
Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232–8.
Wasserberg N, Feinmesser M, Schachter J, Fenig E, Gutman H. Sentinel-node guided lymph-node dissection for Merkel cell carcinoma. Eur J Surg Oncol. 1999;25(4):444–6.
Dancey AL, Rayatt SS, Soon C, Ilchshyn A, Brown I, Srivastava S. Merkel cell carcinoma: a report of 34 cases and literature review. J Plastic Reconstr Aesthet Surg. 2006;59(12):1294–9.
Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma: prognosis and management. Arch Surg. 1991;126(12):1514–9.
Shaw JH, Rumball E. Merkel cell tumour: clinical behaviour and treatment. Br J Surg. 1991;78(2):138–42.
Mortier L, Mirabel X, Fournier C, Piette F, Lartigau E. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol. 2003;139(12):1587–90.
Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–9.
Gillenwater AM, Hessel AC, Morrison WH, Burgess M, Silva EG, Roberts D, Goepfert H. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001;127(2):149–54.
Poulsen M. Merkel-cell carcinoma of the skin. Lancet Oncol. 2004;5(10):593–9.
Iyer JG, Storer BE, Paulson KG, Lemos B, Phillips JL, Bichakjian CK, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol. 2014;70(4):637–43.
Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope. 2012;122(6):1283–90.
Arruda EP, Higgins KM. Role of sentinel lymph node biopsy in the management of merkel cell carcinoma. J Skin Cancer. 2012;2012:176173.
Fritsch VA, Camp ER, Lentsch EJ. Sentinel lymph node status in Merkel cell carcinoma of the head and neck: not a predictor of survival. Head Neck. 2014;36(4):571–9.
Mehrany K, Otley KC, Weenig RH, Phillips PK, Roenigk RK, Nguyen TH. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28(2):113–7. discussion 117.
Sadeghi R, Adinehpoor Z, Maleki M, Fallahi B, Giovanella L, Treglia G. Prognostic significance of sentinel lymph node mapping in Merkel cell carcinoma: systematic review and meta-analysis of prognostic studies. Biomed Res Int. 2014;2014:489536.
Shibayama Y, Imafuku S, Takahashi A, Nakayama J. Role of sentinel lymph node biopsy in patients with Merkel cell carcinoma: statistical analysis of 403 reported cases. Int J Clin Oncol. 2015 Feb;20.
Paulson KG, Iyer JG, Byrd DR, Nghiem P. Pathologic nodal evaluation is increasingly commonly performed for patients with Merkel cell carcinoma. J Am Acad Dermatol. 2013;69(4):653–4.
Kachare SD, Wong JH, Vohra NA, Zervos EE, Fitzgerald TL. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21(5):1624–30.
Thompson JF, Hruby G. The role of sentinel lymph node biopsy in patients with merkel cell carcinoma: uncertainty prevails. Ann Surg Oncol. 2014;21(5):1517–9.
Warner RE, Quinn MJ, Hruby G, Scolyer RA, Uren RF, Thompson JF. Management of Merkel cell carcinoma: the roles of lymphoscintigraphy, sentinel lymph node biopsy and adjuvant radiotherapy. Ann Surg Oncol. 2008;15(9):2509–18.
Santamaria-Barria JA, Boland GM, Yeap BY, Nardi V, Dias-Santagata D, Cusack Jr JC. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol. 2013;20(4):1365–73.
Howle JR, Veness MJ. Outcome of patients with microscopic and macroscopic metastatic nodal Merkel cell carcinoma: an Australian experience. Dermatol Surg. 2014;40(1):46–51.
Maza S, Trefzer U, Hofmann M, Schneider S, Voit C, Krössin T, Zander A, Audring H, Sterry W, Munz DL. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. Eur J Nucl Med Mol Imaging. 2006;33(4):433–40.
Fields RC, Busam KJ, Chou JF, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18:2529–37.
Siva S, Byrne K, Seel M, et al. 18F-FDG PET provides high impact and powerful prognostic stratification in the staging of Merkel cell carcinoma: a 15 year institutional experience. J Nucl Med. 2013;54:1223–9.
Victor NS, Morton B, Smith JW. Merkel cell cancer: is prophylactic lymph node dissection indicated? Am Surg. 1996;62(11):879–82.
Ross GL, Shoaib T, Scott J, Soutar DS, Gray HW, MacKie R. The learning curve for sentinel node biopsy in malignant melanoma. Br J Plast Surg. 2002;55(4):298–301.
Schmalbach CE, Nussenbaum B, Rees RS, Schwartz J, Johnson TM, Bradford CR. Reliability of sentinel lymph node mapping with biopsy for head and neck cutaneous melanoma. Arch Otolaryngol Head Neck Surg. 2003;129:61–5.
Wells KE, Stadelmann WK, Rapaport DP, Hamlin R, Cruse CW, Reintgen D. Parotid selective lymphadenectomy in malignant melanoma. Ann Plast Surg. 1999;43:1–6.
Ollila DW, Foshag LJ, Essner R, Stern SL, Morton DL. Parotid region lymphatic mapping and sentinel lymphadenectomy for cutaneous melanoma. Ann Surg Oncol. 1999;6:150–4.
Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001 Aug 15;19(16):3635–48.
Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001 Aug 15;19(16):3635–48.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Smith, O.J., Alkureishi, L.W.T., Ross, G.L. (2016). Sentinel Node Biopsy. In: Bernier, J. (eds) Head and Neck Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-27601-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-27601-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27599-4
Online ISBN: 978-3-319-27601-4
eBook Packages: MedicineMedicine (R0)