Abstract
The article will begin with the discovery of purinergic inhibitory neuromuscular transmission in the 1960s/1970s, the proposal for purinergic cotransmission in 1976 and the recognition that sympathetic nerves release adenosine 5′-triphosphate (ATP), noradrenaline and neuropeptide Y, while non-adrenergic, non-cholinergic inhibitory nerve cotransmitters are ATP, nitric oxide and vasoactive intestinal polypeptide in variable proportions in different regions of the gut. Later, purinergic synaptic transmission in the myenteric and submucosal plexuses was established and purinergic receptors expressed by both glial and interstitial cells. The focus will then be on purinergic mechanosensory transduction involving release of ATP from mucosal epithelial cells during distension to activate P2X3 receptors on submucosal sensory nerve endings. The responses of low threshold fibres mediate enteric reflex activity via intrinsic sensory nerves, while high threshold fibres initiate pain via extrinsic sensory nerves. Finally, the involvement of purinergic signalling in an animal model of colitis will be presented, showing that during distension there is increased ATP release, increased P2X3 receptor expression on calcitonin gene-related peptide-labelled sensory neurons and increased sensory nerve activity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Parasympathetic nerve stimulation that produced atropine-resistant responses of gastrointestinal smooth muscle was recognised early (Langley 1898; McSwiney and Robson 1929; Paton and Vane 1963). However, it was not until the early 1960s that gastrointestinal neuromuscular transmission other than that mediated by the classical transmitters acetylcholine (ACh) and noradrenaline (NA) was recognised (Burnstock et al. 1964; see Burnstock 1969, 2008a). The identification of adenosine 5′-triphosphate (ATP) as the non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in the gut was proposed in 1970 (Burnstock et al. 1970) and the purinergic signalling hypothesis was launched in a Pharmacological Review (Burnstock 1972). This hypothesis was rejected by many people over the next 20 years and it was often ridiculed at international meetings (see Burnstock et al. 2010; Burnstock 2012a). Resistance to the concept was perhaps understandable, because ATP was established as an intracellular energy source involved in the Krebs cycle and it seemed unlikely that such a ubiquitous molecule would also act as an extracellular signaller. It is now clear that ATP, an ancient biological molecule, evolved both as an intracellular energy source and an extracellular signalling molecule. Later, after the cotransmitter hypothesis was published (see Burnstock 1976), it was recognised that nitric oxide (NO) and in some regions vasoactive intestinal polypeptide (VIP) were cotransmitters with ATP in NANC gastrointestinal inhibitory nerves . Strong evidence is now available in support of the purinergic hypothesis (see Olsson and Pearson 1990; Hoyle 1992; Dubyak and El Moatassim 1993; Zimmermann 1994; North 2002; Burnstock 2007a, 2012a; Burnstock et al. 2010; Burnstock and Verkhratsky 2012).
Intestinal motility, secretion and absorption can be influenced by ATP released from intrinsic enteric neurons, sympathetic nerves or sensory-motor nerves during axon reflexes, acting directly on purinoceptors on smooth muscle mediating relaxation or contraction or on epithelial cell secretion. Also, ATP released from mucosal epithelial cells can activate sensory enteric neurons involved in reflex activities. In addition, purine nucleotides and nucleosides can act on blood vessels, glia and interstitial cells of Cajal (ICCs) thereby indirectly modulating motility patterns. After breakdown to adenosine, ATP acts on prejunctional nerve terminals to modify transmitter release from motor and inhibitory neural pathways.
In the late 1980s and 1990s electrophysiological studies established that synaptic purinergic transmission was present between neurons in both myenteric and submucosal enteric plexuses (see LePard et al. 1997; Burnstock 2001a, 2007a; Galligan 2002; Bornstein 2008; Christofi 2008). The turning point for acceptance of purinergic signalling was when receptors for nucleotides and nucleosides were cloned and characterised in the early 1990s. Four subtypes of P1 (adenosine) receptors, 7 subtypes of P2X ion channel nucleotide receptors and 8 subtypes of G protein-coupled receptors were identified (see Ralevic and Burnstock 1998; Burnstock 2007b). RT-PCR and immunohistochemical studies were carried out to show the distribution of purinoceptor subtype mRNA and protein in different neurons and non-neuronal cells in different regions of the gastrointestinal tract of different species, including man (see Burnstock and Knight 2004; Burnstock 2007b, 2008a)
An exciting new field emerged when purinergic mechanosensory transduction in visceral organs was discovered (see Burnstock 1999, 2009). ATP released from mucosal epithelial cells during distension of the gut activates P2X3 receptors on submucosal sensory nerve endings (Wynn et al. 2003). Low threshold intrinsic sensory nerves mediate enteric reflex activity, while high threshold fibres mediate the initiation of pain via extrinsic sensory nerves.
There is increasing interest in the pathophysiology of purinergic signalling in the gastrointestinal tract (see Burnstock 2008b, 2014) and in this article its involvement in inflammatory bowel disease (IBD) (Yiangou et al. 2001; Wynn et al. 2004) will be considered.
The Early Discovery of Purinergic Neuromuscular Transmission
Correlated electrical and mechanical activity was recorded in the guinea pig taenia coli using the sucrose-gap technique (Burnstock and Straub 1958). After stimulation of the intramural nerves in the presence of adrenergic and cholinergic blocking agents, hyperpolarisations and relaxations were reported (Burnstock et al. 1963, 1964; see Burnstock 2004). Tetrodotoxin , a neurotoxin that prevents the action potential in nerves without affecting the excitability of smooth muscle cells blocked the hyperpolarisations (Bülbring and Tomita 1967). This established them as inhibitory junction potentials (IJPs) in response to stimulation of NANC inhibitory nerves. Later NANC neurotransmission was shown to be mediated by intrinsic enteric neurons controlled by vagal and sacral parasympathetic nerves (Burnstock et al. 1966). NANC relaxations were identified at about the same time in the stomach upon stimulation of the vagus nerve (Martinson and Muren 1963; Martinson 1965).
The next step was to try to identify the transmitter released during NANC inhibitory transmission in the gut. Several criteria were postulated by Eccles (1964) and also by Paton (1958) that needed to be satisfied to establish a neurotransmitter: synthesis and storage in nerve terminals; release by a Ca2+-dependent mechanism; mimicry of the nerve-mediated responses by the exogenously applied transmitter; inactivation by ectoenzymes and/or neuronal uptake; and parallel block of responses to stimulation by nerves and exogenously applied transmitter. Different substances were examined in the late 1960s, including amino acids, monoamines and neuropeptides, but none satisfied the criteria. However, hints in a paper by Drury and Szent-Györgyi (1929) showing extracellular actions of purines on heart and blood vessels , a paper by Feldberg and Hebb (1948) showing extracellular actions of ATP on autonomic ganglia and a paper by Holton (1959) showing release of ATP during antidromic stimulation of sensory nerves supplying the rabbit ear artery, led Burnstock and his colleagues to consider ATP and this satisfied all the criteria required to establish it as a transmitter involved in NANC inhibitory neurotransmission (Burnstock et al. 1970). The purinergic neurotransmission hypothesis was proposed in a Pharmacological Review in 1972 (Burnstock 1972).
There was early evidence for ATP as a cotransmitter in sympathetic nerves supplying the guinea pig taenia coli (Su et al. 1971). Periarterial sympathetic nerve stimulation led to release of tritium from guinea pig taenia coli preincubated in [3H]adenosine (which was taken up and converted largely to [3H]ATP) and the release of both tritium and NA was blocked by guanethidine . The proportion of ATP and NA in sympathetic nerves varies in different regions of the gut, between species and during development and ageing. It has been reported that ATP is the sole transmitter in sympathetic nerves supplying arterioles in the submucosal plexus of the intestine, while NA released from these nerves acts as a prejunctional modulator of transmitter release (Evans and Surprenant 1992). ‘Axon reflex’ activity involving sensory-motor nerves is widespread in autonomic effector systems and forms an important physiological component of autonomic control of blood vessels and visceral organs, including the gut (Burnstock 1993; Holzer 2006). ATP and glutamate are cotransmitters in primary afferent sensory nerves. Cotransmission occurs in enteric neurons and the concept of ‘chemical coding ’ was proposed as a consequence of the patterns of co-localisation of neuropeptides defining specific neuron types (Furness et al. 1989). It is now recognised that three major cotransmitters are released from NANC inhibitory enteric nerves: (1) ATP producing fast IJPs; (2) NO also eliciting IJPs, but with a slower time course; and (3) VIP producing slow tonic relaxations (Burnstock 2001a). In some sphincters the NANC inhibitory nerves primarily utilise VIP, in others they utilise NO, and in non-sphincteric regions of the intestine, ATP is prominent. Detailed accounts of purinergic neuromuscular transmission in different regions of the gut are available (Hoyle and Burnstock 1989; Burnstock 2001a, 2014; Burnstock and Verkhratsky 2012).
NANC inhibitory purinergic transmission to intestinal smooth muscle of laboratory animals and humans is mediated by P2Y1 receptors (Wang et al. 2007; Gallego et al. 2008, 2012). α,β-Methylene ATP (α,β-meATP) has a potent relaxant action in some preparations (Johnson and Hourani 1994; Johnson et al. 1996; Pacaud et al. 1996). However, it is likely that α,β-meATP is acting on P2X3 receptors on sensory nerves (Storr et al. 2000; De Man et al. 2003) leading to reflex activation of NANC inhibitory nerves and to P2Y1 receptor-mediated relaxation of smooth muscle (see King and Townsend-Nicholson 2008). Evidence was presented that ATP mediates a non-cholinergic component of the excitatory junction potential and contraction of intestinal smooth muscle (Zagorodnyuk and Maggi 1998). ATP stimulated cholinergic interneurons in the myenteric plexus to cause a fast contraction of rat ileum (Sakai et al. 1979). P2Y2 and/or P2Y4 receptors were shown to mediate smooth muscle contractions in the small intestine of lower vertebrates (Burnstock 1969; Sneddon et al. 1973). Contraction of rat duodenal muscularis mucosae smooth muscle was mediated by P2X receptors (Johnson et al. 1996). P2X receptors mediated contraction of guinea pig ileum (Moody and Burnstock 1982; Ivancheva et al. 2001). Ileal contractions mediated by α,β-meATP were inhibited in P2X1 receptor knockout mice (Vial and Evans 2001). mRNAs for P2X2, P2X3 and P2X4 receptors were expressed by canine colon circular myocytes, while longitudinal myocytes expressed mRNAs for P2X3 and P2X5 receptors (Lee et al. 2005).
Synaptic Purinergic Transmission in the Enteric Plexuses
Elegant electrophysiological studies, carried out during the past 20 years have demonstrated synaptic purinergic transmission between enteric neurons in both myenteric and submucous plexuses in both in situ and tissue culture preparations (see Galligan et al. 2000; Galligan 2002; Hu et al. 2003; Ren et al. 2003; Galligan and North 2004; Ren and Galligan 2005; Burnstock 2007a, 2008a; Bornstein 2008; Ren and Bertrand 2008; Valdez-Morales et al. 2011). Also, extensive immunostaining of the localisation of both P2X and P2Y receptor subtypes in the gastrointestinal tract of guinea pigs, rats and mice was carried out (Gröschel-Stewart et al. 1999; Giaroni et al. 2002, 2006; Van Nassauw et al. 2002, 2006; Xiang and Burnstock 2004a, b, 2005, 2006; Ruan and Burnstock 2005; Yu et al. 2010).
Myenteric Ganglia
The effects of ATP in single myenteric neurons from guinea pig small intestine using intracellular electrodes were first shown by Katayama and Morita (1989). They showed that ATP produced hyperpolarisation in 80 % of AH neurons and depolarisation in 90 % of S neurons. The studies of purinergic signalling in guinea pig myenteric neurons were extended by several groups. Whole-cell and outside-out patch clamp recordings were used to characterise the physiological and pharmacological features of P2X receptors on myenteric neurons of the guinea pig ileum (Barajas-López et al. 1996). Fast excitatory postsynaptic currents (fEPSCs) were recorded in primary cultures of myenteric neurons from guinea pig intestine and hexamethonium-resistant fEPSCs were abolished by the P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (Zhou and Galligan 1996; LePard et al. 1997). Fast excitatory postsynaptic potentials (EPSPs) were mediated in part by P2X receptors in myenteric neurons in both the small and large intestine, but were rare in the gastric corpus (LePard et al. 1997). P2X2 receptors were the dominant subtype shown to be expressed by subpopulations of guinea pig enteric neurons, namely inhibitory motor neurons, vasomotor neurons, cholinergic secretomotor neurons, intrinsic sensory neurons and the endings of vagal afferent fibres in the stomach (Castelucci et al. 2002; Misawa et al. 2010). Using P2X2 receptor knockout mice it was shown that P2X2 receptors contributed to fast synaptic excitation of myenteric neurons in small intestine (Ren et al. 2003). The predominant receptors mediating fast synaptic excitation in the gut appear to be P2X2 homomeric receptors (Galligan 2002; Galligan and North 2004; Ohta et al. 2005), including intrinsic sensory neurons in the gut (Furness et al. 2004b).
P2X3 receptors were expressed by both excitatory and inhibitory motor neurons, ascending interneurons and cholinergic secretomotor neurons (Poole et al. 2002). However, it was claimed that they were not expressed by intrinsic sensory neurons in guinea pig ileum (Van Nassauw et al. 2002). In the small intestine of mice lacking P2X3 receptors peristalsis was impaired (Bian et al. 2003). The distribution of the mRNA and protein of P2X2 and P2X3 receptors were described in the enteric nervous system of the rat (Xiang and Burnstock 2004b). Most myenteric S neurons in guinea pig small intestine expressed P2X3 receptors, about half of which were inhibitory motoneurons (Ren and Galligan 2007). Nerve fibres that enveloped ganglion cell bodies in the myenteric and submucous plexuses in mouse intestine expressed P2X5 receptors, probably as heteromultimers with P2X2 receptors on enteric sensory neurons (Ruan and Burnstock 2005).
Purinergic signalling in dispersed primary cultures of guinea pig myenteric plexus was studied by the group of Mulholland. Different populations of enteric neurons responded to combinations of ATP with ACh, ATP with substance P (SP), ATP with ACh, ATP with ACh and SP, ATP with bombesin or ATP with ACh and bombesin (Kimball and Mulholland 1995). When ACh and ATP acted as cotransmitters, there was an interaction between nicotinic and P2X receptors with cross-inhibition between α3β4 nicotinic receptors and the C-terminal tail of P2X2 receptors (Decker and Galligan 2010). Inhibitory interactions have also been shown to take place between P2X and γ-aminobutyric acid-A receptors on myenteric neurons from the guinea pig small intestine (Karanjia et al. 2006). In excitatory neuro-neuronal transmission in both ascending and descending reflex pathways to the longitudinal and circular muscles of the guinea pig ileum triggered by mucosal stimulation , a major role was played by ATP (Clark et al. 1996; Spencer et al. 2000). P2X receptor-mediated transmission from interneurons to motor neurons in guinea pig ileum underlies descending inhibitory reflexes (Bian et al. 2000; Bornstein et al. 2004).
There is expression of P2Y receptors on enteric neurons in addition to P2X receptors (Xiang and Burnstock 2005, 2006; Van Nassauw et al. 2005; Gao et al. 2006; Wood 2006). In the mouse gastrointestinal tract relaxation is mediated by P2Y1 receptors on NANC myenteric neurons (Giaroni et al. 2002). Slow excitatory synaptic transmission on S-type neurons in the guinea pig enteric nervous system was mediated by P2Y1 receptors (Hu et al. 2003). They also mediated slow excitatory synaptic potentials on interneurons during descending inhibition in guinea pig ileum (Thornton et al. 2013). P2Y2 receptors were expressed by S-type neurons in both myenteric and submucosal plexuses of the guinea pig gut. 40–60 % of P2X3 receptor-immunoreactive neurons were immunoreactive for P2Y2 receptors in the myenteric plexus and all P2X3 receptor-immunoreactive neurons expressed P2Y2 receptors in the submucosal plexus (Xiang and Burnstock 2005). 30–36 % of neurons in ganglia in the myenteric, but not submucosal plexus of the guinea pig gut expressed P2Y6 receptors, while 42–46 % of the neurons in both myenteric and submucosal plexuses were immunoreactive for P2Y12 receptors (Xiang and Burnstock 2006). 28–35 % of P2Y6 receptor-immunoreactive neurons coexisted with NO synthase, while all P2Y12 receptor-immunoreactive neurons were immunopositive for calbindin, on AH intrinsic sensory neurons. P2Y2 and P2Y12 receptors were identified on enteric neurons in the rat distal colon (Van Nassauw et al. 2005). Presynaptic A1 receptors mediated suppression of slow EPSPs and amplified slow inhibitory postsynaptic transmission to myenteric neurons (Christofi and Wood 1993; Kamiji et al. 1994).
Submucosal Ganglia
The non-reversing type of slow excitatory postsynaptic potential recorded in S neurons of the submucous plexus of the guinea pig caecum was mimicked by ATP (Mihara et al. 1985). ATP produced fast transient depolarisation of AH-type neurons (Barajas-López et al. 1994), mediated by P2X receptors (Barajas-López et al. 2000). Neurons in the submucous plexus were immunopositive for P2X3 receptors and were colocalised with calretinin, suggesting labelling of intrinsic sensory neurons (Xiang and Burnstock 2004b). Functional interactions between nicotinic and P2X receptors were demonstrated in dissociated guinea pig submucosal neurons in primary culture (Glushakov et al. 1996; Barajas-López et al. 1998; Zhou and Galligan 1998). Inhibitory interactions between P2X and 5-HT3 receptors in guinea pig submucosal neurons were reported (Barajas-López et al. 2002). Slow, fast and intermediate EPSPs were recorded in neurons of the submucous plexus of the guinea pig ileum (Monro et al. 2004). The slow and intermediate EPSPs were blocked by the P2Y1 receptor selective antagonist MRS2179. P2Y1 receptor signalling involved in synaptic transmission in the human submucous nerve plexus was reported to be predominant (Wunderlich et al. 2008).
Intrinsic Sensory Neurons
Intrinsic sensory neurons are located in the submucosal and myenteric ganglia and their terminals are largely in a subepithelial plexus (Furness et al. 2004a). Intrinsic sensory neurons have been identified electrophysiologically as AH-type and morphologically as Dogiel type II cells. Most AH cells express calbindin and/or calretinin. Synaptic transmission to intrinsic sensory neurons is mediated by P2X receptors (Bertrand and Bornstein 2002), of the P2X2 receptor subtype in guinea pig intestine (Castelucci et al. 2002). Postsynaptic inhibition via P2Y receptors has also been identified on intrinsic sensory nerves (Bertrand 2003, 2004). P2X3 receptors were shown to be expressed by intrinsic sensory nerves in rat ileum and distal colon (Xiang and Burnstock 2004b). P2Y12 receptors were expressed by sensory neurons in guinea pig myenteric plexus (Xiang and Burnstock 2006).
Enteric Glial Cells and Interstitial Cells of Cajal
Enteric glial cells respond to ATP and uridine 5′-triphosphate, increasing intracellular calcium via P2Y2 and/or P2Y4 receptors (Kimball and Mulholland 1996; Sarosi et al. 1998). Immunohistochemical studies also showed expression of P2X7 receptors on enteric glial cells (Vanderwinden et al. 2003) and P2Y4 receptors (Van Nassauw et al. 2006). It was proposed that ATP released from sympathetic nerves activates enteric glia (Gulbransen et al. 2010). Purinergic neuron-glia interactions in the enteric nervous system have been reported, reflecting similar mechanisms in the CNS (Gulbransen and Sharkey 2009). From an electrophysiological study of a mouse enteric neuron-glial culture preparation, it was concluded that neuronal cells primarily express P2X receptors, while glial cells primarily express P2Y receptors (Gade and Akbarali 2013).
ICCs are a specialised cell type that act as pacemakers to regulate the activities of smooth muscle cells in the gut. P2X2 and P2X5 receptors were shown to be expressed on ICC’s in guinea pig intestine (Burnstock and Lavin 2002). Later, P2Y4 receptors were also identified on ICCs in guinea pig gastrointestinal tract (Van Nassauw et al. 2006). It is likely that ATP is released as a cotransmitter from enteric nerves and glial cells to regulate the activities of ICCs (Burnstock and Lavin 2002). Modulation of pacemaker [Ca2+]i activity in ICC’s was mediated by P2X receptors (Furuzono et al. 2005). It was reported that ICCs in human and murine small intestine expressed P2Y1 and P2Y4 receptors (Chen et al. 2007). ‘Fibroblast-like cells’, that form a network of cells distinct from ICCs, located between intestinal circular and longitudinal smooth muscle near terminals of enteric motor neurons and with gap junction connectivity with muscle cells, express P2Y1 receptors (Kurahashi et al. 2011). P2Y1 receptor antagonists blocked the activation of currents and increase in [Ca2+]i by adenosine 5′-diphosphate in these cells. The majority of subserosal ICCs or perhaps fibroblast-like cells in the guinea pig proximal colon responded to ATP via P2Y1 receptors and it was suggested that this may contribute to smooth muscle relaxation (Tamada and Hashitani 2014).
Purinergic Mechanosensory Transduction: Enteric Reflexes and Pain
Both submucosal intrinsic sensory neurons and extrinsic sensory nerves show positive immunoreactivity for P2X3 receptors (Xiang and Burnstock 2004b). It has been proposed that during intestinal distension ATP is released from mucosal epithelial cells to activate P2X3 receptors on both low threshold enteric sensory nerve fibres to mediate enteric reflexes (including peristalsis) and high-threshold extrinsic enteric sensory fibres leading to initiation of nociceptive impulses that pass messages through sensory ganglia to pain centres in the CNS (Burnstock 2001b, 2009). This hypothesis has been supported by experiments on a rat pelvic sensory nerve-colorectal preparation (Wynn et al. 2003). Distension of the colorectum led to increase in release of ATP from mucosal epithelial cells and evoked pelvic sensory nerve excitation. This excitation was mimicked by application of ATP and was attenuated by the selective P2X3 and P2X2/3 antagonist, 2′(3′)-O-(2,4,6-trinitrophenyl) ATP, and by PPADS. The sensory activity in the nerves was potentiated by ARL-67156, an ATPase inhibitor. It has been claimed recently that subepithelial fibroblasts in rat ductal villi also release ATP by mechanical stimuli to activate P2X3 receptors on subepithelial sensory nerves (Furuya and Furuya 2013).
Purinergic Signalling in Inflammatory Gut Disorders
P2X3 receptors are upregulated on enteric sensory neurons in inflammation and hypersensitivity (Wynn et al. 2004). Intestinal inflammation also increased the expression of P2Y6 receptors on epithelial cells and uridine diphosphate, a potent P2Y6 receptor agonist, released CXCL8, a chemokine known for chemoattraction to recruit neutrophils during the acute phase of colitis (Grbic et al. 2008, 2012). ATP may be beneficial in the treatment of intestinal disorders where intestinal permeability changes are involved (Bours et al. 2007). It was reported that P2Y2 receptor expression was upregulated in intestinal epithelial cells by the transcription factor C/EBPβ during inflammation (Degagné et al. 2012).
Expression of P2X3 receptors was increased in enteric plexuses of human IBD, suggesting a role in dysmotility and pain (Yiangou et al. 2001) and the possibility that P2X receptor antagonists could be used for the treatment of irritable bowel syndrome (IBS) was raised (Galligan 2004). It was also suggested that P2X receptors on intrinsic enteric neurons may mediate enhanced gastrointestinal propulsion and secretion and might be used for treating constipation-predominant IBS, while P2X receptor antagonists might be useful for treating diarrhoea-predominant IBS. It has been suggested that sensitisation of P2X3 receptors on vagal and spinal afferents in the stomach may contribute to the development of visceral hyperalgesia (Dang et al. 2005). In inflamed gastrointestinal tract, glial cells proliferate and produce cytokines, indicating that P2X7 receptors may play a role in the response of enteric glia to inflammation (Vanderwinden et al. 2003).
ATP release and P2X3 and P2X2/3 receptor-mediated nociceptive sensory nerve responses were enhanced in the rat trinitrobenzene sulfonic acid (TNBS) model of colitis (Wynn et al. 2004). Different mechanosensory information from the colon to the spinal cord is mediated by lumbar splanchnic (LSN) and sacral pelvic (PN) nerves. It was shown that 40 % of LSN afferents responded to α,β-meATP compared to 7 % of PN afferents (Brierley et al. 2005). There is enhancement of P2X3 receptor-mediated signalling in the TNBS colitis model, which was due, at least in part, to the appearance of P2X3 receptor expression in a greater number of calcitonin gene-related peptide-labelled small nociceptive neurons in the dorsal root ganglia (DRG) (Wynn et al. 2004). There is also increased release of ATP from mucosal epithelial cells with distension in TNBS-treated rats. Purinergic mechanosensory transduction has been shown to contribute to post-infectious mechano-hypersensitivity (Rong et al. 2009). In TNBS-induced colitis in mice, P2X1 receptor expression on colonic submucosal arterioles was increased (Lomax et al. 2007). Propulsive motility was attenuated in the ulcerated region of the TNBS-inflamed colon and this was associated with a decrease in the purinergic component of the descending inhibitory limb of the peristaltic reflex circuit (Strong et al. 2010).
Substances are released from mucosal epithelial cells during distension that often act synergistically to cause sensitisation of afferent nerves to mechanical or chemical stimuli (Wynn and Burnstock 2006). Thus, receptors to a variety of substances including ATP are potential targets for drug treatment for inflamed bowel function and visceral pain (see Kirkup et al. 2001; Holzer 2004). The sensitising effects of P2X3 receptor agonists on mechanosensory function were also demonstrated in oesophagitis (Page et al. 2000). Visceral hyperalgesia was shown to be associated with an increase in ATP activity and enhanced expression of P2X3 receptors in colonic sensory neurons (Xu et al. 2008). Selective P2X3 and P2X2/3 receptor antagonists that are orally bioavailable and do not degrade in vivo are in clinical trials for the treatment of visceral pain (see Gever et al. 2006; Donnelly-Roberts et al. 2008). P2X3 receptor mRNA expression in DRG was significantly decreased in an ovariectomized rat model of colitis, which was reversed by oestrogen (Fan et al. 2009). It was suggested that ATP is a critical autocrine regulator of mechanosensitive 5-hydroxytryptamine (5-HT) release , also involved in the pathogenesis of IBD and it was shown that P2X3 receptors on enterochromaffin cells were down-regulated in ulcerative colitis (Liñán-Rico et al. 2013). CD39 (NPTDase 1) was upregulated in the submucosa during colitis, resulting in compromise of epithelial barrier function (Neshat et al. 2009). It was reported that dysregulation occurs in 59 % of purinoceptor genes in IBD, including P2Y6, P2Y13, P2Y14, P2X5, A2A and A2B receptors (Rybaczyk et al. 2009).
P2X7 receptors play a pivotal role in intestinal inflammation and are involved in the development of visceral hypersensitivity (Keating et al. 2011). Epithelial and immune cells express P2X7 receptors , which are implicated in the pathogenesis of IBD based on the dysregulation of immune responses in (de Campos et al. 2012). Activation of neuronal P2X7 receptor/pannexin 1 mediates death of enteric neurons during colitis (Gulbransen et al. 2012). This supported an earlier study of TNBS-induced colitis, using high-density oligonucleotide microassay analysis and oral N6-(3-iodobenzyl)-adenosine-5-N-methyluronamide, an A3 receptor agonist, blocked the colitis-induced upregulation of P2X1, P2X4, P2X7, P2Y2 and P2Y6 receptors (Guzman et al. 2006). Extracellular ATP largely via P2X7 receptors evoked cell death in human intestinal epithelial cells and the implication of this in inflammatory conditions and immune responses was explored (Souza et al. 2012). It has also been shown that ATP mediated mast cell-dependent intestinal inflammation via P2X7 receptors (Kurashima et al. 2012).
An adenosine A3 agonist has been recommended to be protective in two murine models of colitis (Mabley et al. 2003). A2B receptor expression and signalling in intestinal epithelium in colitis was upregulated by tumour necrosis factor-α (Kolachala et al. 2005). A2B receptor blockade ameliorated mouse colitis (Kolachala et al. 2008a) as did A2B receptor gene deletion (Kolachala et al. 2008b). The inhibitory effects of adenosine on enteric neuromuscular activities were diminished in inflamed colon (Antonioli et al. 2005). Oxidative stress disrupted purinergic neuromuscular transmission in the inflamed colon (Roberts et al. 2013). A2B receptors appear to play a role in the control of T cell-mediated colitis by suppressing the expression of pro-inflammatory cytokines, while sparing anti-inflammatory activity mediated by interleukin (IL)-10 and transforming growth factor-β (Naganuma et al. 2006). A2A receptors were also reported to mediate the inhibitory effects of adenosine on colonic motility in the TNBS model of experimental colitis (Antonioli et al. 2006; Rahimian et al. 2010). Adenosine deaminase inhibition attenuates inflammation in experimental colitis (Antonioli et al. 2007) through the recruitment of A2A and A3 receptors (Antonioli et al. 2010). Adenosine, acting via A3 receptors, has been shown to be involved in intestinal anti-inflammation activities (Guzman et al. 2006; Gessi et al. 2008). A2A receptors were also involved in the anti-inflammatory actions of adenosine (Odashima et al. 2005) and A2A receptor agonists are being developed for the treatment of IBD (El-Tayeb et al. 2011). A2B receptors mediate regulation of 5-HT synthesis and release from hypoxic enterochromaffin cells in IBD (Damen et al. 2013). A2B receptor antagonists were reported to be effective against murine colitis (Kolachala et al. 2008c). The involvement of adenosine A1 and A2A receptors (Antonioli et al. 2011) and A3 receptors (Ren et al. 2011) in colitis has been reported. Recent reviews of the roles of adenosine signalling in gastrointestinal inflammation are available (Estrela and Abraham 2011; Colgan et al. 2013). The involvement of adenosine deaminase in patients with Crohn’s disease has been explored (Maor et al. 2011). Adenosine kinase inhibition by GP515 has also been investigated as a potential target for the treatment of colitis (Siegmund et al. 2001). It was concluded in a review about purinergic receptors in gastrointestinal inflammation (Kolachala et al. 2008a) that P1 (A2A and A2B) and P2Y receptor-based therapy is highly promising for treatment of inflammatory conditions of the gut (see Michael et al. 2010). Serum adenosine deaminase activity has been proposed as a predictor of disease severity in ulcerative colitis (Beyazit et al. 2012). Ecto-nucleoside triphosphate diphosphohydrolase 7 was preferentially expressed in epithelial cells of mouse small intestine (Kusu et al. 2013). ATP released from colonic mucosal epithelial cells of IBS patients excited via P2X receptors enteric cholinergic motor neurons (Balestra et al. 2012). The role of adenosine as an immune modulator of IBD has been considered (Ye and Rajendran 2009). Polymorphisms of CD39 have been linked to Crohn’s disease (Künzli et al. 2011). Release of ATP by activated neutrophils and necrotic intestinal epithelial cells stimulates epithelial cell P2X7 receptors leading to activation of caspase 1 and secretion of IL-1β proinflammatory cytokine (Cesaro et al. 2010).
Concluding Comments
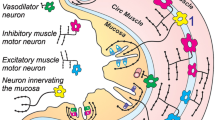
In this brief review, the focus has been on purinergic neuromuscular transmission, synaptic purinergic transmission in the enteric nerve plexuses, purinergic mechanosensory transduction in initiation of enteric reflexes and intestinal nociception and the involvement of purinergic signalling in inflammatory gut disorders. Figure 10.1 summarises the complex distribution of purinoceptor subtypes in the gut. For fuller coverage of the involvement of purinergic signalling in the physiology and pathophysiology of the gastrointestinal system, readers are recommended to refer to the following recent reviews (Burnstock 2008a, b, 2011, 2012b, 2014).
Schematic showing the localisation of receptors to purines and pyrimidines on neurons and non-neuronal effector cells in the gut, although some of the interacting pathways are not yet known. Extrinsic vagal and sacral parasympathetic nerves connect with NANC inhibitory neurons in the myenteric plexus expressing P2X2, P2X3, P2Y1, P2Y6 and A2B receptors, as well as with cholinergic motor neurons; these neurons are also activated by descending interneurons. Extrinsic sympathetic nerves modulate motility via excitatory motor neurons and constrict blood vessels in the gut via P2X1 receptors. Extrinsic sensory nerves arising from cell bodies in dorsal root ganglia and with subepithelial terminals mediate nociception. Intrinsic sensory neurons in both myenteric and submucosal plexuses express P2X2 and P2X3 receptors, while a subpopulation also express P2Y12 receptors; they connect with motor pathways involved in peristalsis. Excitatory motor neurons express P2X2, P2X3, P2X2/3, P2X5 and P2Y2 receptors and connect with both interneurons and secretomotor neurons. Interneurons express P2X2 and P2X3 receptors. Enteric glial cells express P2Y4 and P2X7 receptors, while interstitial cells of Cajal express P2X2, P2X5 and P2Y4 receptors. P2X7 and P1 receptors appear to act as prejunctional modulators of both motor and interneurons. (Reproduced from Burnstock 2008b, with permission.)
References
Antonioli L, Fornai M, Blandizzi C, Salvadorini C, Colucci R, Breschi MC, Del Taca M (2005) The inhibitory effects of adenosine on enteric neuromuscular activity are decreased in inflammed colonic tissues. Gastroenterology 128:A273
Antonioli L, Fornai M, Colucci R, Ghisu N, Blandizzi C, Del Tacca M (2006) A2a receptors mediate inhibitory effects of adenosine on colonic motility in the presence of experimental colitis. Inflamm Bowel Dis 12:117–122
Antonioli L, Fornai M, Colucci R, Ghisu N, Da SF, Natale G, Kastsiuchenka O, Duranti E, Virdis A, Vassalle C, La MC, Mugnaini L, Breschi MC, Blandizzi C, Del Taca M (2007) Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther 322:435–442
Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Da SF, La Motta C, Natale G, Duranti E, Virdis A, Blandizzi C (2010) The blockade of adenosine deaminase ameliorates chronic experimental colitis through the recruitment of adenosine A2A and A3 receptors. J Pharmacol Exp Ther 335:434–442
Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C (2011) Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol 650:639–649
Balestra B, Vicini R, Cremon C, Zecchi L, Dothel G, Vasina V, De GR, Paccapelo A, Pastoris O, Stanghellini V, Corinaldesi R, De Ponti F, Tonini M, Barbara G (2012) Colonic mucosal mediators from patients with irritable bowel syndrome excite enteric cholinergic motor neurons. Neurogastroenterol Motil 24:1118-e570
Barajas-López C, Espinosa-Luna R, Gerzanich V (1994) ATP closes a potassium and opens a cationic conductance through different receptors in neurons of guinea pig submucous plexus. J Pharmacol Exp Ther 268:1397–1402
Barajas-López C, Huizinga JD, Collins SM, Gerzanich V, Espinosa-Luna R, Peres AL (1996) P2x-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Br J Pharmacol 119:1541–1548
Barajas-López C, Espinosa-Luna R, Zhu Y (1998) Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J Physiol 513:671–683
Barajas-López C, Espinosa-Luna R, Christofi FL (2000) Changes in intracellular Ca2+ by activation of P2 receptors in submucosal neurons in short-term cultures. Eur J Pharmacol 409:243–257
Barajas-López C, Montaño LM, Espinosa-Luna R (2002) Inhibitory interactions between 5-HT3 and P2X channels in submucosal neurons. Am J Physiol Gastrointest Liver Physiol 283:G1238–G1248
Bertrand PP (2003) ATP and sensory transduction in the enteric nervous system. Neuroscientist 9:243–260
Bertrand PP (2004) Bursts of recurrent excitation in the activation of intrinsic sensory neurons of the intestine. Neuroscience 128:51–63
Bertrand PP, Bornstein JC (2002) ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci 22:4767–4775
Beyazit Y, Koklu S, Tas A, Purnak T, Sayilir A, Kurt M, Turhan T, Celik T, Suvak B, Torun S, Akbal E (2012) Serum adenosine deaminase activity as a predictor of disease severity in ulcerative colitis. J Crohns Colitis 6:102–107
Bian XC, Bertrand PP, Bornstein JC (2000) Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol 528:551–560
Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ (2003) Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551:309–322
Bornstein JC (2008) Purinergic mechanisms in the control of gastrointestinal motility. Purinergic Signal 4:197–212
Bornstein JC, Costa M, Grider JR (2004) Enteric motor and interneuronal circuits controlling motility. Neurogastroenterol Motil 16(Suppl 1):34–38
Bours MJ, Troost FJ, Brummer RJ, Bast A, Dagnelie PC (2007) Local effect of adenosine 5′-triphosphate on indomethacin-induced permeability changes in the human small intestine. Eur J Gastroenterol Hepatol 19:245–250
Brierley SM, Carter R, Jones W III, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA (2005) Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567:267–281
Bülbring E, Tomita T (1967) Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol 189:299–315
Burnstock G (1969) Evolution of the autonomic innervation of visceral and cardiovascular systems in vertebrates. Pharmacol Rev 21:247–324
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Burnstock G (1976) Do some nerve cells release more than one transmitter? Neuroscience 1:239–248
Burnstock G (1993) Physiological and pathological roles of purines: an update. Drug Dev Res 28:195–206
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–342
Burnstock G (2001a) Purinergic signalling in gut. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology, vol 151/II. Purinergic and pyrimidinergic signalling II – cardiovascular, respiratory, immune, metabolic and gastrointestinal tract function. Springer, Berlin, pp 141–238
Burnstock G (2001b) Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22:182–188
Burnstock G (2004) A moment of excitement. Living history series. The discovery of non-adrenergic, non-cholinergic neurotransmission. Physiol News 56:7–9
Burnstock G (2007a) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Burnstock G (2007b) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Burnstock G (2008a) The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil 20:8–19
Burnstock G (2008b) Commentary. Purinergic receptors as future targets for treatment of functional GI disorders. Gut 57:1193–1194
Burnstock G (2009) Purinergic mechanosensory transduction and visceral pain. Mol Pain 5:69
Burnstock G (2011) Purinergic signaling in the gastrointestinal tract. World J Gastrointest Pathophysiol 2:31–34
Burnstock G (2012a) The Gaddum Lecture. Discovery of purinergic signalling, the initial resistance and current explosion of interest. Br J Pharmacol 167:238–255
Burnstock G (2012b) P2X receptors in the gut. WIREs Membr Transp Signaling 1:269–279
Burnstock G (2014) Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 10(1):3–50
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304
Burnstock G, Lavin S (2002) Interstitial cells of Cajal and purinergic signalling. Auton Neurosci 97:68–72
Burnstock G, Straub RW (1958) A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol 140:156–167
Burnstock G, Verkhratsky A (2012) Purinergic signalling and the nervous system. Springer, Heidelberg/Berlin
Burnstock G, Campbell G, Bennett M, Holman ME (1963) The effects of drugs on the transmission of inhibition from autonomic nerves to the smooth muscle of the guinea pig taenia coli. Biochem Pharmacol 12:134–135
Burnstock G, Campbell G, Bennett M, Holman ME (1964) Innervation of the guinea-pig taenia coli: are there intrinsic inhibitory nerves which are distinct from sympathetic nerves? Int J Neuropharmacol 3:163–166
Burnstock G, Campbell G, Rand MJ (1966) The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol 182:504–526
Burnstock G, Campbell G, Satchell D, Smythe A (1970) Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol 40:668–688
Burnstock G, Fredholm BB, North RA, Verkhratsky A (2010) The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 199:93–147
Castelucci P, Robbins HL, Poole DP, Furness JB (2002) The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol 117:415–422
Cesaro A, Brest P, Hofman V, Hébuterne X, Wildman S, Ferrua B, Marchetti S, Doglio A, Vouret-Craviari V, Galland F, Naquet P, Mograbi B, Unwin R, Hofman P (2010) Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am J Physiol Gastrointest Liver Physiol 299:G32–G42
Chen H, Redelman D, Ro S, Ward SM, Ördög T, Sanders KM (2007) Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol 292:C497–C507
Christofi FL (2008) Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal 4:213–236
Christofi FL, Wood JD (1993) Endogenously released adenosine acts at A1 receptors to suppress slow excitatory transmission (slow EPSP) and enhance slow inhibitory transmission (slow IPSP) in the myenteric plexus of guinea-pig small intestine. Gastroenterology 104:A490
Clark SR, Costa M, Tonini M, Brookes SJ (1996) Purinergic transmission is involved in a descending excitatory reflex in the guinea-pig small intestine. Proc Aust Neurosci Soc 7:176
Colgan SP, Fennimore B, Ehrentraut SF (2013) Adenosine and gastrointestinal inflammation. J Mol Med (Berl) 91:157–164
Damen R, Haugen M, Svejda B, Alaimo D, Brenna O, Pfragner R, Gustafsson BI, Kidd M (2013) The stimulatory adenosine receptor ADORA2B regulates serotonin (5-HT) synthesis and release in oxygen-depleted EC cells in inflammatory bowel disease. PLoS One 8, e62607
Dang K, Bielfeldt K, Lamb K, Gebhart GF (2005) Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol 93:3112–3119
de Campos NE, Marques-da-Silva C, Corrêa G, Castelo-Branco MT, de Souza HS, Coutinho-Silva R (2012) Characterizing the presence and sensitivity of the P2X7 receptor in different compartments of the gut. J Innate Immun 4:529–541
De Man JG, De Winter BY, Seerden TC, De Schepper HU, Herman AG, Pelckmans PA (2003) Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol 140:1108–1116
Decker DA, Galligan JJ (2010) Molecular mechanisms of cross-inhibition between nicotinic acetylcholine receptors and P2X receptors in myenteric neurons and HEK-293 cells. Neurogastroenterol Motil 22:901–908, e235
Degagné É, Turgeon N, Moore-Gagné J, Asselin C, Gendron FP (2012) P2Y2 receptor expression is regulated by C/EBPβ during inflammation in intestinal epithelial cells. FEBS J 279:2957–2965
Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF (2008) Painful purinergic receptors. J Pharmacol Exp Ther 324:409–415
Drury AN, Szent-Györgyi A (1929) The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol 68:213–237
Dubyak GR, El Moatassim C (1993) Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 265:C577–C606
Eccles JC (1964) The Physiology of Synapses. Springer, Berlin, pp 1–316
El-Tayeb A, Michael S, Abdelrahman A, Behrenswerth A, Gollos S, Nieber K, Müller CE (2011) Development of polar adenosine A2A receptor agonists for inflammatory bowel disease: synergism with A2B antagonists. ACS Med Chem Lett 2:890–895
Estrela AB, Abraham WR (2011) Adenosine in the inflamed gut: a Janus faced compound. Curr Med Chem 18:2791–2815
Evans RJ, Surprenant A (1992) Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol 106:242–249
Fan J, Yu L, Zhang W, Zhao T, Yu Y, Gao J, Zou D, Ni X, Ma B, Burnstock G (2009) Estrogen altered visceromotor reflex and P2X3 mRNA expression in a rat model of colitis. Steroids 74:956–963
Feldberg W, Hebb C (1948) The stimulating action of phosphate compounds on the perfused superior cervical ganglion of the cat. J Physiol 107:210–221
Furness JB, Morris JL, Gibbins IL, Costa M (1989) Chemical coding of neurons and plurichemical transmission. Annu Rev Pharmacol Toxicol 29:289–306
Furness JB, Jones C, Nurgali K, Clerc N (2004a) Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72:143–164
Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K (2004b) Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317:1–12
Furuya S, Furuya K (2013) Roles of substance P and ATP in the subepithelial fibroblasts of rat intestinal villi. Int Rev Cell Mol Biol 304:133–189
Furuzono S, Nakayama S, Imaizumi Y (2005) Purinergic modulation of pacemaker Ca2+ activity in interstitial cells of Cajal. Neuropharmacology 48:264–273
Gade AR, Akbarali HI (2013) Electrophysiological characterization of purinergic receptors in mouse enteric neuron-glia culture. FASEB J 27:1093.24
Gallego D, Vanden Berghe P, Farré R, Tack J, Jiménez M (2008) P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil 20:159–168
Gallego D, Gil V, Martínez-Cutillas M, Mañe N, Martín MT, Jiménez M (2012) Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J Physiol 590:1943–1956
Galligan JJ (2002) Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol 2:623–629
Galligan JJ (2004) Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol 141:1294–1302
Galligan JJ, North RA (2004) Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil 16(Suppl 1):64–70
Galligan JJ, LePard KJ, Schneider DA, Zhou X (2000) Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81:97–103
Gao N, Hu HZ, Zhu MX, Fang X, Liu S, Gao C, Wood JD (2006) The P2Y1 purinergic receptor expressed by enteric neurones in guinea-pig intestine. Neurogastroenterol Motil 18:316–323
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117:123–140
Gever J, Cockayne DA, Dillon MP, Burnstock G, Ford APDW (2006) Pharmacology of P2X channels. Pflugers Arch 452:513–537
Giaroni C, Knight GE, Ruan H-Z, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G (2002) P2 receptors in the murine gastrointestinal tract. Neuropharmacology 43:1313–1323
Giaroni C, Knight GE, Zanetti E, Chiaravalli AM, Lecchini S, Frigo G, Burnstock G (2006) Postnatal development of P2 receptors in the murine gastrointestinal tract. Neuropharmacology 50:690–704
Glushakov AV, Melishchuk AI, Skok VI (1996) ATP-induced currents in submucous plexus neurons of the guinea-pig small intestine. Neurophysiology 28:77–85
Grbic DM, Degagné E, Langlois C, Dupuis AA, Gendron FP (2008) Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol 180:2659–2668
Grbic DM, Degagné É, Larriveé JF, Bilodeau MS, Vinette V, Arguin G, Stankova J, Gendron FP (2012) P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in epithelial cells. Inflamm Bowel Dis 18:1456–1469
Gröschel-Stewart U, Bardini M, Robson T, Burnstock G (1999) P2X receptors in the rat duodenal villus. Cell Tissue Res 297:111–117
Gulbransen BD, Sharkey KA (2009) Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136:1349–1358
Gulbransen BD, Bains JS, Sharkey KA (2010) Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 30:6801–6809
Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA (2012) Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18:600–604
Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL (2006) ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis 12:766–789
Holton P (1959) The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol 145:494–504
Holzer P (2004) Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin Ther Targets 8:107–123
Holzer P (2006) Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125:70–75
Hoyle CHV (1992) Transmission: purines. In: Burnstock G, Hoyle CHV (eds) The autonomic nervous system. Autonomic neuroeffector mechanisms. Harwood Academic Publishers, Chur, pp 367–407
Hoyle CHV, Burnstock G (1989) Neuromuscular transmission in the gastrointestinal tract. In: Wood JD (ed) Handbook of physiology, Section 6: The gastrointestinal system, Vol. I: Motility and circulation. American Physiological Society, Bethesda, MD, pp 435–464
Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD (2003) Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550:493–504
Ivancheva C, Rahamimoff R, Radomirov R (2001) Apamin-sensitive nitric oxide- and ATP-mediated motor effects on the guinea pig small intestine. Gen Physiol Biophys 20:97–108
Johnson CR, Hourani SMO (1994) Contractile effects of uridine 5′-triphosphate in the rat duodenum. Br J Pharmacol 113:1191–1196
Johnson CR, Charlton SJ, Hourani SMO (1996) Responses of the longitudinal muscle and the muscularis mucosae of the rat duodenum to adenine and uracil nucleotides. Br J Pharmacol 117:823–830
Kamiji T, Morita K, Katayama Y (1994) ATP regulates synaptic transmission by pre- and postsynaptic mechanisms in guinea-pig myenteric neurons. Neuroscience 59:165–174
Karanjia R, García-Hernandez LM, Miranda-Morales M, Somani N, Espinosa-Luna R, Montaño LM, Barajas-López C (2006) Cross-inhibitory interactions between GABAA and P2X channels in myenteric neurones. Eur J Neurosci 23:3259–3268
Katayama Y, Morita K (1989) Adenosine 5′-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol 408:373–390
Keating C, Pelegrin P, Martinez CM, Grundy D (2011) P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol 187:1467–1474
Kimball BC, Mulholland MW (1995) Neuroligands evoke calcium signaling in cultured myenteric neurons. Surgery 118:162–169
Kimball BC, Mulholland MW (1996) Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem 66:604–612
King BF, Townsend-Nicholson A (2008) Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther 324:1055–1063
Kirkup AJ, Brunsden AM, Grundy D (2001) Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol 280:G787–G794
Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV (2005) TNF-α upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci 62:2647–2657
Kolachala VL, Ruble BK, Vijay-Kumar M, Wang L, Mwangi S, Figler HE, Figler RA, Srinivasan S, Gewirtz AT, Linden J, Merlin D, Sitaraman SV (2008a) Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol 155:127–137
Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV (2008b) A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135:861–870
Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV (2008c) Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol 294:G401–G410
Künzli BM, Berberat PO, Dwyer K, Deaglio S, Csizmadia E, Cowan P, d’Apice A, Moore G, Enjyoji K, Friess H, Robson SC (2011) Variable impact of CD39 in experimental murine colitis. Dig Dis Sci 56:1393–1403
Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM (2011) A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589:697–710
Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H (2012) Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun 3:1034
Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K, Maeda Y, Nishimura J, Arima Y, Atarashi K, Honda K, Murakami M, Kunisawa J, Kiyono H, Okumura M, Yamamoto M, Takeda K (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 190:774–783
Langley JN (1898) On inhibitory fibres in the vagus to the end of the oesophagus and stomach. J Physiol 23:407–414
Lee HK, Ro S, Keef KD, Kim YH, Kim HW, Horowitz B, Sanders KM (2005) Differential expression of P2X-purinoceptor subtypes in circular and longitudinal muscle of canine colon. Neurogastroenterol Motil 17:575–584
LePard KJ, Messori E, Galligan JJ (1997) Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology 113:1522–1534
Liñán-Rico A, Wunderlich JE, Grants IS, Frankel WL, Xue J, Williams KC, Harzman AE, Enneking JT, Cooke HJ, Christofi FL (2013) Purinergic autocrine regulation of mechanosensitivity and serotonin release in a human EC model: ATP-gated P2X3 channels in EC are downregulated in ulcerative colitis. Inflamm Bowel Dis 19:2366–2379
Lomax AE, O’Reilly M, Neshat S, Vanner SJ (2007) Sympathetic vasoconstrictor regulation of mouse colonic submucosal arterioles is altered in experimental colitis. J Physiol 583:719–730
Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C (2003) The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol 466:323–329
Maor I, Rainis T, Lanir A, Lavy A (2011) Adenosine deaminase activity in patients with Crohn’s disease: distinction between active and nonactive disease. Eur J Gastroenterol Hepatol 23:598–602
Martinson J (1965) Studies on the efferent vagal control of the stomach. Acta Physiol Scand Suppl 255:1–24
Martinson J, Muren A (1963) Excitatory and inhibitory effects of vagus stimulation on gastric motility in the cat. Acta Physiol Scand 57:309–316
McSwiney BA, Robson JH (1929) The response of smooth muscle to stimulation of the vagus nerve. J Physiol 68:124–131
Michael S, Warstat C, Michel F, Yan L, Müller CE, Nieber K (2010) Adenosine A2A agonist and A2B antagonist mediate an inhibition of inflammation-induced contractile disturbance of a rat gastrointestinal preparation. Purinergic Signal 6:117–124
Mihara S, Katayama Y, Nishi S (1985) Slow postsynaptic potentials in neurones of the submucous plexus of guinea pig caecum and their mimickry by noradrenaline and various peptides. Neuroscience 16:1057–1066
Misawa R, Girotti PA, Mizuno MS, Liberti EA, Furness JB, Castelucci P (2010) Effects of protein deprivation and re-feeding on P2X2 receptors in enteric neurons. World J Gastroenterol 16:3651–3663
Monro RL, Bertrand PP, Bornstein JC (2004) ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556:571–584
Moody CJ, Burnstock G (1982) Evidence for the presence of P1-purinoceptors on cholinergic nerve terminals in the guinea-pig ileum. Eur J Pharmacol 77:1–9
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177:2765–2769
Neshat S, DeVries M, Barajas-Espinosa AR, Skeith L, Chisholm SP, Lomax AE (2009) Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol 296:G399–G405
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F (2005) Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129:26–33
Ohta T, Kubota A, Murakami M, Otsuguro K, Ito S (2005) P2X2 receptors are essential for [Ca2+]i increases in response to ATP in cultured rat myenteric neurons. Am J Physiol Gastrointest Liver Physiol 289:G935–G948
Olsson RA, Pearson JD (1990) Cardiovascular purinoceptors. Physiol Rev 70:761–845
Pacaud P, Feolde E, Frelin C, Loirand G (1996) Characterization of the P2Y-purinoceptor involved in the ATP-induced rise in cytosolic Ca2+ concentration in rat ileal myocytes. Br J Pharmacol 118:2213–2219
Page AJ, O’Donnell TA, Blackshaw LA (2000) P2X purinoceptor-induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. J Physiol (Lond) 523:403–411
Paton WD (1958) Central and synaptic transmission in the nervous system; pharmacological aspects. Annu Rev Physiol 20:431–470
Paton WD, Vane JR (1963) Analysis of the responses of the isolated stomach to electrical stimulation and to drugs. J Physiol 165:10–46
Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB (2002) The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci 101:39–47
Rahimian R, Fakhfouri G, Daneshmand A, Mohammadi H, Bahremand A, Rasouli MR, Mousavizadeh K, Dehpour AR (2010) Adenosine A2A receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur J Pharmacol 649:376–381
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Ren J, Bertrand PP (2008) Purinergic receptors and synaptic transmission in enteric neurons. Purinergic Signal 4:255–266
Ren J, Galligan JJ (2005) Dynamics of fast synaptic excitation during trains of stimulation in myenteric neurons of guinea-pig ileum. Auton Neurosci 117:67–78
Ren J, Galligan JJ (2007) A novel calcium-sensitive potassium conductance is coupled to P2X3 subunit containing receptors in myenteric neurons of guinea pig ileum. Neurogastroenterol Motil 19:912–922
Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ (2003) P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol 552:809–821
Ren T, Grants I, Alhaj M, McKiernan M, Jacobson M, Hassanain HH, Frankel W, Wunderlich J, Christofi FL (2011) Impact of disrupting adenosine A3 receptors (A3 -/- AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis 17:1698–1713
Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN, Mawe GM (2013) Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol 591:3725–3737
Rong W, Keating C, Sun B, Dong L, Grundy D (2009) Purinergic contribution to small intestinal afferent hypersensitivity in a murine model of postinfectious bowel disease. Neurogastroenterol Motil 21:665–671, e32
Ruan H-Z, Burnstock G (2005) The distribution of P2X5 purinergic receptors in the enteric nervous system. Cell Tissue Res 319:191–200
Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL (2009) New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis 15:971–984
Sakai K, Akima M, Matsushita H (1979) Analysis of the contractile responses of the ileal segment of the isolated blood-perfused small intestine of rats to adenosine triphosphate and related compounds. Eur J Pharmacol 58:157–162
Sarosi GA, Barnhart DC, Turner DJ, Mulholland MW (1998) Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am J Physiol 275:G550–G555
Siegmund B, Rieder F, Albrich S, Wolf K, Bidlingmaier C, Firestein GS, Boyle D, Lehr HA, Loher F, Hartmann G, Endres S, Eigler A (2001) Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther 296:99–105
Sneddon JD, Smythe A, Satchell D, Burnstock G (1973) An investigation of the identity of the transmitter substance released by non-adrenergic, non-cholinergic excitatory nerves supplying the small intestine of some lower vertebrates. Comp Gen Pharmacol 4:53–60
Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MT, Abalo AA, Paiva MM, Coutinho CM, Coutinho-Silva R (2012) Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta 1820:1867–1878
Spencer NJ, Walsh M, Smith TK (2000) Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol 522:321–331
Storr M, Franck H, Saur D, Schusdziarra V, Allescher HD (2000) Mechanisms of α, β-methylene ATPS-induced inhibition in rat ileal smooth muscle: involvement of intracellular Ca2+ stores in purinergic inhibition. Clin Exp Pharmacol Physiol 27:771–779
Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM (2010) Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol 588:847–859
Su C, Bevan JA, Burnstock G (1971) [3H]adenosine triphosphate: release during stimulation of enteric nerves. Science 173:337–339
Tamada H, Hashitani H (2014) Calcium responses in subserosal interstitial cells of the guinea-pig proximal colon. Neurogastroenterol Motil 26:115–123
Thornton PD, Gwynne RM, McMillan DJ, Bornstein JC (2013) Transmission to interneurons is via slow excitatory synaptic potentials mediated by P2Y1 receptors during descending inhibition in guinea-pig ileum. PLoS One 8, e40840
Valdez-Morales E, Guerrero-Alba R, Liñan-Rico A, Espinosa-Luna R, Zarazua-Guzman S, Miranda-Morales M, Montaño LM, Barajas-López C (2011) P2X7 receptors contribute to the currents induced by ATP in guinea pig intestinal myenteric neurons. Eur J Pharmacol 668:366–372
Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans J-P (2002) Neurochemical identification of enteric neurons expressing P2X3 receptors in the guinea-pig ileum. Histochem Cell Biol 118:193–203
Van Nassauw L, Van Crombruggen K, De Jonge F, Burnstock G, Lefebvre RA, Timmermans J-P (2005) Distribution of P2Y receptor subtypes in the rat distal colon. Neurogastroenterol Motil 17:1
Van Nassauw L, Costagliola A, Van Op den bosch J, Cecio A, Vanderwinden J-M, Burnstock G, Timmermans J-P (2006) Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci 126–127:299–306
Vanderwinden JM, Timmermans JP, Schiffmann SN (2003) Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res 312:149–154
Vial C, Evans RJ (2001) Smooth muscles does not have a common P2x receptor phenotype: expression, ontogeny and function of P2x1 receptors in mouse ileum, bladder and reproductive systems. Auton Neurosci 92:56–64
Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD (2007) Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292:G1483–G1489
Wood JD (2006) The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol 6:564–570
Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang Y, Grants I, Mikami DJ, Melvin WS, Cooke HJ, Christofi FL (2008) Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294:G554–G566
Wynn G, Burnstock G (2006) Adenosine 5′-triphosphate and it’s relationship with other mediators that activate pelvic afferent neurons in the rat colorectum. Purinergic Signal 2:517–526
Wynn G, Rong W, Xiang Z, Burnstock G (2003) Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125:1398–1409
Wynn G, Bei M, Ruan H-Z, Burnstock G (2004) Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol 287:G647–G657
Xiang Z, Burnstock G (2004a) Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol 122:111–119
Xiang Z, Burnstock G (2004b) P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol 121:169–179
Xiang Z, Burnstock G (2005) Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol 124:379–390
Xiang Z, Burnstock G (2006) Distribution of P2Y6 and P2Y12 receptor: their colocalization with calbindin, calretinin and nitric oxide synthase in the guinea pig enteric nervous system. Histochem Cell Biol 125:327–336
Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ (2008) P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 57:1230–1237
Ye JH, Rajendran VM (2009) Adenosine: an immune modulator of inflammatory bowel diseases. World J Gastroenterol 15:4491–4498
Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, Williams NS, Anand P (2001) ATP-gated ion channel P2X3 is increased in human inflammatory bowel disease. Neurogastroenterol Motil 13:365–369
Yu Q, Sun J, Guo W, Fu J, Xu X, Burnstock G, He C, Xiang Z (2010) Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol 133:177–188
Zagorodnyuk V, Maggi CA (1998) Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br J Pharmacol 123:122–128
Zhou X, Galligan JJ (1996) P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J Physiol 496:719–729
Zhou X, Galligan JJ (1998) Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol 513:685–697
Zimmermann H (1994) Signalling via ATP in the nervous system. Trends Neurosci 17:420–426
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Burnstock, G. (2016). Purinergic Signalling in the Gut. In: Brierley, S., Costa, M. (eds) The Enteric Nervous System. Advances in Experimental Medicine and Biology(), vol 891. Springer, Cham. https://doi.org/10.1007/978-3-319-27592-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-27592-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27590-1
Online ISBN: 978-3-319-27592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)