Abstract
The distribution of P2Y2 receptor-immunoreactive (ir) neurons and fibers and coexistence of P2Y2 with P2X2 and P2X3 receptors, neuropeptide Y (NPY), calretinin (CR), calbindin (CB) and nitric oxide synthase (NOS) was investigated with immunostaining methods. The results showed that P2Y2-ir neurons and fibers were distributed widely in myenteric and submucous plexuses of the guinea pig stomach corpus, jejunum, ileum and colon. The typical morphology of P2Y2-ir neurons was a long process with strong positive staining on the same side of the cell body. The P2Y2-ir neurons could be Dogiel type 1. About 40–60% P2X3-ir neurons were immunoreactive for P2Y2 in the myenteric plexus and all the P2X3-ir neurons expressed the P2Y2 receptor in the submucosal plexus; almost all the NPY-ir neurons and the majority of CR-ir neurons were also immunoreactive for P2Y2, especially in the myenteric plexus of the small intestine; no P2Y2-ir neurons were immunoreactive for P2X2 receptors, CB and NOS. It is shown for the first time that S type/Dogiel type 1 neurons with fast P2X and slow P2Y receptor-mediated depolarizations could be those neurons expressing both P2Y2-ir and P2X3-ir and that they are widely distributed in myenteric and submucosal plexuses of guinea pig gut.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The P2-receptor family includes P2X receptors equivalent to intrinsic calcium-permeable cation channels and metabotropic P2Y receptors that are G-protein-coupled receptors (Ralevic and Burnstock 1998; Nicholas 2001). Currently, there are seven cloned P2X (P2X1–P2X7) receptor subunits (Khakh et al. 2001) and at least eight P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–P2Y14) (Burnstock 2004). ATP and UTP may be involved in the complex regulation in the enteric nervous system (ENS) via activation of P2 receptors at pre-synaptic or postsynaptic or postjunctional sites in the gut (Burnstock 2001). In the ENS, ATP activates P2Y and P2X receptors to cause slow and fast membrane depolarizations, respectively, in S/type 1 neurons of the submucosal plexus of the guinea pig ileum (Barajas-Lopez et al. 1994; LePard and Galligan 1999; Galligan et al. 2000; Ren et al. 2003; Hu et al. 2003; Monro et al. 2004). Fast and slow calcium transients underlie the fast and slow membrane depolarizations in these neurons (Barajas-Lopez et al. 2000). These data suggest that P2X and P2Y receptors are co-expressed in these neurons.
Immunocytochemical, in situ hybridization and RT-PCR results showed that P2X2, P2X3 and P2X7 receptors subunits were identified in the whole enteric nervous systems of guinea pig, mouse and rat (Hu et al. 2001; Castelucci et al. 2002, 2003; Poole et al. 2002; Van Nassauw et al. 2002; Xiang and Burnstock 2004a, b). The proteins of P2Y1, P2Y2 and P2Y4 receptors in submucosal and myenteric plexuses of rat distal colon and the gene products of P2Y1, P2Y2, P2Y4 and P2Y12 receptors in the submucous plexus were found (Christofi et al. 2004; Cooke et al. 2004). At present there are no morphological data showing P2X and P2Y receptors coexisting in these neurons and no study shows the systematic distribution of P2Y receptors along the whole length of the intestine. In this study, we used single-labeling and double-labeling immunofluorescence methods to study the distribution of P2Y2 receptors and its coexistence with P2X2 and P2X3 receptors, NPY, calbindin, calretinin and NOS in the stomach corpus, jejunum, ileum and colon of the guinea pig.
Materials and methods
Animals and tissue preparation
Breeding, maintenance and killing of the animals used in this study followed principles of good laboratory animal care and experimentation in compliance with Home Office (UK) regulations covering Schedule One Procedures in accordance with the Animals (Scientific Procedures) Act, 1986, governing the use of animals. Protocols were approved by the local animal ethics committee. Five guinea pigs were used. Animals were killed by asphyxiation with CO2 and perfused through the aorta with 0.9% NaCl solution and 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4. Stomach, jejunum, ileum, proximal and distal colon were dissected out. Immediately after the segments of the digestive tract were removed, the contents of the lumen were removed with saline. One end of the segment was knotted with a silk thread and fixative was injected into the lumen to fill it and the open end was also knotted with a silk thread. The fixative-filled segment was then immersed in fixative. This was applied to all segments of the intestine examined, including the stomach, from which the contents were removed via the duodenum which was then closed by a knotted silk thread and filled with fixative from the oesophagus. The oesophagus was then closed such that the stomach resembled an irregular balloon. Once fixed, whole-mount preparations could be prepared from the flattened area of the stomach. Whole-mount preparations were prepared of the myenteric plexus of stomach corpus, jejunum, ileum and distal colon and whole-mount preparations of submucous plexus of jejunum, ileum and distal colon were prepared under a dissection microscope.
Immunocytochemistry
The immunocytochemical method was modified from our previous report (Xiang et al. 1998). The preparations were washed 3×5 min in 0.01 mol/L pH 7.2 phosphate-buffered saline (PBS), then incubated in 1.0% H2O2 for 30 min to block endogenous peroxidase. Preparations were pre-incubated in 10% normal horse serum (NHS), 0.2% Triton X-100 in PBS for 30 min, followed by incubation with P2Y2 antibodies (Alomone Labs, Jerusalem, Israel), diluted 1:300 in antibody dilution solution (10% NHS, 0.2% Triton X-100 and 0.4% sodium azide in PBS) overnight at 4°C. Subsequently, the preparations were incubated with biotinylated donkey-anti-rabbit IgG (Jackson ImmunoResearch Laboratories, PA, USA) diluted 1:500 in antibody dilution solution for 1 h at 37°C and then with streptavidin-HRP (Sigma Chemical Co., Poole, UK) diluted 1:1000 in PBS for 1 h at 37°C. Finally, a nickel-intensified diaminobenzidine (DAB) reaction was used to visualize immunoreactivity. All incubations and reactions were separated by 3×10 min washes in PBS. The preparations were mounted, dehydrated, cleared and covered.
The development and specificity of the P2X2 and P2X3 polyclonal antibodies (Roche Palo Alto, CA, USA) has been reported previously (Oglebsby et al. 1999). Simultaneous detection of two antigens by immunostaining usually requires primary antibodies from two different species. A novel double-labeling immunostaining method for detection of two independent antigens using two antibodies from the same species of animals has been described (Teramoto et al. 1998). The principle of the method was that the first antigen is detected by the first primary antibody that is diluted so extensively that it cannot be detected with conventional methods; a highly sensitive tyramide signals amplification (TSA) system is used to identify this antibody; the second antigen is stained with the secondary primary antibody and detected by conventional immunostaining. The following protocol was modified from this protocol. Endogenous peroxidase was blocked by 1% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% NHS, 0.2% Triton X-100 in PBS for 30 min, followed by incubation with P2Y2, P2X2 and P2X3 receptor antibodies, diluted 1:2,000 in antibody dilution solution (10% NHS, 0.2% Triton X-100 and 0.4% sodium azide in PBS) overnight at 4°C. Subsequently, the sections were incubated with biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) at a dilution of 1:500 in PBS containing 1% NHS for 1 h. The sections were then incubated in ExtrAvidin peroxidase (Sigma) diluted 1:1,000 in PBS for 30 min at room temperature. The immunoreactivity was visualized by the TSA Fluorescein system (NEL701, NEN, USA). After visualization the sections were incubated with a second different primary antibodies to P2X2, P2X3 and P2Y2 receptors diluted 1:300 in antiserum dilution solution overnight at 4°C. Subsequently, the sections were incubated with Cy3 conjugated donkey-anti-rabbit (Jackson ImmunoResearch Laboratories) diluted 1:300 in antiserum dilution solution for 1 h at room temperature. All the incubations and reactions were separated by 3×10 min washes in PBS.
The following protocol was used for double-immunostaining of P2Y2 receptors with either calbindin, calretinin, NOS, NPY or PGP9.5 (Ultraclone Ltd., Wellow, Isle of Wight, UK; used as a general neuronal maker). The preparations were washed 3×5 min in PBS, then pre-incubated in antibody dilution solution for 30 min, followed by incubation with P2Y2 antibody diluted 1:300 and NOS antibody (sheep-anti-rat) diluted 1:1,000 or calbindin (mouse-anti-rat; SWANT, Bellinzone, Switzerland) diluted 1:5,000 or calretinin (mouse-anti-rat; SWANT) diluted 1:2,000 or PGP9.5 antibody (mouse anti-rat) diluted 1:6,000 in antibody dilution solution overnight at 4°C. Subsequently, the preparations were incubated with Cy3 conjugated donkey-anti-rabbit IgG (Jackson ImmunoResearch Laboratories) diluted 1:300 diluted for P2Y2 antibodies and FITC conjugated donkey-anti-mouse or sheep IgG (Jackson ImmunoResearch Laboratories) diluted 1:200 in antibody dilution solution for calbindin, calretinin and NOS for 1 h at room temperature. All the incubations and reaction were separated by 3×10 min washes in PBS. The preparations were evaluated with fluorescence microscopy.
Controls
Control experiments were carried out with P2X2, P2X3 and P2Y2 receptor antibodies pre-absorbed with cognate peptide at a concentration of 25 μg/mL.
Photomicroscopy
Images of immunofluorescence labeling were taken with the Leica DC 200 digital camera (Leica, Switzerland) attached to a Zeiss Axioplan microscope (Zeiss, Germany). Filter sets included the following: for Cy3, excitation 510–550 nm, emission 590 nm; for FITC, 470 nm excitation, 525 nm emission. Images were imported into a graphics package (Adobe Photoshop 5.0, USA). The two-channel readings for green and red fluorescence were merged by using Adobe-Photoshop 5.0.
Quantitative analysis
Whole-mount preparations were used to perform a quantitative analysis, as described previously (Van Nassauw et al. 2002). Briefly, the immunoreactive-positive neurons in the myenteric ganglia were counted per visual field (0.3 mm2) in whole-mount preparations. Ten randomly chosen fields in each whole-mount preparation were studied, and the number of immunoreactive neurons was calculated as a percentage of the total number of neurons as visualized with PGP9.5. A recent paper has provided evidence that the pan-neuronal markers Cuprolinic Blue and anti-HuC/D may be more reliable neuronal markers, visualising a greater number of neurons than PGP9.5, for use in future studies (Phillips et al. 2004).
Results
Localization of P2Y2 receptor immunoreactivity
The P2Y2 receptor immunoreactivity was found in the myenteric plexus of stomach corpus, jejunum, ileum and colon of the guinea pig (see Table 1), since the proximal and distal colon were similar, only findings of the distal colon shall be presented. Most of the positive neurons were shown to have a long process with strong positive staining and most of the positive staining was usually seen on this side of the cell body, while on the other side the staining was much weaker. No staining was observed in the oval nucleus of the positive nerve cells. Two types of positive ganglion neurons, strongly staining and weakly staining nerve cells, were present in myenteric and submucousal plexuses. Most of the strongly stained nerve cells had a long process while the weakly stained nerve cell had no processes. (Fig. 1). In the stomach corpus myenteric plexus, approximately 38% of ganglion cells were positively stained with the P2Y2 antiserum and two types of positive ganglion neurons, strongly staining and weakly staining nerve cells, were present (Fig. 1a). In the small intestine myenteric plexus of whole-mount preparations, P2Y2-ir ganglion neurons were found in all ganglia and approximately 54 and 63% of ganglion cells were positively immunostained for the P2Y2 antibody in the jejunum and ileum myenteric plexuses, respectively (Fig. 1b, c). In the myenteric plexus of distal colon, approximately 65% of ganglion neurons were immunostained intensely by the P2Y2 antiserum (Fig. 1d, e). The P2Y2-ir ganglion cells were also found in the submucosal plexus of those areas of the digestive tract examined. Two types of positive ganglion neurons, strongly staining and weakly staining nerve cells, were also present. Approximately 42, 46 and 45% of ganglion cells were P2Y2-ir in the submucosal plexus of jejunum, ileum and distal colon, respectively (Fig. 1f–h).
P2Y2-ir in nerve cell bodies and processes in myenteric and submucosal plexuses of stomach corpus, small intestine and distal colon of adult guinea pig. Most of the positive cells were shown to have a long process with strong positive staining and most of the positive staining was usually seen in the cytoplasm of the side with the positive process, while in the other side the staining was much weaker. a P2Y2-ir in nerve cell bodies and processes in myenteric plexus of stomach corpus. b P2Y2-ir in nerve cell bodies and processes in myenteric plexus of jejunum. c P2Y2-ir in nerve cell bodies and processes in myenteric plexus of ileum. d P2Y2-ir in nerve cell bodies and processes in myenteric plexus of distal colon at low magnification. e Higher magnification around the area indicated by the arrow. f P2Y2-ir in nerve cell bodies and processes in submucosal plexus of jejunum. g P2Y2-ir in nerve cell bodies and processes in submucosal plexus of ileum. h P2Y2-ir in nerve cell bodies and processes in submucosal plexus of distal colon. Scale bars in a and h=25 μm, in b, c, e, f, g=50 μm, scale bar in d=250 μm
Double-labeling studies
The P2Y2-ir was found to coexist with NPY, calretinin and P2X3 receptors in myenteric plexus of stomach corpus, jejunum, ileum, and distal colon and in submucous plexus of jejunum, ileum, and distal colon.
All the ganglion cells with NPY-ir in both myenteric and submucosal plexuses were also immunoreactive for P2Y2, although only 13, 9, 10 and 11% of the ganglion cells with P2Y2-ir were immunoreactive for NPY in the myenteric plexuses of stomach corpus, jejunum, ileum and distal colon, respectively, and 42, 45 and 48% of the ganglion cells with P2Y2-ir were immunoreactive for NPY in the submucousal plexuses of jejunum, ileum and distal colon, respectively (Fig. 2a–f). Table 2 shows the quantitative analysis of double labeling of P2Y2 receptors with NPY in the myenteric and submucosal plexuses.
Coexistence of P2Y2-ir and NPY-ir in myenteric and submucosal plexuses of guinea pig stomach corpus, small intestine and distal colon. a–c show coexistence (yellow) between P2Y2-ir (red) and NPY-ir (green) in myenteric plexus of stomach corpus, ileum and distal colon respectively. d–f show coexistence in submucous plexus of jejunum, ileum and distal colon, respectively. A blue arrow indicates a single neuron labelled with P2Y2 antibody (red), a white arrow indicates a single neuron labeled with NPY antibody (green) and a yellow arrow indicates a neuron double labeled with both P2Y2 and NPY antibodies (yellow). Scale bars in a–f=50 μm
About 22, 87, 84 and 36% of P2Y2-ir ganglion cells showed immunoreactivity for CR in the myenteric plexuses of the stomach corpus, jejunum, ileum and distal colon, and 45, 47 and 43% P2Y2-ir ganglion cells showed immunoreactivity for CR in the myenteric plexuses of the jejunum, ileum and distal colon, respectively, although there were also CR-ir ganglion cells with no P2Y2 immunoreactivity in both myenteric and submucosal plexuses (Fig. 3a–f). Table 3 shows the quantitative analysis of double labeling of P2Y2 receptors with calretinin in myenteric and submucosal plexuses.
Coexistence between P2Y2-ir and calretinin (CR)-ir in myenteric and submucous plexuses in the guinea pig stomach corpus, small intestine and distal colon. a–d show coexistence between P2Y2-ir and CR-ir in myenteric plexus of stomach corpus, jejunum, ileum and distal colon, respectively; a blue arrow indicates a single neuron labeled with P2Y2 antibody (red), a white arrow indicates a single neuron labeled with CR antibody (green) and a yellow arrow indicates a neuron double labeled with both P2Y2 and CR antibodies (yellow). e and f show coexistence between P2Y2-ir and CR-ir in submucous plexus in ileum and distal colon. Scale bars in a–f=50 μm
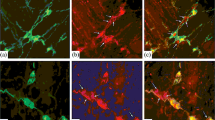
About 46, 52, 52 and 46% P2X3-ir ganglion cells were found to display P2Y2 receptor-ir in the myenteric plexuses of the stomach corpus, jejunum, ileum and distal colon, respectively. However, in the submucous plexus of the jejunum, ileum and distal colon all the P2X3-ir ganglion cells were found to display P2Y2 receptor-ir, but only 30, 37 and 33% of the ganglion cells with P2Y2-ir were immunoreactive for P2X3 receptors (Figs. 4a–f, 5a–f). Table 4 shows the quantitative analysis of double labeling of P2Y2 receptors with P2X3 receptors in the myenteric and submucous plexuses.
Coexistence between P2Y2-ir and P2X3-ir in myenteric plexus in the guinea pig stomach corpus, small intestine and distal colon. a P2Y2-ir neurons and fibers in myenteric plexus in stomach corpus (red). b P2X3-ir neurons and fibers in myenteric plexus in stomach corpus (green). c The merged figure from a and b showing coexistence of P2Y2 and P2X3 receptors (yellow). d, e and f show coexistence (yellow) of P2Y2-ir (red) and P2X3-ir (green) in myenteric plexus of jejunum, ileum and distal colon, respectively. Scale bars in a–f=50 μm
No P2Y2-ir ganglion cells were found to be immunoreactive for P2X2 receptors, calbindin or NOS (Figs. 5a–f, 6f).
Coexistence between P2Y2-ir, P2X2-ir and P2X3-ir in submucous plexus in the guinea pig small intestine and distal colon. a P2Y2-ir neurons in submucous plexus of ileum. b P2X3-ir neurons in submucous plexus of ileum. c The merged figure from a and b showing coexistence of P2Y2 and P2X3 receptors (yellow). d and e show coexistence (yellow) between P2Y2- (red) and P2X3-ir (green) in submucous plexus of proximal and distal colon, respectively. f Co-localization between P2Y2-ir (red) and P2X2-ir (green) in submucous plexus of distal colon, note that there was no double-labeling in neurons and fibers. Scale bars in a–f=50 μm
Co-localizations among P2Y2-ir, calbindin (CB) and NOS in myenteric and submucous plexuses in the ileum and distal colon. a P2Y2-ir neurons and fibers in myenteric plexus of ileum. b CB-ir neurons and fibers in myenteric plexus of guinea pig ileum. c The merged figure from a and b, note that there was no double-labeling. d P2Y2-ir neurons in myenteric plexus of distal colon. e NOS-ir neurons in myenteric plexus of distal colon. f The merged figure from d and e; note that there was no double-labeling. Scale bars in a–f=50 μm
Discussion
The P2X receptors have been shown to play an important role in synaptic transmission within the neural pathways mediating motor activity in the intestine (Katayama and Morita 1989; Kimball et al. 1996; Heinemann et al. 1999; LePard and Galligan 1999; Bian et al. 2000; Galligan et al. 2000; Spencer et al. 2000; Ren et al. 2003) and the non-cholinergic portion of fast excitatory postsynaptic potentials are mediated by P2X receptors, with similarities to P2X4 and P2X6 receptor subunits in the submucous and myenteric plexuses (Barajas-Lopez et al. 1994, 2000; Galligan and Bertrand 1994; LePard et al. 1997; Zhou and Galligan 1998; Burnstock 2001). In addition to fast responses, ATP activates a P2Y receptor subunit to cause a slow membrane depolarization in a subset of S/type 1 neurons of the submucous plexus of the guinea pig ileum (Barajas-Lopez et al. 1994, 2000; Hu et al. 2003). Not all S/type 1 neurons have both fast and slow membrane depolarization responses. Three subsets of cells could be distinguished: those with both fast and slow responses, those with only fast responses and those with only slow responses (Barajas-Lopez et al. 2000). These results suggested that a subset of S/type 1 neurons exist in the submucous plexus of the guinea pig that express both P2X and P2Y receptors. In this study, we used a double-labeling immunofluorescence technique to demonstrate a subset of neurons expressing both P2X3 and P2Y2 receptors in myenteric and submucousal plexuses of stomach corpus, jejunum, ileum and distal colon. This subset of neurons could be the candidate for this functional subset of S/type 1. Furthermore, the subunit of P2X receptor that coexists with P2Y2 receptors is of the P2X3, but not the P2X2 receptor subunit. In the present study, all the P2X3-ir neurons were found to also express P2Y2 receptors in submucous plexus neurons. So there must be at least one other P2X receptor subunit in the submucosal plexus since three functional subsets of neurons have been distinguished (Barajas-Lopez et al. 2000). In fact, other subunits of P2X receptors (P2X2 and P2X7) have been identified in submucous plexus by immunofluorescence (Hu et al. 2001; Castelucci et al. 2002, 2003; Xiang and Burnstock 2004b).
The shape of all the P2Y2-ir neurons demonstrated in this study in both myenteric and submucosal plexuses of all the regions of guinea pig gut that we examined was characteristic, with only one axon-like process present. This result suggests that most of P2Y2-ir neurons in guinea pig gut have Dogiel type 1 neuron morphology. Most of the Dogiel type 1 neurons are S neurons with electrophysiological properties of monophasic depolarizations, no slow after hyperpolarization, and fast excitatory postsynaptic potentials in response to fiber tract stimulation (Nurgali et al. 2004). Our results together with those of Nurgali et al. (2004) imply that some P2Y2-ir neurons in both myenteric and submucosal plexuses in all regions of guinea pig gut are likely to be S/Dogiel type 1 neurons.
The P2Y2-ir neurons in the myenteric and submucosal plexuses are not intrinsic primary afferent neurons as they do not express calbindin, which is believed to be a marker for intrinsic sensory neurons in the guinea pig intestine (Furness et al. 1990; Quinson et al. 2001). The morphology of intrinsic sensory neurons in the guinea pig ileum is distinct; their shape and projection patterns fit with those of Dogiel type-2 neurons (Furness et al. 1998).
The P2Y2-ir neurons were found not to express NOS-ir, although NOS-ir neurons have been found to label S/type 1 neurons in guinea pig myenteric plexus exhibiting uniaxonal and Dogiel type 1 morphology (McConalogue and Furness 1993). These data imply that a subpopulation of S/type 1 neurons in the myenteric and submucosal plexuses of guinea pig do not express P2Y2-ir.
In this study, we observed that there were obvious regional differences in the percentages of coexistence of P2Y2 receptors with CR, NPY and P2X3 receptors. For example, 84–87% P2Y2 receptor-ir neurons were also immunoreactive for CR in the myenteric plexus of the small intestine while only 36% were CR-ir in the distal colon. In the myenteric plexus only 9–11% P2Y2 receptor-ir neurons were found to express NPY-ir while in the submucousal plexus almost half the P2Y2 receptor-ir neurons were also immunoreactive for NPY. Since the function of the digestive tract varies with region, so too does the morphology and the neurochemical coding of their nerves (Furness 2000). Such regional differences have been observed in this study. The density of NPY-ir neuronal cell bodies and fibers in the submucosal plexus was high in the ascending colon and progressively declined in an anal direction, immunoreactive cell bodies being rare in the rectum. The potentially important regional differences in the functions of neuropeptide Y as an antisecretory peptide in the local regulation of chloride transport in the mucosa and as a modulator of ganglionic transmission has been proposed (Cunningham and Lees 1995).
In the myenteric and submucosal plexuses of the guinea pig gastrointestinal tract, especially, the small intestine, the majority of the P2Y2-ir neurons were also immunoreactive for calretinin in this study. Calretinin is believed to be a marker for cholinergic neurons in the guinea pig intestine, being Dogiel type 1 neurons, which project to the longitudinal muscle, the submucosal vasculature and mucosal glands (Brookes et al. 1991) and control the physiological functions of these structures. Thus, extracellular ATP may regulate the function of these cholinergic neurons and thereby indirectly regulate the physiological functions of smooth muscle, submucosal blood vessels and mucosal glands.
In the present study almost all the NPY-ir neurons were also immunoreactive for P2Y2 receptors. The NPY-ir neurons were localized in myenteric and submucosal plexuses, which project to the circular muscle, the longitudinal muscle and the mucosa; these NPY-ir neurons also show Dogiel type 1 neuron morphology, except those projecting to mucosa which have fine branching processes (Uemura et al. 1995). These data imply that the physiological function of NPY-ir neurons in myenteric and submucosal plexuses can be regulated by extracellular ATP.
In summary, P2Y-ir neurons and fibers were found to be distributed widely in myenteric and submucosal plexuses in the guinea pig stomach corpus, jejunum, ileum and colon. The typical morphology of P2Y-ir neuron is that they have one long process and the distribution of positive staining in the cell body is polarized. The P2Y-ir neurons are Dogiel type 1. Double-labeling studies showed that 40–60% of P2X3-ir neurons were immunoreactive for P2Y2 receptors in the myenteric plexus and all the P2Y2-ir neurons were immunoreactive for P2X3 receptors in the submucosal plexus; almost all the NPY-ir neurons and the majority of CR-ir neurons were also immunoreactive for P2Y2 receptors. However, no P2Y2-ir neurons were immunoreactive for P2X2 receptors, calbindin or NOS. It is shown for the first time that S/Dogiel type 1 neurons with fast P2X and slow P2Y receptor-mediated depolarizations are those neurons, which express both P2Y2-ir and P2X3-ir and that they are widely distributed in myenteric and submucosal plexuses of guinea pig gut. This appears to be in contrast to mouse intestine where P2X3 receptor knockout studies suggest that P2X3 subunits are localised on AH (sensory), but not S neurons (Bian et al. 2003).
References
Barajas-Lopez C, Espinosa-Luna R, Gerzanich V (1994) ATP closes a potassium and opens a cationic conductance through different receptors in neurons of guinea pig submucous plexus. J Pharmacol Exp Ther 268:1397–1402
Barajas-Lopez C, Espinosa-Luna R, Christofi FL (2000) Changes in intracellular Ca2+ by activation of P2 Rs in submucosal neurons in short-term cultures. Eur J Pharmacol 409:243–257
Bian X, Bertrand PP, Bornstein JC (2000) Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol 528:551–560
Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford APDW, Galligan JJ (2003) Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551:309–322
Brookes SJ, Steele PA, Costa M (1991) Calretinin immunoreactivity in cholinergic motor neurones, interneurones and vasomotor neurones in the guinea-pig small intestine. Cell Tissue Res 263:471–481
Burnstock G (2001) Purinergic signaling in gut. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology. Volume 151/II, Purinergic and pyrimidinergic signaling II, cardiovascular, respiratory, immune, metabolic and gastrointestinal tract function. Springer-Verlag, New York, pp 141–238
Burnstock G (2004) Introduction: P2 receptors. Curr Topics Med Chem 4:793–803
Castelucci P, Robbins HL, Poole DP, Furness JB (2002) The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem Cell Biol 117:415–422
Castelucci P, Robbins HL, Furness JB (2003) P2X2 purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res 312:167–174
Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N, Cooke H (2004) Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol 469:16–36
Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, Javed N, Christofi FL (2004) Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol 469:1–15
Cunningham SM, Lees GM (1995) Neuropeptide Y in submucosal ganglia: regional differences in the innervation of guinea-pig large intestine. J Auton Nerv Syst 55:135–145
Furness JB, Trussell DC, Pompolo S, Bornstein JC, Smith TK (1990) Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell Tissue Res 260:261–272
Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC (1998) Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54:1–18
Furness JB (2000) Types of neurons in the enteric nervous system. J Auton Nerv Syst 81:87–96
Galligan JJ, Bertrand PP (1994) ATP mediates fast synaptic potentials in enteric neurons. J Neurosci 14:7563–7571
Galligan JJ, LePard KJ, Schneider DA, Zhou X (2000) Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 81:97–103
Heinemann A, Shahbazian A, Bartho L, Holzer P (1999) Different receptors mediating the inhibitory action of exogenous ATP and endogenously released purines on guinea-pig intestinal peristalsis. Br J Pharmacol 128:313–320
Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD (2001) P2X7 receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol 440:299–310
Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao YX, Xia Y, Wood JD (2003) Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550:493–504
Katayama Y, Morita K (1989) Adenosine 5′-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol 408:373–390
Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA (2001) International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev 53:107–118
Kimball BC, Yule DI, Mulholland MW (1996) Extracellular ATP mediates Ca2+ signaling in cultured myenteric neurons via a PLC-dependent mechanism. Am J Physiol 270:G587–G593
LePard KJ, Galligan JJ (1999) Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am J Physiol 276:G529–G538
LePard KJ, Messori E, Galligan JJ (1997) Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology 113:1522–1534
McConalogue K, Furness JB (1993) Projections of nitric oxide synthesizing neurons in the guinea-pig colon. Cell Tissue Res 271:545–553
Monro RL, Bertrand PP, Bornstein JC (2004) ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556:571–584
Nicholas RA (2001) Identification of the P2Y12 receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides. Mol Pharmacol 60:416–420
Nurgali K, Stebbing MJ, Furness JB (2004) Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol 468:112–124
Oglebsby IB, Lachnit WG, Burnstock G, Ford APDW (1999) Subunit specificity of polyclonal antisera to the carboxy terminal regions of P2X receptors P2X1 through P2X7. Drug Dev Res 47:189–195
Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL (2004) Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods 133: 99–107
Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB (2002) The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci 101:39–47
Quinson N, Robbins HL, Clark MJ, Furness JB (2001) Calbindin immunoreactivity of enteric neurons in the guinea-pig ileum. Cell Tissue Res 305:3–9
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford APDW, Galligan JJ (2003) P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mice small intestine. J Physiol 552:809–812
Spencer NJ, Walsh M, Smith TK (2000) Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol 522:321–331
Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, Klein G (1998) Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Methods 73:89–97
Uemura S, Pompolo S, Furness JB (1995) Colocalization of neuropeptide Y with other neurochemical markers in the guinea-pig small intestine. Arch Histol Cytol 58:523–536
Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP (2002) Neurochemical identification of enteric neurons expressing P2X3 receptors in the guinea-pig ileum. Histochem Cell Biol 118:193–203
Xiang Z, Burnstock G (2004a) Development of nerves expressing P2X3 receptors in the myenteric plexus of rat stomach. Histochem Cell Biol 122:111–119
Xiang Z, Burnstock G (2004b) P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol 121:169–179
Xiang Z, Bo X, Burnstock G (1998) Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Letts 256:105–108
Zhou X, Galligan JJ (1998) Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol 513:685–697
Acknowledgments
The authors thank Dr. Gillian E. Knight and Dr. Chrystalla Orphanides for editorial assistance. This study was supported by Welcome Trust of UK (064931/Z/01/A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, Z., Burnstock, G. Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol 124, 379–390 (2005). https://doi.org/10.1007/s00418-005-0043-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-005-0043-7