Abstract
Preterm birth is a significant yet poorly understood public health problem that may arise in part from maternal exposure to chemicals in the environment. This review explores the state of the knowledge on prematurity in relation to: (1) Organic pollutants, including persistent organic pollutants, such as dichlorodiphenyltrichloroethane, polychlorinated biphenyls, and perfluorinated compounds, disinfection byproducts, such as trihalomethanes, non-persistent pesticides, such as atrazine, and non-persistent organics of emerging concern, such as phthalates and bisphenol-A; (2) Metals and metalloids, including lead, cadmium, arsenic, and mercury; and (3) Air pollutants, including EPA criteria air contaminants, environmental tobacco smoke, and polycyclic aromatic hydrocarbons. We also highlight pervasive study limitations as well as important directions for future research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Preterm birth, defined commonly as birth before 37 weeks completed gestation, is a complex and poorly understood disease that is highly prevalent in the US and elsewhere. Preterm newborns are at a much higher risk of mortality and various morbidities, and longitudinal studies have linked being born preterm to a range of morbidities in childhood and later in life, such as asthma and metabolic disorders as well as neurodevelopment delays. The combined cost of caring for preterm infants plus addressing subsequent complications was estimated at 26.2 billion dollars in the US in 2005 (Behrman and Butler 2007). Preventing preterm birth is a priority of the March of Dimes Foundation, the Surgeon General, and the Institute of Medicine. Despite concerted efforts, factors that are known to cause preterm birth, and their underlying mechanistic pathways, are few, and current strategies are likely to decrease preterm birth rates minimally by 2015 (Chang et al. 2013).
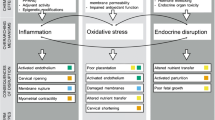
Of emerging concern is the contribution of environmental exposures to preterm birth. Women are exposed to a chemical milieu throughout life, and the gestational time period is no exception. Furthermore, many chemicals are capable of infiltrating the placenta and accumulating in the fetus. These exposures may precipitate preterm birth through several hypothesized pathways, such as inducing inflammation, prostaglandin release, hormonal changes, or oxidative stress in the maternal-fetal compartment, or through mechanisms that remain unexplored.
Examining environmental contributors to prematurity in an animal model is complicated by the fact that rodents do not naturally deliver preterm, but only do so with specific gene knockouts or with high doses of lipopolysaccharides injection (Cha et al. 2013; Kaga et al. 1996). Thus, evidence linking environmental contaminants to preterm birth largely comes from epidemiologic investigations. These studies have been rigorously examined in a number of substantive reviews (Ferguson et al. 2013; Wigle et al. 2008) and meta-analyses (Nieuwenhuijsen et al. 2013). In this chapter we highlight some of the more robust and influential of these studies as well as more recently published results (summarized by pollutant category in Tables 9.1, 9.2 and 9.3).
2 Organic Pollutants
Of the organic pollutants, persistent compounds have historically received the most attention in the study of preterm birth. These include chemicals such as dichlorodiphenyltrichloroethane (DDT) and other persistent pesticides, polychlorinated biphenyls (PCBs) and perfluorinated compounds previously used in various industrial applications, and brominated flame retardants such as polybrominated diphenyl ethers (PBDEs) which are persistent in the environment and human tissue. Parent compounds and in some instances metabolites can be measured reliably in serum or plasma as well as the placenta, which has enabled study designs with accurate subject-specific exposure metrics. While use of some of these compounds continues, most have been restricted in the US and other highly developed countries; thus, attention to lower levels of exposure may be particularly important. However, overall, the body of evidence suggests that higher doses of these compounds are more clearly linked to prematurity.
DDT and its metabolites dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) were examined in a number of small (N < 60) case-control studies published in the 1980s and 1990s in relation to preterm birth. Evidence was largely conflicting, but associations were clearly stronger in studies with higher exposure levels (Berkowitz et al. 1996; Procianoy and Schvartsman 1981; Saxena et al. 1981; Wassermann et al. 1982). The most rigorous study to address this relationship measured concentrations of DDT, DDE, and DDD in maternal serum collected in the 3rd trimester of pregnancy in the Collaborative Perinatal Project in the US (N = 2380) (Longnecker et al. 2001). These women were recruited from 1959 to 1965 and consequently had higher levels of exposure compared to those observed today. A strong increase in odds of delivering preterm was observed for mothers with high (>60 μg/L) levels of serum DDE. However, a smaller study (N = 455) with slightly higher median exposure levels failed to detect any effects (Farhang et al. 2005). More recent studies with lower exposure levels in various countries have shown conflicting results, but in general more associations have been detected in populations with higher exposure levels (Bergonzi et al. 2011; Pathak et al. 2009; Ribas-Fitó et al. 2002; Torres-Arreola et al. 2003; Wojtyniak et al. 2010; Wood et al. 2007).
Other persistent pesticides examined in relation to preterm birth include hexachlorobenzene (HCB) , hexachlorocyclohexane (HCH) or lindane, heptachlor or heptachlor epoxide and aldrin or dieldrin. These studies showed some suggestive associations but were largely inconclusive (Bergonzi et al. 2011; Fenster et al. 2006; Pathak et al. 2009; Ribas-Fitó et al. 2002; Saxena et al. 1981; Torres-Arreola et al. 2003; Wassermann et al. 1982). A large study in Spain (N = 1568) conducted recently also failed to detect any significant association between 1st trimester maternal HCB levels and preterm birth (Basterrechea et al. 2014). Interestingly, however, a small gene-environment interaction study (N = 156 cases, 151 controls) examining polymorphisms in the genes responsible for organochlorine pesticide metabolism and detoxification found significant interactions between a number of these compounds, particularly HCH, and risk of delivering preterm (Mustafa et al. 2013), suggesting some women may be more susceptible to these effects than others. Additionally, high levels of exposure may be more likely to have an effect. A study of mothers from the French West Indies, where chlordecone use is common, plasma levels measured at delivery were associated with increased odds of prematurity (Kadhel et al. 2014).
In the Collaborative Perinatal Project described above, Longnecker and colleagues also examined the association between exposure the industrial lubricants and insulators PCBs and preterm birth (Longnecker et al. 2005). Although this was one of the largest (N = 1034) and best designed studies to examine this relationship no associations were detected, and any suggestive associations were diminished after models were adjusted for DDT exposure levels. As with DDT, a number of smaller case control studies have shown some evidence for associations between prenatal PCB exposures and preterm birth; however, most of the studies to date have shown no associations (Bergonzi et al. 2011; Berkowitz et al. 1996; Govarts et al. 2012; Ribas-Fitó et al. 2002; Wassermann et al. 1982).
Perfluorinated compounds (PFCs) , including perfluorooctanoic acid (PFOA) or perfluorooctane sulfonic acid (PFOS) were used until recently for industrial and some consumer product applications as repellants of oil, grease, and water. Human exposure occurs primarily through consumption of contaminated food and drinking water or through inhalation of dusts in the US and other populations. Several studies have examined PFOA and/or PFOS concentrations in cord or maternal serum in association with preterm birth in populations with exposure levels consistent with those observed currently in the US. Results from these studies have been conflicting, with most reporting null or even protective associations (Apelberg et al. 2007; Fei et al. 2007; Hamm et al. 2010; Whitworth et al. 2012), yet some evidence for increased odds of preterm birth or reduced gestational age at delivery in relation to PFOS concentrations specifically (Arbuckle et al. 2012; Chen et al. 2012).
Incidences of drinking water contamination have resulted in higher than average PFC exposures to some populations. In the US, industry contamination of water sources in the Mid-Ohio valley resulted in PFOA human exposure levels 5 times higher than in subjects from the National Health and Nutrition Examination Survey (NHANES) , a nationally representative sample (Frisbee et al. 2009). The C8 Health Project was designed to investigate exposure levels in this population and to conduct epidemiologic studies of potential health consequences. Despite large sample sizes (N > 1000) and a variety of sophisticated modeling techniques, no associations were detected between PFOA or PFOS exposures and preterm birth in that study population (Nolan et al. 2009; Savitz et al. 2012a, b; Stein et al. 2009). In a more recent study in the same population utilizing biomarkers of exposure measured somewhat proximally to pregnancy, no associations between PFOA or PFOS and preterm birth were detected either (Darrow et al. 2013). However, in a Chinese population with elevated exposures due to environmental contamination by electronic waste, PFOA in maternal serum was associated with preterm birth (Wu et al. 2012).
Finally, in the realm of persistent organic pollutants, few studies exist measuring associations between preterm birth and either dioxin or PBDEs. Suggestive but generally null relationships have been observed with dioxin (Le and Johansson 2001; Lin et al. 2006; Revich et al. 2001), even in populations with high exposures resulting from a chemical explosion in Seveso, Italy (Eskenazi et al. 2003; Wesselink et al. 2014). One study observed higher cord blood levels of PBDE in newborns with an adverse birth outcome but that definition was not specific to preterm birth (Wu et al. 2010).
Disinfection byproducts (DBPs) , formed from chlorine used to treat drinking water, have been examined in a number of studies in relation to preterm birth. These include trihalomethanes (THMs) , such as chloroform, as well as haloacetic acids (HAAs) such as trichloroacetic acid (TCAA) . A recent review and meta-analysis of 9 studies by Grellier and colleagues concluded that insufficient evidence exists for a relationship between THMs or HAAs on preterm birth (Grellier et al. 2010; Dodds et al. 1999; Gallagher et al. 1998; Hoffman et al. 2008; Kramer et al. 1992; Lewis et al. 2007; Savitz et al. 1995; Wright et al. 2003, 2004; Yang et al. 2007). Since the publication of these findings several additional studies have been published, primarily with null findings. In areas of drinking water contamination, two large (N > 1000) studies assigning exposure based on THM levels measured in drinking water paired with questionnaire data on drinking water use found no association with preterm birth (Patelarou et al. 2011; Villanueva et al. 2011). Another study with similar exposure assessment methods found an association between total organic halide exposure and preterm birth in an area of brominated disinfection byproduct contamination (Horton et al. 2011). One study utilizing urinary biomarkers of exposure to TCAA also failed to detect any associations with PTB, although detection in samples was quite low (Costet et al. 2012). However, the most recent study to examine the relationship between DBPs and preterm birth observed significantly increased crude odds ratios across all exposure categories for most DBPs measured in public drinking water systems in the Boston area (N = 712,394) (Rivera-Núñez and Wright 2013). Most associations were attenuated with adjustment for pertinent covariates; relationships remained statistically significant or suggestive for chloroform, TCAA, and summed DBPs.
Trichloroethylene (TCE) and tetrachloroethylene (PCE) are also common, though unintentional, organic drinking water contaminants. Three studies examining associations between these compounds and preterm birth have utilized drinking water concentrations in areas of contamination and paired these data with information on maternal residence to assess exposures. None found significant associations despite large sample sizes (Aschengrau et al. 2008; Bove et al. 2002; Sonnenfeld et al. 2001). Additionally, one study examined air concentrations in an area with contaminated soil and found no association with preterm birth (Forand et al. 2011). Benzene, like TCE and PCE, is a volatile organic compound, but exposure occurs more typically through inhalation. Two studies examining the relationship between ambient air monitoring levels of benzene observed significantly increased odds of preterm birth in association with exposure levels (Llop et al. 2010; Wilhelm et al. 2011). Finally, one study examined the relationship between maternal formaldehyde exposure during pregnancy and prematurity and did not observe any statistically significant associations (Maroziene and Grazuleviciene 2002).
Non-persistent pesticides are also a drinking water contaminant of concern. Although not bioaccumulative or persistent in the environment, these compounds have longer half-lives in drinking water which can results in population-wide exposures, particularly in agricultural areas. Atrazine is one such pesticide that has been examined in a number of studies in association with preterm birth. Most studies have utilized drinking water measurements paired with information on maternal residence and in some instances drinking water use. However, detection in drinking water sources is typically low, and while one study observed a slight increase in odds of preterm birth (Villanueva et al. 2005) other findings have been null (Ochoa-Acuña et al. 2009; Rinsky et al. 2012). Two other studies have examined the non-persistent organophosphate pesticides in association with preterm birth in agricultural populations. One utilized biomarkers of parent compounds or their metabolites in urine samples collected during pregnancy and found no evidence of an association; although the authors did observe that decreased maternal cholinesterase activity, which is related to an overall increase in exposure to organophosphate pesticides, was associated with increased odds of preterm birth (Eskenazi et al. 2004). However, within the Agricultural Health Study, self-reported pesticide use was not associated with preterm birth (Sathyanarayana et al. 2010).
Finally, the non-persistent endocrine disrupting compounds phthalates and bisphenol-a (BPA) have received recent attention for their potential contribution to preterm birth. As with the persistent organic pollutants, most studies investigating these compounds have utilized personal exposure measurements; however, as these are metabolized rapidly in the human body, levels are measured in urine samples and are less stable over time. The first study to examine phthalate exposure in relation to length of gestation measured di-2-ethylhexyl phthalate (DEHP ) and its primary metabolite mono-2-ethylhexyl phthalate in cord blood of preterm and term newborns and found that concentrations were associated with earlier delivery (Latini et al. 2003). Another study measuring a panel of 9 phthalates in cord blood also observed an association with increased odds of preterm birth (Huang et al. 2014). Most other studies have utilized a single spot urine sample, in most instances collected from mothers in the third trimester of pregnancy. While some of these studies reported increased odds of preterm birth or decreased gestational age at delivery in association with several phthalate metabolites, particularly those of DEHP (Meeker et al. 2009; Whyatt et al. 2009; Latini et al. 2003), others reported null (Suzuki et al. 2010) or even protective effects (Adibi et al. 2009; Wolff et al. 2008). A more recent study examined associations with average levels of up to three phthalate metabolite measurements per subject over the course of pregnancy and observed significantly elevated odds of preterm birth , and even stronger associations when spontaneous preterm births, defined as deliveries following spontaneous preterm labor and/or preterm premature rupture of the membranes, were examined alone (Ferguson et al. 2014b). When associations were examined with phthalates measured at each individual time point during pregnancy, relationships for spontaneous preterm birth were stronger when concentrations were measured later in pregnancy (Ferguson et al. 2014a). Only one study has examined the relationship between BPA exposure and preterm birth. Concentrations in 3rd trimester urine samples were associated with a suggestive increase in odds of preterm birth, and the relationship was stronger when the case definition was restricted to deliveries at 36 weeks or less (Cantonwine et al. 2010b). This relationship remained after adjustment for urinary phthalate metabolite concentrations.
3 Metals and Metalloids
Some of the most convincing evidence for a relationship between an environmental exposure and preterm birth comes from the literature on lead. Early findings, primarily from smaller case-control studies, are strongly indicative of a relationship between prenatal lead exposure and preterm birth, and suggest a dose-dependent effect (Andrews et al. 1994). Most of these studies also examined effects of high levels of exposure. More recent research on highly exposed populations has demonstrated associations with preterm birth as well (Torres-Sánchez et al. 1999; Jelliffe-Pawlowski et al. 2006). Following the removal of lead from gasoline the general population in the US has experienced much lower levels of exposure (Pirkle et al. 1994), thus heightening the interest in studying levels at lower concentrations (e.g., blood levels <10 μg/dL). Studies examining lower levels of lead during pregnancy have been less consistent in establishing a relationship with preterm birth. No associations were observed in studies in populations with median maternal blood lead levels of approximately 1–2 μg/dL (Sowers et al. 2002; Zhu et al. 2010), while three other studies measuring levels in 1st trimester maternal blood (Vigeh et al. 2011), mid pregnancy red blood cells (Taylor et al. 2014), and placenta at delivery (Falcon et al. 2003), also with low exposure levels, did detect statistically significant associations with preterm birth.
Some recent studies of preterm birth in association with lower levels of lead exposure illustrate that to detect more subtle effects additional attention must be paid to timing of exposure and specific characteristics of the pregnancy. One study in Mexico City showed significant associations between lead levels measured in the 2nd trimester, but no associations with levels measured in the 1st or 3rd trimesters (Cantonwine et al. 2010a). Another study in eastern Massachusetts found that low maternal blood lead levels during pregnancy were strongly associated with preterm birth of male infants but not females (Perkins et al. 2014). Thus, a relationship may exist at lower levels of exposure but more care must be paid in statistical analysis in order to detect effects.
Other toxic metals have been studied much less intensively in relation to preterm birth. An early ecologic study suggested that mothers residing in an area with elevated cadmium contamination during pregnancy were not more likely to have a preterm birth compared to women in non-contaminated areas (Landgren 1996). A small case-control study measuring cadmium levels in maternal blood, cord blood, and placenta likewise observed no significant associations between cadmium levels and preterm birth (Zhang et al. 2004). Two studies, however, suggest that a relationship may exist. Fagher and colleagues observed elevated maternal blood cadmium concentrations in mothers who delivered preterm compared to term in a small case-control study (Fagher et al. 1993). Additionally, a study that assessed exposure using urine biomarkers demonstrated a significant association between maternal gestational cadmium exposure and preterm birth in an area of high contamination in Japan (Nishijo et al. 2002). Thus, for cadmium there may be a threshold effect or, as with lower levels of lead exposure, the relationship with preterm birth may be more subtle in magnitude requiring more careful exploration in epidemiologic studies with attention to fetal gender, etiology, and timing of exposure.
Arsenic exposure has been examined in association with prematurity in several studies, all of which have assigned exposure based on drinking water contamination levels in areas of relatively high exposure. Most of these studies showed no association between maternal drinking water levels and preterm birth (Mukherjee et al. 2005; Myers et al. 2010; Yang et al. 2003), although small study in Bangladesh observed a significant association (Ahmad et al. 2001).
Finally, some evidence exists for a relationship between mercury exposure during pregnancy and preterm birth. One study demonstrated an association with elevated hair mercury concentrations representative of exposure during gestation (Xue et al. 2007). A second ecologic study observed higher rates of preterm birth among African American mothers residing in areas with high levels of mercury contamination in fish in South Carolina (Burch et al. 2014). However, a third study utilizing maternal urinary mercury concentrations during pregnancy as well as umbilical cord blood mercury levels did not detect any significant associations with prematurity in a population from Brooklyn, New York (Bashore et al. 2014). Associations with mercury may be particularly difficult to assess, as one of the major exposure sources is through consumption of contaminated fish, which have other characteristics (e.g., high concentration of omega-3 fatty acids ) which may be beneficial to pregnancy. Additional studies with attention to adjustment for this confounding may be necessary to clarify this relationship.
4 Air Pollutants
A large number of studies have examined the relationship between air pollutant exposures and preterm birth, and likewise there have been many systematic reviews summarizing this information (Glinianaia et al. 2004; Shah and Balkhair 2011; Sram et al. 2005; Stillerman et al. 2008; Stieb et al. 2012; Bonzini et al. 2010; Nieuwenhuijsen et al. 2013; Lai et al. 2013). Most of the literature in this area has focused on EPA criteria air pollutants, identified by the Clean Air Act as common US exposures with need for regulation. These include ozone, particulate matter (including PM2.5 and PM10), carbon monoxide, nitrogen oxides, and sulfur dioxide. (Lead is also an EPA criteria air pollutant, as exposure prior to elimination from gasoline was common via inhalation routes.) The primary results from these reviews and meta-analyses conclude that there is strong evidence for a relationship between sulfur dioxide and PM2.5 and preterm birth, but other exposures have only weak to moderate evidence for an association. The most recent of these published by Stieb et al. in 2012 calculated pooled estimates of preterm birth risk in association with each of these exposures in 62 studies meeting their inclusion criteria. The authors found that associations with exposures assessed in the third trimester of pregnancy were most precise, but were generally small in magnitude (odds ratios 0.80–1.15). Significantly increased odds of preterm birth were found in association with carbon monoxide and PM10 exposures. The limitations from these and other criteria air pollutant studies have been described in the aforementioned reviews, and also in more depth in a report from the International Workshop on Air Pollution and Human Reproduction (Slama et al. 2008). Generally, recommendations for research in this area include utilization of prospective study designs, consideration of a uniform set of confounders across studies (importantly, including season of exposure and delivery and maternal diet), promotion of subject-specific exposure assessment methods (e.g., biomarkers, personal air monitoring), and investigation of mechanism of air pollutant action using molecular epidemiology (Ferguson et al. 2013; Slama et al. 2008).
There are a number of non-criteria pollutants with primarily inhalation exposure routes that have been measured more frequently in epidemiologic studies on the individual level. These exposures include environmental tobacco smoke (ETS ) and polycyclic aromatic hydrocarbons (PAH) , and will be examined in more detail here. Studies on ETS likely stemmed from the strong evidence that active maternal smoking during pregnancy increases odds of delivering preterm (Ion and Bernal 2015). Results from research on ETS effects are less conclusive. A recent meta-analysis demonstrated that maternal ETS exposure was associated with only a slight increase in risk of preterm birth, and associations were not significant in adjusted models (Salmasi et al. 2010). Likewise, another meta-analysis concluded no significant association between ETS and gestational age at delivery (Leonardi-Bee et al. 2008). However, systematic reviews by other groups conclude that there is an association (Stillerman et al. 2008; Wigle et al. 2008).
PAH exposure can result inhalation exposure in ambient (industrial combustion, automobile emissions, etc.) as well as indoor (from heating or cooking emissions) air contamination. Additionally individuals can be exposed through consumption of certain foods, particularly grilled and smoked meats (ATSDR 1995). Several studies have examined the relationship between PAH exposure and preterm birth using personal exposure methods, including personal air monitoring or measurement of biomarkers. In New York City, one study observed that increased total PAH exposure measured using personal air monitors in the third trimester of pregnancy were associated with significantly increased odds of preterm birth in African American, but not Dominican mothers (Choi et al. 2008). Studies using biomarkers suggest an association as well. One small case-control study measured PAH concentrations in placental tissue and observed significantly higher levels of individual PAH compounds in cases compared to controls (Singh et al. 2008). In another study where PAH were measured in cord blood in a highly contaminated region of China, increased levels were also significantly associated with adverse birth outcomes, including preterm birth, low birth weight, congenital malformations, and stillbirth (Guo et al. 2012). Looking at length of gestation more specifically, there was an association between PAH exposure and decreased gestational age at delivery as well. Studies using ambient air monitoring for assessment purposes also suggest that maternal PAH exposure is associated with a significantly increased risk of having a preterm birth (Padula et al. 2014; Vassilev et al. 2001; Wilhelm et al. 2011).
5 Conclusions and Research Needs
Overall, these data suggest an important role of environmental chemical exposures in the etiology of preterm birth. Of the organic pollutants studied, the strongest evidence for a relationship with prematurity exists for DDT. Notably, however, most of the evidence for this association comes from studies where exposure levels were relatively high. This may be relevant to countries that continue to utilize DDT as an insecticide but less so in developed nations. Additionally, although research is nascent, there is strongly suggestive evidence for a relationship between phthalate exposure during pregnancy and preterm birth. While lead exposure, particularly at high concentrations, is strongly associated with preterm birth, other metals have received very little attention in this area of research. This may be an important area to expand upon in future studies, particularly as biomarkers of metals exposures are available and maternal exposures at relatively low levels have been linked to other adverse reproductive and developmental outcomes. Finally, reviews and meta-analyses of air pollution effects show somewhat conflicting evidence for relationships between criteria air pollutants and ETS on preterm birth. As previously mentioned, this may be due in large part to use of ambient air monitors to assess effects and poor ability to examine exposure levels on an individual basis. PAH exposure during pregnancy, which has been studied using ambient and personal air monitors and biomarkers, shows strong evidence for an effect, despite the fact that research in this area, as with phthalates, has been limited to the last decade.
Absence of statistically significant associations in many of these studies may be due to a true lack of relationship with preterm birth, or, alternatively, to issues with study design. Preterm birth is a complex disease that is defined typically, especially in studies of environmental contributors, by a cutoff point of 37 weeks gestation, rather than by diagnostic markers or by etiology. This broad diagnosis may be problematic in several ways. Attention to 37 weeks as a cutoff point, which is typical because of the association that this dichotomy has with adverse effects on the infant, may be inappropriate. Recent evidence shows that early term (e.g., 37–39 weeks gestation) delivery is also associated with increased risk of neonatal mortality and various morbidities (Boyle et al. 2012). Additionally, while cutoffs by time in gestation (e.g., <37 weeks, or early preterm birth, <32 weeks) are commonly examined in relation to exposures, these do not separate preterm cases by etiology. Many causes contribute to early delivery (e.g., spontaneous preterm labor, preterm premature rupture of the membranes, intrauterine growth restriction, preeclampsia, etc.) and, though there may be some overlap, these conditions arise through different mechanisms (McElrath et al. 2008; Savitz 2008). While research in the field of obstetrics generally characterizes preterm birth into categories based on etiology (e.g., considering only spontaneous preterm births in a given analysis), few studies in the realm of environmental health sciences examine this distinction and thus may be observing diluted effects.
Additional study design issues may contribute to the inconsistency of some effects observed in this literature. First, many studies examining the relationships with preterm birth were small or were designed with other objectives in mind and consequently have low power to detect effects. Studies with case-control designs, particularly with attention to the aforementioned subtypes of preterm birth, may be more appropriate for this area of research. Second, most studies fail to examine multiple exposure windows during pregnancy. Depending on the type of preterm birth, exposures early or late in pregnancy may be more relevant to this outcome. For example, if preterm birth originates from impaired placentation, exposures early in pregnancy may be more relevant. Conversely, if preterm birth originates from spontaneous preterm labor, exposures late in pregnancy may be the most significant predictors. Finally, some of the exposure assessment methods employed in this research may be inadequate to detect effects. For example, BPA is a rapidly metabolized and excreted compound and concentrations in urine show low reliability over time (Fisher et al. 2014). Spot urine samples may not fully characterize a mother’s exposure either at the time point or over the course of gestation. Thus, greater attention to exposure assessment methods must be paid in this area of research as well.
Future research in the field of environmental health should address these study design limitations, their characterization of preterm birth, but additionally the effects of combined exposures to multiple pollutants during pregnancy and mechanism of toxicant action. Mothers are exposed to a complex milieu of environmental toxicants during gestation (Woodruff et al. 2011), and it is highly plausible that some of these compounds may act through similar pathways or through mechanisms that exacerbate one another to cause prematurity. Thus, study of effects of combined exposures should be a research priority.
In addition to improving and expanding epidemiologic studies, translation of research findings to public health practice is essential, especially for those compounds with well-demonstrated associations with preterm birth. There are two routes to this end. First, with a better understanding of mechanism of toxicant, interventions may be developed to stop the pathway from exposure to prematurity. For example, if it is clear that a compound is leading to preterm birth by inducing maternal oxidative stress during pregnancy, supplementation with antioxidants could be a useful intervention. Unfortunately, understanding mechanisms by which exposures lead to preterm birth is difficult to assess. Animal models of preterm birth are limited, as rodents rarely deliver prematurely except with high doses of lipopolysaccharide injection or with gene knock-outs (Cha et al. 2013; Kaga et al. 1996). However, such models may be useful in some circumstances if the limitations are fully acknowledged (Elovitz and Mrinalini 2004) or if specific mechanisms known to be relevant to human prematurity, such as inflammation, are the outcomes of interest. Additionally, mechanisms of action can be examined in human studies using biomarkers of intermediate effect.
Nevertheless, attempting to stop pathological processes connecting exposure and preterm birth may not be practical, as decades of research devoted to understanding mechanisms of prematurity have been relatively ineffective in developing successful interventions (Chang et al. 2013). A second and potentially much more feasible strategy for ameliorating effects of environmental chemicals is preventing maternal exposures during pregnancy. For compounds that have clearly demonstrated links with preterm birth, development of clinician recommendations and studies examining effectiveness for reducing exposure will be an important next step.
In summary, there is much evidence to suggest a relationship between environmental contaminant exposures and preterm birth, although additional work is necessary to fully assess the effect of individual chemicals. Nevertheless, this is an important area of future research, as maternal exposure to many chemicals may be modifiable, as opposed to other factors simultaneous under investigation, such as genetic polymorphisms. Also, identifying exposures that may be prevented prior to or during pregnancy may be more effective than interventions that can be implemented at delivery, which have shown low potential for reduction of preterm prevalence thus far (Chang et al. 2013). Thus, further investigation of the role of environmental exposures in the etiology of prematurity is a promising line of research in the initiative to prevent this significant public health problem.
References
Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH (2009) Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol 169(8):1015–1024
Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK (2001) Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect 109(6):629–631
Andrews KW, Savitz DA, Hertz-Picciotto I (1994) Prenatal lead exposure in relation to gestational age and birth weight: a review of epidemiologic studies. Am J Ind Med 26(1):13–32
Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR (2007) Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676
Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, Wen SW, Arnold DL (2012) Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int J Hyg Environ Health 216(2):184–194
Aschengrau A, Weinberg J, Rogers S, Gallagher L, Winter M, Vieira V, Webster T, Ozonoff D (2008) Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of adverse birth outcomes. Environ Health Perspect 116(6):814–820
ATSDR (1995) Toxicological profile for polycyclic aromatic hydrocarbons. Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25. Accessed 26 Sept 2014
Bashore CJ, Geer LA, He X, Puett R, Parsons PJ, Palmer CD, Steuerwald AJ, Abulafia O, Dalloul M, Sapkota A (2014) Maternal mercury exposure, season of conception and adverse birth outcomes in an urban immigrant community in Brooklyn, New York, U.S.A. Int J Environ Res Public Health 11(8):8414–8442
Basterrechea M, Lertxundi A, Iñiguez C, Mendez M, Murcia M, Mozo I, Goñi F, Grimalt J, Fernández M, Guxens M (2014) Prenatal exposure to hexachlorobenzene (HCB) and reproductive effects in a multicentre birth cohort in Spain. Sci Total Environ 466:770–776
Behrman RE, Butler AS (eds) (2007) Preterm birth: causes, consequences, and prevention. The National Academies Press, Washington, DC
Bergonzi R, De Palma G, Specchia C, Dinolfo M, Tomasi C, Frusca T, Apostoli P (2011) Persistent organochlorine compounds in fetal and maternal tissues: evaluation of their potential influence on several indicators of fetal growth and health. Sci Total Environ 409(15):2888–2893
Berkowitz GS, Lapinski RH, Wolff MS (1996) The role of DDE and polychlorinated biphenyl levels in preterm birth. Arch Environ Contam Toxicol 30(1):139–141
Bonzini M, Carugno M, Grillo P, Mensi C, Bertazzi PA, Pesatori AC (2010) Impact of ambient air pollution on birth outcomes: systematic review of the current evidences. Med Lav 101(5):341–363
Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE (1995) Public drinking water contamination and birth outcomes. Am J Epidemiol 141:850–862
Bove F, Shim Y, Zeitz P (2002) Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect 110(Suppl 1):61–74
Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, Quigley MA (2012) Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ 344:e896
Burch JB, Wagner Robb S, Puett R, Cai B, Wilkerson R, Karmaus W, Vena J, Svendsen E (2014) Mercury in fish and adverse reproductive outcomes: results from South Carolina. Int J Health Geogr 13:30
Cantonwine D, Hu H, Sanchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, Mercado-Garcia A, Hernandez-Avila M, Wright RO, Tellez-Rojo MM (2010a) Critical windows of fetal lead exposure: adverse impacts on length of gestation and risk of premature delivery. J Occup Environ Med 52(11):1106–1111
Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, Fortenberry GZ, Calafat AM, Tellez-Rojo MM (2010b) Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health 9:62
Cha J, Bartos A, Egashira M, Haraguchi H, Saito-Fujita T, Leishman E, Bradshaw H, Dey SK, Hirota Y (2013) Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest 123(9):4063–4075
Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE, Born Too Soon Preterm Prevention Analysis Group (2013) Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 381(9862):223–234
Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC, Hsieh WS (2012) Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One 7(8):e42474
Choi H, Rauh V, Garfinkel R, Tu Y, Perera FP (2008) Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ Health Perspect 116(5):658–665
Costet N, Garlantezec R, Monfort C, Rouget F, Gagniere B, Chevrier C, Cordier S (2012) Environmental and urinary markers of prenatal exposure to drinking water disinfection by-products, fetal growth, and duration of gestation in the PELAGIE birth cohort (Brittany, France, 2002–2006). Am J Epidemiol 175(4):263–275
Darrow LA, Stein CR, Steenland K (2013) Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect 121(10):1207
Dodds L, King W, Woolcott C, Pole J (1999) Trihalomethanes in public water supplies and adverse birth outcomes. Epidemiology 10(3):233–237
Elovitz MA, Mrinalini C (2004) Animal models of preterm birth. Trends Endocrinol Metab TEM 15(10):479–487
Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG Jr (2003) Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect 111(7):947–953
Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Furlong CE, Holland NT (2004) Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect 112(10):1116–1124
Fagher U, Laudanski T, Schutz A, Sipowicz M, Akerlund M (1993) The relationship between cadmium and lead burdens and preterm labor. Int J Gynaecol Obstet 40(2):109–114
Falcon M, Vinas P, Luna A (2003) Placental lead and outcome of pregnancy. Toxicology 185(1–2):59–66
Farhang L, Weintraub JM, Petreas M, Eskenazi B, Bhatia R (2005) Association of DDT and DDE with birth weight and length of gestation in the Child Health and Development Studies, 1959–1967. Am J Epidemiol 162(8):717–725
Fei C, McLaughlin JK, Tarone RE, Olsen J (2007) Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682
Fenster L, Eskenazi B, Anderson M, Bradman A, Harley K, Hernandez H, Hubbard A, Barr DB (2006) Association of in utero organochlorine pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect 114(4):597–602
Ferguson KK, O’Neill MS, Meeker JD (2013) Environmental contaminant exposures and preterm birth: a comprehensive review. J Toxicol Environ Health B Crit Rev 16(2):69–113
Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD (2014a) Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int 70:118–124
Ferguson KK, McElrath TF, Meeker JD (2014b) Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67
Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, Koniecki D, Ramsay T, Provencher G, Berube R, Walker M (2014) Bisphenol a and phthalate metabolite urinary concentrations: daily and across pregnancy variability. J Expo Sci Environ Epidemiol 25(3):231–239
Forand SP, Lewis-Michl EL, Gomez MI (2011) Maternal exposure to tetrachloroethylene and trichloroethylene through soil vapor intrusion and adverse birth outcomes in New York State. Environ Health Perspect 120:616–621
Frisbee SJ, Brooks AP Jr, Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C (2009) The C8 health project: design, methods, and participants. Environ Health Perspect 117(12):1873–1882
Gallagher MD, Nuckols JR, Stallones L, Savitz DA (1998) Exposure to trihalomethanes and adverse pregnancy outcomes. Epidemiology 9(5):484–489
Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D (2004) Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 15(1):36–45
Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, Chevrier C, Eggesbo M, Guxens M, Kramer U, Legler J, Martinez D, Palkovicova L, Patelarou E, Ranft U, Rautio A, Petersen MS, Slama R, Stigum H, Toft G, Trnovec T, Vandentorren S, Weihe P, Kuperus NW, Wilhelm M, Wittsiepe J, Bonde JP, Obelix E (2012) Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect 120(2):162–170
Grellier J, Bennett J, Patelarou E, Smith RB, Toledano MB, Rushton L, Briggs DJ, Nieuwenhuijsen MJ (2010) Exposure to disinfection by-products, fetal growth, and prematurity: a systematic review and meta-analysis. Epidemiology 21(3):300–313
Guo Y, Huo X, Wu K, Liu J, Zhang Y, Xu X (2012) Carcinogenic polycyclic aromatic hydrocarbons in umbilical cord blood of human neonates from Guiyu, China. Sci Total Environ 427–428:35–40
Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I (2010) Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol 20(7):589–597
Hoffman CS, Mendola P, Savitz DA, Herring AH, Loomis D, Hartmann KE, Singer PC, Weinberg HS, Olshan AF (2008) Drinking water disinfection by-product exposure and duration of gestation. Epidemiology 19(5):738–746
Horton BJ, Luben TJ, Herring AH, Savitz DA, Singer PC, Weinberg HS, Hartmann KE (2011) The effect of water disinfection by-products on pregnancy outcomes in two southeastern US communities. J Occup Environ Med 53(10):1172–1178
Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, Wang L, Tan Y, Luo J, Qiu Z, Chen JA, Shu W (2014) Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One 9(2):e87430
Ion R, Bernal AL (2015) Smoking and Preterm birth. Reprod Sci 22(8):918–926
Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V (2006) Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J Perinatol 26(3):154–162
Kadhel P, Monfort C, Costet N, Rouget F, Thome JP, Multigner L, Cordier S (2014) Chlordecone exposure, length of gestation, and risk of preterm birth. Am J Epidemiol 179(5):536–544
Kaga N, Katsuki Y, Obata M, Shibutani Y (1996) Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol 174(2):754–759
Kramer MD, Lynch CF, Isacson P, Hanson JW (1992) The association of waterborne chloroform with intrauterine growth retardation. Epidemiology 3(5):407–413
Lai H-K, Tsang H, Wong C-M (2013) Meta-analysis of adverse health effects due to air pollution in Chinese populations. BMC Public Health 13(1):360
Landgren O (1996) Environmental pollution and delivery outcome in southern Sweden: a study with central registries. Acta Paediatr 85(11):1361–1364
Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P (2003) In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect 111(14):1783–1785
Le TN, Johansson A (2001) Impact of chemical warfare with agent orange on women’s reproductive lives in Vietnam: a pilot study. Reprod Health Matters 9(18):156–164
Leonardi-Bee J, Smyth A, Britton J, Coleman T (2008) Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 93(5):F351–F361
Lewis C, Suffet IH, Hoggatt K, Ritz B (2007) Estimated effects of disinfection by-products on preterm birth in a population served by a single water utility. Environ Health Perspect 115(2):290–295
Lin CM, Li CY, Mao IF (2006) Birth outcomes of infants born in areas with elevated ambient exposure to incinerator generated PCDD/Fs. Environ Int 32(5):624–629
Llop S, Ballester F, Estarlich M, Esplugues A, Rebagliato M, Iniguez C (2010) Preterm birth and exposure to air pollutants during pregnancy. Environ Res 110(8):778–785
Longnecker MP, Klebanoff MA, Zhou H, Brock JW (2001) Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet 358(9276):110–114
Longnecker MP, Klebanoff MA, Brock JW, Guo X (2005) Maternal levels of polychlorinated biphenyls in relation to preterm and small-for-gestational-age birth. Epidemiology 16(5):641–647
Maroziene L, Grazuleviciene R (2002) Maternal exposure to low-level air pollution and pregnancy outcomes: a population-based study. Environ Health 1(1):6
McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred EN, Leviton A, Investigators ES (2008) Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 168(9):980–989
Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Tellez-Rojo MM (2009) Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect 117(10):1587–1592
Mukherjee SC, Saha KC, Pati S, Dutta RN, Rahman MM, Sengupta MK, Ahamed S, Lodh D, Das B, Hossain MA, Nayak B, Mukherjee A, Chakraborti D, Dulta SK, Palit SK, Kaies I, Barua AK, Asad KA (2005) Murshidabad–one of the nine groundwater arsenic-affected districts of West Bengal, India. Part II: dermatological, neurological, and obstetric findings. Clin Toxicol 43(7):835–848
Mustafa M, Banerjee B, Ahmed RS, Tripathi A, Guleria K (2013) Gene–environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol Hum Reprod 19(1):35–42
Myers SL, Lobdell DT, Liu Z, Xia Y, Ren H, Li Y, Kwok RK, Mumford JL, Mendola P (2010) Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. J Epidemiol Community Health 64(4):325–329
Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M (2013) Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health 12(1):6
Nishijo M, Nakagawa H, Honda R, Tanebe K, Saito S, Teranishi H, Tawara K (2002) Effects of maternal exposure to cadmium on pregnancy outcome and breast milk. Occup Environ Med 59(6):394–396; discussion 397
Nolan LA, Nolan JM, Shofer FS, Rodway NV, Emmett EA (2009) The relationship between birth weight, gestational age and perfluorooctanoic acid (PFOA)-contaminated public drinking water. Reprod Toxicol 27(3–4):231–238
Ochoa-Acuña H, Frankenberger J, Hahn L, Carbajo C (2009) Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ Health Perspect 117(10):1619–1624
Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB, Shaw GM (2014) Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res 135:221–226
Patelarou E, Kargaki S, Stephanou EG, Nieuwenhuijsen M, Sourtzi P, Gracia E, Chatzi L, Koutis A, Kogevinas M (2011) Exposure to brominated trihalomethanes in drinking water and reproductive outcomes. Occup Environ Med 68(6):438–445
Pathak R, Ahmed RS, Tripathi AK, Guleria K, Sharma CS, Makhijani SD, Banerjee BD (2009) Maternal and cord blood levels of organochlorine pesticides: association with preterm labor. Clin Biochem 42(7–8):746–749
Perkins M, Wright RO, Amarasiriwardena CJ, Jayawardene I, Rifas-Shiman SL, Oken E (2014) Very low maternal lead level in pregnancy and birth outcomes in an Eastern Massachusetts population. Ann Epidemiol 24(12):915–919
Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD (1994) The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 272(4):284–291
Procianoy RS, Schvartsman S (1981) Blood pesticide concentration in mothers and their newborn infants. Relation to prematurity. Acta Paediatr Scand 70(6):925–928
Revich B, Aksel E, Ushakova T, Ivanova I, Zhuchenko N, Klyuev N, Brodsky B, Sotskov Y (2001) Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere 43(4–7):951–966
Ribas-Fitó N, Sala M, Cardo E, Mazon C, De Muga ME, Verdu A, Marco E, Grimalt JO, Sunyer J (2002) Association of hexachlorobenzene and other organochlorine compounds with anthropometric measures at birth. Pediatr Res 52(2):163–167
Rinsky JL, Hopenhayn C, Golla V, Browning S, Bush HM (2012) Atrazine exposure in public drinking water and preterm birth. Public Health Rep 127(1):72–80
Rivera-Núñez Z, Wright JM (2013) Association of brominated trihalomethane and haloacetic acid exposure with fetal growth and preterm delivery in Massachusetts. J Occup Environ Med 55(10):1125–1134
Salmasi G, Grady R, Jones J, McDonald SD (2010) Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 89(4):423–441
Sathyanarayana S, Basso O, Karr CJ, Lozano P, Alavanja M, Sandler DP, Hoppin JA (2010) Maternal pesticide use and birth weight in the agricultural health study. J Agromedicine 15(2):127–136
Savitz DA (2008) Invited commentary: disaggregating preterm birth to determine etiology. Am J Epidemiol 168(9):990–992; discussion 993–994
Savitz DA, Andrews KW, Pastore LM (1995) Drinking water and pregnancy outcome in central North Carolina: source, amount, and trihalomethane levels. Environ Health Perspect 103(6):592–596
Savitz DA, Stein CR, Bartell SM, Elston B, Gong J, Shin HM, Wellenius GA (2012a) Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 23(3):386–392
Savitz DA, Stein CR, Elston B, Wellenius GA, Bartell SM, Shin HM, Vieira VM, Fletcher T (2012b) Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the Mid-Ohio Valley. Environ Health Perspect 120:1201–1207
Saxena MC, Siddiqui MK, Seth TD, Krishna Murti CR, Bhargava AK, Kutty D (1981) Organochlorine pesticides in specimens from women undergoing spontaneous abortion, premature of full-term delivery. J Anal Toxicol 5(1):6–9
Shah PS, Balkhair T (2011) Air pollution and birth outcomes: a systematic review. Environ Int 37(2):498–516
Singh VK, Singh J, Anand M, Kumar P, Patel DK, Krishna Reddy MM, Javed Siddiqui MK (2008) Comparison of polycyclic aromatic hydrocarbon levels in placental tissues of Indian women with full- and preterm deliveries. Int J Hyg Environ Health 211(5–6):639–647
Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, Glinianaia S, Hoggatt KJ, Kannan S, Hurley F, Kalinka J, Sram R, Brauer M, Wilhelm M, Heinrich J, Ritz B (2008) Meeting report: atmospheric pollution and human reproduction. Environ Health Perspect 116(6):791–798
Sonnenfeld N, Hertz-Picciotto I, Kaye WE (2001) Tetrachloroethylene in drinking water and birth outcomes at the US Marine Corps Base at Camp Lejeune, North Carolina. Am J Epidemiol 154(10):902–908
Sowers M, Jannausch M, Scholl T, Li W, Kemp FW, Bogden JD (2002) Blood lead concentrations and pregnancy outcomes. Arch Environ Health 57(5):489–495
Sram RJ, Binkova B, Dejmek J, Bobak M (2005) Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect 113(4):375–382
Stein CR, Savitz DA, Dougan M (2009) Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am J Epidemiol 170(7):837–846
Stieb DM, Chen L, Eshoul M, Judek S (2012) Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 117:100–111
Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ (2008) Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci 15(7):631–650
Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H (2010) Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int 36(7):699–704
Taylor C, Golding J, Emond A (2014) Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: a prospective birth cohort study. BJOG 122(3):322–328
Torres-Arreola L, Berkowitz G, Torres-Sanchez L, Lopez-Cervantes M, Cebrian ME, Uribe M, Lopez-Carrillo L (2003) Preterm birth in relation to maternal organochlorine serum levels. Ann Epidemiol 13(3):158–162
Torres-Sánchez LE, Berkowitz G, Lopez-Carrillo L, Torres-Arreola L, Rios C, Lopez-Cervantes M (1999) Intrauterine lead exposure and preterm birth. Environ Res 81(4):297–301
Vassilev ZP, Robson MG, Klotz JB (2001) Outdoor exposure to airborne polycyclic organic matter and adverse reproductive outcomes: a pilot study. Am J Ind Med 40(3):255–262
Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, Yunesian M (2011) Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med 68(3):231–234
Villanueva CM, Durand G, Coutte MB, Chevrier C, Cordier S (2005) Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestational-age status. Occup Environ Med 62(6):400–405
Villanueva CM, Gracia-Lavedan E, Ibarluzea J, Santa Marina L, Ballester F, Llop S, Tardon A, Fernandez MF, Freire C, Goni F, Basagana X, Kogevinas M, Grimalt JO, Sunyer J (2011) Exposure to trihalomethanes through different water uses and birth weight, small for gestational age, and preterm delivery in Spain. Environ Health Perspect 119(12):1824–1830
Wassermann M, Ron M, Bercovici B, Wassermann D, Cucos S, Pines A (1982) Premature delivery and organochlorine compounds: polychlorinated biphenyls and some organochlorine insecticides. Environ Res 28(1):106–112
Wesselink A, Warner M, Samuels S, Parigi A, Brambilla P, Mocarelli P, Eskenazi B (2014) Maternal dioxin exposure and pregnancy outcomes over 30 years of follow-up in Seveso. Environ Int 63:143–148
Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Cupul-Uicab LA, Brantsaeter AL, Longnecker MP (2012) Perfluorinated compounds in relation to birth weight in the Norwegian mother and child cohort study. Am J Epidemiol 175:1209–1216
Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, Perera FP, Andrews H, Just AC, Hoepner L, Tang D, Hauser R (2009) Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics 124(6):e1213–e1220
Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D (2008) Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B 11(5–6):373–517
Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B (2011) Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health 10:89
Wojtyniak BJ, Rabczenko D, Jonsson BA, Zvezday V, Pedersen HS, Rylander L, Toft G, Ludwicki JK, Goralczyk K, Lesovaya A, Hagmar L, Bonde JP (2010) Association of maternal serum concentrations of 2,2′,4,4′5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p′-DDE) levels with birth weight, gestational age and preterm births in Inuit and European populations. Environ Health 9:56
Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM (2008) Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116(8):1092–1097
Wood SL, Jarrell JJ, Swaby C, Chan S (2007) Endocrine disruptors and spontaneous premature labor: a case control study. Environ Health 6:35
Woodruff TJ, Zota AR, Schwartz JM (2011) Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–885
Wright JM, Schwartz J, Dockery DW (2003) Effect of trihalomethane exposure on fetal development. Occup Environ Med 60(3):173–180
Wright JM, Schwartz J, Dockery DW (2004) The effect of disinfection by-products and mutagenic activity on birth weight and gestational duration. Environ Health Perspect 112(8):920–925
Wu K, Xu X, Liu J, Guo Y, Li Y, Huo X (2010) Polybrominated diphenyl ethers in umbilical cord blood and relevant factors in neonates from Guiyu, China. Environ Sci Technol 44(2):813–819
Wu K, Xu X, Peng L, Liu J, Guo Y, Huo X (2012) Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int 48:1–8
Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L (2007) Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect 115(1):42–47
Yang CY, Chang CC, Tsai SS, Chuang HY, Ho CK, Wu TN (2003) Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res 91(1):29–34
Yang CY, Xiao ZP, Ho SC, Wu TN, Tsai SS (2007) Association between trihalomethane concentrations in drinking water and adverse pregnancy outcome in Taiwan. Environ Res 104(3):390–395
Zhang YL, Zhao YC, Wang JX, Zhu HD, Liu QF, Fan YG, Wang NF, Zhao JH, Liu HS, Ou-Yang L, Liu AP, Fan TQ (2004) Effect of environmental exposure to cadmium on pregnancy outcome and fetal growth: a study on healthy pregnant women in China. J Environ Sci Health A Tox Hazard Subst Environ Eng 39(9):2507–2515
Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM (2010) Maternal low-level lead exposure and fetal growth. Environ Health Perspect 118(10):1471–1475
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ferguson, K.K., Meeker, J.D. (2016). The Role of Environmental Exposures in Preterm Birth. In: Hughes, C., Waters, M. (eds) Translational Toxicology. Molecular and Integrative Toxicology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-27449-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-27449-2_9
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-27447-8
Online ISBN: 978-3-319-27449-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)