Abstract
Over the last few years, the development of nanotechnology has resulted in generation of new materials and innovation for a wide range of applications and products. Among these applications, textile industry is expected to hold a considerable potential for the development of advanced nano-based materials. For example, nanotechnology enabled the production of novel smart “multifunctional” textiles with combined properties in one fabric. Conductive textiles represent a key class of smart textiles with promising future’s applications in areas such as electronic textiles, display devices, health monitoring devices, thermal and moisture management, flexible energy storage, and power generation devices. Recently, a remarkable attention has been devoted to the development of textile supercapacitor for energy storage and wearable electronics applications. Supercapacitor textiles offer advantages such as lightweight, flexibility, stretchability, and ease of integration with electronic textiles. Different approaches have been investigated for fabrication of smart conductive textiles for supercapacitor applications. Among these approaches, textiles coated with electrically conducting polymers (ECPs) are one of the most promising and facile approaches for fabrication of textile supercapacitors. ECP-coated textiles are characterized with high specific capacitance through fast redox reaction ease of integration into planar, flexible, and stretchable textile substrates with various shapes and large areas, thin film fabrication with controlled nanostructured morphology, and applicability for fabrication of composite and asymmetric textile supercapacitors. This chapter highlights the recent advances and developments in the fabrication of ECP-based textile supercapacitors, including different types of pure ECPs and their composites with other conducting materials for preparation of hybrid supercapacitors with superior performance for textile supercapacitor applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
21.1 Introduction
21.1.1 Smart Textiles
Smart textiles are defined as textile products such as fibers, filaments, and yarns together with woven, knitted, or nonwoven structures, which can interact with the environment/user [1]. Smart textiles are attracting a great interest due to their versatile functionalities and capabilities of sensing, actuating, responding to external stimuli, communicating, power generation, and storage, whereby health monitoring, safety, and protection can be endorsed. Indeed, increasing competition in the textile industry has been observed after the introduction of the concepts “smart textiles” and “ultrasmart textiles.” During the period between 1995 and 2011, the global growth of technical textiles market was estimated to be 133 billion USD [2]. This extraordinary growth is attributed to the recent development and integrated expertise on nanotechnologies, material science, electronics, and manufacturing processes. As depicted in Fig. 21.1, smart textiles have been utilized for a wide range of applications including stimuli-responsive textiles [3, 4], antibacterial textiles [5, 6], flame retardant textiles [7–10], shape-memory textiles [11–13], textiles for engineered membranes [14], personal thermal management textiles [15], moisture management textiles [16–18], self-cleaning textiles [19–22], and smart conductive textiles [23, 24].
21.1.2 Smart Conductive Textiles
As can be inferred from Fig. 21.1, smart conductive textiles represent an important class of smart functional textiles due to their wide range of possible applications and technologies including luminescent textiles [25–27], photovoltaic devices and dye-sensitized solar cells [28–31], Li-ion batteries [32–37], supercapacitors [38–45], transistors [46–49], sensors [50–53], biosensor [54–56], display devices [57–59], water purification [60–65], and personal thermal management [66, 67]. The key requirement for successful use of smart conductive textiles in each of the above-listed applications depends on their electrical conductivity .
According to their electrical conductivity, textiles can be classified into conductive and nonconductive categories. Conductive textiles are those textiles which exhibit inherent electrical conductivity such as metal-based textiles (mainly knitted wire meshes) and carbon-based textiles, whereas the nonconductive textiles include natural and synthetic polymeric fibers and textiles. Metal textiles have high electrical conductivities and lack important features such as flexibility, stretchability, lightweight per unit area, and resistance to atmospheric oxidation. The aforementioned missing features are inconsistent with the materials and geometries that are required for conductive textiles, because the incorporation of metal wires within textiles increases stiffness and reduces elasticity [1]. Therefore, metal-based textiles are expected to have limited applications and uses in areas such as wearable electronic textiles (“e-textiles”) and flexible textiles for energy and environmental applications.
The most used and commercialized carbon-based textiles are made from graphitic carbon and are known as carbon cloth (CC) [68, 69]. Compared to metals, carbon-based conductive textiles offer features such as flexibility and stability with respect to atmospheric oxidation.
Textiles made from natural or synthetic polymer fibers are prepared by using different technologies such as gel spinning, melt spinning, wet spinning, dry spinning, and electrospinning techniques. Natural and synthetic polymeric fibers offer advantages such as a wide range of selection, low cost, flexibility, stretchability, durability, lightweight, porosity, good tensile and elastic properties, good moisture management, and thermal management properties. However, natural and synthetic fibers/textiles are nonconductive and do not allow for the flow of electricity through their structure. The insulation feature of these textiles can be recognized from the electrical properties of the corresponding neat polymers that exhibit high electrical resistivity values and low dielectric constants [70, 71]. The inability of nonconductive textiles to transport electrical current is accompanied by their ability to generate static electricity that allows electrical charges to remain fixed on the textiles’ surface. The stored static electricity can cause problems during textile processing and end-user applications due to the electrostatic discharge. The introduction of conductive textile materials provides a solution to the aforementioned problem through transport and dissipation of the electrical charges.
Conductive textile can be made directly by converting a nonconductive woven/nonwoven textile into a conductive one or indirectly by using different conductive fibers and converting them into conductive textiles through a knitting or weaving process. A general overview for various direct and indirect techniques used for preparation of conductive textiles will be highlighted in Sect. 21.2.
21.1.3 Smart Conductive Textiles for Energy Applications
Recently, the interest in smart electronic textiles for medical, sports, military, and energy applications has increased markedly [1, 72]. Electronic textiles offer advantages such as low cost, lightweight, stretchability, and flexibility to be integrated into variously shaped structures that would otherwise be impossible with traditional electronics technology. Currently, most wearable e-textiles are made by the attachment of electronic devices onto the textile surface [1]. However, such detachable devices still need to be flexible. This limitation can be overcome by the direct fabrication of devices into the textile/fabric. The rough surface of textiles makes this task extremely challenging for fabrication of many electronic devices [73]. Regardless, the large surface roughness property of textiles is preferred by other energy devices such as supercapacitors and Li-ion batteries [73]. In such devices, the surface roughness and porosity of the conductive textile electrodes provide an ideal situation for manipulation of ion movements between two sides of the fabricated device.
This chapter provides a brief description of various methods for preparation of conductive textiles (Sect. 21.2), including the use of metals, carbons, electrically conducting polymers (ECPs), and other recent proposed technologies for preparation of functional conductive textiles. Section 21.3 provides a general background on electrochemical supercapacitors as an emerging technology for energy storage devices. Section 21.4 highlights the recent advances in the utilization of ECPs for preparation and fabrication of flexible supercapacitor textiles for various applications.
21.2 Preparation of Conductive Textiles
21.2.1 Coated Conductive Fibers and Textiles
Electrically conductive fibers and textiles can be produced by coating the fibers with conductive materials such as metals, metallic salts, metal oxides, or ECPs. Coatings can be applied to the surface of fibers, yarns, or even fabrics to create electrically conductive textiles. The main textile coating methods include electroless plating, chemical vapor deposition (CVD), atomic layer deposition (ALD), and sputtering as well as coating with ECPs and conductive inks.
21.2.1.1 Sputtering
Sputtering is an established technique that enables the use of a variety of metals, polymers, metal oxides, or mixtures thereof. However, the sputtering process for functionalization of textiles is expensive and usually performed on the side facing the target which prevents the deposition of the functional coating onto the inner layers of thick textile materials [74]. In sputtering process, a good adhesion between the sputter-coated layer and the fibers can be obtained by increasing the thickness of the deposited layers which may be critical for the final use and application in cases such as that of wearable electronic textiles [74].
21.2.1.2 Evaporative Deposition Techniques
Evaporative deposition techniques , such as CVD and ALD, offer a practical way for preparation of textiles with conductive functionality [74]. However, these techniques are expensive when scaling-up the technology and require the use of relatively high temperatures which may not be suitable for the commodity textiles used in daily life applications [74]. The thickness of the deposited film is very critical for the adhesion of the deposited conductive film. Higher thicknesses are susceptible to cracking and lower thicknesses may not provide uniform conductivity [75]. One of the challenges in case of the ALD technique is the chemistry and requirements of the employed precursors. For example, the precursors must chemisorb onto the substrate to be coated, and they must have a reasonably high vapor pressure to allow saturation of the chamber volume upon dosing [76]. The precursors must have a good thermal stability so as to avoid decomposition in the subsequent steps [76].
21.2.1.3 Electroless Plating Technique
Electroless plating technique is the simplest one and does not require electrical energy. However, the electroless plating process is carried out under high- or low-pH conditions which impose a deterioration effect on the treated fibers and textiles [74]. The presence of impurities during the electroless plating process can cause pitting and reduced ductility, adhesion, and roughness [74]. The adhesion of the metal particles exhibits a moderate strength on the surface of the fabric which may be peeled off when the plated metal is exposed to the air. The whole process of electroless plating is a multistage process which requires the use of complex chemicals and generates a lot of chemical waste.
21.2.1.4 Electrically Conducting Polymer Coatings
Coatings offer advantages such as their suitability for different synthetic and natural fibers as well as good electrical conductivity without significantly altering existing key substrate properties such as density, flexibility, and handling. However, in case of metal coatings discussed above, the adhesion between the metal and the coated fibers/textiles as well as corrosion resistance are challenging for the final use and application.
ECPs offer an alternative approach for coating various types of textiles, fibers, and fabrics. The coating process is simple and fast and does not require complicated or long procedures. The coating of ECPs is cost-effective; does not have restrictions related to substrate shape, type, surface chemistry (i.e., hydrophobic/hydrophilic), and fiber type (i.e., woven/nonwoven); and can be done at temperatures less than or equal to room temperature. Compared to the evaporative deposition techniques, the coating process of ECPs covers all the internal microfibrils of the coated fibers/textile. This advantage ensures the homogeneity of the whole coating and provides a more uniform coating which is critical for its performance during the end-use applications. The mechanical properties and physical appearance of the ECP-coated fibers and textiles are not affected, and they are not susceptible to cracking like metal and metal oxide coatings. Hence, the flexibility and integrity of the coated fibers and textiles are not affected by physical forces such as bending and stretching. Although the conductivity of the ECP coatings is much less than that of metal coatings, the conductivity of the ECP-coated textiles can be controlled and tailored by controlling parameters such as concentrations of monomers, temperature, doping agents, and the nanostructured morphology of the ECPs (e.g., arrays of nanowires) [77]. All the abovementioned advantages of ECP coatings make them suitable candidates for the preparation of functional smart conductive textiles for various applications and device fabrication.
ECPs can be prepared mainly by two methods, electrochemical polymerization and oxidative chemical polymerization (OCP) [78]. However, electrochemical polymerization can be used only for coating conductive substrates such as carbon-based materials and metals [79, 80]. Also, electrochemical polymerization is not suitable for the bulk production of conductive polymers and is not suitable for controlled preparation of films with thicknesses above 100 μm. However, the electrochemical polymerization methodology can be employed using different scan rates for obtaining ECPs with different nanostructured morphologies [81, 82]. On the other hand, the OCP, which is often called redox polymerization, is more versatile and can be used for both conductive and nonconductive substrates, which makes it a good choice for the preparation of a wide range of conductive textiles. OCP offers the advantage of controlling the morphology of the nanostructured conductive coating by adjusting the nucleation and growth chemistry during the chemical polymerization process [77, 83]. Since the ECPs are polyelectrolytes, textiles can be coated with ECP polyelectrolytes through the layer-by-layer coating methodology [84, 85]. In addition, ECPs can be prepared by using an interfacial polymerization technique which is very useful for deposition of thin film coatings with controlled nanofiber morphology [86–88]. ECPs with a nanofiber morphology can be obtained by approaches such as the use of template polymerization [82, 89–92], templateless polymerization [93], seeding polymerization [94], oligomer-assisted polymerization [95], dilute polymerization [96, 97], and surfactant-assisted polymerization [98] and using different oxidants such as vanadic acid [99] and silver nitrate [61]. Similarly, ECPs with nanowire morphology can be obtained by using template or templateless polymerization methods [77]. In addition to chemical and electrochemical polymerization, ECPs can be incorporated into various textiles by the vapor phase polymerization (VPP) technique [40, 100–102].
21.2.2 Conductive Ink Coatings
Compared to metal coatings, the use of conductive Nanoink , such as carbon nanotubes and graphene, allows for retaining the porous structure of the textile without blocking the external surface of treated textiles [73]. Conductive inks can be used for preparation of conductive textiles and fibers by one the following methods.
21.2.2.1 Dip-Dry Coating
Dip-dry coating is a very simple and quick method that can be applied to different types of textiles and fibers. The target textile must possess a good porosity to absorb the maximum amount of the ink solution. Cotton-based conductive textiles, which are highly porous and hydrophobic, are easily prepared by the dipping-drying procedure. The ink is prepared from a dispersion containing the conductive ingredient (e.g., carbon nanotubes, graphene oxide, graphene, graphite, or carbon black) with dispersing agent or surfactant and the usual solvent used is water. The conductivity of the textile substrate depends on the number of dipping cycles. The final step requires washing out the employed dispersing surfactant to maintain the maximum conductivity and to clean up the coated substrate.
21.2.2.2 Inkjet Printing
Direct printing with an inkjet printer is a material-saving, high-speed, and low-cost process. The inkjet printing process is more challenging than simple conformal coating with dispersible ink. Different types of conductive inks can be used including colloidal suspensions of nanoparticles [103], organometallic compounds in solution [104], conductive polymer dispersions [105–107], carbon nanotube dispersions [108–110], graphene [107, 111–113], and graphene oxide [114] dispersions. Regardless of the type of ink employed, the inks should satisfy the following requirements to be used for conductive inkjet printing [1, 113, 115, 116]:
-
High electrical conductivity.
-
Stability toward oxidation in air (e.g., silver and gold are stable; copper and nickel are easily oxidized in air).
-
Dry out without clogging the nozzle during printing.
-
Good adhesion to the substrate.
-
Ink shall not agglomerate and clog the nozzles (stable dispersion).
-
Solvent properties (nontoxic solvent, solvent with reasonable viscosity at room temperature).
-
Solvent must readily evaporate once deposited but not so fast that it dries out at the nozzle when idle, causing nozzle clogging.
-
Fluid properties of the formulated ink such as viscosity, density, and surface tension.
21.2.2.3 Reactive Inkjet Printing
An alternative way to fabricate conductive textiles and fibers using inkjet printing involves the deposition of two inks, which react to form the conductive material [115]. The main advantage of this reactive inkjet printing technique is that it allows different materials to be selectively produced or removed [115]. For example, Li et al. [117] have utilized the reactive inkjet printing methodology for the preparation of copper conductive ink. The reactive ink solution was prepared from a copper citrate solution acting as a metal precursor and a sodium borohydride solution acting as a reducing agent. Recently, Walker and Lewis employed the reactive ink technique for the preparation of silver ink with high conductivity similar to that of bulk silver [116]. The preparation procedure was simple and based on the modified Tollens’ process [116].
21.2.2.4 Screen Printing
Screen printing is useful for fabricating electrics and electronics due to its ability to produce patterned, thick layers from paste-like conducting materials. The screen printing technique, a stencil process, includes the printing of a viscous paste through a patterned fabric screen which is then followed by a drying step. However, screen printing using thick conductive ink pastes for the production of conductive textiles has not been thoroughly investigated or employed due to it being both labor and capital intensive and may cause production delays when designs are modified or changed over [118]. Recently, Kazani et al. [119] employed silver ink paste for screen printing on different conductive woven textile substrates including cotton, polyester, polyamide, and viscose. It was found that the square resistance of the printed samples remained high enough even after the washing process. After 20 washes, the printed conductive layer that was covered by a protective polyurethane (PU) thin layer was stable compared with the uncovered samples which showed cracks and peeling of the printed conductive layer.
21.2.3 Conductive Textiles from Conductive Fibers
An alternative approach for preparation of conductive textiles is by the knitting and weaving of conductive fibers. Various conductive fibers can be used such as carbon-based conductive fibers, ECP-based conductive fibers, and metal fibers. The main factor that limits the applicability of certain conductive fibers for the fabrication of conductive textiles is their ability to withstand the weaving/knitting processes without being broken apart. This means that the conductive fibers should have enough tensile strength without being brittle when contorted through the knitting machine.
21.2.3.1 Carbon-Based Conductive Fibers
Due to the recent advances in nanotechnologies, different types of carbon-based fibers have been fabricated such as carbon nanotube fibers (CNFs) [120–122], graphene fibers (GFs) [123, 124], graphene oxide fibers (GOFs) [125], graphene-carbon nanotube composite fibers [126, 127], and silver-doped graphene fibers [128]. The former processes are expensive, complicated, and not yet scaled up to a commercial level. A detailed description and discussion for the preparation of carbon-based conductive fibers can be found in literature [129].
21.2.3.2 ECP-Based Conductive Fibers
ECPs are a class of polymers with a fully conjugated aromatic backbone structure. The full delocalization of π electrons provides the unique feature of electrical conductivity for this class of polymers. However, due to the high aromaticity of ECPs, they possess a rigid backbone structure, which makes them available in relatively low molecular weight forms, so much so that the elasticity of their solutions is generally insufficient for the direct spinning or electrospinning of their fibers. However, there are some reports describing the preparation of ECP fibers by spinning, coaxial spinning, and electrospinning [130–136].
21.2.3.3 Twisted Conductive Fibers
Conductive textiles can be made by the integration of conductive yarns in a textile structure [1]. This was achieved by twisting metal wires into a fabric textile. However, the integration of conductive yarns in a fabric structure is a complex process, and the resulting conductive fabrics were hard, rigid, and uncomfortable for electronic textiles and wearing applications [1].
21.2.3.4 Biscrolled Conductive Fibers
A new technology was recently developed for preparation of smart functional fibers based on the biscrolling technique by incorporating functional guests into yarns [137, 138]. The fabrication process of biscrolled yarns involves the twist-based spinning of CNT sheets (the host) that are overlaid with a layer of up to 99 wt% of one or other functional materials (the guest). The guest-host bilayers were scrolled into biscrolled yarn in such a way that the minor CNT sheet concentration confined guest powders down to nanometer-scale proximity in the scroll galleries. The guest material can be deposited into the host CNT sheets by electron beam evaporation, sputtering, exposure to an aerosol formed by gas-phase reaction, or simple filtration-based guest deposition. The main advantage of this technology relies on its ability to incorporation versatile functional guest materials in different applications [137]. The biscrolled yarns can be knotted and sewn with potential applications in various smart textiles.
21.2.3.5 Welded Conductive Fibers
Recently, Jost et al. [139] employed a technology called “natural fiber welding ” [140] (NFW) for the preparation of conductive yarns in knittable textile supercapacitor applications. As illustrated in Fig. 21.2, the NFW process employs activated carbon as the conductive material embedded into cellulose yarns (cotton, linen, bamboo, or viscose) being swelled in an ionic liquid (IL) solution. This step is followed by removal of the IL by washing it in water as anti-solvent and, finally, twisting the activated carbon-NWFs with a highly conductive stainless steel yarn. The prepared conductive cotton yarns were too brittle and broke apart during the knitting process. In contrast, conductive yarns made from linen, bamboo, and viscose were knitted successfully into fabric textiles and utilized for making a knitted stretchable supercapacitor device as shown in Fig. 21.3.
Schematic illustration of the natural fiber welding process for preparation of conductive fibers. Reproduced with permission from [139]
Photographs of knitted samples: (a) photograph of a flat knitted supercapacitor, (b) photograph of knitted supercapacitor while stretched. Reproduced with permission from [139]
21.3 Capacitors for Energy Storage Devices
21.3.1 Conventional Capacitors
Conventional capacitors consist of two conducting electrodes separated by an insulating dielectric material. When a voltage is applied to a capacitor, opposite charges accumulate separately on the surfaces of each electrode, which results in the generation an electric field that allows the capacitor to store energy. The ratio of the stored (positive) charge (Q) to the applied voltage (V) is called capacitance (C):
For conventional capacitors, C is directly proportional to the surface area (A) of the electrodes and inversely proportional to the distance (D) between the electrodes:
where
ε 0 = dielectric constant “permittivity” of the space and
ε r = dielectric constant of the separating “dielectric” material.
The main attributes of the capacitor are “energy density (E)” and “power density (P).” For both (E) and (P), the density can be calculated as quantity per unit mass or unit volume. The stored energy (E) is defined as:
where P is the energy consumed per unit time. In order to determine the power, one has to consider that the capacitor is represented as a circuit in series, with an external load/resistance (R). Also, there is a contribution from the internal components of the capacitor (e.g., current collectors, electrodes, and dielectric materials), which is measured in aggregate by a quantity known as the equivalent series resistance (ESR). The voltage during discharge is determined by these resistances. When measured at matched impedance (R = ESR), the maximum power for the capacitor P max is
From Eq. (21.4), it is clear that as resistance increases, the capacitor becomes less efficient providing lower power densities.
Compared with electrochemical batteries and fuel cells, conventional electrostatic capacitors exhibit relatively high power densities but relatively low energy densities (Fig. 21.4). Hence, a battery can store more total energy than a capacitor, but it cannot deliver it very quickly, which means its power density is low. On the other hand, capacitors store relatively less energy per unit mass or volume, but the stored energy can be discharged rapidly to produce a lot of power, so their power density is usually high.
Ragone plots showing the specific power against specific energy for various electrical energy storage systems [141], copyright 2008, Nature Publishing Group
21.3.2 Supercapacitors
Supercapacitors , often called electrochemical capacitors (ECs), are governed by the same basic principles as conventional electrostatic capacitors. However, they incorporate electrodes with much higher surface areas (A) and much thinner dielectrics that decrease the distance (D) between the electrodes. Thus, from Eqs. (21.2) and (21.3), it can be inferred that supercapacitors are able to reach higher values for both capacitance and energy. In addition, supercapacitors can reach comparable power densities to those of the conventional capacitors by maintaining low values of ESR. Supercapacitors have several advantages over electrochemical batteries and fuel cells, including higher power density, shorter charging times, and longer cycle and shelf lives [142].
The electrochemical performance of supercapacitors can be described by specific capacitance, which can be considered the most important characteristic of a supercapacitor indicating its capability of storing charges. The specific capacitance (Csp) is calculated from the charge–discharge curve according to the following equation:
where m is the mass, which is usually specified to active materials, one electrode or entire device; i is the charge and discharge current and usually fixed during the tests; and ΔV is the applied voltage window during tests. The upper limit of voltage depends on the type of the employed electrolyte. Generally, a water-based electrolyte has a voltage maximum of 1.2 V (and usually charged to 1.0 V) which is the decomposition voltage of water. However, organic electrolytes allow for higher charge voltages. The capacitance is impervious to voltage window but dictated by the scanning rate, ΔV/Δt, which is the slope of the charge–discharge curve.
21.3.3 Electrochemical Capacitors
Different than conventional electrostatic capacitors (sometimes called film capacitors or film dielectric capacitors) which store charges in an electric field imposed across a thin layer of dielectric material, ECs store charges at the electrochemical interfaces between the high surface area, porous electrode material, and the electrolyte. The effective capacitance of ECs is typically a few orders of magnitude higher than those resulting from electrostatic capacitors which is attributed to the large specific surface area of the porous electrodes (≈500–2000 m2 g−1 for ECs) and the shorter path length between the electrode and the electrolyte ions (in order of nanometer) [143]. According to the used energy storage mechanism, ECs can be classified into the following three categories.
21.3.3.1 Electrochemical Double-Layer Capacitors
In case of electrochemical double-layer capacitors (EDLCs ) , charges are stored electrostatically via ion absorption at the electrode/electrolyte interface. Carbon-based materials with high surface areas are the most commonly used electrode materials for EDLCs [73, 144–146]. Due to their fast and near-surface electrochemical process, EDLCs have the ability to provide very high power and possess an excellent cycle life. However, the energy stored in EDLCs is often limited by the finite electrical charge separation at the interface of electrode/electrolyte and by the working voltages that is primarily determined by the stable potential window of the used electrolyte.
21.3.3.2 Pseudocapacitors
In contrast to EDLC, pseudocapacitors employ fast and reversible faradic processes (redox reactions) at the surface of electroactive materials for charge storage. Faradic electrodes provide higher specific pseudocapacitance values (≈300–1000 F g−1) that exceed the specific capacitance of double-layer charge storage devices using carbon-based materials (100–259 F g−1). Typical active pseudocapacitive materials are transition metal oxides [147–150] and ECPs such as polyaniline (PANI), polypyrrole (PPY), and polythiophene (PTH) [77, 151, 152].
21.3.3.3 Hybrid Capacitors
Hybrid capacitors try to exploit the advantages and mitigate the disadvantages of EDCs and pseudocapacitors to achieve better performance characteristics. Utilizing both Faradaic and non-Faradaic processe s for charge storage, hybrid capacitors have achieved greater energy and power densities than EDCs without affecting the cycling stability and affordability that have limited the success of pseudocapacitors. Depending on the used electrode configuration, three different types of hybrid capacitors can be recognized which are composite, asymmetric, and battery type, respectively.
21.3.3.3.1 Composite ECs
Composite electrodes utilize a combination of two or more different materials of the following main categories: ECPs, metal oxides, and carbon-based materials. The hybrid composite materials integrate both physical and chemical charge storage mechanisms in a single electrode. The carbon-based materials provide a capacitive double layer of charge with an accessible high surface area that increases the contact between the deposited pseudocapacitive materials and electrolyte. The pseudocapacitive materials provide increases in the capacitance through Faradaic reactions.
21.3.3.3.2 Asymmetric ECs
Asymmetric hybrids combine Faradaic and non-Faradaic processes by coupling an EDC electrode with a pseudocapacitor electrode. In this case, the negative electrode will use carbon-based materials and the second positive electrode will use ECPs or metal oxides. The lack of an efficient, negatively charged, conducting polymer material has limited the success of conductive polymer pseudocapacitors. The implementation of a negatively charged, activated carbon electrode attempts to avoid this problem. Asymmetric hybrid capacitors that couple these two electrodes are expected to achieve higher energy and power densities than comparable EDCs along with better cycling stability than comparable pseudocapacitors.
21.3.3.3.3 Battery-Type ECs
Battery-type hybrids couple two different electrodes, a supercapacitor electrode with a battery electrode. This specialized configuration addresses the need for higher-energy supercapacitors and higher-power batteries, combining the energy characteristics of batteries with the power, cycle life, and recharging times of supercapacitors.
21.4 Materials for Textile Supercapacitors
As discussed previously (Sect. 21.3.3), the research efforts for making supercapacitors were directed into utilizing three categories of materials : highly conductive carbon-based materials with a large surface area, transition metal oxides, and ECPs. Various methods for preparation of conductive textiles utilizing different carbon-based material and ECPs were also discussed earlier (Sect. 21.2). The use of nanomaterials , such as CNTs and graphene, is expected to improve energy storage devices because the size reduction of materials will increase the contact surface area between the electrode and the electrolyte and decrease the length of the transport path for both electrons and ions [77]. Recently, several smart conductive textiles based on carbon materials have been investigated as flexible supercapacitors including spun carbon nanotube (CNT) yarns [44], CNT-based smart textiles [73, 153], CNF-based flexible supercapacitors [154], and CNT-based composite textile supercapacitors [155–157]. However, the performance of these ECs is limited by their inherently low specific capacitance [158]. On the other hand, pseudocapacitors based on transition metal oxides and ECPs showed very high capacitance behavior (Fig. 21.5). This section discusses the recent advances in utilizing ECP-based textiles for the preparation of flexible and lightweight high-performance supercapacitors.
Capacitive performance of various electrode materials reported in the literature [159] (reproduced with permission of The Electrochemical Society)
21.4.1 ECP-Based Textile Supercapacitors
Electrically conducting polymers (ECPs) were discovered in 1976 by Heeger, MacDiarmid, and Shirakawa, for which they were awarded the Nobel Prize in chemistry in 2000 [160]. ECPs belong to the class of polymers which have π conjugation along the polymer backbone such as polyaniline (PANI), polypyrrole (PPY), and polythiophene (PTH). One of the unique characteristics of ECPs is their ability to undergo oxidation–reduction reactions by gaining or losing electrons from the surrounding environment. This feature enables the application of ECPs in different areas such as in smart self-healing coatings for corrosion protection [78], chromatic display devices [58, 59], electrochemical mechanical actuators, electrochemical batteries [161, 162], and electrochemical supercapacitors [142]. As ECPs can be doped and dedoped rapidly to high charge density, they can be applied as active materials for pseudocapacitors. The most commonly used conducting polymers for supercapacitor applications include polyaniline (PANI), polypyrrole (PPY), poly[3,4-ethylenedioxythiophene] (PEDOT), and their derivatives [163]. High charge densities can be achieved as the charge–discharge process occurs through the volume of the ECP and not on the external surface area (Fig. 21.6). ECP-based pseudocapacitors with advantages such as high-redox active capacitance, high conductivity, and, essentially, high intrinsic flexibility have promised the most for high-performance portable, planar, and flexible supercapacitor applications [158].
One of the attractive features for utilizing ECP-based textiles for supercapacitor applications is the simple and diverse approaches for their preparation and incorporation in the flexible textile devices, with an ability to control their nanostructured morphology. The following section discusses briefly the preparation of pseudocapacitor textiles based on pure ECPs.
21.4.1.1 Polyaniline -Based Textile Supercapacitors
Among the ECPs, PANI has attracted much interest due to its low cost, good mechanical and environmental stability, and adjustable conductivity. PANI can be synthesized chemically by oxidative polymerization or electrochemically by employing a cyclic voltammetry technique. PANI can be represented in three different convertible forms: the oxidized form (leucoemeraldine), the reduced form (pernigraniline), and the emeraldine base (EB). The most useful structure is the nonconducting EB which can be converted into the conducting emeraldine salt (ES) by acid treatment through a process known as “doping.” The associated ionic materials/electrolytes are called “dopants.” Although the protonated form of polyaniline (ES) is reported to have poor conductivity, PANI is one of the most studied ECPs for EC applications due its environmental stability and the high doping level of 0.5 (i.e., two monomer units per dopant). Theoretically, PANI has a maximum specific capacitance of up to 2000 (F g−1) [164]. However, the reported specific capacitance in literature varies significantly according to parameters such as the nanostructured morphology, polymerization process, dopant type, dopant concentration, and the ionic diffusion length of the electroactive material. The essential requirements for achieving high capacitance using PANI-based pseudocapacitive electrodes are high surface area, controlled nanostructured morphology, and optimized type as well as concentration of the doping electrolyte [77, 165]. Table 21.1 shows the effect of various PANI morphologies on the obtained specific capacitance. The capacitance of polyaniline nanowire arrays is much larger than both compact film and disordered nanowire networks as electrodes for supercapacitors which is attributed to both a reduced path for ion diffusion and a lower ion diffusion resistance [77].
Kim et al. [173] fabricated a highly stable flexible supercapacitor electrode by coating PANI nanofibers on gold-coated polyvinylidene fluoride-co-hexafluoropropylene (PVDF-co-HFP) membranes. PANI nanofibers were prepared by rapid mixing of aniline monomer and APS initiator, with a 4:1 ratio, in 1 M sulfuric acid solution. A symmetric capacitor cell was prepared by sandwiching the two identical PVDF electrodes between Nafion membrane soaked with 0.5 M sulfuric acid. The assembled cell showed the performance of a practical flexible pseudocapacitor of the composite planar and bent electrode .
21.4.1.2 Polypyrrole-Based Textile Supercapacitors
Polypyrrole (PPY) is one of the most promising ECPs for pseudocapacitor applications due to its distinctive features such as high conductivity, fast charge–discharge mechanism, good thermal stability, low cost, and high energy density [81, 174–176]. Similar to PANI, PPY nanostructured morphology affects the performance of the fabricated supercapacitor. Compared with the specific capacitance obtained from PPY with nanobelt (296 F g−1) and nanobrick (357 F g−1) morphologies, PPY with a nanosheet morphology provided the highest specific capacitance (586 F g−1) for a PPY-coated stainless steel pseudocapacitor [81]. The high capacitance of PPY nanosheets was attributed to the porous structure and high BET surface area of the nanosheets being 37.1 m2 g−1 as compared with nanobricks and nanobelts with BET surface areas of 26.4 m2 g−1 and 22.6 m2 g−1, respectively.
Yuan et al. [177] have fabricated PPY-coated paper through a simple “soak and polymerization” methodology. The PPY-coated paper flexible electrodes showed a capacitance of 0.42 F cm−2 with high energy density of 1 mW h cm−3 at a power density of 0.27 W cm−3. The conductance of the PPY-coated paper remained almost constant after 100 cycles of bending. This approach provides a practical and low-cost method for large-scale production of conductive paper-based electrodes for energy storage devices and flexible electronics.
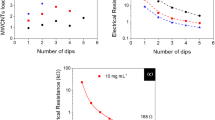
Yue et al. [39] prepared a stretchable electrode for supercapacitor application by coating nylon lycra fabric with polypyrrole ECP. PPY was coated on the fabric by a simple oxidative polymerization employing ammonium persulfate (APS) as a redox initiator and naphthalene-2, 6-disulfonic acid disodium salt (Na2NDS), at 4 °C for 2 h. The surface resistance of the PPY-coated fabric was 149 Ω/Sq. The electrical resistance decreased during stretching of the fabric and increased during fabric relaxation. This behavior was attributed to the better surface–surface contact within the yarns upon stretching, which improved the conductivity by penetration of the polymer into the fabric yarns. The electrochemical properties of the electrode were evaluated by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) employing 1.0 M NaCl as the electrolyte solution. At scan rate of 10 mV s−1, the PPY-coated electrode exhibited a nearly rectangular CV behavior which indicated that the charge–discharge responses of the electric double layer were highly reversible and kinetically facile. However, the rectangular CV shape became distorted when applying scan rates of ≥25 mV s−1. This was attributed to the slow diffusion of the counter ions during the insertion/ejection process compared to the faster electron transfer process at high scan rates. Using the three-electrode system, the specific capacitance (Csp) of the PPY-coated nylon lycra fabric was calculated using the following equation:
where Csp is the specific capacitance, A is the integral area of the cyclic voltammogram loop, \( f \) is the scan rate, \( v \) is the voltage window, and \( m \) is the mass of electroactive material (PPY). The delivered specific capacitance was found to be 123.3, 100.7, 69.7, and 39.4 F g−1 at a scan rate of 10, 25, 50, and 100 mV s−1, respectively. When the two-electrode system was used, the discharge capacitance (C m ) was calculated using the following equation:
where C m , I, t, ΔV, and m are the discharge capacitance per electrode, the current of charge–discharge, time of discharge, charge/discharge potential windows, and the amount of active materials on one electrode, respectively. The specific capacitance obtained from PPY-coated fabric without strain applied was 108.5 F g−1, with an energy density of 6.7 Wh kg−1 and power density of 753.4 W kg−1. The capacitance increased to 117.6, 119.6, and 125.1 F g−1 with an elongation of 20 %, 40 %, and 60 %, respectively. It was found that the PPY-coated nylon lycra preserved its electrochemical properties with less than 10 % specific capacitance loss after being stretched to 100 % for 1000 times.
In a later study, Yue et al. [178] reported the fabrication of a PPY-coated fabric electrode for a supercapacitor application through the electrochemical polymerization method. PPY was polymerized electrochemically on the conductive gold-coated fabric substrate. Acetonitrile was employed as the polymerization solvent, and p-toluenesulfonic (p-TS) acid was used as the organic acid dopant. The conductive textile electrode sustained up to 140 % strain without electric failure. Using 1.0 M NaCl as the electrolyte, the flexible electrode delivered a high specific capacitance of 254.9 F g−1 at a scan rate of 10 mV s−1 and maintained this almost unchanged up to 50 % applied strain, accompanied with improved cycling stability.
Recently, super-high-rate stretchable polypyrrole-based supercapacitors with excellent cycling stability were reported [179]. The pseudocapacitor device was fabricated using electrochemical polymerization of purified pyrrole monomers on smartly tailored stretchable stainless steel meshes (Fig. 21.7). The capacitance of the fabricated supercapacitors increased from the initial 170 F g−1 at a relaxed state to 214 F g−1 at a 20 % strain at a specific current of 0.5 A g−1. Surprisingly, it was found that the solid-state supercapacitors can be operated at super high rates of up to 10 Vs−1, being 1–2 orders of magnitude higher than most scan rates for PPY electrodes measured, even in aqueous electrolytes. The fabricated PPY pseudocapacitors achieved capacitance retention of 98 % under 0 % strain and 87 % under a strain of 20 % applied after 10,000 cycles at a very high specific current of 10 A g−1.
Schematic illustration: (a) preparation of a stretchable steel mesh by cutting along the dashed black lines; (b) stretch the mesh biaxially; (c) PPY electrode position on the stretchable steel mesh; (d) supercapacitor device assembly by coating the H3PO4/PVA electrolyte [179] (reproduced with permission, copyright 2015, Elsevier)
Babu et al. [180] investigated the capacitance of different PPY-coated textiles including cotton, viscose, linen, and polyester fabrics. A unit cell was fabricated to investigate the capacitive behavior by assembling two symmetric textile electrodes separated by a solid polymer electrolyte membrane (PVA/1 M H2SO4 gel). The textile electrodes prepared with PPY-cotton and PPY-viscose exhibited the highest specific capacitance values of 268 F g−1 and 244 F g−1, respectively, at a scan rate of 5 mV s−1. This was attributed to the strong ionic cross-linking through hydrogen bonding in case of PPY coating with cellulosic fabrics, providing uniform adsorption of pyrrole monomers and homogeneous polymer coating for the entire fabric .
21.4.1.3 Polythiophene-Based Textile Supercapacitors
Unlike PANI and PPY, which are p-type ECPs, polythiophene (PTH) and its derivatives can be both p-type and n-type ECPs. Compared with PANI and PPY, polythiophene exhibits much lower electrical conductivities and lower capacitance. However, the p-doped PTHs have advantages such as higher stability in air and humidified environments as well as their electrodes can be operated in a comparatively higher potential window (≈1.2 V), which enables the fabrication of asymmetric supercapacitors with one ECP-based electrode. Among all PTH derivatives, poly (3, 4-ethylenedioxythiophene) (PEDOT), with high environmental stability, has been investigated thoroughly for supercapacitor and pseudocapacitor applications [181–184].
Laforgue reported the fabrication of an all-textile flexible supercapacitor using electrospun PEDOT nanofibers [38]. The nanofiber mats with diameters around 350 nm demonstrated good electrochemical properties due to their ultraporous structure and very high electrical conductivity (60 ± 10 S cm−1). The mats were incorporated into an all-textile flexible supercapacitor, by using carbon cloths as current collectors and the electrospun PAN nanofibrous membranes as a separator. The supercapacitor device was fabricated by stacking and embedding the textile layers in a solid electrolyte containing an ionic liquid and PVDF-co-HFP as the host polymer. The pseudocapacitor showed a specific capacitance of 20 F g−1, with limited cycling stability. This was attributed to the increased cell resistance caused by the turning of the identical electrodes into its nonconductive (undoped) state when the cell is at its maximum voltage. This drawback of using ECP-based electrodes can be circumvented by designing composite supercapacitor electrodes using another type of active materials such as carbon nanotubes, carbon nanofibers, graphene nanosheets, and metal oxides.
21.4.2 Hybrid Textile Supercapacitors
The main shortcomings for utilizing pure ECPs in supercapacitors are discussed as follows: (1) The obtained practical specific capacitance is lower than theoretical prediction because the inner layer of the thick electrode cannot be fully used. (2) ECPs usually possess poor cyclic stability in long-term charge–discharge processes. The poor cyclic stability of conducting polymer electrodes could be attributed to the following three reasons [77]: (a) Poor mechanical stability due to reversible swelling/shrinkage caused by volumetric changes during the doping–dedoping process as a result of repeated insertion/de-insertion of ions during charging and discharging (Fig. 21.6) (b) Loss of the active material as a result of conducting polymers peeling off from the current collector or dissolving into the electrolyte (c) Over-oxidative degradation due to the limited working potential range
The above challenges of using pure ECP pseudocapacitive materials can be mitigated by fabrication of hybrid composite electrodes containing other types of materials with different capacitive performances and mechanisms. The following section highlights the recent progress in the fabrication of composite supercapacitor textiles using ECPs with carbon nanotubes, carbon cloths, graphene, and metal oxides.
21.4.2.1 Textile Supercapacitors Based on ECPs/CC Composites
Compared to textiles and fibers based on CNTs and GNSs, CC offers an alternative material as a current collector for supercapacitor devices due to their lower cost, porous 3D structure, high surface area, chemical oxidative stability, good electrical conductivity, and flexibility. Hence, a combination of CC as a conductive porous current collector with pseudocapacitive ECPs is expected to provide a supercapacitor with superior performance compared to a device with either CC or ECP alone.
Horng et al. [79] prepared a flexible supercapacitor based on conductive carbon cloth (CC)/PANI nanowires (PANI-NWs) composite electrodes. PANI-NWs were deposited on the CC by electrochemical polymerization using HCl as dopant ion. A symmetric supercapacitor device was fabricated by sandwiching two CC/PANI-NWs composite electrodes between a cellulose film separator and 1 M H2SO4 as the electrolyte. The textile supercapacitor demonstrated high flexibility, with only 0.05 % capacitance loss during the bending test. At 1.73 A g−1 discharge current, the gravimetric capacitance of 1079 F g−1 was obtained at a specific energy of 100.9 W kg−1 and a specific power of 12.1 kW kg−1. The cycling performance of the PANI-NWs/CC electrode was studied at a current density of 8.65 A g−1 in 1 M H2SO4 aqueous electrolyte. After 2100 cycles, the initial gravimetric capacitance was reduced by a factor of 14 %, which indicated the long-life electrochemical stability of the PANI-NWs/CC composite electrode.
Cheng et al. [80] investigated an electro-etched carbon fiber cloth coated with PANI-NWs as an alternative material for supercapacitor electrodes. It was found that the surface of carbon fibers becomes more hydrophilic after the etching process, which provided a more uniform and thin polyaniline coating around the carbon fibers, allowing them to be more accessible while also facilitating electrolyte transport among PANI-NWs and through various carbon fibers. The obtained mass-normalized specific capacitance was 1026.8 and 265.9 F g−1 at the PANI coating density of 1.8 and 9 mg cm−2, respectively. The reduction in capacitance at a higher PANI coating density was attributed to the fact that only the PANI on or near the surface could participate to the redox reactions. Hence, it was concluded that some of the PANI was not utilized when the PANI coating layer was thick. Achieving both high mass-normalized capacitance and area-normalized capacitance for electrodes is crucial for practical applications. At the optimized conditions of 30 min coating time and 5.4 mg cm−2 PANI coating density, the mass-normalized specific capacitance and area-normalized specific capacitance were 673 F g−1 and 3.5 F cm−2, respectively.
21.4.2.2 Textile Supercapacitors Based on ECPs/Graphene Composites
Graphene is a 2D single-atom-thick carbon allotrope tightly arranged in honeycomb lattices and has inspired an enormous amount of research [185, 186]. Due to its unique structure, graphene possesses ultrahigh theoretical specific surface area (SSA ≈ 2630 m2 g−1) and extraordinary electronic, mechanical, thermal, and optical properties. Hence, graphene is proposed as novel future material with great promise for potential applications in high-performance supercapacitors [142, 146, 187]. However, the serious aggregation and restacking of graphene is considered to be one of the major obstacles significantly inhibiting the commercial application of graphene in supercapacitors [142]. This problem can be overcome by incorporating pseudocapacitive materials such as ECPs into graphene as spacers to form composites, which can effectively prevent graphene agglomeration [142]. When ECPs are used as spacers, they can provide effective suppression for the irreversible restacking and, hence, maintain the intrinsic high SSA and provide more active sites to form EDLs. In addition, the use of ECPs/graphene composite electrodes provides accessibility for the pseudocapacitance mechanism (Faradic reactions) which may contribute to the overall specific capacitance. More importantly, the presence of highly conductive graphene with high SSA increases the contact between the deposited pseudocapacitive ECP material and the electrolyte, which alleviates the cycling stability drawback of the ECP-based electrodes. Yan et al. [188] synthesized a graphene/PANI composite using in situ polymerization which provided a maximum specific capacitance of 1046 F g−1 compared to 115 F g−1 for pure PANI. The large improvement of specific capacitance was attributed to the synergetic scenario between PANI and graphene nanosheets. PANI nanoparticles prevented the agglomeration of graphene nanosheets as well as reduced the ion diffusion path during charge/discharge processes. The cycling stability was enhanced remarkably by the addition of 1 % CNTs into the graphene/PANI composite which provided 94 % retention after 1000 cycles, compared with 48 % for graphene/PANI composite due to the improved mechanical properties and presence of a highly conductive path during the doping/dedoping processes [189].
Xu et al. [190] employed a screen printing methodology for the fabrication of a screen-printable thin film supercapacitor device utilizing graphene/PANI ink. Flexible conductive carbon fabrics were used as substrates for screen printing which can function as electrodes for direct assembly of the supercapacitor. After 1000 cycles, the flexible textile supercapacitor provided a maximum specific capacitance ratio of 352 F g−1 at discharge rate of 1 A g−1. The improved electrochemical stability was attributed to the combination of high graphene conductivity along with PANI reversible redox properties which is regarded as necessary to achieve supercapacitors with long cycle life. In a later study, Xu et al. [107] reported the utilization of graphene/PANI ink for preparation supercapacitors through an inkjet printing methodology. Electrochemical measurements with a 1 M H2SO4 electrolyte yielded a maximum specific capacitance of 82 F g−1, power density of 124 kW kg−1, and energy density of 2.4 Wh kg−1 when a scan rate of 20 mV s−1 was applied. The fabricated supercapacitors were flexible and showed a long cycle life over 1000 cycles.
Fan et al. [191] reported the use of self-assembling sulfonated graphene (SG)/PANI nanocomposite paper for high-performance supercapacitors. Sulfonated graphene was found to act as a dopant for PANI. The SG/PANI nanocomposite papers had thin, lightweight, and flexible characteristics, and its supercapacitor devices showed excellent electrochemical performance with a ratio capacitance of 478 F g−1 at a discharge rate of 0.5 A g−1 and its capacitance retention rate maintained 88 % of its original capacitance after 2000 cycles.
21.4.2.3 Textile Supercapacitors Based on ECP/CNT Composites
Pan et al. [192] prepared a flexible and transparent wearable supercapacitor device based on aligned CNT fiber textiles and polyaniline (CNTFT/PANI) (Fig. 21.8). PANI was deposited on the CNTFT using an electrochemical polymerization method. The weight percentage of PANI was determined by the electron transfer numbers and controlled by the electrodepositing time during synthesis.
Schematic illustration of the fabrication of a supercapacitor textile based on CNT/PANI composite fiber. Reproduced with permission from [192]
The deposited PANI filled the voids among aligned CNTs with the critical weight of 50 %, above which the PANI started to aggregate into bulk particle material. The CNTFT/PANI composite was coated with PVA-H3PO4 gel electrolyte and two textile electrodes were stacked into a supercapacitor. It was found that the threshold weight percentage of PANI that provided the maximum capacitance is 50 % with observation of capacitance reduction above this value due to the pseudocapacitance property of PANI at high loads. The specific capacitance of the bare CNTFT was 7.7 F g−1, whereas the specific capacitances of the CNTFT/PANI composites were 108.5, 152.8, and 201.8–272.7 F g−1 corresponding to 15 %, 30 %, 40 %, and 50 % PANI in the composite, respectively. When the PANI weight percentage reaches 60 %, the specific capacitance was slightly reduced to 240.6 F g−1. The increased specific capacitance below 60 % was ascribed to the pseudocapacitive nature of PANI. However, beyond the critical point (60 %), PANI aggregates into particles which decrease the contact with aligned CNTs and, consequently, a reduction in charge transport. There was no obvious reduction in the capacitance of supercapacitor textiles when the current densities were increased, and hence, the supercapacitor can effectively work at a wide range of current density. The supercapacitor provided over 90 % retention of its capacity after 2000 charge/discharge cycles, which was attributed to the remarkable mechanical and electronic properties from aligned CNTs.
Furthermore, the supercapacitor textile was integrated to create a new energy textile that can convert solar energy to electrical energy instead of storing it, and a high entire photoelectric conversion and storage efficiency of 2.1 % was achieved. A combination of both photoelectric conversion (PC) and electrochemical storage was utilized for the fabrication of a wearable energy textile by stacking the PC part with the supercapacitor textile as illustrated in Fig. 21.9.
(a) Photograph of multilayered clothes; (b) schematic illustration of the integrated energy textile. The enlarged view shows the working mechanism. Reproduced with permission from [192]
21.4.3 Supercapacitor Textiles from Supercapacitor Fibers
Supercapacitors can be fabricated using fiber-shaped devices or textile devices. Compared to fiber-shaped devices, textiles provide the feasibility of making planar devices such as planar flexible supercapacitor textiles. Planar textile devices can provide specific mass capacitance of ≈3000 F g−1, while the fiber-shaped counterpart achieves ≈300 F g−1, 10 times lower than planar textile device [193]. For practical applications, planar textile supercapacitor devices provide enhanced performance in terms of stability, durability, and lifetime [193]. For example, a uniform layer of gel electrolyte is critical for fiber-shaped ECs. If the layer is too thick or not uniform, the two electrodes will make contact with each other, and hence, the fiber-shaped device cannot be operated normally. Regardless, as mentioned previously (Sect. 21.2), conductive textiles can be made by weaving or knitting supercapacitor fibers.
Wang et al. [194] reported the fabrication of high-performance two-ply yarn supercapacitor based on a CNT/PANI-NW array [194]. A CNT yarn was coated with PANI-NWs by dilute polymerization, which was followed by coating it with PVA gel electrolyte. In the last step, two CNT@PANI@PVA yarns were twisted together to form a two-ply yarn, the final threadlike supercapacitor. The two-ply yarn is a solid-state supercapacitor that retains the characteristics of pure CNT yarn for conventional textile processing, such as weaving and knitting. As shown in Fig. 21.10a, a plain weave model fabric was manually constructed from six two-ply yarn supercapacitors. Figure 21.10b shows the co-woven two-ply yarn supercapacitor with conventional textile yarns. The model fabric is composed of four conventional two-ply cotton yarns and four two-ply CNT@PANI@PVA yarn supercapacitors. The performance of the composite flexible supercapacitor was almost constant at different bending cycles. At the current density of 0.01 mA cm−2, the CNT@PANI yarn-based supercapacitor showed a capacitance of 38 mF cm−2, while the areal capacitance of the pure CNT yarn-based supercapacitor was only ≈2.3 mF cm−2. Due to improved capacitance, flexibility, and feasibility of integration with textiles, the two-ply yarn supercapacitor might be used for power storage in electronic textiles for applications that require conventional fabric-like breathability and durability.
Optical microphotographs: (a) a model woven energy storage device consisting of six two-ply yarn supercapacitors (reflection mode); (b) yarn supercapacitors are co-woven with conventional cotton yarns to form a flexible electronic fabric with self-sufficient power source. Reproduced with permission [194]
ECPs have been proposed for electrochromic applications due to its properties such as low operational voltage, ease in the fabrication of large area, the use of flexible substrates, high quality, optically transparent thin films, and excellent coloration contrast with matching rapid coloration rates [195]. Among the stated advantages, the possibility of using ECPs for the preparation of flexible electrochemical devices is very attractive for applications such as display devices and, particularly, flexible textile display devices [196].
Kelly et al. [58] reported the employment of PANI as solid-state electrochromic material in the application of flexible textile display. Nonwoven PET and viscose textiles were coated with PANI by in situ oxidative chemical polymerization using p-dodecylbenzenesulfonate as a doping agent. The electrochromic flexible display device was constructed by using four-layered sandwiched structure without the need for extra liquid electrolyte layers. The use of a solid electrochromic material offers a practical solution by preventing the leakage of liquid-phase electrochromic materials that require sufficient sealing for the fabricated device.
Recently, Chen et al. [197] reported the fabrication of a flexible electrochromic fiber-shaped supercapacitor. The fabrication process is illustrated in Fig. 21.11. Initially, the CNT sheet was wound onto an elastic rubber fiber. After that, PANI was electrochemically deposited onto the CNT sheet. The maximum specific capacitance based on the weight of CNTs and PANI was obtained at 70 % weight PANI (255 F g−1 at charge/discharge rate of 1 A g−1). At higher weight percentage of PANI (e.g., 90 %), the excess PANI was found coated on the outer surface of the CNT/PANI composite fiber electrode and could not effectively make contact with the aligned CNTs, which eventually led to slower and less effective ion diffusion. Figure 21.12 shows the utilization of the wire-shaped electrochromic supercapacitors for the fabrication of a woven textile that can act as displayer.
Schematic illustration of the structure and display function of the electrochromic, wearable fiber‐shaped supercapacitor. Reproduced with permission from [197]
Chromatic transitions during the charge–discharge process. (a) An energy storage textile woven from electrochromic fiber‐shaped supercapacitors during the charge–discharge process; (b and c) electrochromic fiber‐shaped supercapacitors that have been designed and woven to display the signs “+” and “F,” respectively. Reproduced with permission from [197]
Lee et al. [40] reported the utilization of a biscrolling technique for the fabrication of PEDOT/CNT composite supercapacitors for textiles and microdevice applications. The fabricated PEDOT/CNT redox supercapacitor yarn electrodes were made by a process called biscrolling (Sect. 21.2.3.4), which involves inserting a twist in a host sheet that is overlaid with the guest (Fig. 21.13). The biscrolled yarns were prepared by twist insertion in hundred-nanometer-thick ECP infiltrated MWNT sheets. The ECP yarn guest was deposited on MWNT sheets by vapor phase polymerization (VPP), which is a well-known technique for providing uniform coatings of highly ECP layers on substrates. The volumetric capacitance of the plied biscrolled yarn/Pt wire device was up to 179 F cm−3. The complete supercapacitor could be wound or sewn for 10,000 cycles with over 90 % performance retention rate (Fig. 21.14).
(a) Schematic illustration showing the fabrication of a biscrolled PEDOT/MWNT yarn. (b) Optical microscope image of the spinning wedge, which shows the wedge edges being twisted to form a dual-Archimedean scroll yarn, which is schematically illustrated in the inset. Scale bar, 200 mm [40]. Copyright 2013, Nature Publishing Group
Cyclic life and stability of solid-electrolyte biscrolled yarn supercapacitors when weaved into a glove (the yarn supercapacitor was 5 cm long). Reprinted with permission from [40]. Copyright 2013, Nature Publishing Group
Recently, Huang et al. [198] reported a novel method for the fabrication of large wearable energy storage textiles from industrially weavable and knittable highly conductive yarns. The highly conductive yarns were made from 316 L stainless steel thin fibers by the twist-bundle drawing technique (Fig. 21.15a). Stainless steel 316 L was selected due to its anticorrosion capability and thermal stability and flexibility of its micrometer-sized fibers compared to other types of stainless steel such as the brittle 304 stainless steel microfibers. The twisted long yarns have a small diameter of 180–250 μm and were found to be very soft and flexible as well, comparable to those conventional cotton yarns with higher tensile strength (>700 MPa), which facilitated the weaving and knitting process. The main advantage behind using metal-based conductive textiles is the ability of metal-based wires/yarns to provide effective long-distance electron transport [199]. As illustrated in Fig. 21.15b, the highly conductive yarns were prepared through a three-stage fabrication process. Initially, reduced graphene oxide (rGO) was introduced, which was followed by the electrodeposition of pseudocapacitive MnO2 and PPY conductive layers in the subsequent steps, respectively. The PPY layer is expected to serve as a stress buffer during the various deformations that are usually faced by textiles [179]. A freestanding all solid-state yarn supercapacitor was made from two such parallel yarn electrodes using a PVA/H3PO4 gel electrolyte that exhibited a long cycle life (>92 % device capacitance retention over 4950 cycles). The possibility of the practical use of the hierarchically structured conductive yarns for preparation of energy storage textiles through weaving (Fig. 21.15c) and knitting (Fig. 21.15d) processes was successfully demonstrated.
(a) Schematics of yarn fabrication; (b) illustration of the three-stage fabrication process of the hierarchically structured conductive yarn; (c and d) photographs of the energy storage textiles made of yarns (the 15 cm × 10 cm woven clothes can light 30 LEDs) (c), and a wristband knitted with a pattern (inset shows the pattern powering a LED) (d). Reproduced with permission from [198]
21.5 Conclusion
Among the different approaches for the preparation of smart conductive textiles, ECPs have been proposed as a promising material for the preparation of conductive textiles without affecting their original properties such as flexibility, stretchability, and tensile strength. ECP-based textiles can be utilized for different applications (Fig. 21.1) including flexible textile electrodes for supercapacitors and energy storage applications. In the case of ECP-based textile supercapacitors, the energy storage is provided by a pseudocapacitive mechanism through reversible Faradic redox reactions during fast doping–dedoping processes (Fig. 21.6). In general, ECP-based textiles can be prepared directly by a simple coating onto various shaped textile substrates or indirectly by knitting or weaving ECP-based composite fibers. In the latter case, the limiting factors for fabrication of conductive textile are the tensile strength and flexibility of the conductive composite fibers that reflect the ability of fibers to withstand during the knitting/weaving process without being broken apart.
Like other material, the use of ECPs for textile supercapacitors has both advantages and challenges. The main characteristics of ECP-based textile supercapacitors are (1) their inherent flexibility, (2) ease of coating into various types and shapes of textiles, (3) diverse methods for polymerization and embedding into various conductive/nonconductive textile substrates, (4) possibility of controlling their nanostructured morphology with nanoscale dimensions, (5) possibility of use without electrolyte, (6) high theoretical specific capacitance, (7) relatively low cost for industrial scale-up process, (8) possibility for a wide range of working potential window based on the employed electrolyte, (9) possibility for integration into high-performance composite/asymmetric hybrid supercapacitors, and (10) the possibility of use in flexible textile display devices.
The main challenges for utilization of ECP-based textile supercapacitors are (1) their limited cycling stability due to the swelling/shrinkage during charging/discharging process (Fig. 21.6), (2) lower conductivity when compared to metals and metal nanoparticle, and (3) lower capacitance when employed with gel electrolytes compared to liquid electrolytes.
21.6 Future Outlook
The above limitations of using ECP-based textile supercapacitors can be resolved by: (1) Increasing the specific capacitance of pure ECP-based textile supercapacitors by designing nanostructured polymer coatings with controlled nanoscale dimensions, porous structure, and high surface area for achieving both reduced path for ion diffusion and lower ion diffusion resistance. (2) Improving the limited cycling stability by using composite materials such as ECP/CC, ECP/graphene, ECP/CNTs, and ECP/metal oxides. (3) Preparation of ECP-based textile supercapacitors indirectly from ECP-coated fibers. This approach is expected to provide conductive fibers without affecting their flexibility and tensile properties, and hence, it offers an alternative choice for preparation of textile supercapacitors through weaving and knitting process. Various fibers can be explored including natural and synthetic fibers, metal fibers, and carbon-based fibers. (4) Preparation of ECP-based textile supercapacitors through different technologies such as inkjet/screen printing or preparation of ECP-based conductive fibers through fiber welding and biscrolling methodologies. The latter two approaches allow for incorporation of ECPs with a wide range of guest materials for enhancing conductivity, redox properties, or other essential properties that might enhance the performance of the target textile supercapacitor.
References
Stoppa M, Chiolerio A (2014) Wearable electronics and smart textiles: a critical review. Sensors 14(7):11957–11992
http://www.textileworld.com/Articles/2015/February/The_Rupp_Report-Successful_Technical_Textiles_Part_II (Last Accessed Dec 10, 2015)
Jinlian H et al (2012) A review of stimuli-responsive polymers for smart textile applications. Smart Mater Struct 21(5):053001
Stuart MAC et al (2010) Emerging applications of stimuli-responsive polymer materials. Nat Mater 9(2):101–113
Li Z et al (2014) Ag nanoparticle–ZnO nanowire hybrid nanostructures as enhanced and robust antimicrobial textiles via a green chemical approach. Nanotechnology 25(14):145702
Kowal K et al (2014) Biocidal effect and durability of nano-TiO2 coated textiles to combat hospital acquired infections. RSC Adv 4(38):19945–19952
Li Y-C et al (2010) Flame retardant behavior of polyelectrolyte−clay thin film assemblies on cotton fabric. ACS Nano 4(6):3325–3337
Horrocks AR (2011) Flame retardant challenges for textiles and fibres: new chemistry versus innovatory solutions. Polym Degrad Stab 96(3):377–392
Cain AA et al (2014) Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol Mater Eng 299(10):1180–1187
Carosio F et al (2014) Flame retardancy of polyester and polyester–cotton blends treated with caseins. Indus Eng Chem Res 53(10):3917–3923
Hu J (2007) Chapter 10 – Shape memory textiles. In: Hu J (ed) Shape memory polymers and textiles. Woodhead Publishing, Cambridge, pp 305–337
Mattila H (2015) Chapter 15 – Yarn to fabric: intelligent textiles. In: Sinclair R (ed) Textiles and fashion. Woodhead Publishing, Cambridge, pp 355–376
Hu J (2013) Chapter 10 – Shape memory finishing treatments for smart textiles. In: Hu J (ed) Advances in shape memory polymers. Woodhead Publishing, Cambridge, pp 259–280
Gugliuzza A, Drioli E (2013) A review on membrane engineering for innovation in wearable fabrics and protective textiles. J Membr Sci 446:350–375
Hsu P-C et al (2015) Personal thermal management by metallic nanowire-coated textile. Nano Lett 15(1):365–371
Wang X et al (2014) Smart hydrogel-functionalized textile system with moisture management property for skin application. Smart Mater Struct 23(12):125027
Yu D et al (2014) Modifying surface resistivity and liquid moisture management property of keratin fibers through thiol-ene click reactions. ACS Appl Mater Interfaces 6(2):1236–1242
Wang X et al (2015) Effect of surface modifications on the thermal and moisture behavior of wool fabric. Appl Surf Sci 342:101–105
Qi K et al (2006) Self-cleaning cotton. J Mater Chem 16(47):4567–4574
Qi K et al (2007) Facile preparation of anatase/SiO2 spherical nanocomposites and their application in self-cleaning textiles. J Mater Chem 17(33):3504–3508
Bedford N, Steckl A (2010) Photocatalytic self cleaning textile fibers by coaxial electrospinning. ACS Appl Mater Interfaces 2(8):2448–2455
Afzal S, Daoud WA, Langford SJ (2014) Superhydrophobic and photocatalytic self-cleaning cotton. J Mater Chem A 2(42):18005–18011
Lina MC, Alison BF (2014) Smart fabric sensors and e-textile technologies: a review. Smart Mater Struct 23(5):053001
Hang Q, Oleg S, Maksim S (2015) Flexible fiber batteries for applications in smart textiles. Smart Mater Struct 24(2):025012
Saeed SE-S et al (2014) Novel chitosan-ZnO based nanocomposites as luminescent tags for cellulosic materials. Carbohydr Polym 99:817–824
Zhang P et al (2015) Luminescent golden silk and fabric through in situ chemically coating pristine-silk with gold nanoclusters. Biomaterials 36:26–32
Yang H, Lightner CR, Dong L (2011) Light-emitting coaxial nanofibers. ACS Nano 6(1):622–628
Lv Z et al (2012) Highly efficient and completely flexible fiber-shaped dye-sensitized solar cell based on TiO2 nanotube array. Nanoscale 4(4):1248–1253
Yun MJ et al (2014) Highly flexible dye-sensitized solar cells produced by sewing textile electrodes on cloth. Sci Rep 4:5322
Liu Z et al (2015) Flexible electronics based on inorganic nanowires. Chem Soc Rev 44(1):161–192
Singh MK (2011) Flexible photovoltaic textiles for smart applications. INTECH Open Access, Rijeka, Croatia
Hu L et al (2011) Lithium‐ion textile batteries with large areal mass loading. Adv Energy Mater 1(6):1012–1017
Liu B et al (2012) Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett 12(6):3005–3011
Liu Y et al (2012) Flexible, solid electrolyte-based lithium battery composed of LiFePO4 cathode and Li4Ti5O12 anode for applications in smart textiles. J Electrochem Soc 159(4):A349–A356
Luo Y et al (2012) Seed-assisted synthesis of highly ordered TiO 2@ α-Fe 2 O 3 core/shell arrays on carbon textiles for lithium-ion battery applications. Energy Environ Sci 5(4):6559–6566
Cheng Q et al (2013) Folding paper-based lithium-ion batteries for higher areal energy densities. Nano Lett 13(10):4969–4974
Zhou X et al (2014) Cotton-templated fabrication of hierarchical SnO2 mesoporous microtubes as the anode material of lithium ion battery. Mater Lett 120:279–282
Laforgue A (2011) All-textile flexible supercapacitors using electrospun poly (3, 4-ethylenedioxythiophene) nanofibers. J Power Sources 196(1):559–564
Yue B et al (2012) Polypyrrole coated nylon lycra fabric as stretchable electrode for supercapacitor applications. Electrochim Acta 68:18–24
Lee JA et al (2013) Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nature Commun 4:1970–1977
Meng Y et al (2013) All-graphene core‐sheath microfibers for all‐solid‐state, stretchable fibriform supercapacitors and wearable electronic textiles. Adv Mater 25(16):2326–2331
Ma TY et al (2014) Phosphorus-doped graphitic carbon nitrides grown in situ on carbon-fiber paper: flexible and reversible oxygen electrodes. Angew Chem 54(15):4646–4650
Yang P et al (2014) Worm-like amorphous MnO2 nanowires grown on textiles for high-performance flexible supercapacitors. J Mater Chem A 2(3):595–599
Zhang D et al (2014) Core spun carbon nanotube yarn supercapacitors for wearable electronic textiles. ACS Nano 8(5):4571-4579
Wu Q et al (2010) Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 4(4):1963–1970
Bonfiglio A et al (2005) Organic field effect transistors for textile applications. IEEE Trans Inf Technol Biomed 9(3):319–324
Hamedi M, Forchheimer R, Inganas O (2007) Towards woven logic from organic electronic fibres. Nat Mater 6(5):357–362
Hamedi M et al (2009) Fiber‐embedded electrolyte‐gated field‐effect transistors for e‐textiles. Adv Mater 21(5):573–577
Bubnova O, Berggren M, Crispin X (2012) Tuning the thermoelectric properties of conducting polymers in an electrochemical transistor. J Am Chem Soc 134(40):16456–16459
Collins GE, Buckley L (1996) Conductive polymer-coated fabrics for chemical sensing. Synth Met 78(2):93–101
Babel A et al (2005) Electrospun nanofibers of blends of conjugated polymers: morphology, optical properties, and field-effect transistors. Macromolecules 38(11):4705–4711
Jung YS et al (2008) Nanowire conductive polymer gas sensor patterned using self-assembled block copolymer lithography. Nano Lett 8(11):3776–3780
Castano LM, Flatau AB (2014) Smart fabric sensors and e-textile technologies: a review. Smart Mater Struct 23(5):053001
Forzani ES et al (2004) A conducting polymer nanojunction sensor for glucose detection. Nano Lett 4(9):1785–1788
Shim BS et al (2008) Smart electronic yarns and wearable fabrics for human biomonitoring made by carbon nanotube coating with polyelectrolytes. Nano Lett 8(12):4151–4157
Shyamkumar P et al (2014) Wearable wireless cardiovascular monitoring using textile-based nanosensor and nanomaterial systems. Electronics 3(3):504–520
Cochrane C et al (2011) Flexible displays for smart clothing: Part I-Overview. Indian J Fibre Textile Res 36(4):422
Kelly FM et al (2013) Polyaniline: application as solid state electrochromic in a flexible textile display. Displays 34(1):1–7
Wang P-C et al (2013) Transparent electrodes based on conducting polymers for display applications. Displays 34(4):301–314
Abu-Thabit N, Basheer R (2014) Nanostructured conductive composite filter electrodes for water sterealization by application of low electrical current. In: Proceedings of the 1st international electronic conference on materials, 26 May–10 June 2014. Sciforum Electronic Conference Series, vol 1, b015. doi:10.3390/ecm-1-b015
Abu-Thabit NY, Basheer RA (2014) Synthesis of highly conductive cotton fiber/nanostructured silver/polyaniline composite membranes for water sterilization application. Mater Res Express 1(3):035010
Basheer R, Abu-Thabit N (2012) High-speed water sterilization using conducting polymer-metal nanoparticles composite. Abstracts of papers of the American Chemical Society, Washington, DC
Liu C et al (2013) Conducting nanosponge electroporation for affordable and high-efficiency disinfection of bacteria and viruses in water. Nano Lett 13(9):4288–4293
Liu C et al (2014) Static electricity powered copper oxide nanowire microbicidal electroporation for water disinfection. Nano Lett 14(10):5603–5608
Schoen DT et al (2010) High speed water sterilization using one-dimensional nanostructures. Nano Lett 10(9):3628–3632
Wang F et al (2010) A review of technology of personal heating garments. Int J Occup Saf Ergonom 16(3):387–404
Hsu P-C et al (2014) Personal thermal management by metallic nanowire-coated textile. Nano Lett 15(1):365-371
Matsunaga T et al (1992) Disinfection of drinking water by using a novel electrochemical reactor employing carbon-cloth electrodes. Appl Environ Microbiol 58(2):686–689
Carrott P et al (2001) Preparation of activated carbon fibres from acrylic textile fibres. Carbon 39(10):1543–1555
Ren Y, Lam DCC (2008) Properties and microstructures of low-temperature-processable ultralow-dielectric porous polyimide films. J Electron Mater 37(7):955–961
Mark JE (2009) Polymer data handbook, vol 27. Oxford University Press, New York, NY
Zeng W et al (2014) Fiber-based wearable electronics: a review of materials, fabrication, devices, and applications. Adv Mater 26(31):5310–5336
Hu L, Cui Y (2012) Energy and environmental nanotechnology in conductive paper and textiles. Energy Environ Sci 5(4):6423–6435
Wei Q (2009) Surface modification of textiles.Woodhead Publishing Limited, Cambridge, UK
Jur J et al (2011) Atomic layer deposition of conductive coatings on cotton, paper, and synthetic fibers: conductivity analysis and functional chemical sensing using “all‐fiber” capacitors. Adv Funct Mater 21(11):1993–2002
Marichy C, Bechelany M, Pinna N (2012) Atomic layer deposition of nanostructured materials for energy and environmental applications. Adv Mater 24(8):1017–1032
Wang K et al (2014) Conducting polymer nanowire arrays for high performance supercapacitors. Small 10(1):14–31
Abu-Thabit NY, Makhlouf ASH (2014) Chapter 17 – Recent advances in polyaniline (PANI)-based organic coatings for corrosion protection. In: Makhlouf ASH (ed) Handbook of smart coatings for materials protection. Woodhead Publishing, Cambridge, pp 459–486
Horng Y-Y et al (2010) Flexible supercapacitor based on polyaniline nanowires/carbon cloth with both high gravimetric and area-normalized capacitance. J Power Sources 195(13):4418–4422
Cheng Q et al (2011) Polyaniline-coated electro-etched carbon fiber cloth electrodes for supercapacitors. J Phys Chem C 115(47):23584–23590
Dubal DP et al (2012) Porous polypyrrole clusters prepared by electropolymerization for a high performance supercapacitor. J Mater Chem 22(7):3044–3052
Kuila BK et al (2009) Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties. Chem Commun 38:5749–5751
Stejskal J, Sapurina I, Trchova M (2010) Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog Polym Sci 35:1420–1481
Agarwal M, Lvov Y, Varahramyan K (2006) Conductive wood microfibres for smart paper through layer-by-layer nanocoating. Nanotechnology 17(21):5319
Jeon J-W, Kwon SR, Lutkenhaus JL (2015) Polyaniline nanofiber/electrochemically reduced graphene oxide layer-by-layer electrodes for electrochemical energy storage. J Mater Chem A 3(7):3757–3767
Guan H et al (2010) Polyaniline nanofibers obtained by interfacial polymerization for high-rate supercapacitors. Electrochim Acta 56(2):964–968
Hao Q et al (2011) Morphology-controlled fabrication of sulfonated graphene/polyaniline nanocomposites by liquid/liquid interfacial polymerization and investigation of their electrochemical properties. Nano Res 4(4):323–333
Zhang X et al (2004) Nanofibers of polyaniline synthesized by interfacial polymerization. Synth Met 145(1):23–29
Wu C-G, Bein T (1994) Conducting polyaniline filaments in a mesoporous channel host. Science 264(5166):1757–1759
Menon VP, Lei J, Martin CR (1996) Investigation of molecular and supermolecular structure in template-synthesized polypyrrole tubules and fibrils. Chem Mater 8(9):2382–2390
Choi SJ, Park SM (2000) Electrochemical growth of nanosized conducting polymer wires on gold using molecular templates. Adv Mater 12(20):1547–1549
Zhong W et al (2005) Synthesis of large‐area three‐dimensional polyaniline nanowire networks using a “soft template”. Macromol Rapid Commun 26(5):395–400
Pillalamarri SK et al (2005) Radiolytic synthesis of polyaniline nanofibers: a new templateless pathway. Chem Mater 17(2):227–229
Zhang X, Goux WJ, Manohar SK (2004) Synthesis of polyaniline nanofibers by “nanofiber seeding”. J Am Chem Soc 126(14):4502–4503
Li W, Wang H-L (2004) Oligomer-assisted synthesis of chiral polyaniline nanofibers. J Am Chem Soc 126(8):2278–2279
Chiou NR, Epstein AJ (2005) Polyaniline nanofibers prepared by dilute polymerization. Adv Mater 17(13):1679–1683
Zhang X et al (2006) Fibrillar growth in polyaniline. Adv Funct Mater 16(9):1145–1152
Zhang X, Manohar SK (2004) Polyaniline nanofibers: chemical synthesis using surfactants. Chem Commun 20:2360–2361
Li G, Jiang L, Peng H (2007) One-dimensional polyaniline nanostructures with controllable surfaces and diameters using vanadic acid as the oxidant. Macromolecules 40(22):7890–7894
Winther-Jensen B, West K (2004) Vapor-phase polymerization of 3, 4-ethylenedioxythiophene: a route to highly conducting polymer surface layers. Macromolecules 37(12):4538–4543
Najar SS, Kaynak A, Foitzik RC (2007) Conductive wool yarns by continuous vapour phase polymerization of pyrrole. Synth Met 157(1):1–4
Dall’Acqua L et al (2006) Vapour phase polymerisation of pyrrole on cellulose-based textile substrates. Synth Met 156(5):379–386
Lee H-H, Chou K-S, Huang K-C (2005) Inkjet printing of nanosized silver colloids. Nanotechnology 16(10):2436
Perelaer J et al (2009) One-step inkjet printing of conductive silver tracks on polymer substrates. Nanotechnology 20(16):165303
Jang J, Ha J, Cho J (2007) Fabrication of water‐dispersible polyaniline‐poly (4‐styrenesulfonate) nanoparticles for inkjet‐printed chemical‐sensor applications. Adv Mater 19(13):1772–1775
Chiolerio A, Bocchini S, Porro S (2014) Inkjet printed negative supercapacitors: synthesis of polyaniline‐based inks, doping agent effect, and advanced electronic devices applications. Adv Funct Mater 24(22):3375–3383
Xu Y et al (2014) Inkjet-printed energy storage device using graphene/polyaniline inks. J Power Sources 248:483–488
Kaempgen M et al (2009) Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett 9(5):1872–1876
Chen P et al (2010) Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res 3(8):594–603
Tortorich RP, Choi J-W (2013) Inkjet printing of carbon nanotubes. Nanomaterials 3(3):453–468
Huang L et al (2011) Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res 4(7):675–684
Kong D et al (2012) Temperature-dependent electrical properties of graphene inkjet-printed on flexible materials. Langmuir 28(37):13467–13472
Li J et al (2013) Efficient inkjet printing of graphene. Adv Mater 25(29):3985–3992
Le LT et al (2011) Graphene supercapacitor electrodes fabricated by inkjet printing and thermal reduction of graphene oxide. Electrochem Commun 13(4):355–358
Cummins G, Desmulliez MP (2012) Inkjet printing of conductive materials: a review. Circuit World 38(4):193–213
Walker SB, Lewis JA (2012) Reactive silver inks for patterning high-conductivity features at mild temperatures. J Am Chem Soc 134(3):1419–1421
Li D et al (2009) Conductive copper and nickel lines via reactive inkjet printing. J Mater Chem 19(22):3719–3724
Meoli D, May-Plumlee T (2002) Interactive electronic textile development: a review of technologies. J Textile Apparel Technol Manage 2(2):1–12
Kazani I et al (2012) Electrical conductive textiles obtained by screen printing. Fibres Textiles East Eur 90(1):57–63
Behabtu N et al (2013) Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 339(6116):182–186
Dalton AB et al (2003) Super-tough carbon-nanotube fibres. Nature 423(6941):703
Zhang M, Atkinson KR, Baughman RH (2004) Multifunctional carbon nanotube yarns by downsizing an ancient technology. Science 306(5700):1358–1361
Cong H-P et al (2012) Wet-spinning assembly of continuous, neat, and macroscopic graphene fibers. Sci Rep 2, 613
Dong Z et al (2012) Facile fabrication of light, flexible and multifunctional graphene fibers. Adv Mater 24(14):1856–1861
Xu Z et al (2013) Ultrastrong fibers assembled from giant graphene oxide sheets. Adv Mater 25(2):188–193
Cheng H et al (2013) Textile electrodes woven by carbon nanotube–graphene hybrid fibers for flexible electrochemical capacitors. Nanoscale 5(8):3428–3434
Sun H et al (2014) Novel graphene/carbon nanotube composite fibers for efficient wire‐shaped miniature energy devices. Adv Mater 26(18):2868–2873
Xu Z et al (2013) Highly electrically conductive ag‐doped graphene fibers as stretchable conductors. Adv Mater 25(23):3249–3253
Peng H (2015) Electrically conducting fiber. In: Fiber-shaped energy harvesting and storage devices. Springer, Berlin, pp 7–38
Bowman D, Mattes B (2005) Conductive fibre prepared from ultra-high molecular weight polyaniline for smart fabric and interactive textile applications. Synth Met 154(1):29–32
Pomfret SJ et al (2000) Electrical and mechanical properties of polyaniline fibres produced by a one-step wet spinning process. Polymer 41(6):2265–2269
Pomfret SJ et al (1998) Inherently electrically conductive fibers wet spun from a sulfonic acid–doped polyaniline solution. Adv Mater 10(16):1351–1353
Mottaghitalab V, Spinks GM, Wallace GG (2005) The influence of carbon nanotubes on mechanical and electrical properties of polyaniline fibers. Synth Met 152(1):77–80
Okuzaki H, Ishihara M (2003) Spinning and characterization of conducting microfibers. Macromol Rapid Commun 24(3):261–264
Okuzaki H, Harashina Y, Yan H (2009) Highly conductive PEDOT/PSS microfibers fabricated by wet-spinning and dip-treatment in ethylene glycol. Eur Polym J 45(1):256–261
Jalili R et al (2011) One-step wet-spinning process of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) fibers and the origin of higher electrical conductivity. Adv Funct Mater 21(17):3363–3370
Lima MD et al (2011) Biscrolling nanotube sheets and functional guests into yarns. Science 331(6013):51–55
Fang S et al (2012) Fabrication of biscrolled fiber using carbon nanotube sheet. US Patent No 8,968,756. Washington, DC: U.S. Patent and Trademark Office
Jost K et al (2014) Natural fiber welded electrode yarns for knittable textile supercapacitors. Adv Energy Mater 5(4):1–8
Haverhals LM et al (2010) Natural fiber welding. Macromol Mater Eng 295(5):425–430
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Yan J et al (2014) Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv Energy Mater 4(4):1-43
Yu G et al (2013) Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2(2):213–234
Zhang LL, Zhao X (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38(9):2520–2531
Frackowiak E, Beguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39(6):937–950
Zhu Y et al (2011) Carbon-based supercapacitors produced by activation of graphene. Science 332(6037):1537–1541
Cottineau T et al (2006) Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl Phys A 82(4):599–606
Deng W et al (2011) Electrochemical capacitors utilising transition metal oxides: an update of recent developments. RSC Adv 1(7):1171–1178
Lang X et al (2011) Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat Nanotechnol 6(4):232–236
Dubal D et al (2015) Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem Soc Rev 44(7):1777–1790
Qu Q et al (2012) Core–shell structure of polypyrrole grown on V2O5 nanoribbon as high performance anode material for supercapacitors. Adv Energy Mater 2(8):950–955
Zhang K et al (2015) Flexible and all-solid-state supercapacitors with long-time stability constructed on PET/Au/polyaniline hybrid electrodes. J Mater Chem A 3(2):617–623
Hu L et al (2010) Stretchable, porous, and conductive energy textiles. Nano Lett 10(2):708–714
Huang Y et al (2015) High-performance flexible supercapacitors based on mesoporous carbon nanofibers/Co3O4/MnO2 hybrid electrodes. RSC Adv 5(24):18952–18959
Jiang Y et al (2015) Flexible of multiwalled carbon nanotubes/manganese dioxide nanoflake textiles for high-performance electrochemical capacitors. Electrochim Acta 153:246–253
Zhang Z et al (2015) Superelastic supercapacitors with high performances during stretching. Adv Mater 27(2):356–362
Dong L et al (2015) High-performance compressible supercapacitors based on functionally synergic multiscale carbon composite textiles. J Mater Chem A 3(8):4729–4737
Shown I et al (2015) Conducting polymer‐based flexible supercapacitor. Energy Sci Eng 3(1):2–26
Naoi K, Simon P (2008) New materials and new configurations for advanced electrochemical capacitors. J Electrochem Soc 17(1):34–37
Shirakawa H, McDiarmid A, Heeger A (2003) Twenty-five years of conducting polymers. Chem Commun 2003(1):1–4
Novák P et al (1997) Electrochemically active polymers for rechargeable batteries. Chem Rev 97(1):207–282
Li FS et al (2015) A mechanically robust and highly ion‐conductive polymer‐blend coating for high‐power and long‐life lithium‐ion battery anodes. Adv Mater 27(1):130–137
Peng C et al (2008) Carbon nanotube and conducting polymer composites for supercapacitors. Prog Nat Sci 18(7):777–788
Li H et al (2009) Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J Power Sources 190(2):578–586
Chen W, Rakhi RB, Alshareef HN (2013) Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J Mater Chem A 1(10):3315–3324
Fusalba F et al (2001) Electrochemical characterization of polyaniline in nonaqueous electrolyte and its evaluation as electrode material for electrochemical supercapacitors. J Electrochem Soc 148(1):A1–A6
Tan YT et al (2013) Synthesis and electrochemical properties of hollow polyaniline microspheres by a sulfonated polystyrene template. J Appl Polym Sci 127(3):1544–1549
Lu YF et al (2007) Direct electrochemistry and bioelectrocatalysis of myoglobin at a carbon nanotube-modified electrode. Acta Phys Chim Sin 23(1):5–11
Yang H-S, Zhou X, Zhang Q (2005) Electrochemical performances of supercapacitor with polyaniline particles with hierarchy as active electrode material. Acta Phys Chim Sin 21(04):414–418
Xiong SX et al (2012) Covalently bonded polyaniline/fullerene hybrids with coral-like morphology for high-performance supercapacitor. Electrochim Acta 85:235–242
Gupta V, Miura N (2005) Electrochemically deposited polyaniline nanowire’s network – a high-performance electrode material for redox supercapacitor. Electrochem Solid State Lett 8(12):A630–A632
Wang K, Huang JY, Wei ZX (2010) Conducting polyaniline nanowire arrays for high performance supercapacitors. J Phys Chem C 114(17):8062–8067
Kim BC et al (2010) Preparation and enhanced stability of flexible supercapacitor prepared from Nafion/polyaniline nanofiber. Synth Met 160(1–2):94–98
Sharma R, Rastogi A, Desu S (2008) Pulse polymerized polypyrrole electrodes for high energy density electrochemical supercapacitor. Electrochem Commun 10(2):268–272
Zhang D et al (2011) Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J Power Sources 196(14):5990–5996
Ingram MD, Staesche H, Ryder KS (2004) ‘Activated’ polypyrrole electrodes for high-power supercapacitor applications. Solid State Ion 169(1):51–57
Yuan L et al (2013) Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ Sci 6(2):470–476
Yue B et al (2013) Electrochemically synthesized stretchable polypyrrole/fabric electrodes for supercapacitor. Electrochim Acta 113:17–22
Huang Y et al (2015) Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 11:518–525
Firoz Babu K, Siva Subramanian SP, Anbu Kulandainathan M (2013) Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr Polym 94(1):487–495
Carlberg J, Inganäs O (1997) Poly (3, 4‐ethylenedioxythiophene) as electrode material in electrochemical capacitors. J Electrochem Soc 144(4):L61–L64
Ryu KS et al (2004) Poly (ethylenedioxythiophene)(PEDOT) as polymer electrode in redox supercapacitor. Electrochim Acta 50(2):843–847
Stenger-Smith JD et al (2002) Poly (3, 4-alkylenedioxythiophene)-based supercapacitors using ionic liquids as supporting electrolytes. J Electrochem Soc 149(8):A973–A977
Alvi F et al (2011) Graphene–polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim Acta 56(25):9406–9412
Zhu Y et al (2010) Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater 22(35):3906–3924
Geim AK (2009) Graphene: status and prospects. Science 324(5934):1530–1534
Liu C et al (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10(12):4863–4868
Yan J et al (2010) Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 48(2):487–493
Yan J et al (2010) Preparation of graphene nanosheet/carbon nanotube/polyaniline composite as electrode material for supercapacitors. J Power Sources 195(9):3041–3045
Xu Y et al (2013) Screen-printable thin film supercapacitor device utilizing graphene/polyaniline inks. Adv Energy Mater 3(8):1035–1040
Fan T et al (2015) Self-assembling sulfonated graphene/polyaniline nanocomposite paper for high performance supercapacitor. Synth Met 199:79–86
Pan S et al (2014) Novel wearable energy devices based on aligned carbon nanotube fiber textiles. Adv Energy Mater 5(4):1–8
Peng H (2015) Fiber-shaped energy harvesting and storage devices. Springer, Berlin
Wang K et al (2013) High‐performance two‐ply yarn supercapacitors based on carbon nanotubes and polyaniline nanowire arrays. Adv Mater 25(10):1494–1498
Lu W et al (2002) Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 297(5583):983–987
Meunier L et al (2011) Flexible displays for smart clothing: Part II-Electrochromic displays. Indian J Fibre Textile Res 36(4):429
Chen X et al (2014) Electrochromic fiber‐shaped supercapacitors. Adv Mater 26(48):8126–8132
Huang Y et al (2015) From industrially weavable and knittable highly conductive yarns to large wearable energy storage textiles. ACS Nano. doi:10.1021/acsnano.5b00860
Lee MR et al (2009) Solar power wires based on organic photovoltaic materials. Science 324(5924):232–235
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Abu-Thabit, N.Y., Makhlouf, A.S.H. (2016). Smart Textile Supercapacitors Coated with Conducting Polymers for Energy Storage Applications. In: Hosseini, M., Makhlouf, A. (eds) Industrial Applications for Intelligent Polymers and Coatings. Springer, Cham. https://doi.org/10.1007/978-3-319-26893-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-26893-4_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26891-0
Online ISBN: 978-3-319-26893-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)