Abstract
Tropical forests are known centers of amphibian richness and endemicity. Within these forests, embedded wetlands, particularly streams and other lotic environments, are focal areas of amphibian diversity. Are specific streams or sections of streams relatively more important than others as reservoirs of species richness or endemicity? To address this question, we studied stream-dwelling amphibian assemblages within Gunung Mulu National Park, Sarawak, East Malaysia (Borneo). Six streams were selected, ranging from the headwaters of Sungei Tapin (1,800 m asl) to low elevation streams within the Sungei Melinau system, both tributaries of Sungei Tutoh. A 100 m transect was established at each stream, and standardized visual encounter surveys conducted along each transect at night. A cumulative sampling effort from 35 nights yielded a total of 262 individuals representing 41 amphibian species. Our results indicate greater endemicity in lower order streams (headwaters highest- 80 %) compared to higher order streams at lower elevations (500 m asl, 75 %; <200 m, 42–57 %). In contrast, species diversity and species richness were significantly greater at larger streams. Species composition at lower elevation streams were more similar to one another, but could be separated into discrete large stream- and small stream- assemblages. These results suggest that species composition of stream-dwelling amphibians is affected by stream elevation or stream width. Our results underline the importance of riparian habitats, especially forested headwaters, in harboring Bornean endemics, a number of which are on the global list of threatened species. These findings support prioritization of stream types and stream segments, especially within forested headwaters, as a regional conservation strategy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The island of Borneo is known as a mega-biodiversity center, harboring a biota that is remarkably lineage-rich (MacKinnon et al. 1996; Sodhi et al. 2004). At present, around 180 amphibians are known from the island, consisting 174 species of anuran amphibians and 6 species of caecilians (Das et al. 2014; Frost 2015). These figures reveal that Bornean amphibians are exceedingly rich and show high levels of endemism, with nearly 70 % (113 species) not recorded elsewhere. The island is also the type locality for 141 species of anuran amphibians, some widely distributed, others restricted to small parts of the island. An earlier study reported that a third of the fauna as specialists of montane/submontane environments, above 750 m asl (Inger and Stuebing 2005).
The island’s biodiversity is severely threatened by logging, conversion of forests into agricultural and other developments, which have destroyed large swathes of lowland rainforests (MacKinnon et al. 1996; Achard et al. 2002; Fuller et al. 2003; Curran et al. 2004). Ninety-eight species (60 %) are facing population declines, 27 species (17 %) show stable populations, and 38 species (23 %) are unassessed (IUCN 2014). These figures reveal that Bornean amphibian populations face high levels of decline, and 24.2 % of the species are classified in a threat category.
Tropical amphibians may utilize a wide range of microhabitat types, with some species showing specialisations for rocky stream, hilly terrain and non-riparian forested areas (Inger et al. 2002). Further, most highland frog species use similar microhabitats (Ramlah et al. 2002). Species may be segregated into ecological guilds in such tropical environments. Keller et al. (2009) recognised three distinct habitat guilds, comprising large stream species, waterfall and stream species, and calm stream (presumably slow-flowing) species. Faunal turnover with elevation is also known, with increasing elevation known to be related to both species diversity and richness (Das et al. 2007).
Located in northern Sarawak, East Malaysia, Gunung Mulu National Park is arguably the most spectacular National Park on Borneo. The Park, covering ca. 52,865 ha of forest, encompasses a remarkable diversity of vegetation, with 17 recognized vegetation zones. Elevational range in the Park is from 45 to 2,376 m asl. The region is drained by tributaries of two major river systems, the Limbang and the Baram, formed by numerous subcatchments (Walsh 1982) with numerous accessible established trails. The area is known to be home to over 100 species of amphibians (Malkmus 2002; Pui unpubl), making it arguably the richest amphibian hotspot in the Old World, and matching species-rich sites in the Neotropics (Donnelly and Guyer 1994). A large number of amphibian species on Mulu are, in fact, endemic to the mountain massifs (Dring 1983a, b, 1987; Inger et al. 1995; Dehling 2008, 2010, 2011). Despite the fact that trails are well established, the numerous perennial streams, intermittent and ephemeral water bodies, and the presence of intact vegetation and high amphibian diversity, spatial aspects of amphibian diversity remain unstudied. Even small-scale habitat assessment information is important for amphibian conservation because most these species are not widely distributed. This study addressed the following question: Are specific streams or sections of streams are more important than others as reservoirs of species richness or endemicity?
2 Methodology
2.1 Field Site
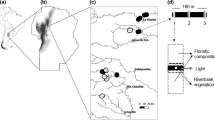
Field work was carried out at Gunung Mulu National Park (headquarters at 04.02.30.3N; 114.48.46.1E; 49 m asl; datum WGS84; Fig. 1) between 28 February 2008 and 31 March 2009. A total of six transect lines were established along streams (ca. 100 m long) (Fig. 2). Detailed descriptions of the survey sites are provided below following the forest classification of Anderson and Chai (1982). The Park’s vegetation was described by Anderson and Chai (1982) and Hazebroek and Abang Morshidi (2002). Its geology and geomorphology have been described by Waltham and Webb (1982) and Osmaston and Sweeting (1982), based on data from the Royal Geographical Society (RGS) Expedition to the Park in 1977–1978. GPS data were obtained with a Garmin® GPSMAP 60CSx device.
-
Site 1- Melinau Paku Stream, Camp 5. This site was located in the vicinity of Camp 5, with the 100 m stream transect established at the Melinau Paku Stream (starting point: N04o07.820′, E114o52.396′; 125 m asl). The stream has an average width of 15 m, clear water with moderately fast flowing current, and a rather open canopy along the established transect, although partially shaded along some portions of the stream. The stream bed was dominated by pebbles, and the banks covered by shrubs and saplings
-
Site 2- Sungei Nipa, Camp 1. This site was located in the vicinity of Camp 1 ca. 15 m from the Camp 1 hut. A 100 m transect was established at Sungei Nipa (starting point: N04o02.765′, E114o51.373′; 250 m asl). The stream had an average width of 12 m and a gradient of ca. 6–7o, with an open canopy and clear and fast-flowing water currents. It is surrounded by undisturbed mixed diterocarp forest. The stream substrate consists of gravel, boulders, and pebbles. The stream banks are of moderate height, often covered by saplings and fallen logs. This stream is referred to as ‘Likoh Nipa’ by the Berawan ethnic group (Proctor 1982).
-
Site 3- Small Stream, Camp 1. A 100 m length of stream transect was established along a small stream that is located at Camp 1 (starting point: N04o03.077′, E114o51.699′; 250 m asl). The stream is surrounded by undisturbed mixed dipterocarp forest on moderately hilly terrain, with an average width of 1.5 m; large forest-floor with numerous permanent small pools are found at 80–90 m along the transect. The stream has a shaded canopy, and the banks dominated by shrubs and saplings.
-
Site 4- Small Stream, Sarawak Chamber Trail. An ca. 100 m transect was established along a small stream that cuts across the Sarawak Chamber Trail (starting point: N04o03.572′, E114o51.451′; 245 m asl). The stream is surrounded by undisturbed mixed dipterocarp forest, with average width of 3 m, and has slow-flowing current with clear water and substrate composed of sand, leaf litter, gravel, and pebbles. The stream banks are abundantly clad with shrubs and saplings, and the canopy extensive, producing a substantial amount of shade along the transect.
-
Site 5- Small Hill Stream, Camp 2. This site is located in the vicinity of Camp 2. A ca. 50 m stream transect was established. This stream could be characterized as a moderate gradient (8–9o) hill stream (starting point: N04o02.401′, E114o52.403′; 540 m asl). The stream width averaged 2 m, minor torrents and shallows, and containing pebbles and pools. Bedrock is exposed along the stream, the banks dominated by shrubs and saplings, the canopy moderate, resulting in partial shade.
-
Site 6- Headwaters of Sungei Tapin, Camp 4. This site is located in the vicinity of Camp 4, where a ca. 100 m length of transect was established along the stream (starting point: N04o02.323, E114o54.542; 1,600–1,700 m asl). The stream is small, steep, and rocky, and has an average width of 2 m, with clear and moderately fast current. The streambed is dominated by bedrock and gravel, with a few fallen logs, the banks dominated by herbaceous and epiphytes, and a few saplings with branches that overhung the stream. The stream canopy was extensive, and the site was rather shaded along the established transect.
2.2 Data Collection and Analysis
Standardised visual encounter surveys (VES) were conducted along each transect between 1900 and 2200 h, in order to determine the species diversity, species richness, and relative abundance of amphibians within the selected streams. A species relative abundance (RA) was estimated by total number of individuals per species divided by the total number of captures. During these surveys, amphibians were observed by walking slowly along the established transect line using headlights; all amphibians encountered were collected (see Crump and Scott 1994; Heyer et al. 1994). Nomenclature of species follows Frost (2015). Voucher specimens have been deposited at the Museum of the Institute of Biodiversity and Environmental Conservation, UNIMAS.
The occurrence of amphibian species during each census at each sites was used to generate species accumulation curves. The total number of species richness was estimated from the census data using Jackknife and Chao estimators by carrying out 100 random re-ordering of censuses in Estimate S 9.1.0 (Colwell et al. 2004). PAST (Palaeontological Statistics) version 1.96 (Hammer et al. 2001) was used to generate the diversity indices (Shannon-Wiener Index and the Simpson’s Diversity Index), and diversity t-tests were used to detect significant differences in species diversity among the sites. Unweighted Pair-Groups Method Average (UPGMA) cluster analysis was conducted following hierarchical agglomerative clustering, based on the Bray-Curtis Similarity Coefficient (Bray and Curtis 1957) on presence/not detected data of species composition across sites, using the Multivariate Statistical Package (MVSP) program by Kovach (1998).
3 Results and Discussion
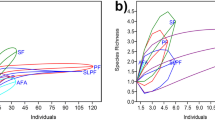
The cumulative 35 night transect sampling effort yielded a total of 262 individual amphibians comprising 41 species belonging to 7 families at the 6 sites: Megophryidae with 7 species; Microhylidae with 1 species; Ranidae with 11 species; Rhacophoridae with 5 species; and Bufonidae and Dicroglossidae with 8 species each (Table 1). Species accumulative curves are shown in Fig. 2 for each sampling sites, suggested additional species (in the range of 1–3 species) are expected from each site. Species richness was greatest at Sungei Nipa (S2) with estimated of 18 species. The Melinau Paku (S1) with 17 species, followed by the headwaters of Sungei Tapin (13 species), the hilly small stream at Camp 2 and the small stream at Sarawak Chamber (9 species) and the small stream at Camp 1 (8 species). Estimated species richness in this study are in the range 8–17, therefore species richness in most of the sites match those studied by Inger and Voris (1993).
In terms of overall species relative abundance (RA), Staurois latopalmatus had the greatest percentage of relative abundance (RA = 12.21 %). This species was followed by Feihyla kajau (RA = 7.25 %), Ansonia hanitschi (RA = 6.49 %), and Chalcorana raniceps, Limnonectes ibanorum and L. kuhlii (each with RA = 5.34 %). At some of the survey sites, certain species showed relatively high abundance and dominate the amphibian community. For instance, S. latopalmatus was abundant at both large streams (Sungei Nipa, S2; and, Melinau Paku Stream, S1). In contrast, the small stream at Camp 1 (S3) at low elevation was dominated by Feihyla kajau, and the small stream on the Sarawak Chamber trail (S4) was dominated by Leptobrachella juliandringi. Furthermore, the headwaters of Sungei Tapin (S6, 1,600 m asl) was dominated by Ansonia hanitschi (Table 1). The hilly small stream at Camp 2 appeared had no obvious dominant species, with species being more evenly distributed (S5; evenness = 0.9137).
When the local distributions of threatened or endemic species of each stream were evaluated, the endangered species Ansonia platysoma was found at the hilly small stream at Camp 2 (S5). Another vulnerable species, Leptobrachella brevicrus, occurred in the headwaters of Sungei Tapin (S6), while Leptobrachella parva was found to occur at the Sungei Nipa at Camp 1 (S2). For endemism, the headwaters of Sungei Tapin, located at high elevations (1,600 m), had the greatest percentage of endemism (80 %) compared to sites at lower elevations, including a stream at Camp 2 (500 m asl, 75 %) and other low elevation streams (>200 m, 42–57 %). High percentages of endemism (80 %) at high elevational streams (1,600 m) suggest that amphibian endemicity at Mulu is most likely concentrated along higher elevation forested streams.
In terms of species diversity, Sungei Nipa (S2) was found to be the highest (H′ = 2.333, D-1 = 0.8544), with the Melinau Paku stream (S1) slightly lower for Shannon-Wiener’s (H′), but higher for Simpson’s (1-D) (H′ = 2.198, D-1 = 0.857). They were, in turn, followed by the small hill stream at Camp 2 (S5) (H′ = 1.989, D-1 = 0.857), the headwaters of Sungei Tapin (S6) (H′ = 1.935, D-1 = 0.7975), the small stream at the Sarawak Chamber Trail (S4) (H′ = 1.677, D-1 = 0.759), and the small stream at Camp 1 (S3) (H′ = 1.236, D-1 = 0.5848). The Shannon diversity t-test indicate that only the small stream at Camp 1 (S3) differed significantly in diversity from the other streams (p < 0.05), except for the small stream at the Sarawak Chamber Trail (S4) (p = 0.055). This study demonstrate that large streams have greater species diversity and richness compared to small streams (Fig. 3). This may be attributed to the ability of large stream landscapes to provide favorable breeding habitats for numerous species, as well as for tadpole microhabitat/diet requirements. Some species (such as Huia cavitympanum and Meristogenys spp.) breed only in swift-flowing waters and their tadpoles live in the strongest of currents. Large streams are able to hold water for longer periods, which is important for species with long period of tadpole development, such as in species of Limnonectes and Hylarana, and S. latopalmatus that are restricted to clear, swift and rocky streams (Parris and McCarthy 1999; Inger and Stuebing 2005; Keller et al. 2009). Many of these species are found abundant along stream banks, and two of these, Pedostibes hosii and Leptobrachella parva, occasionally form large calling groups. This suggests that the availability of breeding habitats is a major causal factor for high species diversity and richness regionally. Nevertheless, other factors need to be considered. Forest landscapes, disturbance levels, availability of resources, competition, elevational and habitats types are known to influence species diversity and species composition (Duellman 1997; Faruk et al. 2013; Konopik et al. 2015).
Amphibian assemblages in selected streams at Mulu are found to be distinctly clustered into two major ecological groups (similarity coefficient = 0; Fig. 4). The fauna of survey sites at large streams (S1 and S2) are closely related to each other, and separate from smaller streams at lower elevations. The amphibian assemblage at the headwaters of Sungei Tapin, a first order stream, located at 1,600 m, shows a complete species turnover compared to those of higher order, or streams at lower elevations (below 500 m). These results suggest that species composition of higher order streams are more similar to each other, albeit differentiable into discrete large stream- and small stream- assemblages. Thus, species composition of stream-dwelling amphibians is affected by stream elevation and/or stream width.
4 Conclusion
This study highlights the role of upland forests streams as important refuges for Bornean endemic amphibians, and a majority of the endemic species at Mulu were found in such regions. These results coincided with numerous works that document highlands as refuges for many endemic amphibians globally. Species composition of stream-dwelling amphibians is affected by both stream elevation and stream width. Our results underline the importance of riparian habitats, especially low order streams in forested headwaters, in harbouring Bornean endemics, a number of which are on the global list of threatened species. They further support prioritization of stream types and stream segments, especially within the forested headwaters, as a regional conservation strategy.
References
Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreu J-P (2002) Determination of deforestation rates of the world’s humid tropical forests. Science 297:999–1002
Anderson JA, Chai PP-K (1982) Vegetation. Sarawak Mus J 51:196–206
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27(4):325–349
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Crump ML, Scott NJ (1994) Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek L-AC, Foster MS (eds) Measuring and monitoring biological diversity, standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 84–92
Curran LM, Trigg SN, McDonald AK, Astiani D, Hardiono YM, Siregar P, Caniago I, Kasischke E (2004) Lowland forest loss in protected areas of Indonesian Borneo. Science 303:1000–1003
Das I, Jankowski A, Makmor MI, Haas A (2007) Species diversity, elevational distribution and reproductive modes in an amphibian community at the Matang Range, Sarawak (Borneo). Mitt Zool Mus Hamburg 104:141–174
Das I, Tuen AA, Pui YM, Ong JJ (2014) The Bornean Frog Race- raising conservation awareness on amphibians of Sarawak and Malaysia. Herpetol Rev 45:66–73
Dehling JM (2008) A new treefrog (Anura: Rhacophoridae: Rhacophorus) from Gunung Mulu, Borneo. Salamandra 44:193–205
Dehling JM (2010) A new bush frog (Anura: Rhacophoridae: Philautus) from Gunung Mulu National Park, East Malaysia (Borneo). Salamandra 46:63–73
Dehling JM (2011) A new karst-dwelling species of Kalophrynus (Anura: Microhylidae) from Gunung Mulu National Park, Borneo, Malaysia. Zootaxa 2737:49–60
Donnelly MA, Guyer C (1994) Patterns of reproduction and habitat use in an assemblage of neotropical hylid frogs. Oecologia 98:291–302
Dring J (1983a) Frogs of the genus Leptobrachella (Pelobatidae). Amphibia-Reptilia 4:89–102
Dring J (1983b) Some new frogs from Sarawak. Amphibia-Reptilia 4:103–115
Dring J (1987) Bornean treefrogs of the genus Philautus (Rhacophoridae). Amphibia-Reptilia 8:19–47
Duellman WE (1997) Amphibians of La Escalera Region, southeastern Venezuela: taxonomy, ecology, and biogeography. Sci Pap Nat Hist Mus Univ Kansas 2:1–52
Faruk A, Belabut D, Ahmad N, Knell RJ, Garner TWJ (2013) Effects of oil‐palm plantations on diversity of tropical anurans. Conserv Biol 27:615–624
Frost DR (2015) Amphibian species of the world: an online reference. Version 6.0. Electronic database. American Museum of Natural History, New York. Available from: http://research.amnh.org/vz/herpetology/amphibia/index.php. Accessed 5 Aug 2015
Fuller DO, Jessup TC, Salim A (2003) Loss of forests cover Kalimantan, Indonesia, since the 1997–1998 El Niño. Conserv Biol 18:249–254
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: palaeontological statistics software package for education and data analysis. Palaentologica Electonica 4:1–9
Hazebroek HP, Abang Morshidi AK (2002) A guide to Gunung Mulu National Park: a world heritage site in Sarawak, Malaysia Borneo. Natural History Publications (Borneo), Kota Kinabalu
Heyer WR, Donnelly MA, McDiarmid RW, Hayek L-AC, Foster MS (1994) Measuring and monitoring biological diversity. Standard methods for amphibians. Smithsonian Institution Press, Washington, DC
Inger RF, Stuebing RB (2005) A field guide to the frogs of Borneo, 2nd edn. Natural History Publications (Borneo) Sdn Bhd, Kota Kinabalu
Inger RF, Voris HK (1993) A comparison of amphibian communities through time and from place to place in Bornean forests. J Trop Ecol 9:409–433
Inger RF, Stuebing RB, Tan F-L (1995) New species and new records of anurans from Borneo. Raffles Bull Zool 43:115131
Inger RF, Tan F-L, Yambun P (2002) The frog fauna of three parks in Sabah, Malaysia—Kinabalu Park, Crocker Range Park, and Tawau Hills Park. Sabah Parks Nat J 3:728
IUCN (2014) The IUCN red list of threatened species. Version 2015.2. Electronic database. IUCN- The World Conservation Union. Available from: http://www.iucnredlist.org. Accessed 5 Aug 2015
Keller A, Rödel M-O, Linsenmair KE, Grafe TU (2009) The importance of environmental heterogeneity for species diversity and assemblage structure in Bornean stream frogs. J Anim Ecol 78:305–314
Konopik O, Ingold S-D, Grafe TU (2015) Effects of logging and oil palm expansion on stream frog communities on Borneo, Southeast Asia. Biotropica 47:636–643
Kovach WL (1998) MVSP- A multivariate statistical package for Windows, ver. 3.0. Konvack Computing Services, Pentraeth
MacKinnon K, Hatta G, Halim H, Mangalir A (1996) The ecology of Indonesia series volume III: the ecology of Kalimantan, Indonesia Borneo. Periplus Editions Ltd, Hong Kong
Malkmus R (2002) Die Amphibien des Mulu-Nationalparks in Sarawak/Malaysia. Natur Mus 32:93–105
Osmaston HA, Sweeting MM (1982) Geomorphology. Sarawak Mus J 51:75–94
Parris KM, McCarthy MA (1999) What influences the structure of frog assemblages at forest streams? Aust J Ecol 24:495–502
Proctor J (1982) Place name. In: Jermy AC, Kavanagh P (eds) Gunung Mulu National Park, Sarawak: an account of its environment and biota being the results of The Royal Geographical Society/Sarawak Government Expedition and Survey 1977–1978 (Part I). Sarawak Mus J 52:17–27
Ramlah Z, Wasly L, Ali H (2002) An account of anuran at Crocker Range National Park, Sabah, Malayia. In: Ismail G, Ali L (eds) A scientific journey through Borneo. Crocker Range National Park, vol 1, Natural ecosystem and species components. ASEAN Academic Press, London, pp 137–146
Sodhi NS, Koh LP, Brooke BW, Ng PKL (2004) Southeast Asian biodiversity; an impending disaster. Trends Ecol Evol 19(12):654–660
Sweeting M (1980) Symposium on the geomorphology of the Mulu Hills. Geogr J 146(1):1–50
Walsh RP (1982) Hydrology and water chemistry. Sarawak Mus J 51(2):121–182
Waltham AC, Webb B (1982) Geology. Sarawak Mus J 51:68–74
Acknowledgments
This study was funded by a Fundamental Research Grant, FRGS/06(10)667/2007(32), from the Ministry of Higher Education, Government of Malaysia. We thank the Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, for support. At Mulu, we remain grateful to Brian and Sue Clarke and their staff at the Park Headquarters, and to the Sarawak Forest Department for a research permit (NPW907.4.2 (IV)–7). We are grateful to our colleague, Jongkar Grinang for his assistance in preparing Fig. 1. Finally, we thank C. Kenneth Dodd and Ulmar Grafe for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pui, Y.M., Das, I. (2016). Streams in Forested Headwaters as Reservoirs of Endemicity in Bornean Amphibians. In: Das, I., Tuen, A. (eds) Naturalists, Explorers and Field Scientists in South-East Asia and Australasia. Topics in Biodiversity and Conservation, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-319-26161-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-26161-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26159-1
Online ISBN: 978-3-319-26161-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)