Abstract
Logging and human-induced conversion of natural forests into agricultural areas are major drivers of biodiversity loss in the tropics. Anuran larvae can be highly diverse, can reach high biomass and can play important roles in tropical streams; yet, compared to the adult frog communities, relatively little is known about how larval communities respond to disturbance. Information on larvae is highly relevant for amphibian conservation because larvae represent direct evidence of breeding and thus provide a good indicator of species persistence in disturbed habitats. We studied tadpole assemblages in Ranomafana, southeastern part of Madagascar, in streams in a disturbed forest (previously logged forest), at “forest edge” (streams embedded in matrix nearby forest blocks), and compared these to communities in a primary forest. We sampled tadpoles at the microhabitat level (“pools” and “riffles”) in 9 streams. We recorded 27 species with a maximum of 17 species/stream recorded at edge. The three habitats harbored different assemblages, but, as could be expected, more similarities existed among forest habitats than between forest and non-forest habitats. The most and the least diverse communities were recorded at edge and in the disturbed forest, respectively. Assemblages were dominated by one generalist species, and changes in communities were mostly driven by changes in forest specialists, which either decreased in disturbed forest or were replaced by edge specialists outside forest. Although species richness varied, relative abundances were maintained among habitats, suggesting potential compensatory mechanisms in tadpole biomass. Community structure changed at the microhabitat level: pool environments usually harbored relatively higher species richness and abundance than riffles. Our study highlights the relevance of edge habitats for maintaining amphibian diversity and the pronounced negative effects of past logging activities on tadpole communities. Given the diverse roles of tadpoles in streams, changes in community structure potentially affect critical stream ecosystem processes. The study has strong implications for designing buffer zones around protected areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the extent of primary forests is shrinking throughout the tropics (Gibson et al. 2011), and given the insufficient protection provided by reserves (Coad et al. 2019), there is increasing interest in quantifying the biodiversity values of disturbed habitats (Edwards et al. 2014; Laurance et al. 2014). This has particularly been the case for amphibians (e.g., Cushman 2006; Kurz et al. 2014; Riemann et al. 2015; Ndriantsoa et al. 2017) in lights of their alarming global population declines and considering that at least 30% of species in this taxonomic group are facing extinction (Stuart et al. 2004).
Most studies on amphibian disturbance ecology have typically tended to focus on the adult stage (Ernst and Rödel 2005, 2008; Gardner et al. 2007; Riemann et al. 2015; Ferreira et al. 2016). Relatively next to nothing is known about the effects of disturbance on larval communities, or, reciprocally, the values of disturbed habitats for amphibian breeding and maintenance. In general, studies on tropical tadpole community ecology are scarce (see review in Borges Júnior and Rocha 2013), and still little information is available for the larval stage of many tropical amphibians, even for some purportedly abundant species (Wells 2010). Moreover, there has been an idiosyncratic assumption that tadpole communities would simply match the adult community present at a site; this argument may explain the difference in research pace between these two communities. Although reciprocal influences on communities of adult and larval stages have been documented (Inger et al. 1986), this assumption is not satisfactory because adults can be observed from habitats where no breeding takes place (Skelly and Richardson 2010) and not all frog species, in the tropics in particular, have their tadpoles develop in water bodies (Wells 2010).
Larvae represent concrete evidence of breeding and provide good indicators of species persistence in modified landscape. Thus, information on larvae is highly relevant for assessing the quality of disturbed habitats. Larval surveys are less likely to overestimate breeding distribution (Skelly and Richardson 2010) and can provide critical information on population trajectories and the factors that may affect abundance, distribution, and assemblages (Skelly and Richardson 2010). In contrast to the adults that can be cryptic and for which detection rate can considerably vary with sampling efforts, climate, or calling activities (Vonesh et al. 2010), tadpoles’ detection rate can be relatively high in a relatively defined small area (Skelly and Richardson 2010), making studies on larvae highly pertinent for characterizing amphibian community.
The tropical forests of Madagascar are among the most biologically rich and unique of the world (Harper et al. 2007). More than 90% of Madagascar endemic animal species live exclusively in forest and woodland habitats (Irwin et al. 2010). As for other tropical countries (Burivalova et al. 2014; Laurance et al. 2014), habitat loss, mainly due to logging and forest conversion into agricultural areas (e.g., slash-and-burn agriculture), is a major threat to this biodiversity (Irwin et al. 2010).
Ranomafana, in the southeastern part of Madagascar, represents a model system for studies on the effects of habitat disturbance on biological communities (Razafimahaimodison 2004; Tecot 2008; Herrera et al. 2011; Gerber et al. 2012; Riemann et al. 2015). One part of Ranomafana National Park was selectively logged approximately 30 years ago but has become a protected area ever since. Selective logging negatively impacted forest structure by reducing basal area (m2/ha) by 53%, mean crown volume (m3) by 17%, and average tree height by 12% (Ramaharitra 2006; Tecot 2008). The other parts of the park are relatively less disturbed and could be still considered as primary forests (Tecot 2008). Adjacent to the park are matrix, namely agricultural areas dominated by rice paddy fields, rainfed crops, and banana plantations. Matrix, although it is often highly disturbed, might provide valuable habitat for some amphibian species (Ndriantsoa et al. 2017) and, hence, could be an important component of biodiversity maintenance on a landscape scale. The differences in ecological conditions over short distances in Ranomafana makes it an ideal study site as small-scale contrasts are more sensitive at detecting ecological determinants than comparisons made on larger scales that were often performed in previous amphibian studies (Parris 2004; Ernst et al. 2006).
Ranomafana is characterized by its high amphibian diversity with no less than 112 candidate frog species (Vieites et al. 2009), and at least 45% of frogs of Ranomafana National Park reproduce in forest streams to form the world richest stream tadpole assemblages (Strauß et al. 2010), with up to 25 species found within a single stream (Strauß et al. 2013). We took advantage of this established scenario to study the changes in tadpole assemblages in streams in a disturbed forest (formerly logged forest), a habitat matrix (streams at the interface between forest block and agricultural landscape), and compared these to assemblages in streams in a primary forest. Because isolating the effects of logging from other confounding disturbance effects (e.g., tourism, invasive species, cyclone) is difficult, we arbitrary referred this habitat as “disturbed forest” although logging has been known to be the major disturbance recorded in that forest (Tecot 2008).
Small changes in vegetation structure can create significant alterations to amphibian communities (Cortés-Gómez et al. 2013), and logging, even when conducted selectively, can bear dramatic effects on amphibians, especially on forest specialists for which life-history typically rely on forest habitats (Burivalova et al. 2014; Ferreira et al. 2016). We asked the following questions: do the three habitats harbor similar assemblages? Do communities change at the microhabitat level within and among habitats? Do assemblages seasonally vary across habitats? We expected to find the most diverse community (high species richness and relative abundance) in the least disturbed habitat, i.e., primary forest and the least diverse at edge because of the relative high frequency of disturbance (e.g., frequent slash-and-burn). We also predicted that assemblages would change at the microhabitat level as suggested by earlier habitat-relationship models (Strauß et al. 2013). Last, we expected to find different assemblages at different sampling periods, as previously recorded for tadpole communities in Ranomafana (Strauß et al. 2016).
Materials and methods
Ranomafana National Park (RNP) comprises 43,500 ha of continuous mid-altitude mountain rainforest (500–1300 m a.s.l.). Precipitations are high, with alternating periods of low and heavy rains with an annual precipitation between 1700 and 4300 mm (Wright and Andriamihaja 2003). Periods of heavy rains typically occur between January and April. As a result of slash and burn agriculture, landscapes outside RNP consist of forest fragments embedded in a matrix of cultivated land (e.g., banana and rice paddy fields) and secondary vegetation (i.e., grasslands with bush and shrub vegetation).
Sampling procedures
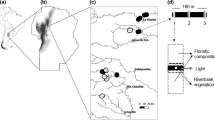
We sampled tadpoles in 9 streams, 3 at each habitat: primary forest (Vatoharanana), disturbed forest (Talatakely, previously logged forest), and in “matrix” (Ambatolahy) (Fig. 1). “Matrix streams” consisted of streams that crossed nonforested areas, embedded in agricultural areas (rice fields, rainfed crops, banana fields), with adjacent riparian vegetation consisting of small trees, bushes, and strawberry guava (generally less than 5 m on each bank). Nearest forest habitat to these matrix streams was at least 50 m aerial distance. To avoid ambiguity, we refer this type of habitat as “edge” in this study. Note that “edge” here may differ from its classical ecological definition because these streams were not directly adjacent to forest habitat but were more embedded in matrix habitats. These replicates per habitat were realistically the maximum number of streams that met the objectives of the study and were within the same range of altitude (900–1020 m a.s.l). Samplings were conducted in late October–early November 2014 and in March 2015, namely at the beginning and in the later part of the rainy season (Strauß et al. 2016).
The streams were second-order streams and were 2.1–3.05 m width. Each stream was distant of at least 200 m with no direct connection between them; so, tadpoles from one stream could not be washed away to another stream. Mean water temperatures were 18–19 °C during the study.
The general sampling procedure followed the methods of Keller et al. (2009) in which we studied assemblages at the microhabitat level (stream section). This was done because amphibian assemblage can strongly vary within few meters in streams (Keller et al. 2009), and our field observations along with previous studies indicated that microhabitat heterogeneity can strongly structure tadpole assemblages (Inger et al. 1986; Eterovick and Barata 2006; Afonso and Eterovick 2007; Eterovick et al. 2010; Borges Júnior and Rocha 2013; Strauß et al. 2013). In each stream, we sampled tadpoles in 4 pools and 4 riffles, each representing section of 2.5 m with at least 10-m stream distance separating two consecutive “microhabitat-sites”. Pools represented sections with debris loading and slow-flowing water; riffles designated habitats with relatively fast flowing stream section with the substrate dominated by pebbles. The “microhabitat-sites” were not chosen systematically (i.e., fixed distance between microhabitats) but rather at random with irregular intervals to cover habitat heterogeneity (substrate, water velocity, canopy openness, water depth, characteristics of the surrounding vegetation). We sampled tadpoles using dipnets of different sizes, adjusted to obtain optimal sampling results for each microhabitat. An important component of the fieldwork was to standardize sampling effort that would allow estimating tadpole relative abundance. Sampling consisted of dipnetting tadpoles in microhabitats within 4 min (time was stopped during sample processing), to provide per-unit-time density estimates following (Werner et al. 2007). We assumed that all tadpoles within the microhabitat were caught because we often did not catch any more individuals at the end of each sampling. Samplings were always conducted in the morning. We ensured that no tadpoles moved from one microhabitat to the next one by always starting sampling downstream.
The tadpoles were kept alive and were brought back to the laboratory. The tadpoles were sorted into series based on morphological differentiation. Because of the high number of species and our inability to distinguish all species, we assigned series provisional numbers. We took specimen of each series and after anesthetization by Tricaine Methanesulfonate (MS-222), took a fragment of tadpole tail that was used in DNA analysis for species identification. DNA barcoding was based on a fragment of the mitochondrial 16SrRNA gene (modified 16Sar (550 bp) (5′-CGCCTGTTTAYCAAAAACAT-3′) and modified 16Sbr (550 bp) (5′-CCGGTYTGAACTCAGATCAYGT-3′) following Bossuyt and Milinkovitch (2000). PCR products were prepared for sequencing using BigDye Terminator sequencing chemistry (Applied Biosystems, CA, USA).
Environmental characterization
We characterized the adjacent forest and riparian vegetation, representing habitat relevant for the adults. Two 5 × 10 m plots, with the longer side parallel to the stream, were randomly established on each side of a stream (then 4 plots at each stream). We recorded Diameter at Breast Height (DBH) of trees > 5 cm to estimate basal area of riparian vegetation. Within each 5 × 10 m plot, we had a 5 × 5 m subplot, in which the number of trees DBH < 5 cm (shrubs) was counted. Canopy openness of the habitat was estimated at the center of each plot. Two random 1 × 1 quadrats were set in each 5 × 5 plot to measure understory height (3 measurements) and litter depth (3 measurements). Measurements were averaged within each 1 m2 quadrat. Heights of hanging vegetation were also recorded at 2-m interval along a 5 × 10 m plot. At the center of each plot, we estimated canopy openness using a fish-eye lens mounted on a digital camera. Canopy openness was estimated using the CanopOn2 software (http://takenaka-akio.org/etc./canopon2/index.html). These measurements were conducted in October–November 2014.
Data analysis
Species diversity, species richness and relative abundance
We used Shannon’s H′ and Simpson’s indexes to measure species diversity in each habitat. These indexes were computed as follows:
where pi is the proportion of individuals belonging to the ith species in the habitat.
where n = the total number of individuals of a particular species and N = the total number of individuals of all species. The value of this index also ranges between 0 and 1, and the greater the value, the greater the sample diversity.
We conducted two types of analysis that focused on the stream and on the microhabitat levels for species richness and relative abundance. Species richness corresponded to the maximum number of species found in each stream (stream level) or in each microhabitat (pool or riffle level); relative abundance represented the total number of tadpoles sampled from each stream or from each microhabitat. At the stream level, we analyzed the effects of habitat and time of sampling on species richness and relative abundance using linear mixed-effects models with the function “lmer” in the package “lmerTest” in R (Kuznetsova et al. 2017). In these models, “habitat” and “year” were the factors; “stream” was the random factor. At the microhabitat level, these analyses involved “habitat”, “microhabitat”, and “year” as explanatory variables and “stream” as random factor. P values from these models were obtained by F tests based on Satterthwaite’s method.
Community analysis
We used non-metric multidimensional scaling (NMDS) to visualize and evaluate patterns of dissimilarity in species composition at the microhabitat level between the three habitats for each sampling period. NMDS can handle data with many zeros, ranked and non-normal data better than classical ordination methods (e.g., PCA, CCA), and is well suited for ecological data. Unlike methods that attempt to maximize the variance or correspondence between objects in an ordination, NMDS represents, as closely as possible, the pairwise dissimilarity between objects in a low-dimensional space. That is, microhabitats that are projected closer to each other on the NMDS coordinate system are more likely to harbor similar species than more distant ones. The number of axis was selected based on the lowest stress. As a rule of thumb, a stress value lower than 0.2 represents a good fit of the data (Clarke 1993). The ordination was constructed from a Jaccard dissimilarity matrix using species presence/absence data. NMDS was performed with function “metaMDS” from R package “vegan” (Oksanen et al. 2016).
We conducted a three-way perMANOVA with the function “adonis2” from R package “vegan” (Oksanen et al. 2016) to test for differences in species composition at the microhabitat and at the habitat levels across the two sampling periods (“habitat”, “microhabitat”, and “year”). perMANOVA is a powerful permutation method to detect changes in community structure (Anderson and Walsh 2013). The three-way perMANOVA was based on Bray–Curtis dissimilarity and 9999 permutations.
We conducted SIMPER analysis (Clarke 1993) with the presence–absence data to break down the contribution of each species to the observed dissimilarity between the habitats. The function performs pairwise comparisons of groups of sampling units and finds the average contributions of each species to the average overall Bray–Curtis dissimilarity.
Riparian vegetation structure
We analyzed changes in riparian vegetation structure (understory height, litter cover, vegetation cover, canopy cover, basal area, shrub density, riparian vegetation height) between the three habitats using linear mixed-effects models with the function “lmer” in the package “lmerTest” in R (Kuznetsova et al. 2017). We entered “plot” nested in “stream” as random factors and computed the afore-mentioned environmental parameters as response variables. P values from these models were obtained by F tests based on Satterthwaite’s method. Posthoc tests were conducted using least-square means; results of these tests are directly displayed on the figures. Data were log-transformed before analysis.
Mixed-effects models, NMDS ordination, and perMANOVA were performed in R 3.3.3 (R Core Team 2017). The SIMPER analysis and the graphs were made on PAST 3.0 (Hammer et al. 2001).
Results
We recorded 4444 individuals of 27 species (“Appendix 1”) and 2764 individuals of 16 species of the family Mantellidae recorded in the beginning (October–November 2014) and in the later part (March 2015) of the rainy season, respectively.
Species diversity and richness
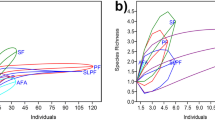
Primary forest and edge were the most diverse communities with edge having higher H′ value than primary forest. Diversity varied with the period of sampling and in 2015, primary forest harbored the highest species diversity (Table 1). For both diversity indexes, the disturbed forest had the lowest values across the two sampling periods.
In 2014, edge habitats harbored the highest species richness with 21 species (range = 14–17 species/stream), followed by primary forest with 17 species (range = 11–15 species/stream). The lowest species richness was recorded in the disturbed forest with 12 species (range = 8–10 species/stream). Species richness was lower in 2015 and the highest diversity was then recorded in primary forest with 13 species (range = 7–11 species/stream), disturbed forest with 10 species (range = 4–7 species/stream). The lowest species richness was recorded at edge with 9 species (range = 5–9 species/stream) (Table 1; Fig. 2).
Tadpole relative abundances in streams (n = 3 for each habitat) at edge, in disturbed forest, and in primary forest at the beginning (2014) and in the later part (2015) of the rainy season. Genus names were abbreviated in the graph as follows: Boophis (B.), Mantidactylus (M.), Spinomantis (S.), Gephyromantis (Ge.), Guibemantis (Gu.)
At the stream level, species richness significantly differed between the three habitats and sampling period; the interaction between the two factors was marginally significant (Table 2). At the microhabitat level, habitat, microhabitat, and sampling periods influenced species richness (Table 3). Species richness significantly differed between microhabitats within the same habitat (Table 3). Pools generally harbored higher species richness than riffles (average species number per microhabitat, 2014: 7.62 vs 5.83, 2015: 4.75 vs. 4.25).
Relative abundance
At the stream level, tadpole relative abundance did not significantly differ among the three habitats, though there was tendency for primary forest to harbor more tadpole individuals in streams. This was because there was high variation in tadpole relative abundance among streams (Table 1). At the microhabitat level, microhabitat and sampling period influenced tadpole abundance. Pools significantly harbored higher number of tadpoles (average number of tadpole individual per microhabitat, 2014: 87.51 vs. 38.36; 2015: 55.87 vs. 30.5). Significantly higher number of tadpoles was recorded in October 2014 than in March 2015 (Fig. 2; Tables 1, 2).
Tadpoles of the genus Boophis dominated the assemblages in all habitats (Fig. 2). The genus Gephyromantis and Guibemantis were represented by one species, respectively. Patterns of species abundance show that by far the most abundant species were Boophis quasiboehmei, B. madagascariensis, and B. reticulatus (Fig. 2). These species were ubiquitous in all streams with B. quasiboehmei being the dominant species in all streams and were abundant in all sampling periods (Fig. 2). B. andohahela, B. tasymena, and B. sp37 were exclusively recorded at edge where B. picturatus was also rare. Spinomantis perraccae and S. aglavei were absent outside forest and could be considered forest specialists.
Community diversity and structure
The general stress coefficients of NMDS models were 0.119 and 0.105 in 2014 and 2015, respectively, indicating good preservation of ordering relationships of the multidimensional among-microhabitat dissimilarities. Primary forest and disturbed showed overlaps in community structure (Fig. 3).
Non-metric multidimensional scaling (NMDS) showing differences in species composition between microhabitats in streams at edge (red), disturbed forest (blue), and in primary forest (green) at the beginning (2014, NMDS stress = 0.119) and in the later part (2015, NMDS stress = 0.105) of the rainy season. Ordination was based on Jaccard dissimilarity using presence–absence data (color figure online)
Results of three-way perMANOVA (Table 4) indicated that species assemblages changed at the microhabitat (pools and riffles) and at the habitat levels, and between sampling periods. Tadpole communities significantly changed between microhabitats within each habitat and between sampling periods within each habitat (for all pairwise tests P < 0.001, following Bonferoni P value corrections, “Appendix 2”).
SIMPER analyses revealed that overall dissimilarity between the primary forest and the disturbed forest was 35.43% (vs. 41.55% in 2015), 61.25% (vs. 66.88% in 2015) between the primary forest and the edge, and 54.19% (vs. 62.67% in 2015) between the disturbed forest and the edge. “Specialists” (i.e., species that were only recorded in forest habitats or at edge) mostly explained these dissimilarities (“Appendix 3”).
Riparian habitat structure
For the parameters we measured, riparian vegetation mainly differed in basal area, litter depth and canopy cover (Table 5); we did not find significant differences in any other variables. As could be expected, forest habitats had higher basal area, thicker litter, and lower canopy openness. Disturbed forest tended to have higher density of shrubs per unit of area compared to the other habitats but this was not significantly different from the other habitats.
Discussion
As for many other tropical countries, logging and conversion of natural forests to agricultural areas are major threats to biodiversity in Madagascar. Given that current protected areas may not be sufficient in maintaining all extant species in the long term (Coad et al. 2019), it is important to quantify the conservation values of disturbed habitats around protected areas (Irwin et al. 2010). We found that tadpole community structures in disturbed forest and at edge markedly differed from the ones recorded in primary forest.
We expected the highest species diversity in primary forest, but in contrast to our predictions, the highest and the lowest species diversity were recorded at edge and in the disturbed forest, respectively. Logging activities occurred in 1989 and had simplified forest structure by reducing tree basal area (by 53%) and crown volume (by 17%) in this part of the forest of Ranomafana National Park (Ramaharitra 2006; Tecot 2008). The effects of selective logging on tropical forests are often negative (see review in Burivalova et al. 2014) and can halve amphibian richness, especially those forest specialists, at logging intensities of 63 m3/ha (Burivalova et al. 2014). Though it is difficult to compare this value with data available on logging intensity in Ranomafana, the disturbed forest harbored a significantly lesser number of species (species richness 12 vs. 17) and markedly lower abundance of forest specialist species (Spinomantis species and B. picturatus) than the primary forest, suggesting that logging could be one driver of community dissimilarity between the two habitats.
Spinomantis often call from canopies of large trees and are known to be restricted to undisturbed habitats (Glaw and Vences 2007); thus, they are likely very sensitive to logging. Species in this genus partly explained the community dissimilarity between the forest habitats by having lower incidence (here presence or absence in microhabitat) and lower abundance in the disturbed forest. Structure of the riparian vegetation may have little influence on community dissimilarity (Table 5), which could be not surprising because logging majorly targeted the upper part of the forest. It would be misleading, however, to assume that logging is the only source of disturbance in this forest. In fact, high tourism activity and the invasion of strawberry guava Psidium cattleianum in this part of Ranomafana National Park are potential factors that may affect frog populations; their effects on amphibians are unknown though.
Edge harbored the highest species richness with up to 21 species in streams. This number is much lower than the 34 species recorded by Ndriantsoa et al. (2017) in matrix streams in Ranomafana. Two reasons may explain this difference; first, Ndriantsoa et al. (2017) surveyed more streams (5 streams vs. 3 streams in this study) and focused on the adult populations using call surveys. In this respect, they were likely to detect higher number of species if frogs call from habitats where no breeding occurs (thus no larvae). Second, not all frog species have their tadpoles develop in streams. The question is why relatively more species were detected outside forest. Earlier studies suggested that factors for the maintenance of amphibian diversity in disturbed habitats are vegetation structure and more importantly the availability of breeding habitats (Bickford et al. 2010; Riemann et al. 2015). In matrix and fragmented landscapes in Ranomafana, the presence of stream is an important factor of high species richness (Riemann et al. 2015; Ndriantsoa et al. 2017) independently of the surrounding forest type. Diversity in degraded habitats can be equal (Riemann et al. 2015) or can even be higher than of primary forests (this study). This is interesting because edge effects on amphibians are not always positive. For example, (Schneider-Maunoury et al. 2016) reported decreased abundance in three-quarter of amphibian species with proximity to edge in a neotropical fragmented landscape. However, the authors noted that species-specific edge effects were not always consistent and some species can have opposite edge responses when measured in different landscapes (Schneider-Maunoury et al. 2016). This could be because species have different tolerance to modified habitats (Laurance 1991). For example, frog species richness was higher in forest fragments compared to forest block in Amazonia because some species were associated with matrix habitats and many of primary-forest species used these habitats as breeding sites (Gascon et al. 1999).
The high species richness at edge is suggested to be result of shared species between forest and edge habitats (increase of generalists), and because of some species that were only recorded at edge (prevalence of edge specialists) (Lövei et al. 2006). As forest specialists declined (e.g., Spinomantis species, B. picturatus), other species with niches better suited to the new environmental conditions composed communities at edge, eventually helping diversity to be maintained (Riemann et al. 2015) or even higher at edge (this study). Species that were only recorded at edge were species in the genus Boophis: B. andohahela, B. tasymena, B. elenae, B. luteus, B. luciae, B. periegetes, and B. sp37. Many species in the genus Boophis are most abundantly in open areas in altered habitats (Andreone 1994), but probably not all of these afore-mentioned species are edge specialists because an earlier study recorded at least B. luteus in continuous forest (Riemann et al. 2015). Glaw and Vences (2007) described B. andohahela as a forest specialist, but along with Strauß et al. (2013) we found that this species, at least its larvae, can also adapt to degraded habitats.
The relatively high species richness at edge is intriguing. Edge habitats are characterized by higher temperatures, increased wind speed, and decreased relative humidity (Lehtinen et al. 2003), to which amphibians are particularly sensitive. Species at edge could be adapted to open habitats and may even be specialized on disturbed habitats. It is possible that species that were only detected at edge may also occur inside RNP, but some indeed may be restricted to edge habitats. The eastern rainforest belt of Madagascar was originally completely forested and thus, the majority of the species in this study should be forest species. However, frequent natural disturbance such as cyclones influence forest structure and microclimate and may have favored amphibian adaptation to disturbed habitats. Thus, species adapted to natural disturbance may have better ability to cope with anthropogenic disturbance (Riemann et al. 2015). Andreone (1994) hypothesized that stream-dwelling species depend less on the microclimatic conditions of the forest floor and may adapt to disturbed environments.
Assemblages changed at the microhabitat level in streams, species richness and abundances were relatively higher in pools than in riffles. The tadpoles of many frog species have affinity to still and slow-flowing stream sections where leaf litter accumulates (Wells 2010). Litter can represent important refuge and food resources for tadpoles (Ramamonjisoa and Natuhara 2018). Even in riffles where gravels represent the main substrate, the tadpoles mainly occupied the slow-running parts of these microhabitats. Indeed, few tadpole species have evolved adaptation to riffle microhabitats; the tadpoles of B. picturatus are characterized by an extremely derived oral disc without any keratodonts and with completely reduced jaw sheaths and are known to ingest sand particles (Grosjean et al. 2011). The tadpoles of B. andohahela and B. marojejiensis have enlarged suctorial mouthparts (nozzle-shaped oral disk) allowing these species attach to rocks and boulders, likely an adaptation to circumventing strong current (Wells 2010).
An interesting aspect of the tadpole communities in Ranomafana is the dominance of one species B. quasiboehmei. Although species richness differed among habitats, relative abundances were maintained among the three habitats. B. quasiboehmei seems to compensate for decline in abundance of other species in forest habitats while outside forest, increases in abundance of other “edge” species allowed abundances to be maintained (Fig. 2). These indicate some signals of compensatory mechanisms in which declines in biomass by some species are compensated for by increases in others, eventually allowing distributions of abundance to be maintained (Brown et al. 2001; Morgan Ernest and Brown 2001; Dornelas 2010). This has an important implication for ecosystem functioning and stability given that changes in tadpole biomass can have significant effects of stream ecosystem processes (Ramamonjisoa and Natuhara 2018).
It is unclear from this study whether the populations recorded at edge were simply tadpoles that were flushed downstream after heavy rains from forested parts on higher altitude. However, this might not be the case because earlier studies in the same study site (Riemann et al. 2015; Ndriantsoa et al. 2017) reported similar diversity outside forest, suggesting that species that were recorded at edge could be already established populations. Moreover, heavy rains typically occur between January and February in Ranomafana (Strauß et al. 2016). Thus, we believe that at least our first sampling in October 2014 provided a good characterization of tadpole community structure. Communities in the earlier part of the rainy season were more diverse (higher number of species and higher relative abundance) than the ones recorded in the second sampling period. Community at edge exhibited the biggest change in species composition, going from having the richest to the lowest species richness across years among the three habitats. We do not have a clear explanation for this result but it is possible that because samplings were conducted after the period of heavy rains, the tadpoles could have been flushed due to strong currents in streams at edge. Another explanation is that tadpoles at edge may have metamorphosed earlier due to relatively higher water temperature (18 vs. 19 °C) and likely higher resources availability (Ramamonjisoa, unpublished data). Both factors are known to influence growth and metamorphosis in tadpoles (Alvarez and Nicieza 2002).
Conclusions and conservation implications
Primary forests are often labeled “irreplaceable” for sustaining tropical biodiversity (Gibson et al. 2011). However, increasing loss of natural habitats and the limitation of current established protected areas in conserving biodiversity in the tropics have called for the need to assess the values of human-modified landscapes and evaluate the relevance of degraded habitats for amphibian conservation (Irwin et al. 2010; Riemann et al. 2015). Considerable number of threatened and data-deficient amphibian species is currently outside protected areas for many of which distribution is limited to very small area (Ramamonjisoa et al. 2013; Nori and Loyola 2015). Edge habitats represent typical “hotspots” because of their high species richness and high level of disturbance, and represent priority habitats in conservation planning. The values of edge habitats in maintaining amphibian diversity are nevertheless likely to depend on the distance from forest block due to dispersal limitation of the adults, suggesting that the quality of matrix is of paramount importance for the conservation of amphibians in degraded habitats (Ndriantsoa et al. 2017).
While studies on amphibian disturbance ecology have typically focused on the adult phase, we call for more studies focusing on the larvae. Larvae are good indicators of the quality of disturbed habitats as they represent direct evidence of breeding and species persistence; thus, surveys limited to adult populations may be less informative for predicting population dynamics. Moreover, given that tadpoles can influence critical ecosystem processes in tropical streams (Ranvestel et al. 2004; Colón-Gaud et al. 2008; Rugenski et al. 2012; Ramamonjisoa and Natuhara 2018), information on larvae is needed.
References
Afonso LG, Eterovick PC (2007) Microhabitat choice and differential use by anurans in forest streams in southeastern Brazil. J Nat Hist 41:937–948
Alvarez D, Nicieza A (2002) Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol 16:640–648
Anderson MJ, Walsh DC (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 83:557–574
Andreone F (1994) The amphibians of Ranomafana rain forest, Madagascar—preliminary community analysis and conservation considerations. Oryx 28:207–214
Bickford D, Ng TH, Qie L, Kudavidanage EP, Bradshaw CJA (2010) Forest fragment and breeding habitat characteristics explain frog diversity and abundance in Singapore. Biotropica 42:119–125
Borges Júnior V, Rocha CF (2013) Tropical tadpole assemblages: which factors affect their structure and distribution? Oecologia Australis 17:217–228
Bossuyt F, Milinkovitch MC (2000) Convergent adaptive radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. PNAS 97:6585–6590
Brown JH, Whitham TG, Ernest SM, Gehring CA (2001) Complex species interactions and the dynamics of ecological systems: long-term experiments. Science 293:643–650
Burivalova Z, Şekercioğlu ÇH, Koh LP (2014) Thresholds of logging intensity to maintain tropical forest biodiversity. Curr Biol 24:1893–1898
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J of Ecol 18:117–143
Coad L, Watson JE, Geldmann J, Burgess ND, Leverington F, Hockings M, Knights K, Di Marco M (2019) Widespread shortfalls in protected area resourcing undermine efforts to conserve biodiversity. Front Ecol Environ 17:259–264
Colón-Gaud C, Peterson S, Whiles MR, Kilham SS, Lips KR, Pringle CM (2008) Allochthonous litter inputs, organic matter standing stocks, and organic seston dynamics in upland Panamanian streams: potential effects of larval amphibians on organic matter dynamics. Hydrobiologia 603:301–312
Cortés-Gómez AM, Castro-Herrera F, Urbina-Cardona JN (2013) Small changes in vegetation structure create great changes in amphibian ensembles in the Colombian Pacific rainforest. Tropi Conserv Sci 6:749–769
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Dornelas M (2010) Disturbance and change in biodiversity. Philos Trans Royal Soc B 365:3719–3727
Edwards DP, Tobias JA, Sheil D, Meijaard E, Laurance WF (2014) Maintaining ecosystem function and services in logged tropical forests. Trends Ecol Evol 29:511–520
Ernst R, Rodel M-O (2005) Anthropogenically induced changes of predictability in tropical anuran assemblages. Ecology 86:3111–3118
Ernst R, Rödel M-O (2008) Patterns of community composition in two tropical tree frog assemblages: separating spatial structure and environmental effects in disturbed and undisturbed forests. J Trop Ecol 24:111–120
Ernst R, Linsenmair KE, Rödel M-O (2006) Diversity erosion beyond the species level: dramatic loss of functional diversity after selective logging in two tropical amphibian communities. Biol Conserv 133:143–155
Eterovick PC, Barata IM (2006) Distribution of tadpoles within and among Brazilian streams: the influence of predators, habitat size and heterogeneity. Herpetologica 62:365–377
Eterovick PC, Rievers CR, Kopp K, Wachlevski M, Franco BP, Dias CJ, Barata IM, Ferreira AD, Afonso LG (2010) Lack of phylogenetic signal in the variation in anuran microhabitat use in southeastern Brazil. Evol Ecol 24:1–24
Ferreira RB, Beard KH, Crump ML (2016) Breeding guild determines frog distributions in response to edge effects and habitat conversion in the Brazil’s atlantic forest. PLoS ONE 11:e0156781
Gardner TA, Ribeiro-Junior MA, Barlow JOS, Ávila-Pires TCS, Hoogmoed MS, Peres CA (2007) The value of primary, secondary, and plantation forests for a neotropical herpetofauna. Conserv Biol 21:775–787
Gascon C, Lovejoy TE, Bierregaard RO Jr, Malcolm JR, Stouffer PC, Vasconcelos HL, Laurance WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Gerber BD, Karpanty SM, Randrianantenaina J (2012) The impact of forest logging and fragmentation on carnivore species composition, density and occupancy in Madagascar’s rainforests. Oryx 46:414–422
Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJ, Laurance WF, Lovejoy TE (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378
Glaw F, Vences M (2007) A field guide to the amphibians and reptiles of Madagascar, 3rd edn. Frosch Verlag, Cologne
Grosjean S, Randrianiaina R-D, Strauß A, Vences M (2011) Sand-eating tadpoles in Madagascar: morphology and ecology of the unique larvae of the treefrog Boophis picturatus. Salamandra 47:75–88
Hammer Ř, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F (2007) Fifty years of deforestation and forest fragmentation in Madagascar. Environ Conserv 34:325–333
Herrera JP, Wright PC, Lauterbur E, Ratovonjanahary L, Taylor LL (2011) The effects of habitat disturbance on lemurs at Ranomafana National Park, Madagascar. Int J Primatol 32:1091–1108
Inger RF, Voris HK, Frogner KJ (1986) Organization of a community of tadpoles in rain forest streams in Borneo. J Trop Ecol 2:193–205
Irwin MT, Wright PC, Birkinshaw C, Fisher BL, Gardner CJ, Glos J, Goodman SM, Loiselle P, Rabeson P, Raharison J-L, Raherilalao MJ, Rakotondravony D, Raselimanana A, Ratsimbazafy J, Sparks JS, Wilmé L, Ganzhorn JU (2010) Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol Conserv 143:2351–2362
Keller A, Rödel M-O, Linsenmair KE, Grafe TU (2009) The importance of environmental heterogeneity for species diversity and assemblage structure in Bornean stream frogs. J Anim Ecol 78:305–314
Kurz DJ, Nowakowski AJ, Tingley MW, Donnelly MA, Wilcove DS (2014) Forest-land use complementarity modifies community structure of a tropical herpetofauna. Biol Conserv 170:246–255
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw. https://doi.org/10.18637/jss.v082.i13
Laurance WF (1991) Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conserv Biol 5:79–89
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116
Lehtinen RM, Ramanamanjato J-B, Raveloarison JG (2003) Edge effects and extinction proneness in a herpetofauna from Madagascar. Biodivers Conserv 12:1357–1370
Lövei GL, Magura T, Tóthmérész B, Ködöböcz V (2006) The influence of matrix and edges on species richness patterns of ground beetles (Coleoptera: Carabidae) in habitat islands. Glo Ecol Biogeogr 15:283–289
Morgan Ernest S, Brown JH (2001) Homeostasis and compensation: the role of species and resources in ecosystem stability. Ecology 82:2118–2132
Ndriantsoa SH, Riemann JC, Raminosoa N, Rödel M-O, Glos JS (2017) Amphibian diversity in the matrix of a fragmented landscape around Ranomafana in Madagascar depends on matrix quality. Trop Conserv Sci 10:1–16
Nori J, Loyola R (2015) On the worrying fate of data deficient amphibians. PLoS ONE 10:e0125055
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2016) Package ‘vegan’. Community Ecology Package 2:1–295
Parris KM (2004) Environmental and spatial variables influence the composition of frog assemblages in sub-tropical eastern Australia. Ecography 27:392–400
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramaharitra T (2006) The effects of anthropogenic disturbances on the structure and composition of rainforest vegetation. Tropical Resources Bulletin 25:32–37
Ramamonjisoa N, Natuhara Y (2018) Contrasting effects of functionally distinct tadpole species on nutrient cycling and litter breakdown in a tropical rainforest stream. Freshwater Biol 63:202–213
Ramamonjisoa N, Rakotonoely H, Thomas H (2013) Spatial and temporal distribution of call activities of two Gephyromantis species (Mantellidae) along forest—farmbush habitat in Ranomafana, Madagascar. Afr J Herpetol 62:90–99
Ranvestel AW, Lips KR, Pringle CM, Whiles MR, Bixby RJ (2004) Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biol 49:274–285
Razafimahaimodison JCRA (2004) Impacts of habitat disturbance, including ecotourism activities, on breeding behavior and success of the pitta-like ground roller, Atelornis pittoides, an endangered bird species in the eastern rainforest of Ranomafana National Park, Madagascar
Riemann JC, Ndriantsoa SH, Raminosoa NR, Rödel M-O, Glos J (2015) The value of forest fragments for maintaining amphibian diversity in Madagascar. Biol Conserv 191:707–715
Rugenski AT, Murria C, Whiles MR (2012) Tadpoles enhance microbial activity and leaf decomposition in a neotropical headwater stream. Freshwater Biol 57:1904–1913
Schneider-Maunoury L, Lefebvre V, Ewers RM, Medina-Rangel GF, Peres CA, Somarriba E, Urbina-Cardona N, Pfeifer M (2016) Abundance signals of amphibians and reptiles indicate strong edge effects in Neotropical fragmented forest landscapes. Biol Conserv 200:207–215
Skelly DK, Richardson JL (2010) Larval sampling. In: Dodd CK Jr (ed) Amphibian ecology and conservation. Oxford University Press, New York, pp 55–70
Strauß A, Reeve E, Randrianiaina R, Vences M, Glos J (2010) The world’s richest tadpole communities show functional redundancy and low functional diversity: ecological data on Madagascar’s stream-dwelling amphibian larvae. BMC Ecol 10:12
Strauß A, Randrianiaina RD, Vences M, Glos J (2013) Species distribution and assembly patterns of frog larvae in rainforest streams of Madagascar. Hydrobiologia 702:27–43
Strauß A, Guilhaumon F, Randrianiaina RD, Valero KCW, Vences M, Glos J (2016) Opposing patterns of seasonal change in functional and phylogenetic diversity of tadpole assemblages. PLoS ONE 11:e0151744
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Tecot S (2008) Seasonality and predictability: the hormonal and behavioral responses of the red-bellied lemur, Eulemur rubriventer, in Southeastern Madagascar. SUNY Stony Brook, New York, p 544
Vieites DR, Wollenberg KC, Andreone F, Köhler J, Glaw F, Vences M (2009) Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. PNAS 106:8267–8272
Vonesh JR, Mitchell JC, Howell K, Crawford AJ (2010) Rapid assessments of amphibian diversity. In: Dodd CK Jr (ed) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press, Oxford, pp 263–280
Wells KD (2010) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Werner EE, Skelly DK, Relyea RA, Yurewicz KL (2007) Amphibian species richness across environmental gradients. Oikos 116:1697–1712
Wright PC, Andriamihaja B (2003) The conservation value of long-term research: a case study from the Parc National de Ranomafana. In: Goodman SM, Benstead JP (eds) The natural history of Madagascar. University of Chicago Press, Chicago
Acknowledgements
This study was supported by JSPS KAKENHI No. 26640137, the Graduate School of Environmental Studies of Nagoya University, and the Ministry of Education, Sports and Culture of the Government of Japan. The study was conducted under the research permit 256/15/MEEMF/SG/DGF/DAPT/SCBT. We are indebted to Justin Solo for assisting in the fieldwork. Yoda Ken and Kenichiro Sugitani provided valuable comments on earlier drafts of the manuscript. We thank Rio Heriniaina for making the map. We are grateful to the Ethology lab of Kyoto University for hosting N. Ramamonjsoa at the time of finalizing the manuscript. We thank Eileen Larney, MICET/ICTE, and Centre Valbio Ranomafana for logistic supports. We are grateful to Madagascar National Parks and the Ministry of Forest and Environment Madagascar for their collaboration.
Author information
Authors and Affiliations
Corresponding authors
Appendices
Appendix 1
Species of tadpole recorded in streams in primary forest, disturbed forest (selectively logged forest), and at forest edge in Ranomafana.
Mouthpart cluster | IUCN status | |
|---|---|---|
Boophis albilabris | Boophis–generalized | LC |
Boophis andohahela | Suctorial | VU |
Boophis elenae | Boophis–generalized | NT |
Boophis luciae | Suctorial | LC |
Boophis luteus | Boophis–generalized | LC |
Boophis madagascariensis | Boophis–generalized | LC |
Boophis marojezensis | Suctorial | LC |
Boophis narinsi | Boophis–generalized | EN |
Boophis periegetes | Boophis–generalized | NT |
Boophis picturatus | Sand-eater | LC |
Boophis quasiboehmei | Boophis–generalized | NA |
Boophis reticulatus | Boophis–generalized | LC |
Boophis sp. 37 (aff. elenae) | Boophis–generalized | DD |
Boophis tasymena | Boophis–generalized | LC |
Guibemantis liber | Gu.–podgy | LC |
Gephyromantis ventrimatulatus | Ge.–non-feeding | LC |
Mantidactylus aerumnalis | Funnel mouthed | LC |
Mantidactylus betsileonis | Md. generalized | LC |
Mantidactylus majori | Reduced teeth | LC |
Mantidactylus melanopleura | Funnel mouthed | LC |
Mantidactylus opiparus | Md.–funnel mouthed | NA |
Mantidactylus sp. 47 (aff. mocquardi) | Md.–reduced teeth | NA |
Mantidactylus sp. 28 (aff. betsileanus) | Md.–generalized | NA |
Mantidactylus sp. 48 (aff. cowani small) | Md.—fossorial | NA |
Spinomantis aglavei | Spinomantis–generalized | LC |
Spinomantis peraccae | Spinomantis–generalized | LC |
Spinomantis sp2 (fimbriatus) | Spinomantis–generalized | DD |
Appendix 2
Pairwise differences following per MANOVA on species composition between the three habitats.
2014
Primary forest | Disturbed forest | Edge | |
|---|---|---|---|
Primary forest | 0.0003 | 0.0003 | |
Disturbed forest | 0.0003 | 0.0003 | |
Edge | 0.0003 | 0.0003 |
2015
Primary forest | Disturbed forest | Edge | |
|---|---|---|---|
Edge | 0.0003 | 0.0003 | |
Disturbed forest | 0.0003 | 0.0003 | |
Primary forest | 0.0003 | 0.0003 |
Appendix 3
SIMPER analyses on species compositional similarities between primary forest, disturbed forest, and edge.
2014
Primary versus disturbed forests | Primary forest versus edge | Disturbed forest versus edge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Overall average dissimilarity 35.43 | Overall average dissimilarity 61.25 | Overall average dissimilarity 54.19 | |||||||||
Taxon | Av. dissim | Contrib. % | Cumulative % | Taxon | Av. dissim | Contrib. % | Cumulative % | Taxon | Av. dissim | Contrib. % | Cumulative % |
M. sp47 | 3.885 | 10.97 | 10.97 | B. picturatus | 7.041 | 11.49 | 11.49 | B.andohahela | 7.095 | 13.09 | 13.09 |
B. reticulatus | 3.704 | 10.45 | 21.42 | B. andohahela | 5.839 | 9.533 | 21.03 | B.picturatus | 6.178 | 11.4 | 24.49 |
M. sp28 | 3.627 | 10.24 | 31.66 | B. elenae | 4.777 | 7.798 | 28.82 | B.elenae | 5.35 | 9.872 | 34.37 |
Spinomantisaglavei | 3.394 | 9.579 | 41.24 | M. sp47 | 3.513 | 5.735 | 34.56 | M.melanopleura | 3.81 | 7.03 | 41.4 |
M. majori | 3.211 | 9.063 | 50.3 | B. reticulatus | 3.487 | 5.693 | 40.25 | M.sp28 | 3.693 | 6.814 | 48.21 |
S. peraccae | 3.103 | 8.758 | 59.06 | M. melanopleura | 3.448 | 5.628 | 45.88 | B.tasymena | 3.604 | 6.651 | 54.86 |
G. liber | 2.401 | 6.775 | 65.83 | B. tasymena | 3.244 | 5.297 | 51.18 | B.madagascariensi | 3.373 | 6.225 | 61.09 |
M. opiparus | 2.322 | 6.553 | 72.38 | B. madagascariensis | 3.005 | 4.905 | 56.08 | M.sp47 | 3.356 | 6.192 | 67.28 |
M. melanopleura | 2.188 | 6.174 | 78.56 | M. majori | 2.988 | 4.879 | 60.96 | B.reticulatus | 2.9 | 5.351 | 72.63 |
B. madagascariensis | 2.121 | 5.987 | 84.55 | S. peraccae | 2.822 | 4.606 | 65.57 | B.sp37 | 2.517 | 4.645 | 77.27 |
B. picturatus | 1.793 | 5.061 | 89.61 | M. sp28 | 2.581 | 4.213 | 69.78 | S.aglavei | 2.396 | 4.422 | 81.7 |
M. sp48 | 1.017 | 2.87 | 92.48 | G. liber | 2.46 | 4.016 | 73.8 | B.marojejiensis | 1.993 | 3.677 | 85.37 |
M. aerumnalis | 0.9716 | 2.742 | 95.22 | S. aglavei | 2.455 | 4.007 | 77.8 | B.luteus | 1.927 | 3.556 | 88.93 |
B. andohahela | 0.7137 | 2.014 | 97.23 | M. opiparus | 2.269 | 3.704 | 81.51 | B.periegetes | 0.9834 | 1.815 | 90.74 |
S. sp2 | 0.4375 | 1.235 | 98.47 | B. sp37 | 2.246 | 3.667 | 85.18 | M.opiparus | 0.7639 | 1.41 | 92.15 |
B. albilabris | 0.2847 | 0.8035 | 99.27 | B. marojejiensis | 1.792 | 2.926 | 88.1 | B.luciae | 0.7536 | 1.391 | 93.55 |
G. ventrimaculatus | 0.2585 | 0.7296 | 100 | B. luteus | 1.738 | 2.837 | 90.94 | B.narinsi | 0.6946 | 1.282 | 94.83 |
B. tasymena | 0 | 0 | 100 | M. sp48 | 0.9557 | 1.56 | 92.5 | B.albilabris | 0.6642 | 1.226 | 96.05 |
B. sp37 | 0 | 0 | 100 | M. aerumnalis | 0.8982 | 1.466 | 93.96 | Guibemantisliber | 0.6642 | 1.226 | 97.28 |
B. narinsi | 0 | 0 | 100 | B. periegetes | 0.8855 | 1.446 | 95.41 | M.majori | 0.5797 | 1.07 | 98.35 |
B. marojejiensis | 0 | 0 | 100 | B. albilabris | 0.8192 | 1.337 | 96.75 | M.betsileonis | 0.345 | 0.6366 | 98.98 |
B. quasiboehmei | 0 | 0 | 100 | B. luciae | 0.6677 | 1.09 | 97.84 | S.peraccae | 0.2842 | 0.5244 | 99.51 |
M. betsileonis | 0 | 0 | 100 | B. narinsi | 0.622 | 1.015 | 98.85 | G.ventrimaculatus | 0.2658 | 0.4906 | 100 |
B. periegetes | 0 | 0 | 100 | S. sp2 | 0.3935 | 0.6423 | 99.5 | S.sp2 | 0 | 0 | 100 |
B. luciae | 0 | 0 | 100 | M. betsileonis | 0.3092 | 0.5048 | 100 | M.aerumnalis | 0 | 0 | 100 |
B. luteus | 0 | 0 | 100 | G. ventrimaculatus | 0 | 0 | 100 | B.quasiboehmei | 0 | 0 | 100 |
B. elenae | 0 | 0 | 100 | B. quasiboehmei | 0 | 0 | 100 | M.sp48 | 0 | 0 | 100 |
2015
Primary versus disturbed forests | Primary forest versus edge | Disturbed forest versus edge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Overall average dissimilarity 41.55 | Overall average dissimilarity 66.88 | Overall average dissimilarity 62.67 | |||||||||
Taxon | Av. dissim | Contrib. % | Cumulative % | Taxon | Av. dissim | Contrib. % | Cumulative % | Taxon | Av. dissim | Contrib. % | Cumulative % |
B. msis | 6.568 | 15.81 | 15.81 | B. andohahela | 10.72 | 16.03 | 16.03 | B. andohahela | 13.22 | 21.1 | 21.1 |
M. sp47 | 4.673 | 11.25 | 27.05 | B. elenae | 9.232 | 13.8 | 29.84 | B. elenae | 11.34 | 18.09 | 39.19 |
B. reticulatus | 4.64 | 11.17 | 38.22 | B. picturatus | 9.21 | 13.77 | 43.61 | B. picturatus | 9.602 | 15.32 | 54.51 |
G. liber | 4.548 | 10.94 | 49.16 | M. melanopleura | 6.039 | 9.029 | 52.64 | M. melanopleura | 8.228 | 13.13 | 67.64 |
M. melanopleura | 4.465 | 10.74 | 59.91 | B. msis | 5.893 | 8.811 | 61.45 | B. reticulatus | 5.731 | 9.145 | 76.79 |
S. aglavei | 3.999 | 9.623 | 69.53 | B. reticulatus | 4.727 | 7.068 | 68.52 | B. msis | 5.362 | 8.556 | 85.34 |
S. peraccae | 3.955 | 9.517 | 79.05 | G. liberH | 4.235 | 6.332 | 74.85 | M. sp47 | 4.407 | 7.032 | 92.37 |
B. picturatus | 2.9 | 6.979 | 86.03 | M. sp47 | 3.964 | 5.926 | 80.78 | B. quasiboehmei | 1.715 | 2.737 | 95.11 |
M. majori | 1.485 | 3.575 | 89.6 | S. peraccae | 3.691 | 5.519 | 86.3 | M. sp48 | 1.641 | 2.618 | 97.73 |
M. sp48 | 1.362 | 3.278 | 92.88 | S. aglavei | 3.599 | 5.382 | 91.68 | S. aglavei | 0.5134 | 0.8192 | 98.55 |
B. quasiboehmei | 1.161 | 2.795 | 95.67 | M. sp48 | 1.85 | 2.766 | 94.44 | M. opiparus | 0.4553 | 0.7266 | 99.27 |
M. opiparus | 1.028 | 2.473 | 98.15 | M. majori | 1.378 | 2.061 | 96.5 | G. ventrimaculatus | 0.4553 | 0.7266 | 100 |
G. ventrimaculatus | 0.4085 | 0.983 | 99.13 | B. quasiboehmei | 1.363 | 2.038 | 98.54 | M. sp28 | 0 | 0 | 100 |
M. sp28 | 0.3613 | 0.8696 | 100 | M. opiparus | 0.6333 | 0.947 | 99.49 | S. peraccae | 0 | 0 | 100 |
B. elenae | 0 | 0 | 100 | M. sp28 | 0.341 | 0.5098 | 100 | G. liber | 0 | 0 | 100 |
B. andohahela | 0 | 0 | 100 | G. ventrimaculatus | 0 | 0 | 100 | M. majori | 0 | 0 | 100 |
Appendix 4
Sampling design
In each habitat, we sampled three streams. In each stream, we sampled tadpoles in 4 pools and in 4 riffles.

Rights and permissions
About this article
Cite this article
Ramamonjisoa, N., Sakai, M., Ndriantsoa, S.H. et al. Hotspots of stream tadpole diversity in forest and agricultural landscapes in Ranomafana, Madagascar. Landscape Ecol Eng 16, 207–221 (2020). https://doi.org/10.1007/s11355-020-00407-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-020-00407-w