Abstract
It is strongly believed that the wide genetic variability within the cotton (Gossypium spp.,) increases their chance for adaptation to changing harmful environments, and thus upsurge the likelihood of long-term survival of such unusual and important cash crop in the world. Given, the importance of cotton in the world economy and its usefulness to the human, cotton genetic resources should be conserved effectively and managed wisely, since such cotton genetic resources are used as the raw material for breeding new cultivars and act as a reservoir, and/or buffer against ecological and economic changes. However, the trend is reverse as there has been significant loss of genetic diversity during the past couple of decades, and the process of genetic erosion continues. Although, the narrow genetic diversity that exists in cotton has been noticed for more than two decades, there is little data on its amount and extent. Besides the threatening genetic base of future cotton breeding programs, erosion of cotton genetic resources could pose a severe threat to the world’s natural fiber production in the long-term, since loss of genetic variation may decrease the potential for a species to persist in the face of abiotic and biotic environmental changes. Future progress in the improvement of cotton largely depends on discovery, collection, and immediate conservation of genetic resources such as wild progenitors and landraces of Gossypium for their effective and sustainable utilization in the cotton breeding program. This chapter describes the challenges to cotton genetic diversity, presents the strategies that are being implemented to reverse the erosion of that diversity, outlines several gaps in our knowledge, and describes strategies that must be addressed to make such approaches more effective. Deployment of biotechnological tools in the study and conservation of cotton genetic resources are also highlighted in this chapter. Integration of the knowledge about evolution and natural population structure of domesticated Gossypium species combined with emerging sequence and functional genomics information will lead to the better management of cotton germplasm resources and more efficient utilization of natural variation for cotton genetic improvement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cotton (Gossypium spp.)
- Genetic diversity
- Cotton germplasm conservation
- Genetic erosion
- Molecular markers
- Pollen culture
- Transgenic technology
12.1 Introduction
12.1.1 Cotton: Single Crop—Multiple Uses
Cotton is uncommon among major commercial crops since it has a huge impact in the global economic, industrial, and social sectors. Cotton’s primary organ of commerce, seed-borne lint fiber, is still the most preferred natural fiber in the world and greatly diminishes the dependence on synthetic fibers that are derived from highly depleting resources of petrochemicals. It is estimated that cotton fiber with improved uniformity, durability, and strength can effectively replace synthetic fibers that need ∼230 million barrels of petroleum per year in the USA alone (Holt et al. 2003). Further, cotton production has enormous economic benefits in the cotton-growing countries. It is being cultivated on about 2.5 % of arable land, approximately 150 countries are involved in cotton import and export, provides income for approximately 100 million family units and sustains the textile industry, with a worldwide aggregate influence estimated annually at more than $500 billion (Kranthi 2013). Moreover, cotton is a major economic driver in several developing countries. For example, Uzbekistan produces ∼4 million tons of raw cotton per year and exports ∼$900 million worth cotton fiber (Chen et al. 2007). Similarly, the cultivation alleviates poverty in West Africa, where it represents from 25 to 51 % of the exportations in Burkina Faso, Chad, Benin, and Mali (Vitale et al. 2009).
All parts of the cotton plant are useful and it has hundreds of uses. During the celebration of the International Year of Natural Fibres in 2009, the usefulness of cotton fiber received topmost attention among plant and animal fibers (http://www.naturalfibres2009.org/; accessed on 15th December, 2014). In addition to their widely known uses in clothing or apparels and home furnishings, fiber-derived products are used in plastics and in many industrial goods such as digital screens. Furthermore, cotton is an important source of feed, foodstuff, and oil. Cotton seed oil and by-products of fiber processing are also used as raw materials for biofuel production.
Each cotton fiber is a single and unusually elongated cell from epidermal layer of the ovule, with about 25,000 per seed. It has been shown that there are only a few cells in the plant kingdom that are as blown up in their size or composition as cotton fibers and some of the cotton fiber cells can reach lengths of over 6 cm or one-third the height of an Arabidopsis plant (Kim and Triplett 2001). Therefore, besides its economic importance, cotton fiber is an outstanding model for the study of plant cell elongation, cell wall, and cellulose biosynthesis. The fiber is composed of nearly the pure cellulose, the largest component of plant biomass. Compared to lignin, cellulose is easily convertible to biofuels (Chen et al. 2007). Therefore cotton is considered as an attractive target crop in public and private sectors. Further genetic improvements that enhance the economics of production and sustainability and fiber processing characteristics will ensure competitiveness in the market of this natural-renewable product with nonrenewable petroleum-derived synthetic fibers. It is also worth here to mention that modifications to expand the use of seed derivatives for food and feed could profoundly benefit the diets and livelihoods of millions of people in food-challenged economies. Such improvements have more economic, health and ecological, and thus societal impacts on both national and international boundaries.
12.1.2 Importance of Genetic Diversity in Cotton and Its Multiple Perspectives
The improved cotton cultivars developed during the past few decades led to spectacular increases in fiber yields. On the other hand, they also led many farmers to abandon the age-old practice of planting a mix of traditional varieties as insurance against adverse conditions. For example, in India which has largest cotton cultivation area in the world (Navarro and Hautea 2014), it can be easily predicted from the current trend in cotton cultivation that less than 20 hybrids will soon cover more than 80 % of the total cotton area, replacing thousands of different cotton cultivars that were once grown there. As a consequence, vast areas of land are now planted to a small number of high-yielding cotton hybrids, which require enormous inputs of fertilizer, pesticide, and water.
Past history of agriculture clearly demonstrated that use of small number of cultivars in the given environment has led to huge loss in agricultural production. One example of this occurred in the USA in 1970, when the uniformity of the maize crop enabled a blight to destroy almost US$1000 million of maize and reduced the yields by as much as 50 %. After spending lot of time, scientific efforts and money, resistance to the blight was finally found in the genes of an African maize variety called Mayorbella (http://www.worldbank.org/html/cgiar/25years/gene.html; accessed on 15th December, 2014). As urban development destroys habitats, and farmers abandon traditional varieties in favor of modern uniform types, the resulting loss of diversity has serious implications for long-term fiber production. Further, because of deployment of few cultivars, cotton cultivation now is more vulnerable to attacks from pests and diseases and more prominently to climate changes such as water stress.
Thus, during the past few decades it is increasingly believed that meeting the natural fiber needs of the world’s growing population depends, to a large extent, on the conservation and use of the world’s remaining cotton genetic resources (Boopathi et al. 2014). The conservation and use of genetic resources is as old as agriculture itself. For over thousands of years farmers have conserved seeds for future planting, domesticated wild cotton species and selected, and bred thousands of different cotton cultivars to suit their specific needs and conditions. Several centuries ago, the tetraploid cultivated cotton species Gossypium hirsutum and G. barbadense have been independently domesticated in Mexico and Peru (Campbell et al. 2010).
Yet much of this cotton genetic diversity has now been lost. As stated above, of the several thousand cotton cultivars used in the past for fiber production, only hundreds are cultivated today in the world. The most significant loss of cotton diversity had taken place in recent decades due to the introduction of new, high-yielding cotton cultivars that began on a large-scale in the late 1950s and 1960s. In addition, cotton production is increasingly market-oriented, making farmers less inclined to select for crop characteristics that once were important for local customs and culture. All the studies that were intended to estimate the genetic diversity exist in the currently cultivating cotton cultivars and the germplasm accessions used in cotton breeding program evidently revealed that there was a very narrow genetic diversity in the investigated materials (Lacape et al. 2007 and references therein; Boopathi et al. 2008; Thiyagu et al. 2011; Ravikesavan et al. 2014).
International recognition of the importance of cotton genetic diversity and the increasing threat of genetic erosion grew significantly and the scientific principles, which underlie strategies and methodologies for collecting, conserving, evaluating, and documenting cotton genetic resources were comprehensively addressed recently (Abdurakhmonov 2014). As countries became aware of the danger of genetic erosion and the need for conservation, greater priority was given for collecting the cotton genetic resources in the field and establishing in situ and ex situ gene banks (see below).
If the adoption and use of improved cultivar and hybrids and other farming technology bring significant benefits to cotton farmers, why should we be concerned with the loss of landraces and the preservation of cotton genetic diversity? There are several important reasons. Genetic diversity is the elementary factor of evolution in Gossypium species. It is the foundation of sustainability because it provides raw material for adaptation, evolution, and survival of species and individuals, especially under changed environmental, disease, and social conditions and it will allow them to respond to the challenges of the next century (Hammer and Teklu 2008). As it is evident from the past history, many advances in modern cotton breeding have been possible because of the wide range of genetic source material provided by cotton germplasm. Therefore, the future of global fiber supply depends on the exploitation of genetic recombination and allelic diversity that exists in the cotton germplasm resources. The considerable genetic diversity of cotton traditional varieties is the most immediately useful and economically valuable part of cotton germplasm. Subsistence cotton farmers use landraces as a key component of their cropping systems. In addition, landraces are the basic raw materials used by cotton breeders for developing modern varieties. Landraces are a complex and continually evolving collection of local cotton varieties that reflect interactions with wild species, adaptations to changing farming conditions and responses to the economic and cultural factors that shape farmer’s priorities. Over the past few decades, awareness of the rich diversity of exotic or wild germplasm has also increased. This has led to a more intensive use of such germplasm resources in regional cotton breeding programs aiming to increase fiber yields in an unprecedented way.
In addition, the preservation and utilization of cotton genetic diversity is of particular importance to the more marginal, diverse agricultural environments where modern plant breeding tools and technologies have had much less success. For example, in India, >60 % cotton is cultivated under rainfed environments by resource-poor farmers (Choudhary and Laroia 2001). Farmers in these areas tend to be poorly served by public research and extension systems. These areas are often centers of diversity for many Gossypium species (for example, karunganni cotton in South Tamil Nadu, India), but increasing poverty is forcing many of these farmers to place more dependence on nonfarm sources of income, with consequent reduction in their capacity to grow and maintain the range of cotton local varieties they have been adapted to manage. More importantly, the maintenance of a wide and evolving range of regional cotton landraces is threatened by the advent of intellectual property protection for crop varieties, accelerated by the formation of the World Trade Organization. The increasing application of plant breeder’s rights has several implications for cotton genetic diversity. For a new variety to be legally protected, it must be subject to very precise description, including the requirement that it be distinct, uniform, and stable (Cooke and Reeves 2003). This is a limiting element to the promotion of inherently diverse landraces or of varietal mixtures. An additional debate concerns farmer’s privilege, the ability of farmers to save the seed of a variety, to exchange it with neighbors and to adapt it to their own growing conditions. These practices could be challenged by the seed companies when cotton hybrids/varieties are sold under strict legal protection. It is even possible to envisage situations, where varieties that originated in farmer’s fields may be legally protected and then denied to the farmers responsible for having developed them (Hammer 2004). Hence the advent of plant variety protection lends added urgency to the search for solutions to the conservation of cotton genetic diversity.
Nevertheless there are several decisions that must be made in designing cotton germplasm conservation projects. First, because landraces are not static entities, decisions have to be taken with regard to the nature of human intervention in the selection process. Whose criteria are to be used in the selection and adaptation of new materials? Are local farmers’ criteria the only ones to be applied in deciding what is conserved or should scientists’ interests also play a role in determining the direction of conservation? Since there is no successful example in this case, it is imperative to develop a large multinational collaborative project to test and develop techniques for cotton germplasm conservation. In addition, farmers generally seek germplasm from other sources to complement their own landraces. To what extent should this be allowed or encouraged in an in situ conservation project? To what extent is the objective to build a fence around an area of genetic diversity in order to protect traditional crop development processes from outside influences and to what extent is the objective closer to that of community development? In the latter instance, resources and information are provided to farming communities to empower them to make more informed decisions about the management of local varieties and the utilization of recently released cultivars in other crops (Hammer and Teklu 2008).
There are also a number of efforts under way to encourage a wider scope for farmer participation in formal plant breeding (Boopathi 2013). Possibilities include greater farmer representation in priority setting for crop breeding programs, more explicit attention to the crops and varietal characteristics of importance to these farmers, the transfer of significant aspects of plant breeding research to farmer’s fields and the organization, and training of farmers to take a more active part in the variety testing and selection process. There is a growing literature on methods to encourage farmer participation (Bhargav et al. 2014). The innovations include rapid rural appraisal techniques to understand farmer varietal preferences, the organization of various types of adaptive on-farm research to test varieties under field conditions, the wider use of landraces in formal breeding programs and the establishment of mechanisms for contact between farmers and experiment station personnel. Some plant breeders see the possibility of an integrated system that incorporates the strengths of both formal and informal plant breeding techniques. However, such efforts in cotton are found to be very scarce.
The challenges of cotton genetic resource conservation also highlight the dilemma of balancing between development and conservation in the light of policy implications. The dilemma is evident in choices of conservation strategies as well as in the design of development programs. The identification of an optimum mix of development and conservation initiatives is one of the most difficult tasks which will be faced policy makers in the next decade and the necessity to develop location-specific strategies adds to the complexity of the challenge (Hammer and Teklu 2008). Much more effort is required to develop adequate analytical tools to enable policy makers to explicitly address the trade-offs and consequences of particular decisions. It also requires clear policy decisions about the appropriate mix of public, commercial, and voluntary contributions.
Commercial cotton seed multinational companies are now replacing public cotton seed operations and are also making an increasing contribution to plant breeding and variety development (Tripp and Heide 1996). In many instances, cotton seed companies could able to respond more effectively to farmers’ needs than the public sector. But the commercial sector will not be likely to address the special growing conditions that are important to resource-poor farmers, nor will they be likely to play a prominent role in the conservation activities. Adequate legal protection for commercial varieties should be balanced against the assurance of farmer’s privilege to save and adapt their own seed. Therefore, new approaches to plant breeding, plant genetic resource conservation will increasingly involve farmer participation and promotion of long-term, productive collaboration between public agricultural research and organizations active at the community level.
Another challenging feature of conservation programs is their unavoidable long-term nature. Both national policy makers and external donors who wish to support conservation programs must assure that funding is available for an extended period to include the necessary research, training, and implementation. Further, good policy always depends on worthy information and this is particularly true for genetic resource conservation (Tripp and Heide 1996). Despite rapid progress, serious gaps (see below) in our knowledge are likely to constrain the informed management of plant genetic diversity for at least the next decade. The issues demand interdisciplinary collaboration among social and biological scientists. They also require increasing collaboration between researchers and the members of grassroots development initiatives. The policy must direct national research and academic institutions to give priority to collaborative research on conservation issues and should take responsibility for establishing appropriate forum, where different perspectives can be presented, debated, and synthesized.
12.2 Genetic Erosion in Cotton
Genetic erosion refers to the loss of individual genes or combinations of genes, such as those found in locally adapted landraces and wild species (Brush 1995). Genetic erosion also denotes that the normal addition and disappearance of genetic variability in a population is altered so that the net change in diversity is negative (Scarascia-Mugnozza and Perrino 2002). Thus, genetic erosion in cotton can be simply defined as the loss of variability (heterogeneity of alleles, morphotypes, and phenotypes) in Gossypium populations.
Several approaches have been employed to estimate the degree of genetic erosion that Gossypium faces in a certain region over a given time. Such strategies generally rely on any one or combination of the following methods: (i) analysis of molecular data (such as allozymes, DNA or RNA based marker analysis) (ii) comparison between the number of species/cultivars still in use by farmers at the present time to those found in previous studies (iii) using the genetic assessment model, and (iv) using a checklist of risk factors. Among them, the most widely used figures in estimating genetic erosion are indirect, i.e., the diffusion of modern crop varieties released from crop breeding programs (Hammer and Teklu 2008 and references therein).
Therefore, the obvious cause of genetic erosion is the dissemination of modern varieties from cotton improvement programs. With the advances in cotton breeding, high-quality and homogenous new cotton cultivars were quickly and widely distributed, and suppressed the use of cotton landraces. Improved fiber yield (or yield potential) is the most important criterion for the choice of a cotton variety by a farmer. Additionally, update in the global culture is placing a range of pressures on wild areas and on traditional cotton cultivating areas and external interests (such as economic and/or political dominations) also strongly affect the regular cotton cultivation practice. The above said forces intensely change the nature of the decision-making process and the farmer is encouraged to grow high-yield varieties in monoculture using inputs of fertilizer and pesticides. Further, in many parts of the world, farmers were given several socioeconomic incentives or rewards to replace varieties that evolved within their agroecosystem with improved/introduced cotton hybrids. Efforts to localize new populations may be effective, as it was thought that G. mustelinum populations in Brazil were threatened to extinction (Barroso et al. 2009) until new populations were found (Alves et al. 2013; Menezes et al. 2014).
Population growth, urbanization, developmental pressures on the land resources, deforestation, changes in land use patterns, lack of recognition of current or future value of genetic resources, poor monitoring and management of genetic resources, lack of sustainable breeding programs, and natural disasters (famine, drought, flooding, typhoons) were other noteworthy factors that are contributing to abundant habitat fragmentation and destruction of the cultivated Gossypium and their wild relatives.
More recently, global warming and high degrees of water and air pollutions have also been recognized as auxiliary causes for the loss of diversity in cotton. For example, droughts of just a single season could result in drastic changes in cotton seed production and stocks, while successive years of drought can prompt changes in cropping patterns and the geographic distribution of cotton. Social disruptions or wars also pose a constant threat of genetic wipe-out of the promising cotton diversity. Overexploitation and introduction of invasive unfamiliar species are the supplementary minor factors contributing to the loss of genetic resources in cotton.
Similarly, genetic drift is also found to reduce cotton biodiversity (Scarascia-Mugnozza and Perrino 2002). Genetic drift is a random change in the allele frequency in cotton that occurs because gametes transmitted from one generation to the next carry only a sample of the alleles present in the parental generation. Genetic drift changes the distribution of genetic variation in two ways: (i) the decrease of variation within populations (loss of heterozygosity and eventual fixation of alleles) and (ii) the increase of differentiation among populations. Every finite population experiences genetic drift, but the effects become more pronounced as population size decreases (Falconer 1989).
Besides, the problem of genetic erosion through inappropriate maintenance of ex situ collections in cotton is also very obvious. Genetic erosion can occur at many stages in the preparation, sub-sampling, exchange, storage, and regeneration of recalcitrant cotton seed during ex situ conservation. It is also worth to highlight that loss of diversity through genetic shifts and convergent selection during regeneration is often unnoticed. In the world collection, beyond the problem of duplication among accessions, the security of ex situ conservation as a whole is endangered. About half of all cotton gene bank accessions maintained in the world require immediate rejuvenation. However, financial problems, lack of staff, and shortage of farms largely affect such urgent action (Abdurakhmonov 2014). The long-term storage strongly reduces the metabolism and therefore highly limits cotton viability and seed vigor.
In conclusion, to reverse this unrestricted genetic erosion trends, conservation of genetic diversity is a fundamental concern in genetic improvement of cotton, as genetic variation is the raw material for evolutionary change within cotton populations. Detecting and assessing genetic erosions have been suggested as the first priority in any major effort to arrest the loss of genetic diversity and efficiently conserve the cotton germplasm. Generally, many national cotton programs have not viewed quantification of genetic erosion as a high priority, as apparent from the paucity of genetic erosion information in cotton (Gore et al. 2014). Although Gossypium species-specific guidelines are not available, the risk of genetic erosion can be minimized in cotton by familiarity with the biology (including breeding system, mode of reproduction, and pattern of genetic diversity) of the affected Gossypium species and landraces.
12.3 Cotton Diversity Assessment: Tools and Methods
Genetic diversity in cotton is conventionally analyzed using agronomically or economically important morphological traits such as growth habit, flower petal color, number of bolls, ginning percentage, seed index, fiber quality traits, scores of disease and pest resistances, and tolerance to abiotic stresses (Thiyagu et al. 2011). However, it was realized later that the variations observed in such morphological phenotypes were also influenced by environmental factors, and hence. it cannot be used to represent the diversity that is caused by genotypes alone. Due to rapid developments in enzymology and molecular biology, isozymes and molecular markers were found to be efficient tools in genetic diversity analysis in cotton in due course (Boopathi 2013). However, it should be noted that there was an uncoupling trends in genetic diversity in cotton when they were analyzed using molecular markers and morphological traits. In general, there was a greater diversity among the cotton accessions when analyzed with phenotypic traits than with the molecular markers.
However, use of molecular markers for cotton genetic diversity analysis is the choice of the researchers. There are several methods and strategies available to study the genetic diversity in cotton using molecular markers. Precise and objective estimate of genetic relationship depends on sampling strategies, use of several marker data sets, selection of genetic distance estimate strategies, clustering procedures or other multivariate methods and their influence on genetic relationship estimation etc. Thus careful combinations of these features and use of appropriate statistical programs and strategies is the key in genetic diversity data analysis (refer Mohammadi and Prasanna 2003 for further details). In general, the data comprises numerical measurements and combinations of different types of variables. Further, pedigree data, passport data, morphological data, biochemical data, storage proteins data, and nucleus and/or chloroplast-based DNA/RNA marker data are also being used to reliably estimate the genetic relationship. Depending on the objective of the experiment, the level of resolution required, availability of resources and infrastructure facilities and operational cost, and time constraints decide the selection of data sets and each data provide a specific type of information on genetic diversity in cotton.
When we use the molecular data, genetic distance or similarity among individuals of the given germplasm is usually calculated as a quantitative measure that differentiates the two individuals at sequence or allelic frequency level. Wide range of genetic distance measurement are methods available, use of such methods are highly decided by the selection of a software tool that we employ for the analysis (Boopathi 2013). Numerous software programs are available for assessing genetic diversity, such as Arlequin, DnaSP, PowerMarker, MEGA2, PAUP, TFPGA, GDA, GENEPOP, NTSYSpc, Structure, GeneStrut, POPGENE, Maclade, PHYLIP, SITES, CLUSTALW, and MALIGN (Labate 2000). Most are freely available through the Internet. Many perform similar tasks, with the main differences being in the user interface, type of data input and output, and platform. Thus, choosing which to use depends heavily on individual preferences.
Allele mining is another recent tool that can be used to measure the genetic diversity in cotton. Allele mining refers to the identification of naturally occurring allelic variation at agronomically important genetic loci (otherwise called as genes). This can be performed using a variety of approaches including mutant screening, quantitative trait loci (QTL) and advanced backcross QTL (AB-QTL) analysis, association mapping, genome-wide survey for the signature of artificial selection, etc. (Navreet et al. 2010). The successful allele mining procedure is highly dependent on the use of diverse cotton germplasm collections, especially which are rich in wild species. This is because the majority of allelic variations at the gene(s) of interest is largely assumed to occur in the wild relatives of Gossypium due to the unavoidable loss of variation during the domestication process. Despite some efforts, unfortunately, entire cotton germplasm entries have not yet been efficiently characterized for their novel phenotypes due to several challenges including lack of resources for evaluating huge collections.
It is also worth to mention here the role of EcoTILLING in allele mining. A variant of “targeting induced local lesions in genomes (TILLING),” known as EcoTILLING, was developed to identify multiple types of polymorphisms in germplasm collections or breeding materials (Comai et al. 2004). EcoTILLING allows characterization of natural alleles at a specific locus across several germplasm entries in a rapid and affordable way. Recently, geographical information system (GIS)-based data collection from spatial objects and their attributes for species richness and diversity index was proposed for germplasm characterization of wild Abelmoschus species for the collection of diversity on wild okra (Nizar et al. 2014), even though it has not yet reported in cotton.
12.4 Genetic Diversity Analysis in Cotton: Lessons Learnt and What Has Been Neglected?
Irrespective of different methods and tools employed to measure the genetic diversity in cotton, as stated earlier, there was a narrow genetic diversity in cotton. For example, in our study we found that even the cotton cultivars that used different parents for their development in India, they were found to be closely related (which is more than 85 % within the Gossypium species) at molecular level (Fig. 12.1). Interestingly, the cotton accessions in the core germplasm have shown diverse response to water stress that was imposed during the flowering phase. However, they were shown to possess poor genetic diversity when analyzed with SSR markers (Fig. 12.2).
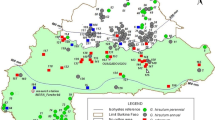
In another study, the diversity of the dooryard plants has been studied in the North Brazil by SSR markers. It is believed that G. barbadense can also be cultivated in dooryards, in urban areas or farms. In Brazil it is used as a medicinal plant, and the effusion of the leaves is believed to have healing properties. It may also be planted just as an ornamental plant, or more rarely it is used to make wicks or swabs. The genetic diversity of height: expected heterozygosity (the probability that two alleles chosen at random from the population are different) among the dooryard G. barbadense was 0.39 (Almeida et al. 2009). This may be explained by the fact that the plants are not cultivars and have not been selected for high production, although at least in North Brazil the healing properties are believed to be stronger in plants with purple leaves than on those with green leaves.
Similarly, in the accessions of Moco cotton (G. hirsutum L. race marie-galante), a landrace reported to be selected by farmers in the Brazilian semiarid region during the second half of the nineteenth century and cultivated until around the year 1980, the expected heterozygosity was 0.52 (Menezes et al. 2010). Such a high value was due to the fact that some of the plants were cultivars and others were landraces. Hence, it can be concluded that smaller amount of genetic diversity exist in the present cultivating cotton cultivars and landraces is of serious concern.
Therefore, the lesson was that, as in other crops, “genetic uniformity will be the basis of vulnerability to epidemics and more generally, to biotic and abiotic stresses” (Scarascia-Mugnozza and Perrino 2002). Although there is undeniable evidence of the erosion of cotton genetic diversity and several innovative responses have been developed, there are important gaps in knowledge (see below) that limit our capacity to decide among the various alternatives. Some of these gaps involve our technical understanding of the nature of cotton genetic diversity, while others are concerned with our understanding of its socioeconomic implications. Appropriate measures for cotton genetic diversity still need to be developed in order to better characterize the current situation and to evaluate the changes in future.
Farmers’ cotton variety classification systems are one place to start, but we do not know how these correspond to actual genetic differences. With few exceptions, there is little research that correlates the variation in conventional taxonomy with genetic differences. When moco cotton was at its peak in the 1970s, it was harvested from 1.8 million ha, and it could be classified by farmers according to its origin and morphological traits (Freire and Moreira 1991). The genetic distance measured by molecular markers is correlated to the places from where moco cotton accessions have been collected, but not with morphological traits (Menezes et al. 2010). The capacity to evaluate genetic material in the laboratory is growing rapidly (Lacape et al. 2007; Boopathi et al. 2008; Thiyagu et al. 2011; Ravikesavan et al. 2014), but these are still expensive techniques and more robust markers and measures are required to ascertain the cotton genetic diversity.
Therefore, when better measures of cotton genetic diversity are devized they will contribute to a clearer understanding of what Gossypium species exactly need to be conserved. Currently, there are only very general ideas about what portion of a cotton population needs to be maintained in order to conserve particular genetic traits. This information is crucial to the efficient design of in situ conservation projects, for instance, in order to know if efforts might be limited to a few farmers’ fields or should instead sample more widely from that agroecosystem.
Additional studies are also required to understand the causes of cotton genetic erosion. The cultural value placed on cotton diversity and local selection techniques are diminishing in many areas and the skills that have contributed to landrace evolution are consequently disappearing. As farmer variety selection skill is a threatened resource, there is also a need to understand how to conserve and enhance it. Although farmers deserve credit for landrace development, there is little knowledge of what the genetic consequences of their selection techniques are, or what specific effects these have on enhancing diversity. Farmers select cotton materials for practical reasons that may not always be compatible with the maintenance of genetic diversity in cotton. Very little interdisciplinary study has been devoted to understand the biological outcome of the application of indigenous technical knowledge and skills in cotton cultivar selection. Further, there is little understanding of whether attempts to improve local selection capacity should focus on individual farmers or on communities. Indeed, we know very little about how cotton varieties and their characteristics are exchanged within communities.
With the introduction of transgenic cotton cultivars/hybrids, concern has also been raised that overall genetic diversity within Gossypium species was decreased since transgenic cotton breeding programs concentrate on a smaller number of economically important cotton accessions (Chakravarthy et al. 2014). Though such effort has the potential and can also increase the genetic diversity, separate methodologies, and evaluation of genetic diversity with specific reference to transgenic cotton require superior attention. There is also a reason to believe that the amount of diversity is sufficient to maintain yields even in the face of most unknown pathogens that might emerge (Ronald 2014). However, the emerging combination of stresses under climate change and the opportunities for new pathogens is unprecedented. The use of Bt cotton is associated with the emergence of Bt resistance and by novel mechanisms in insect pests (Fabrick et al. 2014).
As molecular markers have shown their potential in genetic diversity analysis in cotton, other advances in biotechnology should also be used to develop and refine strategies for duplicate identification, flowering and seed propagation physiology, seed physiology for long-term storage, efficient strategies for pest and pathogen detection, germination testing procedures during long-term storage, accelerated seed aging and seed longevity, population size, and allelic frequency changes on seed rejuvenation, genotype independent tissue culture protocols, identification of somaclonal variation in case of in vitro procedure, employment of efficient methods of cryopreservation for cotton cultivars and wild species, ethical, legal, and social guidelines for exchange of cotton germplasm resources at national and international level.
Monitoring changes in the rate of genetic erosion strictly requires directly comparable, if not identical, measures of the state of a system at several points in time. Alternatively, it is possible to measure the major agents of erosion (e.g., deterioration or destruction of habitat due to urbanization, land clearing, overgrazing, salinization, drought, climate change, etc.). However, such indirect measures are very broad and have other and possibly more profound impacts than causing loss of diversity (Brown 2008). It is also suggested that neutral or trivial changes could mask critical changes when summed over loci, genotypes, populations, or species. A temporal indicator should reveal and be most sensitive to the changes of concern and not be overwhelmed by relatively unimportant changes. For example, the loss of a few alleles at a highly polymorphic microsatellite locus is likely to be of trivial or no importance compared with the loss of disease-resistance alleles. An additional problem lies in stressing combinations of alleles: in sexual species, all multilocus genotypes are unique and ephemeral. Thus, when a claim is made that some percentage of distinct clones or genotypes have been lost from a region or a species, this is not necessarily genetic erosion. A reduction in population size and not increased recombination is the primary agent of erosion (Brown 2008).
Therefore, relevant measures of genetic erosion include some subjective assessments of the significance of any loss, based on expertise and local knowledge. The inclusion of such evaluative information in measuring erosion is desirable. The challenge is to format it in such a way that at least a tentative quantitative treatment is possible. Further research is also needed (1) on the use of GIS technology to monitor genetic diversity in cotton and to predict and minimize genetic erosion and (2) on the incorporation of the resulting information into comprehensive information systems. Additionally, it is also important to understand the nature and extent of possible threats to existing diversity on-farm and in situ. And further care must be given to the many accessions such as tree cotton species, wild species, and land races which do not receive enough attention or investment in terms of conservation research and development.
12.5 Gap Filling Strategies
Currently, there are only very general ideas about what portion of a cotton population needs to be maintained in order to conserve particular genetic traits. This information is crucial to the efficient design of in situ conservation projects in cotton. Monitoring various putative causative factors is clearly one possible approach to assess the risk of future genetic erosion within a gene pool in a given area. Once an association between genetic erosion and different causative and countervailing factor(s) have been investigated in temporal and/or spatial comparisons, a predictive model could be constructed based on the assumptions that the association will continue into the future (Tripp and Heide 1996).
In general, solutions or mitigations for cotton genetic resources conservation have focused on ex situ conservation: seed banks, gene banks, and others. This approach allows genetic diversity to be maintained even if it is not currently represented in agricultural practice. In addition, studies on genetic research compare genetic diversity between modern and historic cultivars or progenitor wild plant species. This information helps to illuminate current or to predict future problems of genetic erosion, allowing an appropriate management response. However, in situ conservation of cotton genetic diversity could be an appropriate parallel conservation strategy, particularly for rare or endangered Gossypium species or those experiencing high mortality or rapid loss of habitat (Guerrant et al. 2004). Thus both ex situ and in situ methods are complementary, rather than alternative, conservation strategies (Rogers 2004).
The five tetraploid species and the cultivated diploid species G. arboreum and G. herbaceum are maintained ex situ with a considerable number of accessions, at least in eight major cotton world germplasm collections in Australia, Brazil, China, France, India, Russia, the United States, and Uzbekistan. The other 18 species of the secondary gene pool are preserved with a small number of accessions by most of these eight collections, but among the 25 species of the tertiary gene pool, five are not preserved in these banks, and two are represented by less than five accessions (Campbell et al. 2010).
Hence, what is needed to be further strengthened is the complementarity between seed conservation in gene banks (ex situ) and in ecosystems, and natural habitats (in situ). It is imperative to better manage cotton diversity in farmers’ fields, develop strategies to protect, collect, and conserve its wild relatives that are under threat, support the use of a wider range of traits for cotton breeding and strengthen seed systems, especially those of locally adapted cotton cultivars. The main focus should be on strengthening the conservation and sustainable use of conserved cotton materials and the crucial linkages between them, through a combination of appropriate policies, use of scientific information, farmers’ knowledge, and action.
Cotton cultivating countries need to establish or strengthen systems for monitoring genetic erosion, including easy-to-use indicators. Some examples of the proposed core indicators include number and kind of threatened and endangered species in cotton, number and kind of wild cotton relatives for in situ conservation, number and kind of protected areas for in situ conservation, number of in situ conservation sites and wild species conserved, number of species and accessions preserved ex situ, medium and long-term storage strategies, degree of genetic integrity of accessions preserved ex situ, and list of major environmental constraints to ex situ conservation. Support should be given to collecting farmers’ varieties/landraces in particularly vulnerable or threatened areas, where these are not already held ex situ, so that these genetic resources can be multiplied for immediate use and conserved for future use. In some countries, the threat of invasive alien species should also be considered, as these may contribute to genetic erosion and support should be provided to establish monitoring mechanisms at all levels. The World Information and Early Warning System (WIEWS) application for remote searching, updating, and reporting on genetic erosion (http://apps3.fao.org/wiews/wiews.jsp; accessed on 15th December, 2014) should also be strengthened with reference to cotton.
In conclusion, what can be done to improve genetic diversity in cotton while maintaining fiber yields and motivate a transition from high input high vulnerability monocultures to sustainable low input high-yield cropping systems? First, annual statistics of on-farm cotton genetic diversity should be collected, especially for the largest farms. These should be collected with relevant biotic and abiotic stress events to create a picture of performance and resilience. Second, on-farm diversity should be encouraged, perhaps by redirecting the subsidy program to support farmers transitioning to higher resilience farming practices with diverse numbers of cotton cultivars. As an example, natural brown and green fibers can be used from G. barbadense and G. hirsutum germplasm. This has dual applications: (i) production of eco-friendly colored cultivars and (ii) increasing the genetic diversity in the cotton field. Third, innovation strategies that promote long-term sustainability and yields, rather than peak quantity, should be introduced. This may require revising or inventing new intellectual property rights (IPR) instruments to maintain private sector incentives or a return to a public breeding and farm extension strategy that does not require capture of a revenue stream from licensing of IPR (Heinemann et al. 2014). It is further highlighted that addressing cotton genetic diversity with modern biotechnological tools, access and benefit sharing through appropriate IPR and biosafety guidelines, and bioethics on socioeconomic development also need additional attention.
Within the past decade the concept of biodiversity and their conservation has passed from the domain of academicians to the widespread attention of the common man. The general public and policy makers are ever more aware of the scope and seriousness of the fading of the genetic heritage. Although much of the debate focuses on animals and wild plant species, there is a growing recognition that the diversity of cultivated cotton species has vastly diminished, affecting the livelihoods of resource-poor farmers and threatening the future of fiber production and development. A number of proposals and policy initiatives are being discussed to address the problem, including preparations for a global plan of action for the conservation and use of cotton genetic resources (Abdurakhmonov 2014).
12.6 Conservation of Cotton Germplasm: Underexploited Treasure Available for Continually Reap the Benefits
Methods for cotton germplasm conservation are determined by a number of factors. One of the first factors to be deliberated when conserving cotton genetic diversity is the efficient and effective selection of the Gossypium genetic resources (Fig. 12.3). Such invaluable and irreplaceable resources are (i) cultivated varieties (cultivars) in current use and newly improved varieties (ii) obsolete cultivars (iii) primitive cultivars (landraces) (iv) wild and weed species, near relatives of cultivated varieties, and (v) special genetic stocks (including elite and current breeder’s lines, recombinant inbred lines, back cross progenies, doubled haploids, cytogenetic stocks, and mutants). Occasionally, genes, DNA fragments, and RNA derived from Gossypium are also included under the purview of genetic resources. The decision must focus that the selected accession is of sufficient importance to warrant active conservation and that the particular gene pool is not adequately conserved in the available cotton germplasm resources. While formulating strategies for such conservation, it is essential to know its areas of distribution and identify regions where both collection and conservation activities could effectively be initiated (Fig. 12.3). Such strategies should also consider any one or combination of the following: high levels of genetic diversity at the site(s), interest of the user community in the specific genetic diversity found at or believed to be found at the site, lack of previous conservation activities, and imminent threat of genetic erosion.
Hence, an ecogeographic survey is the first step in defining the most appropriate conservation strategy and Gossypium accession specific conservation objectives should be formulated, involving both ex situ and in situ components. The collection and analysis of ecogeographic data empower conservationists to make correct decisions on which taxa to be included in the target group, where to find these taxa, which combination of ex situ and in situ conservation to use, what sampling strategy to adapt, and where and how to store the germplasm. Since the ecogeographic data will rarely be sufficiently comprehensive to locate actual populations precisely, the preparatory element of conservation activities should be followed by field exploration (Fig. 12.3) during which the actual populations are located. For example, Central Institute for Cotton Research (CICR) at Nagpur, India has taken the initiative to collect and conserve the landraces of desi and perennial (tree types) cotton with desirable characters that are grown in the home gardens, foothills, and agricultural fields from Maharashtra, Madhya Pradesh, West Bengal, Andhra Pradesh, Mizoram, Meghalaya, Tripura, Gujarat, and Tamil Nadu. The important cotton landraces like Ponduru, Karuganni, Commilla, Uppam, and Wagad were collected from different states of India and conserved in the CICR cotton germplasm unit (Saravanan 2013). Similarly, collections and conservation from dooryard and other areas have also reported in Brazil (Menezes et al. 2014).
As discussed before, there are two primary complementary conservation strategies, ex situ and in situ and each of which includes a range of unlike techniques that can be implemented to achieve the aim of conservation of cotton genetic resources (Fig. 12.3). However, there is a great need to strengthen the conservation and sustainable use of Gossypium species and seed systems through a combination of appropriate policies, use of scientific information, farmers’ knowledge, and action. Recently, it has become clear that the best strategy combines ex situ conservation with on-the-ground (in situ) conservation by farmers in their agroecosystems and in areas where Gossypium wild relatives are protected for their environmental value.
In situ conservation enables cotton species to be conserved under conditions that allow them to continue to evolve. For some species, such as tree cotton species, it is the only feasible method of conservation. The main drawback is the difficulty in characterizing and evaluating the crop’s genetic resources and susceptibility to extreme weather conditions, pests, and disease (Tripp and Heide 1996). In contrast, conservation in ex situ gene banks ensures that the stored materials are readily accessible, can be well documented, characterized, and evaluated and are relatively safe from external threats. The ex situ conservation of genetic resources also allows the reintroduction of cultivars in areas where they have been lost. On the other hand, genetic adaptation and the rate of evolutionary response to selective forces of such stored cotton germplasm depend on inherent levels of genetic diversity present at the time a Gossypium species experiences a threat to its survival.
While such mechanisms are important, the sustainable use of plant genetic resources is equally essential because cotton genetic diversity increases options and provides insurance against future adverse conditions, such as extreme and variable environments and outbreak of new pests and diseases. The existence of variability is essential for breeding as much as for evolution and it must be present to obtain gains in selection. Therefore, conservation without use has little point. On the other hand, use without conservation means neglecting the genetic base needed by farmers and breeders in the future. To be of use, material held in cotton gene banks must be well-documented. This involves maintaining passport data, collection location, site characteristics, species, cultivar name, characterization data, recording highly heritable characteristics that can be used as a basis to distinguish one accession from another and evaluation data (documenting important traits such as yield, fiber quality parameters, phenology, growth habit, and reactions to pest, disease, and abiotic stresses).
If the material stored in cotton gene banks is to be used, it must be accessible with simple efforts. To this end, many cotton germplasm collections have established small subsets of collections, known as core collections, to facilitate research and use (Lacape et al. 2007; Boopathi et al. 2014, Tyagi et al. 2014). Development of core collections aim to include the maximum amount of diversity in a relatively small number of accessions, for example, a subset comprising 10 % of a collection is expected to contain at least 70 % of the total genetic diversity found in the whole collection. Another important method of widening the use of cotton genetic resources is through networks. Networks bring together all those with an interest in cotton genetic resources, including germplasm collectors, curators, researchers, breeders, and other users and provide a means for identifying the genetic resources within a cotton gene pool, and for taking collective action to conserve and use them.
The modern intensive cotton cultivation calls for uniformity in cotton cultivars that favors mechanization. Thus, the cotton production is limited by use of a smaller number of cotton cultivars and hybrids that possess uniform phenological traits and consequently has a narrow genetic base. Though the global cotton area has been increased during the past five decades due to advances in breeding efforts complemented with transgenic technology (Navarro and Hautea 2014), cotton fiber production is threatened by emerging problems such as sucking pest outbreaks, salinization of cotton cultivating area, unpredictable water stress, and so on. To this end, discovery and utilization of new Gossypium diversity is imperative for sustainable cotton production (Boopathi et al. 2014). Similar kind of reports is also seen in other crop plants. It was estimated by FAO that about three-quarters of the genetic diversity of agricultural crops have been lost over the past century and a narrow genetic diversity exists in cultivated crop plants (FAO 2012). The natural ‘genetic bottleneck’ imposed by polyploid formation in cotton has been exacerbated by repeatedly crossing relatively few closely related genotypes to one another to breed new cultivars and using only a few cultivars to deploy transgenes. For example, an impending worldwide water crisis makes it important to identify adaptations that permitted wild cotton accessions to endure periodic drought and temperature extremes, restoring such valuable alleles that may have been left behind during domestication to create cultivars that produce more with less water.
Therefore, to sum-up, it is imperative to conserve genomic resources in cotton gene banks. Such resources provide valuable traits needed for meeting the challenges of adapting cotton varieties. An individual genotype with seemingly useless set of characters today may suddenly become essential tomorrow due to changing climatic conditions or outbreaks of disease. Today, we do not yet know everything about future demands for cotton cultivars. But we know the supply source and it has to be conserved with its full potential. Therefore, it is right to time to realize that let us “conserve all the cotton diversity we have.”
12.7 Trends and Novel Tools in Cotton Germplasm Evaluation, Improvement, and Storage
Cotton diversity evaluation have traditionally been based on phenotypic characters evaluated on living plants managed in seed banks, field gene banks, botanic gardens or in situ reserves or based on dried plants managed in herbarium collections. In the recent past, several cotton germplasm conservation units are turning to DNA technologies to have effective conservation strategies. The DNA bank is an efficient, simple, and long-term method used in conserving genetic resource for biodiversity (Kelleher et al. 2005). Compared to traditional seed or field gene banks, DNA banks lessen the risk of exposing genetic information in natural surroundings. It only requires small sample size for storage and keeps the stable nature of DNA in cold storage. Since whole plants cannot be obtained from DNA, the stored genetic material must be introduced through genetic techniques (DNA Bank 2012). Currently, the plant taxonomy and systematic community have responded to the biodiversity crisis by defining three major challenges: (1) completing the inventory of life, (2) discovering evolutionary relationships through phylogenetic, and (3) providing information via the Internet. DNA collections can help with all three of those activities (Kelleher et al. 2005).
Therefore, DNA sequence analysis is useful in the identification and delimitation of species and higher taxa and is also set to become increasingly important via DNA taxonomy and DNA barcoding (Ronald 2014). Analysis of morphological, chemical, and anatomical characteristics of cotton plant specimens can be used for assessment of genetic variation within and between species, but none of these can claim to offer the same potential as DNA. Genomic DNA samples represent the entire genetic component of the target organism. Therefore, together with the traditional techniques, DNA technologies offer great hope in cotton genetic resources and their diversity analysis.
A range of DNA-and RNA-based molecular markers, from restriction fragment length polymorphism (RFLP) to single nucleotide polymorphisms (SNPs) and insertion–deletion polymorphisms (InDels) and diversity array technology (DArT) are being employed in genetic diversity analysis in cotton. Boopathi (2013) tried to provide a collective depiction of relevant information about the usage of some commonly used markers in cotton and other agriculturally important crops, which help researchers to find out the frequentness and application of different markers and compare their results. Such markers may also serve as a platform and help the intellectuals for the selection and modification of their marker system in cotton diversity analysis. However, use of such markers in cotton genetic diversity analysis has both pros and cons. On the one hand, the designed markers can be well used in diversity studies and tetraploid cotton genetic mapping. On the other hand, the developmental efficiency of markers and polymorphism of designed primers are relatively low (Li et al. 2014a).
It is also equally important to increase the variation in the available cotton germplasm collections and scientists are developing new and more efficient breeding strategies that integrate genomic technologies and high throughput phenotyping to better utilize natural and induced genetic variation. Rapid developments in next generation sequencing (NGS) technologies over the past decade have opened up many new opportunities to explore the relationship between genotype and phenotype with greater resolution than ever before. As the cost of sequencing has decreased, breeders have begun to utilize NGS with increasing regularity to sequence large populations of plants, increasing the resolution of gene and QTL discovery and providing the basis for modeling complex genotype–phenotype relationships at the whole-genome level (Varshney et al. 2014). NGS technology is vitally important as a tool for characterizing cotton genetic resources globally. The vast majority of accessions found in the world’s cotton gene banks are currently poorly characterized and as a result, rarely used. An international effort is essential to take advantage of the low cost and high throughput of NGS, in combination with development of appropriate database of information, large-scale phenotyping, and population development, to help characterize gene bank materials and to provide a rational basis for their utilization.
Breeders using marker-assisted selection (MAS) to introgress a favorable QTL allele from a wild or unadapted donor parent into an elite, adapted line often encounter the problem of linkage drag. The transfer of a large QTL region from a donor plant into a divergent breeding line may introduce undesirable phenotypic effects owing to the presence of linked genes in the introgressed QTL region. These linked genes often have nothing to do with the target trait but can make the new line unacceptable. NGS is vital for quickly identifying the individuals that carry critical recombination breakpoints that break the linkage drag. Because the landraces that served as the breeding donors carried the favorable and the unfavorable alleles in coupling, it took a concentrated effort and deep sequencing within the target region on a large segregating population to identify a recombinant individual in which the linkage had been broken. In such cases, if the causal gene(s) and/or functional polymorphism(s) for the favorable and/or the deleterious trait(s) are known, the breeder can use that information to guide the selection of individuals that carry key recombination events to minimize the effect of linkage drag. Once a recombinant individual is identified, it becomes immediately useful as a donor in breeding and may serve to introduce new genetic variation into a breeding pipeline. Thus, NGS can be extremely helpful to identify the recombinants in breaking linkage drag and liberating new forms of genetic variation for use in cotton breeding. Overcoming linkage drag effects may lead to the achievement of varieties bearing G. hirsutum adaptability and G. barbadense fiber quality.
Specialized genetic stocks, such as bi-parental and multi-parent mapping populations, mutant populations, and immortalized collections of recombinant lines are being generated in cotton to facilitate mapping and gene function analysis via association studies and QTL mapping. Knowledge about the identity and map location of agriculturally important genes and QTL provides the basis for parental selection and MAS in cotton breeding (Boopathi 2013). Alternatively, genotypic and phenotypic datasets on training populations can be used to develop models to predict the breeding value of lines in an approach called genomic selection (Varshney et al. 2014). MAS and marker-assisted back-crossing (MABC) have been valuable for harnessing agriculturally and economically valuable genes and QTLs from wild or unadapted cotton genetic resources, particularly where the phenotype of a wild accession offers little or no insight about its potential value as a breeding parent. Prior to the advent of DNA markers, it was extremely cumbersome and inefficient to try to select for recombinant offspring from interspecific populations that carried the favorable wild allele(s) of interest because many unfavorable alleles that were also inherited from the wild donor typically masked the favorable phenotype. Genomics-assisted breeding has dramatically shifted the way breeders are able to work with unadapted genetic resources. The development of improved breeding lines for commercial cotton cultivation has traditionally been a time consuming and expensive task. With the deployment of genomics-assisted breeding, the generation of such lines is intended to become easier and faster. However, the major limitation is more expensive.
Genome-wide association studies (GWAS) utilize association mapping, also known as linkage disequilibrium (LD) mapping, to map QTLs by taking advantage of historic LD to identify statistically significant phenotype–genotype associations (Varshney et al. 2014). GWAS have been successfully performed in several crop plants, including maize, rice, wheat, soybean, sorghum, and foxtail millet. However, the role of GWAS in cotton has very limited information (Jia et al. 2014). In the future, it is speculated that the use of GWAS will enrich the gene pools of cotton by identifying useful variants that have only rarely been used in modern cotton genetic improvement programs.
Besides, advances in other fields of biotechnology have also generated new opportunities for cotton genetic resources conservation and utilization. Techniques like in vitro pollen culture, DNA banks, and cryopreservation have made it possible to collect and conserve genetic resources, especially of species that are difficult to conserve as seeds. Cryoconservation (storage in extreme deep freeze situations) is accomplished with liquid nitrogen at −196 °C (Hammer 2004). It is also suitable for seeds and leads to a dramatic prolongation of germination rates. It allows for an extremely long storage of many species. For in vitro maintenance cultures, it is the choice of preference because somaclonal variation can be prevented. The problem with cryoconservation is its high cost, especially for technical equipment. A constant supply of liquid nitrogen also has to be available at all times (Hammer 2004). DNA and pollen culture also contribute to ex situ conservation. Since, cotton has shown poor response to tissue culture protocol (Wilkins and Rajasekaran 2000), no single conservation technique can adequately conserve the full range of genetic diversity of a target species or gene pool. Greater biodiversity security can be obtained from the application of a range of complementary ex situ and in situ techniques, (Fig. 12.3) since one technique acts as a backup to the other techniques.
Similarly, recombinant DNA technology increased the possible use of distantly related trait carriers (sometimes completely unrelated, such as microbial and animal biological systems) as donors for the desired characteristics. However, the movement of genes across species boundaries presents many opportunities for both expected and unexpected risks. In addition to food safety issues related to cotton seed oil, other concerns involve ecological risks, such as new or increased resistance to insecticides and weed resistance to herbicides due to hybridization or excessive selection pressure, changes in the ecological competitiveness and the possible loss of genetic diversity in the transgenic areas (Ronald 2014). Transgenes conferring novel traits that enhance survival and reproduction may inadvertently disperse from cultivated plants to wild or weedy populations that lack these traits and might generate similar but unwanted effects in their weedy relatives through gene flow. There is a gain of fitness when G. barbadense plants are crossed to G. hirsutum, showed by a greater seed production of the hybrids when compared to the parents, and transgenic G. barbadense plants harboring Cry1Ac perform better than non-transgenic ones when exposed to Alabama argillacea or Pectinophora gossypiella pests. The in situ preservation of pure G. barbadense can be improved by reproductive isolation, not only from transgenics but also from crossings to traditional upland cotton. Reproductive isolation is not enough to in situ preservation of G. barbadenses in Brazil, since it is used as a medicinal or ornamental plant (Hoffmann et al. 2013). However, it is envisaged that careful deployment of transgenic technology can increase the cotton fiber yield as well as the genetic diversity.
A more recent technology, called genome editing, which makes it possible to precisely alter DNA sequences in living cells, is expected to lead to new crop varieties in the near future (Voytas and Gao 2014). In this technique, targeted double-strand DNA breaks are introduced in the genome at or near the site where a DNA sequence modification is desired using sequence-specific nucleases. The repair of the break can be used to introduce specific DNA sequence changes, DNA deletions, or even serve as an insertion site for arrays of transgenes. Genome editing can thus be used to introduce genetic variation without transgenic technology and can even be used to recreate naturally occurring mutations into elite varieties of crops. For this reason, some scientists and farmers believe that crops generated through this technology will prove to be more socially acceptable elsewhere than those generated by genetic engineering. Genome editing has been successfully used to engineer rice for resistance to the bacterial pathogen, Xanthomonas oryzae pv. oryzae. Researchers created mutations in the promoter of a rice sucrose-efflux transporter gene, which is targeted by a pathogen effector (Voytas and Gao 2014). These mutations, which are mostly DNA deletions, eliminated the transcriptional induction required for pathogen virulence, rendering the plant resistant. However, the role of genome editing in cotton is yet to be demonstrated.
In general, plant breeders recognized three major gene pools based on the degree of sexual compatibility (Huynh et al. 2013). All crop species belong to a primary gene pool together with such material with which they produce completely fertile crosses through hybridisation. In contrast, all those plant groups that contain certain barriers against crossing belong to the secondary gene pool. The tertiary gene pool includes groups that can only be crossed with the help of radically new techniques. Plant breeders have traditionally emphasized closely related, well-adapted domesticated materials within the primary gene pool as sources of genetic diversity. More recently, however, recombinant DNA technology, plant transformation and genomics have led to a new quality which may be defined as a fourth gene pool or as a special case for the third gene pool. Such new tools of biotechnology allow us to bypass sexual incompatibility barriers altogether and introduce new genes into existing cultivars. It should be emphasized here that the major function of such technologies is not the creation of new cultivars but the generation of new gene combinations that can be used in cotton breeding programs.
To this end, crop multi-genotype breeding, which combines the advantages of both old and modern agricultures at the high level of productivity and sustainability, is considered as a promising strategy. The concept, necessity, principle, technical tactics, and characteristics of crop multi-genotype breeding are elucidated in detail and successful case of its application in cotton was documented (Li et al. 2014b). A multi-genotype hybrid variety, Jing-Mi 1 revealed superiority in seeded cotton and lint yields over the check variety in regional trial. Multi-genotype variety could be maintain and recover the genetic diversity in production system. Unlike a set of naturally diverse germplasm, multi-parent advanced generation intercross populations (MAGIC) population is tailor-made for breeders with a combination of useful traits derived from multiple elite breeding lines. The MAGIC populations also present opportunities for studying the interactions of genome introgressions and chromosomal recombination (Li et al. 2014b).
G. longicalyx is a diploid species of the secondary gene pool of G. hirsutum immune to the reniform nematode (Rotylenchulus reniformis). Synthetic tetraploid triple-species hybrids have been crossed four to seven times to cultivated upland cotton, leading to the obtainment of plants bearing the resistance and indistinguishable to G. hirsutum plants under greenhouse conditions (Robinson et al. 2007). Similarly, advanced backcross populations were also constructed in cotton in which transgressive variation, the occurrence of progeny displaying phenotypes more extreme than either parent, was genetically dissected (Lacape et al. 2007).
12.8 Prospects for Sustainable Use of Genetic Resources in Cotton
The prime focus of the cotton breeders has now shifted to addressing problems due to climate change by developing resilient cotton cultivars. This can be achieved by effective utilization of cotton genetic resources and widening the cotton genetic diversity. Each of the three major approaches to increasing genetic diversity—mutagenesis, germplasm introgression, and transformation—have advantages and disadvantages. Interspecific germplasm introgression is particularly attractive in that it utilizes a broad germplasm base, can be targeted to one or more specific traits/genes or modulated to include thousands of genes/even entire genomes and is readily coupled to marker-assisted genome analysis and selection (Saha et al. 2006). Though, QTL mapping and MAS have potential applications in genetic improvement of cotton for higher productivity, their applications are not yet widely documented in cotton breeding program due to poor knowledge on physiological and genetic nature of fiber quality and productivity traits, low, and complex heritability of investigated traits, genotype X environment interactions, etc. (Lacape et al. 2007; Boopathi et al. 2011). Although introgression of genes across species boundaries is difficult, it is quite desirable because the gene pools of cultivated species do not contain all of the desired alleles. Alternatively, mutagenesis and transgenic technology has been proposed. However, currently they have limited applications due to several technical reasons such as nonavailability of novel genes, lack of efficient method to alter/transfer large genetic element, etc. (Wilkins and Rajasekaran 2000). Therefore, sustainable utilization of cotton genetic resources highly demands system-wide, regional, and global focussing programs with strong cooperation among stakeholders for the design, implementation, compliance, and utilization of cotton genetic diversity for breeding new cultivars.
Genetically improved seed, whether derived from conventional genetic modification or newly developed genomics technologies, must be integrated into ecologically based farming systems to maximize their impact on enhancing sustainable fiber production. For example, farmers cannot rely on seed alone to eliminate pests. For example, deployment of a ‘‘refuge strategy’’—creating refugia of crop plants that do not make Bt toxins—promotes the survival of susceptible insects and helps to delay the evolution of pest resistance to Bt crops. Whereas this approach has been successful in the US, where farmers are required to plant refugia, failure to provide adequate refugia appears to have hastened pink bollworm resistance in India (Gujar et al. 2007). It emphasizes the need to deploy crop rotation and diversity to reduce the evolution of insect resistance. Well-funded, long-term, multinational, multidisciplinary collaborations are vital if we continue to make significant progress in developing new crop varieties to enhance cotton fiber production using cotton genetic resources.
12.9 Global Cotton Germplasm Data Management: Annotation, Curating and Dissemination
In order to realize the complete potential of cotton germplasm resources in the future breeding programs, it is essential to develop bioinformatic and database tools to assemble, analyze, and make the information useable to the cotton community. CottonDB (http://cottondb.org; accessed on 15th December, 2014) is one such comprehensive database that was established with the above said aim. Through a website interface, it provides genomic, genetic, and taxonomic information, including germplasm, markers, genetic and physical maps, trait studies, sequences, bibliographic citations. Similarly, the Cotton Portal (http://Gossypium.info; accessed on 15th December, 2014) offers the scientific community a single port of entry to participating Cotton Web resources. The Cotton Diversity Database (http://cotton.agtec.uga.edu; accessed on 15th December, 2014) provides for integrative queries relating to performance trial, phylogenetic, genetic, and comparative data and is closely integrated with comparative physical, EST and genomic sequence data, expression profiling resources and with the capacity for additional integrative queries. Cotton marker database (CMD; http://www.mainlab.clemson.edu/cmd/AboutUs.shtml; accessed on 15th December, 2014) provides centralized access to all publicly available cotton microsatellites and other markers available for genetic diversity analysis and it also contains a core set of markers that are useful for initial genetic diversity analysis in the given cotton germplasm. TropGENE-DB (http://tropgenedb.cirad.fr/en/cotton.html; accessed on 15th December, 2014) integrates a subset of published mapping data.
Besides, several project websites such as cotton functional genomics (http://cottongenomecenter.ucdavis.edu/; accessed on 15th December, 2014), cotton fiber genomics (http://www.cottongenomics.org/; accessed on 15th December, 2014), genetic and physical mapping (www.plantgenome.uga.edu; accessed on 15th December, 2014) the cotton microarray (http://cottonevolution.info/microarray; accessed on 15th December, 2014), Cotton Gene Indices (CGI) (http://www.tigr.org/tigr-scripts/tgi/T_index.cgi?species=cotton; accessed on 15th December, 2014) and Arizona Genomics Institute (http://www.genome.arizona.edu/; accessed on 15th December, 2014) are primarily used for disseminating genomic resources and coordinately distributed genomic resources to the cotton research community.
However, it is increasingly argued that there is a great need to expand bioinformatic infrastructure for managing, curating, and annotating the large amount of cotton genomic information that will be generated in the near future as it is developed in other crops such as Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org/; accessed on 15th December, 2014), Maize Genetics and Genomics Database (MaizeGDB, http://www.maizegdb.org/; accessed on 15th December, 2014), Soybase (http://soybase.agron.iastate.edu/; accessed on 15th December, 2014) and GrainGenes (http://wheat.pw.usda.gov/GG2/index.shtml; accessed on 15th December, 2014). Such cotton database should be able to host and manage information resources in cotton using community-accepted germplasm characterization, genome annotation, nomenclature, and gene ontology.
12.10 Concluding Remarks
A sustainable strategy to provide natural fiber security for a growing population must promote cotton genetic diversity conservation and avoid further habitat loss of natural ecosystems, since the future of cotton production depends on its genetic diversity. However, the greatest challenge facing the cotton community is not collecting and conserving the cotton genetic diversity per se but the conversion of such information to knowledge and utilizing them in routine cotton breeding program for sustainable fiber production in the coming years.
Genetic improvement of fiber production with the new sources of cotton germplasm will ensure that this natural-renewable product will be competitive with petroleum-derived synthetic fibers and reduce the environmental risks. Besides, such efforts will have several other practical ramifications that include increased water use efficiency, other abiotic and biotic stress tolerance/resistance, reduced fertilizer and pesticide requirements, expanded use as specialized fibers. Modifying cottonseed for food and feed could profoundly enhance the nutrition and livelihoods of millions of people in food-challenged economies. Countries that have rich cotton genetic diversity can take advantage of their genetic resources from locally adapted varieties and races and wild relatives of cotton to increase yields. This can be performed by applying biotechnological tools, by implementing bioprospecting activities, and by establishing partnerships with public and private sector institutions in industrial and developing countries. The strategy must also deal with issues of ethics, biosafety, and IPR in the use of new biotechnologies (Krishna et al. 2014).
We can promote cotton germplasm conservation literacy through the establishment of elementary and university curriculums that highlight the social, economic, biological, environmental, and ethical aspects of cotton germplasm conservation. We must also integrate training across scientific fields, including genetics, plant breeding, computer science, mathematics, engineering, biometrics, and bioinformatics and to evolve new forms of communication and professional organization. It is also equally important to have deep discussion with the policy makers, nongovernmental organizations and journalists by providing science-based information in more creative ways—for example, through social media and videography. An engaged, informed public will help us to attain a sustainable cotton cultivation system derived from the available cotton genetic resources that can produce increased and quality fiber in a secure, sustainable, and equitable manner.
References
Abdurakhmonov IY (ed) (2014) World Cotton Germplasm Resources. http://www.intechopen.com/books/world-cotton-germplasm-resources/cotton-germplasm-collection-of-uzbekistan. Accessed 14 Dec 2014
Almeida VC, Hoffmann LV, Yokomizo GK, Costa JN, Giband M, Barroso PAV (2009) In situ and genetic characterization of Gossypium barbadense populations from the states of Pará and Amapá, Brazil. Pesq Agropec Bras 44:719–725
Alves MF, Barroso PAV, Ciampi AY et al (2013) Diversity and genetic structure among subpopulations of Gossypium mustelinum (Malvaceae). Genet Mol Res 12:597–609
Barroso PAV, Hoffmann LV, Freitas RB et al (2009) In situ conservation and genetic diversity of three populations of Gossypium mustelinum Miers ex Watt. Genet Resour Crop Evol 57:343–349
Bhargav DK, Meena HP, Participatory Plant Breeding PPB (2014) Participatory plant breeding: farmers as breeders. Available at http://www.popularkheti.info/documents/Issue-2-1/PK-2-1-2.pdf. Accessed on 15th Dec 2014
Boopathi NM (2013) Genetic mapping and marker assisted selection: Basics Practice and Benefits. Springer, India, p 303
Boopathi NM, Gopikrishnan A, Selvam NJ, Ravikesavan R, Iyanar K, Muthuraman S, Saravanan N (2008) Genetic diversity assessment of G. barbadense accessions to widen cotton (Gossypium spp.,) gene pool for improved fibre quality. J Cotton Res Dev 22(2):135–1384
Boopathi NM, Thiyagu K, Urbi B, Santhoshkumar M, Gopikrishnan A, Aravind S, Swapnashri G, Ravikesavan R (2011) Marker-assisted breeding as next-generation strategy for genetic improvement of productivity and quality: can it be realized in cotton? Int J Plant Genomics 2011:670104. doi:10.1155/2011/67010
Boopathi NM, Sathish S, Dachinamoorthy P, Kavitha P, Ravikesavan R (2014) Usefulness and utilization of Indian cotton germplasm. In: Ibrokhim Y. Abdurakhmonov (ed) World cotton germplasm resources. http://www.intechopen.com/books/world-cotton-germplasm-resources/cotton-germplasm-collection-of-uzbekistan. Accessed 14 Dec 2014
Brown AHD (2008) Thematic background study on “Indicators of genetic diversity, genetic erosion and genetic vulnerability for plant genetic resources for food and agriculture”.A report submitted to FAO. Food and Agriculture Organization of the United Nations, Rome
Brush SB (1995) In situ conservation of landraces in centers of crop diversity. Crop Sci 35:346–354
Campbell BT, Saha S, Percy R et al (2010) Status of the global cotton germplasm resources. Crop Sci 50:1161–1179
Chakravarthy VS, Reddy TP, Reddy VD, Rao KV (2014) Current status of genetic engineering in cotton (Gossypium hirsutum L): an assessment. Critical Rev Biotech 34(2: 144–160
Chen ZJ et al (2007) Toward sequencing cotton (Gossypium) genomes. Plant Physiol 145:1303–1310
Choudhary B, Laroia G (2001) Technological developments and cotton production in India and China. Curr Sci 80:8
Comai L, Young K, Till BJ et al (2004) Efficient discovery of DNA polymorphisms in natural populations by EcoTILLING. Plant J 37:778–786
Cooke RJ, Reeves JC (2003) Plant genetic resources and molecular markers: variety registration in a new era. Plant Genetic Resour Charact Util 1(2–3):81–87
DNA Bank (2012) Crop gene bank knowledge base. http://cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=98&Itemed=202&Lang=English. Accessed on 15th Dec 2014
Fabrick JA et al (2014) Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PLoS ONE 9(5):e97900
Falconer DS (1989) Introduction to quantitative genetics. Wiley, New York
FAO (2012) The second report on the State of the World’s Plant Genetic Resources. Food and Agriculture Organization of the United Nations, Rome. Available at www.fao.org/agriculture/crops/core-themes/theme/seeds-pgr/sow/sow2/en. Accessed on 15th Dec 2014
Freire EC, Moreira JAN (1991) Genetic relationships among moco and other species and races of cotton (Gossypium spp). Brazil J Genetics 14:393–411
Gore MA et al (2014) Linkage map construction and quantitative trait locus analysis of agronomic and fiber quality traits in cotton. Plant Genome 7:1
Guerrant EO, Havens K, Maunder M (2004) Ex Situ plant conservation: supporting species survival in the wild. Island Press, Washington, DC
Gujar GT, Kalia V, Kumari A, Singh BP, Mittal A, Nair R, Mohan M (2007) Helicoverpa armigera baseline susceptibility to Bacillus thuringiensis Cry toxins and resistance management for Bt cotton in India. J Invert Pathol 95(3):214–219
Hammer K (2004) Resolving the challenge posed by agrobiodiversity and plant genetic resources—an attempt. J Agric Rural Dev Tropics Subtropics, Beiheft Nr. 76; DITSL, Kassel university press GmbH, Germany
Hammer K, Teklu Y (2008) Plant genetic resources: selected issues from genetic erosion to genetic engineering. J Agri Rural Dev Tropics Subtropics 109(1):15–50
Heinemann JA, Melanie M, Dorien SC, Sarah ZA, Wen JD (2014) Sustainability and innovation in staple crop production in the US Midwest. Int J Agric Sustain 12(1):71–88
Hoffmann LV, Barroso PAV, Sousa JM, Tibazarwa FI (2013) Fitness of hybrids between Gossypium barbadense and upland cotton and resistance to Pectinophora gossypiella and Alabama argillacea. J Life Sci 7:820–826
Holt G, Simonton J, Beruvides M, Canto AM (2003) Engineering economic analysis of a cotton by-product fuel pellet operation. J Cotton Sci 7:205–216
Huynh BL, Close TJ, Roberts PA, Hu Z, Wanamaker S, Lucas MR, Ehlers JD (2013) Gene pools and the genetic architecture of domesticated cowpea. Plant Genome 6:3
Jia Y, Sun X, Sun J, Pan Z, Wang X et al (2014) Association mapping for epistasis and environmental interaction of yield traits in 323 cotton cultivars under 9 different environments. PLoS ONE 9(5):e95882
Kelleher CT, Hodkinson TR, Douglas GC, Kelly DL (2005) Species distinction in irish populations of Quercus petraea and Q. robur: morphological versus molecular analyses. Ann Bot (Lond) 96:1237–1246
Kim JK, Triplett BA (2001) Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant Physiol 127:1361–1366
Kranthi KR (2013) Cotton production in India. In: Manickam S, Sankaranarayanan K, Prakash AH (eds) Training manual on relevance and techniques of organic cotton production, CICR, Nagpur, India, 21–25 Jan 2013
Krishna V, Qaim M, Zilberman D (2014) Transgenic crops, production risk and agrobiodiversity, ZEF- Discussion Papers on Development Policy No. 186, Center for Development Research, Bonn, February, 2014, pp. 32
Labate JA (2000) Software for population genetic analysis of molecular marker data. Crop Sci 40:1521–1528
Lacape JM, Dessauw D, Rajab M, Noyer JL, Hau B (2007) Microsatellite diversity in tetraploid Gossypium germplasm: assembling a highly informative genotyping set of cotton SSRs. Mol. Breed. 19:45–58
Li X, Li H, Zou Q, Li Z, Wang M, Xu C (2014a) What has been neglected in the green revolution? Developing crop poly-genotype varieties for improving (intra-variety) genetic diversity in agriculture. Open J Ecol 4:394–410
Li X, Gao W, Guo H, Zhang X, Fang DD, Lin Z (2014b) Development of EST-based SNP and InDel markers and their utilization in tetraploid cotton genetic mapping. BMC Genom 15:1046
Menezes IPP, Barroso PAV, Hoffmann LV, Lucena VS, Giband M (2010) In situ and genetic characterization of Gossypium barbadense populations from the states of Pará and Amapá, Brazil. Botany 88:765–773
Menezes IPP, Gaiotto FA, Hoffmann LV et al (2014) Genetic diversity and structure of natural populations of Gossypium mustelinum, a wild relative of cotton, in the basin of the De Contas River in Bahia, Brazil. Genetica 142:99–108
Mohammadi SA, Prasanna BM (2003) Analysis of genetic diversity in crop plants—salient statistical tools and considerations. Crop Sci 43:1235–1248
Navarro MJ, Hautea RA (2014) Adoption and uptake pathways of GM/Biotech crops by small-scale, resource-poor farmers in China, India and the Philippines. ISAAA Brief No. 48. ISAAA, Ithaca, NY
Navreet KB, Zhang Z, Wicker T, Keller B (2010) Wheat gene bank accessions as a source of new alleles of the powdery mildew resistance gene Pm3: a large scale allele mining project. BMC Plant Biol 10:88
Nizar MA, Dikshit N, Sivaraj N (2014) DIVA-Geographic Information System approaches for assessment of diversity and distribution pattern of Abelmoschus species from Maharashtra, India. Adv Appl Res 6(1):28–34
Ravikesavan R, Venkatesan S, Sathish S, Kavitha P, Boopathi NM (2014) Unlocking the genetic potential for improvement of economic traits in large collection of cotton (Gossypium spp) germplasm. J Cotton Res Dev 28(2):175–184
Robinson AF, Bell AA, Dighe ND et al (2007) Introgression of Resistance to Nematode Rotylenchulus reniformis into Upland Cotton (Gossypium hirsutum) from Gossypium longicalyx. Crop Sci 47:1865–1877
Rogers DL (2004) Genetic erosion: no longer just an agricultural issue. Native Plants (Fall) 2004:113–122
Ronald PC (2014) Lab to farm: applying research on plant genetics and genomics to crop improvement. PLoS Biol 12(6):e1001878
Saha S, Jenkins JN, Wu J, McCarty JC, Gutierrez OA, Percy RG, Cantrell RG, Stelly DM (2006) Effects of chromosome-specific introgression in upland cotton on fiber and agronomic traits. Genetics 172:1927–1938
Saravanan M (2013) Tree cotton and cotton perennials of India—a short note. Cotton Innovate 3(9):1
Scarascia-Mugnozza GT, Perrino P (2002) The history of ex situ conservation and use of plant genetic resources. In: Engels JMM, Ramanatha Rao V, Brown AHD, Jackson MT (eds) Managing plant genetic diversity. CABI Publishing, Oxon (UK), pp 1–22
Thiyagu K, Boopathi NM, Nadarajan N, Gopikrishnan A, Selvakumar P, Magadum S, Ravikesavan R (2011) Sampling and exploitation of genetic variation exist in locally adapted accessions using phenotypic and molecular markers for genetic improvement of cotton. Gene Conserve 10(40):129–153
Tripp R, Heide W (1996) The erosion of crop genetic diversity: challenges, strategies and uncertainties. Nat Resour Perspect 7:1–10
Tyagi P, Gore MA, Bowman DT, Campbell BT, Udall JA, Kuraparthy V (2014) Genetic diversity and population structure in the US Upland cotton (Gossypium hirsutum L.). Theor App Genet 127(2):283–295
Varshney RK, Terauchi R, McCouch SR (2014) Harvesting the promising fruits of genomics: applying genome sequencing technologies to crop breeding. PLoS Biol 12(6):e1001883
Vitale JD, Djourra H, Sidib´e A (2009) Estimating the supply response of cotton and cereal crops in smallholder production systems: recent evidence from Mali. Agric Econ 40:519–533
Voytas D, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12:e1001877
Wilkins TA, Rajasekaran KDM (2000) Cotton biotechnology. Critical Rev Plant Sci 15:511–550
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Boopathi, N.M., Hoffmann, L.V. (2016). Genetic Diversity, Erosion, and Population Structure in Cotton Genetic Resources. In: Ahuja, M., Jain, S. (eds) Genetic Diversity and Erosion in Plants. Sustainable Development and Biodiversity, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-319-25954-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-25954-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25953-6
Online ISBN: 978-3-319-25954-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)