Abstract
Systemic lupus erythematosus (SLE) is a complex autoimmune syndrome showing a broad and protean spectrum of clinical and immunological features. Cutaneous involvement is frequently found in SLE. Affected patients may show a variety of different “specific” and “nonspecific” cutaneous manifestations as either an initial leading sign of the disease or as complication in its course. Hence, knowledge of the typical and more unusual cutaneous features associated with SLE is important for the proper diagnosis and management of affected patient.
After articular involvement, the skin represents the second most frequently affected organ in SLE. In fact, approximately 80 % of patients will display skin manifestations during the course of the disease. This chapter will focus on cutaneous involvement in SLE, with special emphasis on classification, diagnostic, and treatment aspects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune syndrome showing a broad and protean spectrum of clinical and immunological features. Cutaneous involvement is frequently found in SLE. Affected patients may show a variety of different “specific” and “nonspecific” cutaneous manifestations as either an initial leading sign of the disease or as complication in its course. Hence, knowledge of the typical and more unusual cutaneous features associated with SLE is important for the proper diagnosis and management of affected patients [1–4].
After articular involvement, the skin represents the second most frequently affected organ in SLE [1]. In fact, approximately 80 % of patients will display skin manifestations during the course of the disease [2, 3].
Cutaneous manifestations may constitute the first sign of SLE in up to 25 % of cases. Therefore, in all patients with newly diagnosed cutaneous lupus erythematosus (CLE), the clinician is invariably faced with the dilemma of whether the observed cutaneous lesions constitute the first sign of SLE or not.
The estimated risk of experiencing a transition from CLE to SLE has been differently estimated with rates up to 25 % [4]. Durosaro et al. reported that in patients with newly diagnosed CLE, the cumulative incidence of SLE among patients was 5 % at 5 years, 10 % at 10 years, 15 % at 15 years, 19 % at 20 years, and 23 % at 25 years after diagnosis, respectively [5]. In a population-based Swedish cohort study, Grönhagen et al. showed that the probabilities of developing SLE in the first and third year after CLE diagnosis are 12.1 % and 20.0 %, respectively. Vice versa, in 24 % of patients with newly diagnosed CLE, there is a current history of SLE [6]. However, the risk of developing SLE differs between subjects with acute CLE and those with localized discoid cutaneous lupus erythematosus (DLE). Therefore, the majority of patients with CLE will never develop any evidence for internal organ involvement.
The Systemic Lupus International Collaborating Clinics (SLICC) group recently revised the ACR-SLE classification criteria to improve their relevance. In the new classification, mucocutaneous signs again constituted 4 of 11 criteria used for SLE classification [7]. Among the SLE-specific skin manifestations, DLE is the most common, followed by subacute CLE (SCLE) and acute CLE (ACLE). The SLE nonspecific skin manifestations include Raynaud’s phenomenon and, more rarely, non-scarring alopecia and cutaneous “vasculitis” [8].
2 Epidemiology of Cutaneous Involvement
Two recent population-based studies reported an incidence of 4 new cases of CLE per 100,000 inhabitants per year in Sweden and the USA [4]. Prevalence of CLE is about 70 cases per 100,000 persons. Discoid chronic CLE (CCLE), the most common subset of CLE, is found in 80 % of cases [5]. Fifteen percent of cases have SCLE, while less than 5 % of cases display other types of CLE, such as lupus profundus. DLE seems to be more common among African Americans [5, 9], whereas SCLE is found more frequently in Caucasians. Finally, there is good evidence indicating that SLE is more common in Asians and African Americans than in Caucasians [10, 11].
3 Pathogenesis
SLE is regarded as heterogeneous group of diseases that develop in genetically susceptible individuals. In those, environmental triggers are thought to lead to the activation of both innate and adaptive immune responses with a loss of tolerance to self-antigens. Development of autoantibodies, activation of the complement system, deficiency in the removal of immune complexes, and other inflammatory processes ultimately lead to cell and tissue injury [12]. Environmental factors include ultraviolet rays, viral infections, or chemicals. Sexual hormones as well as emotional neuro-immunomodulatory factors also contribute to the development of SLE.
CLE shares genetic abnormalities with SLE. Genome-wide association studies (GWASs) have provided evidence for the presence of distinct gene polymorphisms conferring disease susceptibility and which are associated with specific target organ damage [13].
Aberrant clearance of nucleic-acid-containing debris and immune complexes, excessive innate immune activation involving Toll-like receptors (TLRs) and type I interferons (IFNs), and abnormal T- and B-lymphocyte activation constitute pathways involved in disease pathogenesis [14]. For example, patients with SCLE, DLE, and SLE have distinct polymorphisms in the IFN-regulatory factor 5 (IRF5) gene. The latter appears to modulate pathways that mediate production of IFN-1 and the cellular response to IFN-1. In family studies, increased IFN-1 production was found to represent a genetic risk of developing SLE [15]. Type I IFN exerts many biologic effects, including activation of dendritic cells, promotion of the differentiation of monocytes into antigen-presenting cells and B cells into plasma cells, respectively, stimulation of the Th1 pathway, prevention of apoptosis of activated cytotoxic T cells, and suppression of regulatory T cells [16].
Failure to degrade genomic dsDNA represents another major pathway of immune activation as illustrated by TREX1-mediated autoimmune disease [17]. TREX1 contributes to the regulation of PARP1, a nuclear DNA repair enzyme involved in the DNA damage response. Hence, alterations in the function of TREX1 affecting PARP1 activity appear to favor either the development or the progression of autoimmune diseases [18].
UV irradiation (UVR) represents another important trigger for CLE. Patients with DLE, lupus erythematosus tumidus (LET), or SCLE are often photosensitive. Approximately half of CLE patients develop lesions upon exposure to UV light [19]. UV irradiation can result in altered keratinocyte morphology, expression of autoantigens on cell membranes, and cell apoptosis. UV radiation is able to trigger the release of cytokines and chemokines, such as IFN, TNF-α, IL-1, IL-10, and IL-17 from keratinocytes and other cells. The latter contribute to the initiation and amplification of the inflammatory process. In the early phase of UVR-induced CLE skin lesions, there is an accumulation of CD4+ T cells at the dermal-epidermal junction area, whereas in the late phase CD8+ T cells predominate [20]. CCR5 expression is increased, while CCR3 expression is decreased [21]. This shift to Th1-associated chemokine receptor profile might be a marker for the activity of CLE.
4 Classification of Cutaneous Involvement
The spectrum of cutaneous features occurring in the course of SLE is broad and heterogeneous. In a fundamental work of 1981, Gilliam and Sontheimer proposed a classification based on grouping together patients with similar clinical features and similar response to treatment [22]. This classification constituted a progress by identifying cutaneous lesions, which were specific for lupus erythematosus (LE). Specific LE lesions were defined by the presence of interface dermatitis, characterized histopathologically by the presence of vacuolization and necrosis of basal keratinocytes, basal basement membrane thickening, pigment incontinence, and a lymphocytic infiltrate at the dermo-epidermal junction. These specific lesions were classified in acute, subacute, or chronic lesions and were either localized, disseminated, or generalized [23]. This terminology turned out to be misleading and confusing and provided nightmares to generations of medical students, generalists, and specialists for several reasons. First, histologically, the lesions of CLE can often not be classified in one of the three acute, subacute, or chronic subsets. Second, interface dermatitis is also observed in other conditions, such as dermatomyositis, drug reactions, or graft-versus-host disease [24]. Third, entities potentially observed in SLE, such as papulonodular mucinosis of LE and Jessner’s lymphocytic infiltrate of the skin/lupus tumidus or lupus panniculitis, cannot be classified histopathologically as specific LE lesions since they are lacking interface dermatitis. Finally, adjectives referring to chronology such as acute, subacute, or chronic are used to describe morphologic variants and are mixed with ill-defined extent scores such as localized or disseminated referring to topography. Ackerman regarded at the cutaneous changes associated with ACLE, SCLE, and CCLE as the result of the same pathological process [25]. The observed tissue damage may indeed vary according to the intensity of the process and its duration [26]. In this context, we recently proposed a novel classification of lesions of cutaneous signs in patients with LE [26]. This new simple classification is essentially based on findings clinical and from light microscopy studies (Table 7.1). The lesions are classified according to the level of the cellular infiltrate and tissue damage in the epidermis, dermis, and/or subcutis. Furthermore, we highlighted in this classification the clinical very relevant lesions, pointing to the presence of a thrombotic vasculopathy and to distinct inflammatory, neutrophilic-mediated reaction pattern. By taking into consideration these variables, all cutaneous lesions in LE can be easily classified in clinical practice.
4.1 Specific Signs of LE
4.1.1 Dermo-epidermal LE
Dermo-epidermal LE encompasses the classic acute, subacute, chronic, indeterminate, and vesiculobullous forms of LE.
4.1.1.1 Acute Cutaneous Lupus Erythematosus (ACLE)
ACLE presents most commonly as the classic “malar” or “butterfly” rash. The latter, which may occur transiently, can precede the onset of SLE by weeks or months and persist for months without evidence of systemic disease. There are typically small, discrete erythematous macules and papules in the central areas of the face, such as on the nose, chin, front, and then cheeks and malar regions (Fig. 7.1). Earlobes, scalp, and neck may also be involved. In contrast to dermatomyositis, nasolabial folds and periorbital regions are often spared. Lesions may become confluent, with scaling, erosions, and crusting. Severe facial edema may be observed, mimicking dermatomyositis [3, 27]. Erosions and ulcerations of the oral and/or nasal mucosa may complicate ACLE.
Cutaneous lupus erythematosus. Patient with lesions on the cheeks and on the front. The butterfly distribution is typical for acute cutaneous lupus erythematosus. Nevertheless, the intensity and chronicity of the process already resulted in scarring and pigmentary and localized hyperkeratotic changes characteristic for chronic cutaneous lupus erythematosus
In generalized ACLE there are widespread erythematous macular and papular lesions, which are found on the lateral aspect of the arms, elbows, shoulders, knees, and trunk. Lesions predominate on UV-exposed areas. In contrast to dermatomyositis, erythematous lesions are found between the metacarpophalyngeal joints and interphalangeal joints, whereas the knuckles are typically spared. Palms and soles may also be affected.
As ACLE lesions generally occur in the setting of evolving SLE, patients are often treated with steroids and/or immunosuppressors for other reasons such as nephropathy or cytopenia. ACLE then usually regresses without any sequel or leaving transient pigmentary changes, especially in dark-skin people.
4.1.1.2 Subacute Cutaneous Lupus Erythematosus (SCLE)
The lesions of SCLE are typically symmetrically distributed on the upper trunk, shoulders, V area of the neck, and arms. There are erythematous macules or papules which evolve into either scaly papulosquamous psoriasiform lesions or annular patches and plaques in, respectively, half of the patients (Fig. 7.2a). Annular lesions may enlarge and give rise to large polycyclic lesions. Mixed form with both annular and psoriasiform lesions is observed. Healing leads to postinflammatory hyper- and/or hypopigmentation, atrophic scarring grayish, and telangiectasias.
(a) Typical lesions of subacute cutaneous lupus erythematosus with annular and polycyclic configuration. Note the more erythematous and scaly infiltrated borders with central clearing and pigmentary changes. (b) Drug-triggered subacute cutaneous lupus erythematosus. Widespread and confluent erythematous plaques on the neck, shoulders, back, and upper limbs. The lesions are very inflammatory and may result in blistering and erosions
4.1.1.3 Discoid Chronic Cutaneous Lupus Erythematosus (DLE)
DLE is the most common form of CCLE. Lesions affect the face, the scalp, and/or the neck. In disseminated DLE, which occurs in less than 20 % of the cases, lesions spread below the neck. Significant involvement of the back of the hands mainly appears in smokers with a complement deficiency [28]. Patients with disseminated lesions of DLE are considered at increased risk for progression to SLE [29]. DLE is characterized by the presence of a coin-shaped, erythematous plaque of variable size associated with an adherent follicular hyperkeratosis (Fig. 7.3a). There is first an erythema with follicular hyperkeratosis, which then progress to atrophy, pigmentary changes, and scarring. The latter are persistent, contrarily to what is observed in ACLE and SCLE. On the scalp, depending on the severity and duration of the lesions, DLE can lead to scarring alopecia (Fig. 7.3b).
(a) Discoid cutaneous lupus erythematosus. There are erythematous plaques with follicular hyperkeratosis, which have resulted in scarring and pigmentary changes. Certain lesions are still active and show an erythematous inflammatory rim. Note specific involvement of the lips and vermillion. (b) Discoid cutaneous lupus erythematosus of the scalp leading to scarring alopecia
4.1.1.4 Indeterminant LE
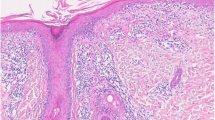
Sontheimer used the term of indeterminant LE to describe long-lasting erythematous lesions and plaques without surface alteration, which however shows a LE typical interface dermatitis (Fig. 7.4) on histopathological evaluation. This type of lesions does not fit into the description of any of the classic subsets originally described.
Histopathology of a LE sample reveals under an interface dermatits a perivascular and periadnexal infitrate in the dermis. The epidermidis shows atrophy and a vacuolar basal cell degeneration with apoptotic keratinocytes and a marked thickening of the basement membrane. Dermis is interested by an edema with mucin deposition
4.1.1.5 LE-Specific Vesiculobullous Disease
Blistering may be a secondary phenomenon in severe CLE. If the interface changes at the dermo-epidermal junction are extensive, the epidermis detaches from the dermis. If the damage involves the entire epidermis and is widespread, the changes may clinically mimic Stevens-Johnson syndrome or toxic epidermal necrolysis.
4.1.2 Dermal LE
Dermal LE includes lupus tumidus and Jessner’s lymphocytic infiltrate, as well as reticular erythematous mucinosis and the papulonodular mucinosis of LE. From a pathological point of view, these entities are characterized by a lymphocytic infiltrate and mucin deposition, respectively.
4.1.2.1 Lupus Erythematosus Tumidus and Jessner-Kanof Lymphocytic Infiltrate of the Skin
Lupus erythematosus tumidus (LET) differs from other variants of CLE. LET has been anecdotally associated with other types of CLE or even SLE. The distinction of LET from benign lymphocytic infiltrate of Jessner-Kanof is virtually impossible and often debated: they most likely represent the same condition [30]. LET is characterized by the development of papular lesions and plaques. The lesions may have a succulent, urticaria-like appearance, with reddish or violaceous smooth surface. The lesions, which may have an arc-shaped and annular appearance, are located on sun-exposed areas, such as the face, upper back, V area of the neck, and extensor aspects of the arms and shoulders [31].
4.1.2.2 Papulonodular Mucinosis
The cutaneous mucinoses are a heterogeneous group of disorders characterized by aberrant accumulation of glycosaminoglycans between collagen in the dermis [32]. The presence of mucin deposition is a relatively common histological finding in connective tissue diseases, such as LE and dermatomyositis [33]. However, mucin accumulation is rarely so abundant to produce clinically visible lesions. The latter appear as skin-colored or slightly red papules and nodules without epidermal changes. Since the first description by Gold [34], several cases of papulonodular mucinosis have been reported in combination with either SLE or DLE. It typically involves the trunk and upper extremities, while face and other areas of the body may also be affected [34].
4.1.3 Lupus Panniculitits (Lupus Profundus)
Lupus panniculitis (LEP) is another relatively rare but typical form of CLE characterized by the development of painful indurated dermo-hypodermal nodules or plaques that result in scarring and skin depression. It typically affects the thighs, the upper arms, or the cheek area of the face. LEP may occur either alone, in association with other forms of CLE, or in the course of SLE. The histological findings include lobular panniculitis with prominent lymphocytic infiltrate and mucin deposition between collagen bundles. Lymphocytic nuclear dust is observed. Differentiation of LEP from a panniculitis-like T-cell lymphoma is sometimes challenging.
4.2 Signs Indicative of a Thrombotic Vasculopathy
The spectrum of cutaneous lesions related and potentially reflecting a thrombotic vasculopathy is wide. It includes Degos’ like papules, atrophie blanche, livedo (racemosa type), non-infiltrated acrally located stellar purpura, splinter hemorrhage, cutaneous necrosis, anetoderma, and thrombophlebitis.
Thrombotic vasculopathy represents an important and prognostic significant sign in SLE, since it may lead to devastating complications. In fact 5 years after initial diagnosis of SLE, thrombotic and ischemic events represent the main cause of morbidity and mortality [35]. Degos’ disease, stellar purpura, splinter hemorrhage, and cutaneous necrosis with retiform purpura may constitute the initial presentation of an antiphospholipid syndrome. Its diagnosis has relevant prognostic and therapeutic implications [26]: affected patients may develop strokes, ischemic attacks, heart valve abnormalities, and hypertension [36, 37]. In these cases, the search and management of additional cardiovascular risk factors are essential.
4.2.1 Livedo Racemosa
Livedo racemosa is one of the most frequent dermatological manifestations in SLE-related antiphospholipid syndrome and is found in approximately 20 % of affected patients [38]. It is characterized by a bluish netlike non-infiltrated discoloration of the skin, which is usually observed in a suspended localization (the buttocks, thighs, trunk, or even face). In some cases, the livedo first affects the hands and feet and then spreads centripetally. In contrast to cutis marmorata, the fishnet has an irregular reticular patter with “broken” circles. In anti-phospholipid antibody-negative patients with Sneddon syndrome, the fishnet is larger and clinically more obvious. In SLE patients with livedo racemosa, additional causes have to be considered, including cholesterol thrombi and calciphylaxis.
4.2.2 Degos-Like Papules
Malignant atrophic papulosis (also called papulosis maligna or Köhlmeier-Degos’ disease) presents with porcelain-white atrophic lesions surrounded by an erythematous rim of less than 1 cm in diameter [39]. Less than 200 cases have been described. The lesions occur on the trunk and upper extremities [40]. The palms, soles, scalp, and face are rarely involved. The early lesion is an erythematous papule, which becomes porcelain white, atrophic, and depressed in the center, while it is surrounded by a slightly elevated red, sometimes telangiectatic border. Histologically, there is a typical wedge-shaped connective tissue necrosis related to the thrombotic occlusion of the small arteries [41]. Patients with generalized disease develop severe neurologic, gastrointestinal, and ocular involvement related to ischemia and infarction. Lesions similar to those observed in Degos’ disease may be found in a subset of SLE and are often located acrally.
4.2.3 Thrombophlebitis
SLE patients who have antiphospholipid antibodies have an increased risk of developing venous thromboembolism [42]. Thrombophlebitis usually occurs within 1 year after the onset of systemic disease, but may also antedate the diagnosis of SLE by several years. Several factors account for the occurrence of thrombophlebitis, such as slow chronic, disseminated intravascular coagulation, vasculitis-triggered platelet activation and aggregation, and prolonged immobility.
4.2.4 Anetoderma
Anetoderma is a relatively uncommon disorder characterized by a focal decrease or loss of elastic tissue in the dermis. Anetoderma presents as a localized herniated or punched-out skin lesions [43]. In practice, after exclusion of an infectious etiology, such as syphilis and tuberculosis, the diagnosis of anetoderma should prompt the exclusion of either a systemic immune-mediated disease, such as antiphospholipid syndrome, or another hypercoagulable state.
4.2.5 Vasculitis of the Skin
Based on a new revised international nomenclature, vasculitis associated with a systemic disease is defined using a prefix specifying the association [41]. The denomination of lupus vasculitis is nevertheless somehow confusing. First, it does not specify the type of vessels or of organs involved. Skin manifestations may be highly variable with palpable purpura, urticarial vasculitis, panarteritis nodosa-like dermo-hypodermal nodules, or ulcerations. Second, the vasculitis may be due to factors independent from SLE, such as drugs and infections [2]. It should be noted that ischemic microinfarcts of the fingertips and splinter hemorrhages are usually not related to vasculitis, but to coagulation defects. In the latter case, anticoagulation therapy may be effective.
4.3 Neutrophilic Cutaneous LE
In patients with SLE and other inflammatory and immune-mediated disorders, a significant infiltration of the skin by neutrophils may be occasionally observed. In addition to bullous LE and urticarial vasculitis, neutrophilic dermatoses such as amicrobial pustulosis of the skin folds, Sweet syndrome, and pyoderma gangrenosum may occur in patients with SLE [44, 45]. The striking accumulation of neutrophils in the skin reflects the activation of the innate immune response in SLE [46].
4.3.1 Amicrobial Pustulosis of Skin Folds
Amicrobial pustulosis of skin folds is characterized by the development of papules and pustules [47], with formation of erosive macerated areas and crusts. The lesions are symmetrically distributed and localized in large body folds such as the axilla and groins. Isolated pustules over the trunk and limbs also occur. External auditory meatus, nares, retroauricular flexures, and interdigital spaces can also be affected as well as the scalp. Histologically, there is spongiform subcorneal pustule formation together with a superficial and deep dermal infiltrate of neutrophils and lymphocytes [48].
4.3.2 Bullous LE
Bullous LE most often occurs in young African Americans with SLE [49]. Vesicles and bullae, which arise on clinically normal-appearing or inflamed skin, occur on sun-exposed areas or are widespread. Lesions may have an arciform or figurate distribution pattern and are accompanied by a burning sensation rather than pruritus [50]. In contrast to epidermolysis bullosa acquisita (EBA), in the vast majority of BSLE patients, scarring and milia formation do not occur and there is further a striking therapeutic response to dapsone [51]. Histologically, subepidermal vesicles, neutrophil microabscesses, nuclear “dust,” and fibrin at the tips of dermal papillae are found. Direct immunofluorescence shows linear deposits of IgG and C3 along the epidermal basement membrane. Affected patients have circulating autoantibodies directed against type VII collagen [51]. Despite its classification as “neutrophilic cutaneous LE” [26], bullous LEs show thus the same immunopathological features of epidermolysis bullosa acquisita, an acquired autoimmune subepidermal bullous disease.
4.3.3 Neutrophilic Urticarial Dermatosis
Neutrophilic urticarial dermatosis (NUD) was recently delineated as a new entity within the spectrum of the neutrophilic dermatoses [52]. NUD is characterized by the development of widespread rose or red macules or slightly elevated papules vanishing within 24 h, associated with fever and joint pain. The histopathological findings consist of a dense perivascular and interstitial infiltrate of neutrophils with leukocytoclasia but without vasculitis. The development of NUD in a patient with known SLE is often mistaken as exacerbation of LE. The therapy of choice of NUD is either dapsone or colchicine rather than increasing immunosuppression [46].
4.4 Other Signs of Yet Uncertain Pathogenesis
There are number of conditions which are (or at least seem to be) epidemiologically more frequent in SLE. These include neurovascular conditions, such as Raynaud’s syndrome, erythromelalgia, as well as granulomatous tissue reactions, such as interstitial granulomatous diseases and rheumatoid nodules. Other lesions such as eruptive fibromas may occur. Their significance in the context of SLE needs to be better assessed.
5 Drug Induced Cutaneous LE
Approximately 10 % of SLE cases can be related to drugs [53]. Drug-induced SLE usually occurs after several months or years of continuous therapy. Compared to “idiopathic” SLE, drug-induced SLE occurs in older people [54]. Arthralgias, myalgias, arthritis, fever, and serositis are often milder than in SLE, whereas malar rash, photosensitivity, and oral ulcers are less common [55]. Drugs involved in drug-induced SLE include typically procainamide, hydralazine, isoniazid, diltiazem, and minocycline [56].
It is important to distinguish drug-induced SLE from a relatively common form of CLE, drug-induced SCLE [57], in which the cutaneous involvement is the leading manifestation. Drug-induced SCLE presents with non-scarring annular or papulosquamous eruptions (Fig. 7.3b). Rarely, pityriasiform, bullous, erythrodermic, poikilodermatous, toxic epidermal necrolysis-like, and erythema multiforme-like presentations have been described [58]. Anti-Ro/SSA antibodies are often detectable [59].
Drug-induced SCLE has been associated with several drugs, including most frequently hydrochlorothiazide, antihypertensive agents, proton pomp inhibitors, and terbinafine. TNF-alpha antagonists are potential triggers of both drug-induced SCLE and DIL. Drug-triggered CLE is rare and typically reported in association with the intake of fluorouracil agents.
It has been proposed to divide drug-induced LE into systemic (DI-SLE) with or without cutaneous manifestations and DI-LE with predominant skin involvement. The latter comprises drug-induced SCLE (DI-SCLE) and drug-induced CCLE (DI-CCLE) [60].
In all cases, when a specific drug is considered as potential trigger for the development or aggravation of cutaneous or systemic manifestations of LE, it should be discontinued whenever possible [61].
6 Photosensitivity
The original SLE classification of the ACR included also photosensitivity as criterion [62]. “Photosensitivity” was defined as a “skin rash as a result of unusual reaction to sunlight by patient history or physician observation.” Unfortunately, this definition is not precise enough. A variety of other benign conditions are associated with light sensitivity, such as polymorphous light eruption, photoallergic contact dermatitis, solar urticaria, or porphyrias. The latter, according to the clinical context, may be wrongly classified as SLE. Furthermore, in patients with CLE, inaccurate assessment of photosensitivity results in an overestimation of SLE [63]. Some authors defined photosensitivity as an induction of skin lesions following sun exposure, while others also considered sunburn and aggravation of the disease in the spring and summer times [64]. Moreover, UV light exposure is not only able to induce and exacerbate lesions of almost all subtypes of CLE [65], but can also trigger significant organ involvement in SLE, including lupus nephritis [66].
The frequency of photosensitivity in the different subtypes of CLE has been variably estimated. This is also due to the lack of well-defined criteria for photosensitivity. Photosensitivity has been reported in 27–100 % of patients with SCLE and in 25–90 % of patients with DLE [67].
Photoprovocation tests with different wavelengths are useful to assess the photosensitivity in patients with CLE. UVB, UVA2, and UVA1 can induce de novo or exacerbate skin manifestations. When compared to other photodermatoses, such as polymorphous light eruption or porphyrias, UV-induced LE-specific lesions usually do not develop immediately, but after 1 week, and persist up to 2 months. For this reason, a number of LE patients do not recognize the association between UV exposure and the induction of skin lesions.
7 CLASI, a Useful Instrument to Assess Cutaneous Activity
The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) is a relatively novel tool that quantifies disease activity and damage in CLE. The activity score is based on the degree of erythema, scale, mucous membrane lesions, and non-scarring alopecia. Unlike other outcome measures, CLASI scores are not based solely on the area of involved skin. Instead, parts of the body that are most visible are weighted more heavily than those that are usually covered [68]. The CLASI has been shown to have good content validity, addressing the most relevant aspects of CLE [69].
CLASI has been found to correlate with the “physicians” and “patients” global assessment of disease activity on a 0–10 visual analog scale [69]. Although the CLASI was firstly designed as an instrument useful in therapeutic trials, it can easily and rapidly be employed in clinical practise.
8 Treatment of Cutaneous Lupus Erythematosus: Basic Principles
The treatment of LE is covered in depth in Chaps. 17 and 18. The basic principles of treating cutaneous LE can be summarized as follows. All patients should use broad-spectrum UVA/UVB sunscreen with SPF > 30. Furthermore, the patients should be informed about appropriate protective measures (sun avoidance, clothing, use of hats or wigs). The first-line treatment of CLE lesions includes antimalarials (mainly hydroxychloroquine and less frequently chloroquine) [4, 70, 71]. Topical steroids or calcineurin inhibitors can be used for isolated lesions in patients without any signs of systemic disease as well as in combination with antimalarials or other drugs. The choice of the second-line treatment varies throughout Europe. Patients who are refractory to antimalarials are usually treated with either methotrexate, azathioprine, or mycophenolate mofetil [71]. Other options include dapsone, acitretin, or thalidomide [71]. Oral steroids and other immunosuppressants are usually not used to treat specific LE lesions, except in severe cases with widespread lesions and risk of significant scarring in specific situations. Photopheresis, belimumab, and rituximab should only be used by very experienced clinicians in exceptional situations [71]. Cessation of cigarette smoking should always be recommended [72].
9 Conclusions
The spectrum of cutaneous manifestations occurring in SLE is broad. Since they can constitute the initial manifestation of the disease, its prompt recognition is important for proper management and workup of affected patients. Furthermore, the development of distinct cutaneous signs, such as thombo-occlusive and vascular complications, has significant prognostic and therapeutic implications. In practice, the first step is to clinically and histologically differentiate between LE-specific and LE-nonspecific lesions [22]. If CLE is diagnosed, activity and damage should be assessed using the CLASI. Appropriate clinical and laboratory exams should be performed to exclude extracutaneous involvement. The patient should be correctly informed about the cutaneous disease and the potential development of SLE – even in the absence of extracutaneous findings. It is important to systematically consider the possibility of drug triggers, which should be eliminated. The patient should be aware of the aggravating factors, such as smoking and sun exposure, and be systematically instructed about sun protective measures, such as use of sunscreens, sun avoidance, and use of appropriate clothing and hats.
References
Obermoser G, Sontheimer RD, Zelger B (2010) Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus 19(9):1050–1070
Rothfield N, Sontheimer RD, Bernstein M (2006) Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol 24(5):348–362
Werth VP (2005) Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev 4(5):296–302
Kuhn A, Ruland V, Bonsmann G (2011) Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol 65(6):179–193
Durosaro O, Davis MD, Reed KB, Rohlinger AL (2009) Incidence of cutaneous lupus erythematosus, 1965–2005: a population-based study. Arch Dermatol 145(3):249–253
Gronhagen CM, Fored CM, Granath F, Nyberg F (2011) Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol 164(6):1335–1341
Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS et al (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8):2677–2686
Grönhagen CM, Gunnarsson I, Svenungsson E, Nyberg F (2010) Cutaneous manifestations and serological findings in 260 patients with systemic lupus erythematosus. Lupus 19(10):1187–1194
Fernández M, Alarcón GS, Calvo-Alén J, Andrade R, McGwin G Jr, Vilá LM, Reveille JD, LUMINA Study Group (2007) A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 57(4):576–584
Chiu Y-M, Lai C-H (2010) Nationwide population-based epidemiologic study of systemic lupus erythematosus in Taiwan. Lupus 19(10):1250–1255
Lee HJ, Sinha AA (2006) Cutaneous lupus erythematosus: understanding of clinical features, genetic basis, and pathobiology of disease guides therapeutic strategies. Autoimmunity 39(6):433–444
Freire EAM, Souto LM, Ciconelli RM (2011) Medidas de avaliação em lúpus eritematoso sistêmico (Assessment measures in systemic lupus erythematosus). Rev Bras Reumatol 51:75–80
Liu Z, Davidson A (2012) Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 18(6):871–882
Deng y, Tsao BP (2010) Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol 6(12):683–692
Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK (2007) High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 8:492–502
Jacob N, Stohl W (2011) Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther 13:228
Tüngler V, Silver RM, Walkenhorst H, Günther C, Lee-Kirsch MA (2012) Inherited or de novo mutation affecting aspartate 18 of TREX1 results in either familial chilblain lupus or Aicardi-Goutières syndrome. Br J Dermatol 167:212–214
Miyazaki T, Kim YS, Yoon J, Wang H, Suzuki T, Morse H (2014) The 3′-5′ DNA exonuclease TREX1 directly interacts with poly(ADP-ribose) polymerase-1 (PARP1) during the DNA damage response. J Biol Chem 289(47):32548–32558
Kuhn A, Wozniacka A, Szepietowski J, Gläser R, Lehmann P, Haust M, Sysa-Jedrzejowska A, Reich A, Oke V, Hügel R, Calderon C, de Vries DE, Nyberg F et al (2011) Photoprovocation in cutaneous lupus erythematosus: a multicenter study evaluating a standardized protocol. J Invest Dermatol 131:1622–1630
Kind P, Lehmann P, Plewig G (1993) Phototesting in lupus erythematosus. J Invest Dermatol 100:53S–57S
Freutel S, Gaffal E, Zahn S, Bieber T, Tüting T, Wenzel J (2011) Enhanced CCR5+/CCR3+ T helper cell ratio in patients with active cutaneous lupus erythematosus. Lupus 20(12):1300–1304
Gilliam JN, Sontheimer RD (1981) Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 4(4):471–475
Gilliam JN, Sontheimer RD (1982) Skin manifestations of SLE. Clin Rheum Dis 8(1):207–218
Sontheimer RD (2009) Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol 129(5):1088–1099
Ackerman AB, Boer A, Benin B, Gottlieb GJ (2005) Histopathological diagnosis of inflammatory skin diseases, 3rd edn. Ardor Scribendi, New York
Lipsker D (2010) The need to revisit the nosology of cutaneous lupus erythematosus: the current terminology and morphologic classification of cutaneous LE: difficult, incomplete and not always applicable. Lupus 19(9):1047–1049
Kuhn A, Lehmann P, Ruzicka T (2004) Clinical manifestations of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, Ruzicka T (eds) Cutaneous lupus erythematosus. Springer, Heidelberg, pp 59–92
Boeckler P, Milea M, Meyer A, Uring-Lambert B, Heid E, Hauptmann G, Cribier B, Lipsker D (2005) The combination of complement deficiency and cigarette smoking as risk factor for cutaneous lupus erythematosus in men; a focus on combined C2/C4 deficiency. Br J Dermatol 152(2):265–270
Tebbe B, Mansmann U, Wollina U, Auer-Grumbach P, Licht-Mbalyohere A, Arsenmeier M, Orfanos CE (1997) Markers in cutaneous lupus erythematosus indicating systemic involvement. A multicenter study on 296 patients. Acta Derm Venereol 77:305–308
Rémy-Leroux V, Léonard F, Lambert D, Wechsler J, Cribier B, Thomas P, Adamski H, Marguery MC, Aubin F, Leroy D, Bernard P (2008) Comparison of histopathologic-clinical characteristics of Jessner’s lymphocytic infiltration of the skin and lupus erythematosus tumidus: multicenter study of 46 cases. J Am Acad Dermatol 58(2):217–223
Kuhn A, Richter-Hintz D, Oslislo C, Ruzicka T, Megahed M, Lehmann P (2000) Lupus erythematosus tumidus e a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol 136:1033–1041
Rongioletti F, Rebora A (1991) The new cutaneous mucinoses. A review with an up-to-date classification of cutaneous mucinoses. J Am Acad Dermatol 24:265–270
Ortize VG, Krishnan RS, Chen LL, Hsu S (2004) Papulonodular mucinosis in systemic lupus erythematosus. Dermatol Online J 10:16
Gold SC (1954) An unusual papular eruption associated with lupus erythematosus. Br J Dermatol 66:429–433
Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO, Chwalinska-Sadowska H, de Ramón E, Fernández-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR, European Working Party on Systemic Lupus Erythematosus (2003) Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82(5):299–308
Francès C, Piette JC (2000) The mystery of Sneddon syndrome: relationship with antiphospholipid syndrome and systemic lupus erythematosus. J Autoimmun 15(2):139–143
Englert HJ, Loizou S, Derue GG, Walport MJ, Hughes GR (1989) Clinical and immunologic features of livedo reticularis in lupus: a case-control study. Am J Med 87:408–410
Francès C, Niang S, Laffitte E, Pelletier F, Costedoat N, Piette JC (2005) Dermatologic manifestations of the antiphospholipid syndrome: two hundred consecutive cases. Arthritis Rheum 52(6):1785–1793
Ball E, Newburger A, Ackerman AB (2003) Degos’ disease: a distinctive pattern of disease, chiefly of lupus erythematosus, and not a specific disease per se. Am J Dermatopathol 25(4):308–320
Theodoridis A, Makrantonaki E, Zouboulis CC (2013) Malignant atrophic papulosis (Köhlmeier-Degos disease) – a review. Orphanet J Rare Dis 8:10
Lipsker D (2012) In: Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, Wolff K (eds) Fitzpatrick’s dermatology in general medicine. McGraw-Hill, New York, pp 2072–2076, Malignant atrophic papulosis (Degos disease)
Wahl DG, Guillemin F, de Maistre E, Perret-Guillaume C, Lecompte T, Thibaut G (1998) Meta-analysis of the risk of venous thrombosis in individuals with antiphospholipid antibodies without underlying autoimmune disease or previous thrombosis. Lupus 7(1):15–22
Venencie PY, Winkelmann RK (1985) Monoclonal antibody studies in the skin lesions of patients with anetoderma. Arch Dermatol 121:747–749
Gusdorf L, Bessis D, Lipsker D (2014) Lupus erythematosus and neutrophilic urticarial dermatosis: a retrospective study of 7 patients. Medicine (Baltimore) 93(29):e351
Mitsias DI, Kapsogeorgou EK, Moutsopoulos HM (2006) Sjögren’s syndrome: why autoimmune epithelitis? Oral Dis 12:523–532
Lipsker D, Saurat JH (2008) Neutrophilic cutaneous lupus erythematosus. At the edge between innate and acquired immunity? Dermatology 216(4):283–286
Marzano AV, Ramoni S, Caputo R (2008) Amicrobial pustulosis of the folds. Report of 6 cases and a literature review. Dermatology 216(4):305–311
Lee HY, Pelivani N, Beltraminelli H, Hegyi I, Yawalkar N, Borradori L (2011) Amicrobial pustulosis-like rash in a patient with Crohn’s disease under anti-TNF-alpha blocker. Dermatology 222:304–310
Fujimoto W, Hamada T, Yamada J, Matsuura H, Iwatsuki K (2005) Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol 32:1021–1027
Camisa C, Sharma HM (1983) Vesiculobullous systemic lupus erythematosus. J Am Acad Dermatol 9:924–933
Hall RP, Lawley TJ, Smith HR, Katz SI (1982) Bullous eruption of systemic lupus erythematosus. Dramatic response to dapsone therapy. Ann Intern Med 197:165–170
Kieffer C, Cribier B, Lipsker D (2009) Neutrophilic urticarial dermatosis; a variant of neutrophilic urticaria strongly associated with systemic disease. Report of 9 new cases and review of the literature. Medicine (Baltimore) 88:23–31
Vedove CD, Del Giglio M, Schena D, Girolomoni G (2009) Drug-induced lupus erythematosus. Arch Dermatol Res 301:99–105
Xiao X, Chang C (2014) Diagnosis and classification of drug-induced autoimmunity (DIA). J Autoimmun 48–49:66–72
Katz U, Zandman-Goddard G (2010) Drug-induced lupus: an update. Autoimmun Rev 10:46–50
Fritzler MJ (1994) Drugs recently associated with lupus syndromes. Lupus 3(6):455–459
Chlebus E, Wolska H, Blaszczyk M, Jablonska S (1998) Subacute cutaneous lupus erythematosus versus systemic lupus erythematosus: diagnostic criteria and therapeutic implications. J Am Acad Dermatol 38(3):405–412
Marzano AV, Lazzari R, Polloni I, Crosti C, Fabbri P, Cugno M (2011) Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. Br J Dermatol 165(2):335–341
Sontheimer RD, Maddison PJ, Reichlin M, Jordon RE, Stastny P, Gilliam JN (1982) Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med 97:664–667
Marzano AV, Vezzoli P, Crosti C (2009) Drug-induced lupus: an update on its dermatologic aspects. Lupus 18:935–940
Rubin RL (2015) Drug-induced lupus. Expert Opin Drug Saf 2:1–18
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725
Kuhn A, Wenzel J, Weyd H (2014) Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol 47(2):148–162
Nyberg F, Hasan T, Puska P, Stephansson E, Häkkinen M, Ranki A, Ros AM (1997) Occurrence of polymorphous light eruption in lupus erythematosus. Br J Dermatol 136(2):217–221
Kim A, Chong BF (2013) Photosensitivity in cutaneous lupus erythematosus. Photodermatol Photoimmunol Photomed 29(1):4–11
Schmidt E, Tony H-P, Bröcker E-B, Kneitz C (2007) Sun-induced life-threatening lupus nephritis. Ann N Y Acad Sci 1108:35–40
Kuhn A, Ruland V, Bonsmann G (2010) Photosensitivity, phototesting, and photoprotection in cutaneous lupus erythematosus. Lupus 19(9):1036–1046
Klein R, Moghadam-Kia S, LoMonico J, Okawa J, Coley C, Taylor L, Troxel AB, Werth VP (2011) Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch Dermatol 147(2):203–208
Bonilla-Martinez ZL, Albrecht J, Troxel AB, Taylor L, Okawa J, Dulay S, Werth VP (2008) The cutaneous lupus erythematosus disease area and severity index: a responsive instrument to measure activity and damage in patients with cutaneous lupus erythematosus. Arch Dermatol 144(2):173–180
Okon LG, Werth VP (2013) Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol 27(3):391–404
Kuhn A, Ruland V, Bonsmann G (2011) Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol 65(6):e195–e213
Kuhn A, Sigges J, Biazar C, Ruland V, Landmann A, Amler S, Bonsmann G, WUSCLE coauthors (2014) Influence of smoking on disease severity and antimalarian therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol 171:571–579
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ribero, S., Lipsker, D., Borradori, L. (2016). The Spectrum of Cutaneous Manifestations in Systemic Lupus Erythematosus and Novel Classification. In: Roccatello, D., Emmi, L. (eds) Connective Tissue Disease. Rare Diseases of the Immune System. Springer, Cham. https://doi.org/10.1007/978-3-319-24535-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-24535-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24533-1
Online ISBN: 978-3-319-24535-5
eBook Packages: MedicineMedicine (R0)