Abstract
Systemic lupus erythematosus (SLE) is considered a typical protean systemic autoimmune disease. It is characterized by multiorgan and multisystem involvement. Virtually, SLE may affect almost any organ during the disease course. Several pathogenic pathways have been reported to sustain inflammation in affected tissues in patients with SLE. Recently, the apoptotic process was thoroughly investigated. The link between apoptotic debris containing autoantigens and innate immunity activation has been also further elucidated. Indeed, as far as the role of lymphocytes in SLE pathogenesis is concerned, the “T-lymphocyte centric” SLE hypothesis has recently been counterbalanced with a newer “B-lymphocyte centric” theory, which was mainly supported by the emerging data arising from B-cell target therapy studies. In this chapter we provide an overview of both the traditional and the more recently discovered immunological pathways that drive inflammation and contribute to organ damage in SLE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
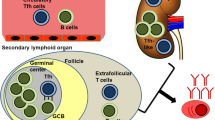
Systemic lupus erythematosus (SLE) is considered a typical protean systemic autoimmune disease. It is characterized by multiorgan and multisystem involvement. Virtually, SLE may affect almost any organ during the disease course, and several pathogenic pathways drive SLE inflammation in affected tissues. Recently, the apoptotic process was thoroughly investigated, and in particular the link between apoptotic debris containing autoantigens, innate immunity activation, and maintaining of inflammation has been further elucidated. A better understanding of the pathogenic mechanisms and of the inflammatory cytokine cascade contributed to the recent development of new biological drugs specifically approved for SLE therapy. In this chapter we provide an overview of both the traditional and the more recently discovered immunological pathways that drive inflammation and contribute to organ damage in SLE (Fig. 3.1).
2 Genetic and Environmental Factors
2.1 Genetic Aberrations
Genes that breach immune tolerance and promote autoantibody production may play a crucial role in SLE development. These genes might act with other genetic factors that augment innate immune signaling and IFN-I production, which in turn can generate an influx of effector leukocytes, inflammatory mediators, and autoantibodies into involved organs, such as the kidneys. Genetic factors influence SLE susceptibility and likely affect disease severity as well. In the last years, genetic susceptibility has been extensively investigated in SLE. However, even if the disease is definitely more frequent in certain families compared to others, identical twins show only 50 % concordance. Some major histocompatibility complex classes, in particular in class II genes (HLA-DR, DQ and DP), have been associated with SLE susceptibility [1]. Recently, several other gene polymorphisms have been found more frequently in patients with a more severe disease course [2].
Some genetic aspects however warrant more accurate discussion in SLE since they probably deeply promote both disease onset and subsequent prolonged inflammation.
The identified genes implicated in SLE can be assigned to one of four functional categories: genes that affect lymphocyte activation, particularly B cells; genes that affect innate immune signaling, (NF-κB activation and IFN-I signaling); genes that might function within the kidneys, potentially promoting renal tissue damage; and genes that influence the handling of apoptotic debris, chromatin, and immune complexes bearing these antigens. These categories have been designated on the basis of a priori analysis regarding the cell types in which the identified genes are expressed and their known molecular functions. However, alternative pathways and models cannot be excluded. Although numerous genes have been implicated in SLE, several questions remain. Firstly, the specific causative mutations and subsequent molecular alterations that contribute to the disease phenotype have not been firmly established for many of the identified candidate genes [2].
Among the others, homozygous C1q deficiency and genetic mutations resulting in low levels of C2 and C4 significantly increase the risk of developing SLE. These complement system deficiencies probably contribute to SLE pathogenesis through defective clearance of the apoptotic material, consequently leading to a significant accumulation of potential autoantigens [3].
Genome-wide microsatellite characterization was recently used to screen large-scale single nucleotide polymorphisms (SNPs) and to identify chromosomal loci that are associated with SLE. Among them IRF5, TYK2, STAT4, IRAK1, and PHPRF1 are linked to type I IFN production or IFN-induced cellular response [2].
IFN-I signaling is important in myeloid cells, including monocytes and dendritic cells, and might also have important functions in resident renal cells. Since direct evaluation of circulating IFN-alpha levels is usually very complex and does not always reliably reflect IFN overexpression, recent studies have shown a good correlation between the expression of IFN-inducible genes in peripheral blood mononuclear cells and SLE activity [4]. Microarray techniques showed that peripheral blood mononuclear cells isolated from patients with active SLE indeed display a high degree of IFN-I activity or “signature” [5, 6]. IFN-alpha hyperactivity is detected, though to a lesser extent, in patients with incomplete lupus syndrome or undifferentiated connective tissue disease, and it is even more blunted in first-degree relatives of patients with SLE [7]. Nowadays, IFN signature represents the most significant genetic discovery in SLE since it potentially implies new therapeutic options.
DNA methylation and histone modifications are key mechanisms of human epigenetic control of gene expression. Patients with active lupus were found to have reduced capacity of DNA methylation of several genes, leading to an increase in the transcription of inflammatory proteins such as CD11a, CD70, and CD40L. Perforin overexpression due to gene hypomethylation is also responsible for abnormal CD4+ T-lymphocyte killing activity [8, 9]. Interestingly, some drugs, such as hydralazine or procainamide, which are well known for being associated with drug-induced, new-onset lupus, may affect DNA hypomethylation as well. Other epigenetic mechanisms potentially affecting pathogenesis of SLE include histone acetylation and microRNA (miRNA) expression. Abnormal patterns of miRNAs have been recently detected in the blood of SLE patients [10].
2.2 Environmental Factors
Many theories and evidence in the past have indeed linked SLE pathogenesis to environmental factors, in particular hormones, since it is well known that SLE predominantly affects women during their childbearing years.
The first reliable mouse model of renal lupus was described in New Zealand black/white female (NZB/WF) mice [11]. Although there have been subsequent descriptions of lupus even in male murine strains, the NZB/WF mouse model provided the very first strong scientific evidence of the influence of sex hormones on SLE pathogenesis [12]. Moreover, increased production of inflammatory cytokines by T and B lymphocytes (probably via NF-kB activation) has been described after exposure to estrogens. On the other hand, progesterone is able to inhibit Toll-like receptor (TLR) 7 signals, inducing a reduction in inflammatory cytokines [13].
Historically, infections have been considered possible triggers for SLE induction in the early phases and for relapse in the course of the disease [14]. Cross-reactivity between self and non-self microbial epitopes is generally considered an appealing mechanism to explain the break in immune tolerance. For instance, a molecular mimicry has been described between EBV nuclear antigen-1 and self-antigens [15].
Ultraviolet (UV) exposure is a well-known risk factor for lupus development and disease flares [16]. UV-B exposure in particular promotes apoptosis of skin cells in SLE patients, with subsequent plasmacytoid dendritic cell (pDC) recruitment into cutaneous lesions, presentation of apoptosis-associated autoantigens to lymphocytes, and triggering of specific humoral and cellular adaptive responses. Increased levels of IFN-alpha triggered by pDCs have been demonstrated in skin specimens of SLE patients after ultraviolet exposure. Similarly, the production of other inflammatory cytokines, such as IL-1, IL-6, and TNF-alpha, by keratinocytes and lymphocytes has been shown to be influenced by UV [17].
The role of vitamin D on the immune system has also been profoundly investigated, since insufficient circulating levels are often detected in patients with several autoimmune diseases, especially with SLE. Besides influencing bone metabolism and protecting from osteoporosis, 1-25 (OH) vitamin D inhibits cellular T-lymphocyte responses and favors Treg differentiation. Vitamin D deficiency is thought to be a potential susceptibility factor for autoimmune diseases [18].
In summary, as far as the role of genetic and environmental factors in SLE is concerned, a combination of genes rather than a single gene seems to predispose to the disease in the majority of patients, in particular when crucial interactions between such genes and environmental factors occur.
3 Apoptosis Disturbances in SLE
Previous research has shown that nuclear antigens are targeted in SLE, which is characterized by a strong serological response to DNA, histones, and ribonuclear proteins. These nuclear antigens are not usually exposed to the immune system as they are sequestered within cellular and nuclear membranes. Consequently, numerous studies have investigated the processes underlying the exposition of nuclear autoantigens and the autoimmune responses associated with SLE. Although several known pathways can lead to cell death, apoptosis remains the dominant mechanism. Apoptosis is a regulated process, which requires energy with ATP consumption, sequential activation of intracellular proteases (caspases), digestion of chromatin and DNA by DNAse enzyme, and lastly cytoskeleton modification through the formation of microparticles from the membrane. Various stimuli, such as DNA damage, UV exposure, or infections, can induce apoptosis in vitro and in vivo [19]. Rapid clearance of apoptotic cells prevents immunogenicity or the ability to initiate an inflammatory response. Experimental evidences suggest that accumulation of apoptotic debris, which can occur as a result of failure of the clearance machinery, is an important contributor to autoimmunity in SLE. Multiple ligands, receptors, opsonins, and other molecules are involved in the clearance of apoptotic cells and their debris. Genetic knockout models and pharmacological studies in mice investigated how genes affecting autoantigen clearance could promote the production of antinuclear autoantibodies and trigger other features of SLE [20]. Murine models with a deficiency of Tyro-3, Axl, and Mertk receptors showed that a decreased capacity in binding to apoptotic cells resulted in autoantibody production, development of arthritis, skin rash, and deposition of immune complexes in glomeruli. Mice deficient in T-cell IgG4 (TIM-4), which binds the phosphatidylserine residues exposed on the surface of apoptotic cells, exhibit anti-dsDNA antibodies and elevated B-cell and T-cell activation. Phosphatidylserine, which is exposed on the external membrane in the early phases of apoptosis, probably plays a pivotal role in phagocyte recognition in SLE. However, the receptors involved in this process are neither completely understood nor have they been fully identified. Noteworthy, the interaction between macrophages and apoptotic cells results in a tolerogenic immunological response that is characterized by the release of TGF-beta and IL-10 into the microenvironment, which ultimately prevents the onset of inflammation and the activation of the immune system.
If phagocytic cells are not effective in removing apoptotic cells, they accumulate and progress into secondary necrosis, which implies an inflammatory reaction. This occurs every time “danger signal” molecules, which are usually enclosed inside the cellular cytoplasm because they could potentially trigger the production of inflammatory cytokines, are released into the extracellular environment after membrane integrity is broken [21]. High-mobility group box protein 1 (HMGB1), which is produced during cell activation and, early, in apoptosis in the attempt to stabilize the nucleosome structure, warrants special consideration. If necrosis occurs, HMGB1 shows strong pro-inflammatory activity when released into the extracellular compartment, acting as an “alarmin” or “danger signal.” HMGB1 activates immune responses by interacting with specific receptors of the innate immunity, in particular with TLR2 and TLR4 [22]. Therefore, the efficient clearance of apoptotic remnants remains a key physiological step for preventing the autoimmune manifestations. Apoptosis-derived extracellular vesicles (exosomes, microvesicles, apoptotic bodies) contain large amounts of digested nuclear compounds which represent a potential source of neoantigens if not promptly recognized and removed by phagocytes [23].
4 Innate Immune System and SLE
The role of the innate component of the immune system has been reevaluated in SLE based on knowledge of the defective apoptotic clearance in SLE, described above. Immature dendritic cells (DCs) normally express self-antigens on their surface in the absence of co-stimulatory signals, thereby inducing a tolerogenic effect on potentially autoreactive lymphocytes. A pro-inflammatory environment, such as infection, can induce DCs’ maturation and the expression of co-stimulatory molecules. In this scenario, self-antigens can be presented to T lymphocytes and an autoimmune response may be potentially triggered [24].
DCs are considered very efficient in recognizing damage-associated molecular patterns (DAMPs) and particularly in recognizing a highly conserved family of pathogen-associated molecular pattern (PAMP) receptors, which can efficiently identify microbiological agents and trigger the earliest inflammatory response.
However, when apoptotic clearance is not effective enough, endogenous components such as DNA, RNA, or ribonucleoproteins may potentially be recognized by specific TLRs. Nucleic acid-sensitive TLRs (TLR3, TLR7, TLR8, and TLR9) are of interest for SLE pathogenesis, since they bind DNA- or RNA-containing antigens. Among these receptors, TLR7 recognizes single-stranded RNA, while TLR9 is considered very efficient in binding unmethylated CpG DNA, both of which are typical of viral genomic material [25].
The strategic location of nuclear-sensitive TLRs inside the cell usually minimizes accidental exposure to endogenous material, so intracellular TLRs are usually activated only by viral- or microbiological-derived DNA or RNA, in particular when they are conjugated with antibodies in the form of immune complexes (ICs).
A second cascade signal coming from an Fc receptor is required to amplify the immunological response. This mechanism is especially relevant in SLE, a condition in which autoantibodies to nuclear antigens are abundantly detected. Many different ICs can activate pDCs, but RNA-containing ICs are probably the best inducers of IFN-alpha secretion owing to the simultaneous recruitment of Fc receptors and intracytosolic TLRs. Both TLR7 and TLR9 efficiently stimulate the production of type I interferon from pDCs using adaptor molecules such as MyD88, an intracellular protein that is critical for IFN-alpha secretion. Increased circulating levels of endogenous DNA, RNA, and nuclear proteins have been observed in predisposed subjects. This apoptotic-derived material or nucleoma is able to activate the IFN-alpha system through TLR7 and TLR9 [26]. It has been also found that HMGB1-DNA compounds and immune complex-containing snRNA can directly activate TLR7 and TLR9 in pDCs in a manner similar to viral RNA or DNA.
The defective apoptotic clearance explains the gap between apoptosis, apoptotic cellular fragments containing nuclear material accumulation, and IFN-alpha production by pDCs [27].
Unlike myeloid dendritic cells (mDCs), pDCs efficiently recognize immune complex containing apoptotic material and are very efficient in producing large amounts of IFN-alpha in response to autoantigen recognition. Although any cell is able to produce type I IFN-alpha in response to certain viral stimuli, pDCs are undoubtedly considered the main producers of this type of cytokine, which indeed encompasses 13 different IFN-alpha isoforms as well as other IFNs. IFN-alpha determines the downstream activation of interferon regulatory factor 5, a transcription factor of pDCs, and the subsequent link to promoter regions and transcription of IFN-alpha target genes [28].
4.1 Modulation of IFN-I Signaling
The type I IFN family is characterized by several immunological functions including promotion of B-cell differentiation, immunoglobulin class switch, production of autoantibodies, and increase in activated B- and T-cell survival. IFN-alpha is endowed with pleiotropic effects to target cells. It activates monocytes, NK cells, cytotoxic CD8+, and CD4 Th1 and induces autoantibody production by B-cell potentiating antiviral response. Conversely, Tregs are usually suppressed by INF-alpha. IFN-gene signature has also been detected in several tissues obtained from SLE patients, such as glomerular, synovial, and cutaneous tissues suggesting a pathogenetic role of type I IFN family in almost all target organs in SLE [29]. Moreover, lupus-like syndrome is a very well-known complication of recombinant IFN-alpha therapy administered for chronic viral hepatitis or during cancer immunotherapy [30].
Under normal circumstances, IFN-alpha release is usually triggered by viral particles through a time-regulated process which is turned off when the infection resolves. This is not the case with SLE since IFN release is independent on viral stimulus [31].
4.2 NETosis
The possible role of neutrophils, the most abundant leukocytes in humans, has been recently suggested in the pathogenesis of SLE. Neutrophils are typically recruited to infection sites during the early phases of inflammatory response and are considered an effective defense against bacterial and fungal infections. They are able to kill pathogens through phagocytosis and release of highly reactive oxygen species or cytotoxic molecules contained in the cytoplasmic granules. Besides, another killing modality has recently been proposed. Neutrophil extracellular traps (NETs) are meshwork structures containing chromatin and peptides with antimicrobial activity, which are externally released from dying cells [32]. This specific form of neutrophil PCD, called NETosis, has been implicated in the pathogenesis of autoimmune diseases [33]. NETosis is a specialized form of cell death that occurs primarily in neutrophils, characterized by NETs’ release. NETs comprise a mesh of DNA and histones, as well as the content of cytoplasmic granules and other mediators. Neutrophil-derived structures containing a significant amount of DNA and ribonucleoproteins could potentially stimulate pDCs (via TLR9) to produce significant amounts of IFN-alpha, similarly to apoptotic-derived nuclear material [34]. Interestingly, neutrophil hyperactivation and NET release have been recently linked to an increased risk of developing deep venous thrombosis in systemic vasculitis and other autoimmune diseases [35].
5 Adaptive or Acquired Immunity in SLE: Focus on T Cells
Both CD4+ or “helper” and CD8+ or “cytotoxic” T cells have been traditionally considered key players in SLE pathogenesis and inflammation.
CD4+ Thelper cells can be subdivided into Th1 and Th2, depending on the pattern of cytokine production and their immunological functions (mainly allergic reactions for Th2 and defense against infections for Th1). IL-12 is the main cytokine driving the differentiation of naïve CD4+ T cells into Th1 cells, which primarily produce IFN-gamma, IL-2, and TNF-alpha. On the other hand, IL-4, IL-5, and IL-13 cytokines produced by Th2 lymphocytes are involved in several T- and B-cell functions including proliferation, activation, and isotype switching.
Previous studies suggested that SLE was a Th2-driven disease since increased levels of IL-10 and IL-4 are usually detected in the lymphocytes isolated from SLE patients [36]. Subsequently, increased levels of both Th1 and Th2 cytokines have been described in humans and mice, underlying the complex heterogeneity of SLE that probably determines the diversity of lymphocyte subsets in the involvement of various organs.
Th1 lymphocytes are probably the key drivers of the inflammatory process in lupus nephritis (LN) [37]. Moreover, T-cell receptor (TCR) hyperactivation after interaction with the MHC-antigen complex has been reported in patients with SLE [38].
Th17 cells are a new subset of T lymphocytes that have been involved in the pathogenesis of a broad spectrum of autoimmune diseases, in particular rheumatoid arthritis, seronegative spondyloarthritis, and inflammatory bowel diseases [39]. They are generated after stimulation of naïve T cells with TGF-beta, IL-6, and IL-23. All these factors act together in preventing the switch of naïve T cells to a Th1 phenotype [40]. Interestingly, besides maintaining ongoing inflammation in target tissues, Th17 cells determine a concomitant downregulation of Treg function and development [41]. Th17 cells and cytokines of the IL-17 family have also been shown to play a crucial role in SLE, and Th17 cells have been detected in the glomerular tissue of patients with active LN [42].
Recently, Savino et al. showed both in mice and in humans a possible role in SLE pathogenesis for Rai, a member of the Src homology 2 domain adapter family. Rai (−/−) mice develop a lupus-like phenotype with spontaneous activation of self-reactive lymphocytes. Moreover, it has been demonstrated that Rai (−/−) mice present Th1 and Th17 cell infiltrates in the kidneys, suggesting that Rai knockout mice (−/−) are more susceptible than normal mice to LN. Finally, a defect in Rai expression has been shown in T cells derived from SLE patients [43].
In summary, several studies on regulatory T cells showed their potential role in the breakdown of immune tolerance, since both quantitative and qualitative abnormalities of peripheral regulatory T lymphocytes (CD4+ CD25 + high) have been described in SLE [44, 45].
6 Adaptive or Acquired Immunity in SLE: Focus on B Cells
Considerable data has been collected regarding the function of B cells and their role in both inflammation and in autoimmune diseases [46].
B-lymphocyte involvement in SLE pathogenesis has traditionally been considered in the light of the production of circulating autoantibodies that are typical of SLE and connective tissue diseases in a broad sense. Noteworthy, such autoantibodies, which are pathogenetic in most cases, are useful to clinicians in the diagnostic work-up and in follow-up [47]. B lymphocytes have been considered fundamental, because of the production of autoantibodies against soluble and cellular components, such as nuclear antigens. However, the role of B cells in autoimmune diseases has recently widely investigated. B lymphocytes are now considered important players of adaptive immunity in the complex chessboard of SLE pathogenesis. It is acknowledged that besides secreting autoantibodies, B lymphocytes efficiently present autoantigens and activate T cells. Therefore, they deeply influence T-cell function and activation [48].
B lymphocytes are classified into two main lineages: B1 and B2 cells. B1 lymphocytes are generated both in the bone marrow and in fetal liver. They are also thought to play a role in removing apoptotic material and debris by linking innate and adaptive immunity together. On the other hand, B2 cells are generated exclusively in the bone marrow, where autoreactive cells are first removed (central tolerance), and then undergo further selection in the spleen microenvironment (peripheral tolerance). After this initial step, B2 cells may follow two different paths. They can become mature follicular cells, migrating to the secondary lymphoid organs and waiting for T-cell-dependent activation, and then they ultimately evolve into plasma cells or memory B cells. Alternatively, B2 cells can colonize the marginal zone (MZ) of the spleen becoming MZ B2 cells, which, similarly to B1 cells, are able to respond to antigens regardless of T-cell help [49].
Although the role of MZ B cells in lupus is still controversial, they are probably involved in some autoimmune cellular responses, such as autoimmune thrombocytopenia, a condition for which splenectomy is usually beneficial [50]. An increased number of MZ B cells have been detected using the NZB/WF1 mouse model of SLE, suggesting an important role for these B lymphocytes in SLE pathogenesis [51]. Interestingly, IFN-alpha is a potent driver of MZ B-cell activation and an efficient enhancer of the co-stimulatory function, making the MZ B cell an important player in the autoantibody response to nuclear autoantigens in the context of pDC hyperactivation [52].
Lastly, B cells can produce important cytokines, such as IL-6 and TNF-alpha and IL-1, therefore contributing significantly to maintaining and amplifying the inflammatory process in SLE. Interestingly, and similar to T cells, BCR hyperactivation has also been described in the B lymphocytes of SLE patients, with increased phosphorylation of several signaling molecules and abnormal calcium influx after antigen recognition [53, 54].
Recently, a regulatory activity has also been described for a subset B lymphocytes. The term regulatory B cells or simply B reg has been used when referring to such cells [55]. The recently described CD24highCD27++B population probably includes the large proportion of human Breg. The main function of regulatory B cells is to produce IL-10, and currently the identification of this cytokine by intracellular staining is the preferred method for isolating Breg [56]. The immunosuppressive properties of IL-10 have been described in animal models of collagen-induced arthritis and experimental autoimmune encephalitis. However, the role of IL-10 in SLE is still controversial since both activating and immunosuppressive properties have been attributed to this cytokine.
Despite such premises, IL-10 is currently regarded mainly as an immunosuppressive cytokine, especially in SLE, and Breg is probably one of the main sources [57].
Interestingly, after depletion of B cells using the anti-CD20 monoclonal antibody rituximab, the subsequent repopulation phase is probably constituted mainly of regulatory B cells, and this is especially true in patients who achieve good clinical response after rituximab [58]. However, further data are necessary to better clarify the role of Breg cells and IL-10 in SLE pathogenesis.
In conclusion, as far as the role of lymphocytes in SLE pathogenesis is concerned, the T-lymphocyte centric hypothesis has recently been counterbalanced with a newer B-lymphocyte centric theory, which has been mainly supported by emerging data from the effects of B-cell target therapies [59].
References
Crow MK (2008) Collaboration, genetic associations and lupus erythematosus. N Engl J Med 358:956–961
Flesher DL, Sun X, Behrens TW, Graham RR, Criswell LA (2010) Recent advances in the genetics of systemic lupus erythematosus. Expert Rev Clin Immunol 6(3):461–479
Bowness P, Davies KA, Norsworthy PJ, Athanassiou P, Taylor-Wiedeman J, Borysiewicz LK, Meyer PA, Walport MJ (1994) Hereditary C1q deficiency and systemic lupus erythematosus. QJM 87(8):455–464
Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK (2005) Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 52(5):1491–1503
Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ et al (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100(5):2610–2615
Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG et al (2004) Coordinate overexpression of interferon-alpha induced genes in systemic lupus erythematosus. Arthritis Rheum 50(12):3958–3967
Li QZ et al (2010) Interferon signature gene expression is correlated with autoantibody profiles in patients with incomplete lupus syndromes. Clin Exp Immunol 159:281–291
Zhao S, Long H, Qiaanjin L (2010) Epigenetic perspectives in systemic lupus erythematosus: pathogenesis, biomarkers and therapeutic potentials. Clin Rev Allergy Immunol 39:3–9
Wen ZK, Xu W, Xu L, Cao QH, Wang Y, Chu YW, Xiong SD (2007) DNA hypomethylation is crucial for apoptotic DNA to induce systemic lupus erythematosus-like autoimmune disease in SLE-non-susceptible mice. Rheumatology (Oxford) 46:1796–1803
Stagakis E, Bertsias G, Verginis P, Nakou M, Hatziapostolou M, Kritikos H, Iliopoulos D, Boumpas DT (2011) Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: mir-21 regulates aberrant T-cells response trough the regulation of PDCD4 expression. Ann Rheum Dis 70:1496–1506
Hudson CA, Cao L, Kasten-Jolly J, Kirkwood JN, Lawrence DA (2003) Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J Toxicol Environ Health A 66(10):895–918
Hughes GC, Clark EA (2007) Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity 40(6):470–481
Tayel SS, Helmy AA, Ahmed R, Esmat G, Hamdi N, Abdelaziz AI (2013) Progesterone suppresses interferon signaling by repressing TLR-7 and MxA expression in peripheral blood mononuclear cells of patients infected with hepatitis C virus. Arch Virol 158(8):1755–1764
Doria A, Canova M, Tonon M, Zen M, Rampudda E, Bassi N, Atzeni F, Zampieri S, Ghirardello A (2008) Infections as trigger and complications of systemic lupus erythematosus. Autoimmun Rev 8:24–28
Poole BD, Scofield RH, Harley JB, James JA (2006) Epstein–Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 39:63–70
Werth VP (2007) Cutaneous lupus: insight into pathogenesis and disease classification. Bull NYU Hosp Jt Dis 65:200–204
Kuhn A, Wenzel J, Weyd H (2014) Photosensitivity, apoptosis, and cytokines in the pathogenesis of lupus erythematosus: a critical review. Clin Rev Allergy Immunol 47(2):148–162
Mathieu C (2011) Vitamin D and the immune system: getting it right. IBMS BoneKEy Rep 8:178–186
Beyer C, Pisetsky DS (2010) The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol 6:21–29
Muñoz LE et al (2010) The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 6:280–289
Mevorach D, Trahtemberg U, Krispin A, Attalah M, Zazoun J, Tabib A, Grau A, Verbovetski-Reiner I (2010) What do we mean when we write “senescence”, “apoptosis”, “necrosis”, or “clearance of dying cells”? Ann N Y Acad Sci 1209:1–9
Pisetsky DS, Erlandsson-Harris H, Andersson U (2008) High mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther 10:209
Rahman A, Isenberg DA (2008) Systemic lupus erythematosus. N Engl J Med 358(9):929–939
Fransen JH, van der Vlag J, Ruben J, Adema GJ, Berden JH, Hilbrands LB (2010) The role of dendritic cells in the pathogenesis of systemic lupus erythematosus. Arthritis Res Ther 12:207
Marshak-Rothstein A (2006) Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6:823–835
Pisetsky DS, Ullal AJ (2010) The blood nucleome in the pathogenesis of SLE. Autoimmun Rev 10:35–37
Theofilopoulos AN et al (2010) Sensors of the innate immune system: their link to rheumatic diseases. Nat Rev Rheumatol 6:146–156
Ronnblom L, Alm GV, Eloranta ML (2011) The type I interferon system in the development of lupus. Semin Immunol 23:113–121
Nzeusseu Toukap A et al (2007) Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum 56(5):1579–1588
Wilson LE et al (2002) Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum 32(3):163–173
Ronnblom L, Elkon KB (2010) Cytokines as therapeutic targets in SLE. Nat Rev Rheumatol 6:339–347
Branzk N, Papayannopoulos V (2013) Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol 35(4):513–530
Knight JS, Kaplan MJ (2012) Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Curr Opin Rheumatol 24:441–450
Garcia-Romo GS et al (2011) Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3:73ra20
Tobias A et al (2012) Neutrophil Extracellular Trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 32:1777–1783
Funauchi M, Ikoma S, Enomoto H, Horiuchi A (1998) Decreased Th1-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol 27(3):219–224
Masutani K, Akahoshi M, Tsuruya K et al (2001) Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum 44(9):2097–2106
Scheinecker C, Bonelli M, Smolen JS (2010) Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun 35:269–275
Truchetet ME, Mossalayi MD, Boniface K (2013) IL-17 in the rheumatologist’s line of sight. Biomed Res Int 2013:295132
Ghoreschi K et al (2011) T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol 32(9):395–401
Chen X, Oppenheim JJ (2014) Th17 cells and Tregs: unlikely allies. J Leukoc Biol 95(5):723–731
Nalbandian A, Crispı’n JC, Tsokos GC (2009) Interleukin-17 and systemic lupus erythematosus: current concepts. Clin Exp Immunol 157(2):209–215
Savino MT, Ulivieri C, Emmi G, Prisco D, De Falco G, Ortensi B, Beccastrini E, Emmi L, Pelicci G, D’Elios MM, Baldari CT (2013) The Shc family protein adaptor, Rai, acts as a negative regulator of Th17 and Th1 cell development. J Leukoc Biol 93(4):549–559
Valencia X, Yarboro C, Illei G, Lipsky PE (2007) Deficient CD4+ CD25 high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 178:2579–2588
Bonelli M et al (2008) Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int Immunol 20:861–868
Crispín JC et al (2010) T cells as therapeutic targets in SLE. Nat Rev Rheumatol 6:317–325
Meroni PL, Biggioggero M, Pierangeli SS, Sheldon J, Zegers I, Borghi MO (2014) Standardization of autoantibody testing: a paradigm for serology in rheumatic diseases. Nat Rev Rheumatol 10(1):35–43
Grammer AC, Lipsky PE (2003) B cell abnormalities in systemic lupus erythematosus. Arthritis Res Ther 5:S22–S27
Dorner T, Radbruch A, Burmester GR (2009) B-cell directed therapies for autoimmune disease. Nat Rev Rheumatol 5(8):433–441
Lopes-Carvalho T, Kearney JF (2005) Marginal zone B cell physiology and disease. Curr Dir Autoimmun 8:91–123
Wither JE, Roy V, Brennan LA (2000) Activated B cells express increased levels of costimulatory molecules in young autoimmune NZB and (NZB 9 NZW)F(1) mice. Clin Immunol 94(1):51–63
Wang JH, Wu Q, Yang P, Li H, Li J, Mountz JD, Hsu HC (2011) Type I interferon-dependent CD86 (high) marginal zone precursor B cells are potent T cell costimulators in mice. Arthritis Rheum 63(4):1054–1064
Jenks SA, Sanz I (2009) Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmun Rev 8(3):209–213
Liossis SN, Kovacs B, Dennis G, Kammer GM, Tsokos GC (1996) B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J Clin Invest 98(11):2549–2557
Goode I, Xu H, Ildstad ST (2014) Regulatory B cells: the new “it” cell. Transplant Proc 46(1):3–8
Iwata Y, Matsushita T, Horikawa M et al (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117:530–541
Matsushita T, Horikawa M, Iwata Y, Tedder TF (2010) Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 185:2240–2252
Sanz I, Lee FE (2010) B cells as therapeutic targets in SLE. Nat Rev Rheumatol 6:326–337
Dörner T et al (2009) B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 5:433–441
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Squatrito, D., Emmi, G., Silvestri, E., Prisco, D., Emmi, L. (2016). SLE Pathogenesis: From Apoptosis to Lymphocyte Activation. In: Roccatello, D., Emmi, L. (eds) Connective Tissue Disease. Rare Diseases of the Immune System. Springer, Cham. https://doi.org/10.1007/978-3-319-24535-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-24535-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24533-1
Online ISBN: 978-3-319-24535-5
eBook Packages: MedicineMedicine (R0)