Abstract
The innate immune system of the lung is a complex network of different cellular and noncellular components protecting the lung from inhaled pathogens. Antimicrobial peptides (AMP) are produced by epithelial and myeloid cells as part of this system. AMPs, such as defensins and cathelicidin, are small cationic peptides with a broad microbicidal activity against respiratory bacteria, viruses, and fungi. However, their functions go beyond antimicrobial activity and include modulation of the innate and adaptive immune response to infection as well as lung repair after injury. Thus, AMPs are involved in pathophysiological processes of many lung diseases, such as acute and chronic lung infection, chronic obstructive pulmonary disease, cystic fibrosis, and lung cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic Obstructive Pulmonary Disease
- Cystic Fibrosis
- Respiratory Syncytial Virus
- Chronic Obstructive Pulmonary Disease Patient
- Alveolar Macrophage
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 The Innate Immune Network of the Lung
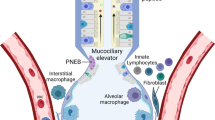
The human lung is continuously exposed to a broad array of airborne pathogens such as bacteria, viruses, and fungi. Given its specific requirement to ensure an effective gas exchange, the lung has a unique structure that is characterized by a large contact surface combined with an extremely thin epithelial layer. Hence, the lung is a potential portal of entry for inhaled microbes. In order to avoid colonization of the lower airways with inhaled pathogens and prevent local and systemic infection, the lung is protected by a complex innate immune network consisting of various cellular and noncellular components.
The first line of defense is represented by the airway epithelium, a pseudostratified epithelium of basal cells, ciliated cells, secretory Clara cells, and goblet cells. Its function within the innate immune system exceeds well-known mechanisms like barrier formation and mucociliary clearance. The epithelium of the respiratory tract is an immunologically active tissue, which exerts functions such as pathogen recognition, pathogen neutralization, and activation of further immune mechanisms. These include resident alveolar macrophages as well as inflowing neutrophils. Those professional immune cells have a high capability to neutralize microbial pathogens. In addition, the release of immunomodulatory mediators from epithelial and myeloid cells induces activation of the adaptive immune response.
Small cationic antimicrobial peptides (AMPs) from the families of defensins and cathelicidins play a key role within the innate immune network of the lung.
3.2 Expression of Antimicrobial Peptides in the Lung
AMPs are expressed by various cell types present in the lung during health and disease. The main AMPs expressed in the lung are α-defensins, β-defensins, and cathelicidins. Table 3.1 gives an overview on AMPs of the human lung.
3.2.1 Defensins
3.2.1.1 α-Defensins
α-Defensins comprise the human neutrophil peptides (HNP) 1–4 and the epithelial α-defensins HD-5 and HD-6 (Doss et al. 2010). Different to other epithelial tissues of the human body, the airway epithelium hardly produces α-defensins; only HD-5 has been detected in airway epithelial cells in small amounts (Frye et al. 2000). The main sources of α-defensins in the human lung are neutrophilic granulocytes. They constitutively express α-defensins, which are stored in azurophilic granula and represent their main protein content. Upon infection, activation of innate immunity triggers the influx of neutrophils into the lung, where they neutralize pathogens through phagocytosis and degranulation of their azurophilic granula (Ganz et al. 1985; Selsted and Ouellette 2005).
In human alveolar macrophages, α-defensins are virtually absent; only rabbits have been found to express α-defensins in their alveolar macrophages (Ganz et al. 1985; Selsted and Ouellette 2005).
3.2.1.2 β-Defensins
The principal β-defensins found in the human lung are human β-defensin (hBD) 1–4 (Doss et al. 2010). Their main sources are epithelial cells and submucous glands (Kao et al. 2003; Bals et al. 1998a). However, β-defensins (hBD-1 and hBD-2) are also released from myeloid cells such as alveolar macrophages and dendritic cells (Duits et al. 2002). The expression of β-defensins shows a gene-specific behavior: hBD-1 is constitutively expressed by the epithelium, ensuring a constant basic antimicrobial activity of the airway surface liquid (McCray and Bentley 1997; Singh et al. 1998). In contrast, hBD-2, hBD-3, and hBD-4 are not released by default, but only if required; high levels can only be detected in the case of infection or inflammation (Hess et al. 2010; Harder et al. 2001; Yanagi et al. 2005; Scharf et al. 2010a, 2012; Benincasa et al. 2009). Compared to hBD-3 and hBD-4, which are predominantly expressed in other epithelial tissues, hBD-2 is mainly expressed in the respiratory tract (Bals et al. 1998a).
3.2.2 Cathelicidin
In the lung, the only human cathelicidin LL-37/hCAP-18 is expressed by different cell types, namely, myeloid cells such as alveolar macrophages (Rivas-Santiago et al. 2008), neutrophils (Rivas-Santiago et al. 2008; Cowland et al. 1995), and mast cells (Di Nardo et al. 2003), as well as airway epithelial cells (Bals et al. 1998b). It can be detected in bronchoalveolar lavage fluid (Agerberth et al. 1999). Similar to α-defensins, cathelicidin is constitutively expressed by neutrophils and stored in granula until release (Larrick et al. 1995). Degranulation is triggered by external stimulation such as TLR or cytokine receptor activation. Since degranulation can also be induced by cathelicidin itself, a feedforward loop is initiated and results in a cathelicidin burst with very high local concentrations (Vandamme et al. 2012). In granula, cathelicidin is stored as an inactive precursor protein that is processed to its active form by extracellular cleavage upon degranulation (Vandamme et al. 2012). The mechanism that activates the cathelicidin precursor protein in airway epithelial cells, which do not store it in granula, has not been identified yet (Vandamme et al. 2012).
3.3 Regulation of Antimicrobial Peptide Expression in the Lung
3.3.1 Defensins
In the lung, β-defensin expression is triggered by common respiratory pathogens including the most common causes of pneumonia. Airway epithelial cells express β-defensins upon stimulation with Streptococcus pneumoniae (Scharf et al. 2012; García et al. 2001), Haemophilus influenzae (Seiler et al. 2013), Legionella pneumophila (Scharf et al. 2010a), and Pseudomonas aeruginosa (García et al. 2001; Harder et al. 2000). β-Defensins are also induced by the most common causes of pulmonary tuberculosis, Mycobacterium tuberculosis and Mycobacterium bovis (Méndez-Samperio et al. 2007; Rivas-Santiago et al. 2005). Viral pathogens resulting in increased expression of β-defensins include respiratory syncytial virus (Kota et al. 2008), rhinovirus (Proud et al. 2004; Duits et al. 2003), and, at least in mice, influenza virus (Chong et al. 2008). Finally, β-defensins are induced by fungal pathogens such as Aspergillus fumigatus (Alekseeva et al. 2009).
The cellular components of lung innate immunity are able to recognize and specifically react to pathogens through detection of conserved microbial structures called pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors. These include transmembrane Toll-like receptors (TLRs) as well as cytosolic NOD-like and RIG-I-like receptors and others. Activation of pattern recognition receptors leads to induction of intracellular signaling pathways like the MAP kinase cascade and activation of transcription factors such as NF-κB, eventually resulting in gene expression. Apart from the constitutively expressed hBD-1, the expression of β-defensins is largely regulated by TLR signaling. The role of cytosolic pattern recognition receptors in the regulation of β-defensins remains to be discovered.
There are ten human members of the TLR family. In the lung, the whole array of TLRs is expressed by airway epithelial cells and resident innate immune cells like alveolar macrophages and neutrophils (Kovach and Standiford 2011; Kawasaki and Kawai 2014; Futosi et al. 2013; Parker and Prince 2011). Many of them have been shown to upregulate β-defensin expression upon stimulation with their specific agonists. In particular, the expression of β-defensins is induced by TLR2 (Hertz et al. 2003; Wang et al. 2003), TLR3 (Seiler et al. 2013; Proud et al. 2004), TLR4 (MacRedmond et al. 2005), TLR5 (Froy 2005), TLR6 (Froy 2005), and TLR9 (Platz et al. 2004). Since the members of this list can be activated by bacterial, mycobacterial, viral, and fungal PAMPs (Akira et al. 2006), the innate immune network of the lung can potently respond to different respiratory infections.
Apart from TLR-related β-defensin upregulation, lung infection also induces the expression of various proinflammatory cytokines derived from epithelial, innate, and adaptive immune cells. TNF-α and IL-1β are released by alveolar macrophages during bacterial infection (Hess et al. 2010) and upregulate hBD-2 in airway epithelial cells in vitro and in vivo (Hess et al. 2010; Harder et al. 2000; Albanesi et al. 2007). hBD-2 expression is also stimulated by IL-17A (Kao et al. 2004), which is derived from Th17 cells during lung infection (Ye et al. 2001), and airway epithelial cell-derived IL-17C (Kusagaya et al. 2014). Upon viral infection, interferon-gamma release is triggered, for example, by TLR3 (Akira 2009) and leads to increased hBD-3 expression (Albanesi et al. 2007).
Stimulation of TLRs and cytokine receptors leads to activation of intracellular signaling processes like mentioned above. Therefore, TLR and cytokine receptors induce β-defensin expression in an NF-κB- or MAP kinase-dependent manner (McCray and Bentley 1997; Scharf et al. 2010b). In fact, hBD-2 has been shown to be a direct target gene of the transcription factors NF-κB and the MAP kinase target AP-1 (Wehkamp et al. 2004; O’Neil et al. 1999). With a vitamin D response element within its promoter sequence, hBD-2 further is a target gene of vitamin D; however, vitamin D reactivity of the hBD-2 gene is not as strong as it is described for cathelicidin (Wang et al. 2004).
3.3.2 Cathelicidin
In contrast to β-defensins, the expression of cathelicidin is not primarily induced upon infectious or inflammatory stimulation but is regulated by vitamin D. Airway epithelial as well as myeloid cells express the vitamin D receptor (Hansdottir et al. 2008; Liu et al. 2006). Stimulation of these cells with the active form of vitamin D, 1,25-dihydroxyvitamin D3, induces the expression of cathelicidin (Wang et al. 2004; Schauber et al. 2007a; Gombart et al. 2005). The promoter of the corresponding gene contains a vitamin D response element, which makes it a direct vitamin D target gene (Wang et al. 2004). Infectious stimuli can also indirectly trigger cathelicidin expression: it has been shown that TLR activation upregulates the expression of the vitamin D receptor in human macrophages, which leads to an increased expression of cathelicidin (Liu et al. 2006). Because the effects of vitamin D rely on its conversion from its inactive proform to 1,25-dihydroxyvitamin D3 by the hydroxylase CYP27B1, cathelicidin expression is largely influenced by modulation of this reaction. TLR stimulation leads to activation of CYP27B1, resulting in increased levels of active vitamin D (Liu et al. 2006). Conversion of vitamin D to its active form is also induced by IFN-γ in alveolar macrophages (Koeffler et al. 1985). 1,25-dihydroxyvitamin D3 further amplifies the innate immune response by upregulation of TLR2 and CD14 (Schauber et al. 2007a, b).
3.4 Microbicidal Functions of Antimicrobial Peptides in the Lung
AMPs exert antimicrobial activity against many common respiratory pathogens, including bacterial, viral, and fungal species. The human β-defensins hBD-2 and hBD-3 are bactericidal against the typic pathogen of community-acquired pneumonia, S. pneumoniae (Scharf et al. 2012). In vitro microbicidal activity against P. aeruginosa and Escherichia coli, frequent causes of nosocomial and ventilator-associated pneumonia, has been shown for hBD-2 (Harder et al. 2000). hBD-3 is further bactericidal against Staphylococcus aureus and Enterococcus faecium (Harder et al. 2001; Chen et al. 2005). hBD-4 has antimicrobial activity against P. aeruginosa (Yanagi et al. 2005).
The antimicrobial spectrum of cathelicidin includes a broad array of bacterial pathogens, including gram-positive species like S. pneumoniae and gram-negative species such as P. aeruginosa (Benincasa et al. 2009; Bals et al. 1998b; Saiman et al. 2001; Felgentreff et al. 2006).
Apart from bacteria, a couple of respiratory viruses are targeted by AMPs: influenza virus, parainfluenza virus, and respiratory syncytial virus (Klotman and Chang 2006; Tripathi et al. 2013). AMPs have also been shown to act against Candida spp. and Aspergillus spp., the most frequent species causing respiratory mycosis (Alekseeva et al. 2009; Aerts et al. 2008).
While there is much evidence for the microbicidal effect of AMPs in vitro, it is hard to assess their impact in the human lung. Due to their chemical structure, salt concentrations and pH in healthy or inflamed lung tissue may alter AMP function in vivo. In addition, physiological concentrations of AMPs may be significantly lower than the minimal inhibitory concentrations assessed by in vitro approaches (Bals et al. 1998b; Bowdish et al. 2005); thus, particular AMPs may not reach microbicidal concentrations in vivo. However, it has to be considered that in the human lung, AMPs are part of a well-orchestrated immune network with many synergistic capacities. AMPs can act in mutual synergism (García et al. 2001; Chen et al. 2005; Tripathi et al. 2013) as well as cooperative with other antimicrobial substances such as lactoferrin and lysozyme (Bals et al. 1998a). The effect of AMPs can also be enhanced by surfactant proteins (Tripathi et al. 2013) and even bacterial components (Iwase et al. 2010). Finally, AMPs have been shown to act synergistic with antibiotic agents (Xiong et al. 1999; Scott et al. 1999a, b; Hancock and Scott 2000).
3.5 Beyond Antimicrobial Activity: Modulatory Functions of Antimicrobial Peptides in the Lung
In primitive invertebrate species like the common model organism Caenorhabditis elegans, there is no cellular immune system. Apart from rather unspecific mechanisms such as pathogen avoidance and physical isolation, their resistance against infectious agents largely depends on the microbicidal activity of their AMPs. In the course of evolution, AMPs became part of increasingly complex immune systems in which their role changed from solely antimicrobial agents to versatile modulators of different physiologic systems.
3.5.1 Modulation of Lung Immunity
Within the innate immune network of the lung, AMPs are one of the first defense mechanisms to face invading pathogens. Apart from their antimicrobial activity, AMPs have many immunomodulatory features that are similar to those of classic cytokines. For example, AMPs act as chemoattractants for different immune cells, recruiting them to sites of inflammation. α-Defensins have been shown as chemotactic factors for monocytes, dendritic cells, and T cells (Yang et al. 2000a, 2001; Chertov et al. 1996). β-Defensins chemoattract monocytes, macrophages, and neutrophils via the chemokine receptor CCR2, immature dendritic cells and T cells via CCR6, and mast cells in a phospholipase C-dependent manner (Soruri et al. 2007; Yang et al. 1999; Röhrl et al. 2010; Niyonsaba 2002). Finally, cathelicidin is capable of recruiting neutrophils, monocytes, and T cells via FPRL1 (Yang et al. 2000b; Kurosaka et al. 2005), and mast cells via phospholipase C (Niyonsaba et al. 2002), and takes part in the regulation of dendritic cell maturation and differentiation (Davidson et al. 2004; Kandler et al. 2006).
In addition to their chemokine nature, AMPs are able to act as endogenous TLR agonists. For example, mouse β-defensin 2 activates TLR4 (Biragyn et al. 2002), and β-defensin 3 is capable of activating TLR1 and TLR2 (Funderburg et al. 2007). By that, AMPs can induce cytokine expression and activation of immune cells: β-defensins induce IL-6, IL-8, IL-10, and MCP-1 in monocytes (Boniotto et al. 2006) and IL-1β and IL-6 in dendritic cells (Biragyn et al. 2002). In myeloid and epithelial cells, IL-8 expression is upregulated by α-defensins (Khine et al. 2006). β-Defensin 2 leads to dendritic cell maturation in mice (Biragyn et al. 2002), and β-defensin 3 activates monocytes and neutrophils (Funderburg et al. 2007). Cathelicidin has been shown to indirectly enhance TLR signaling (Lai et al. 2011). Cathelicidin further induces IL-8 and MCP-1 in a MAPK-dependent manner (Scott et al. 2002; Tjabringa et al. 2003; Bowdish et al. 2004; Yu et al. 2007). Mast cell degranulation and histamine release are mediated by β-defensins and cathelicidin (Schiemann et al. 2009; Niyonsaba et al. 2001).
Excessive activity of the immune system can cause self-damage of the host by immunopathological processes. Therefore, balancing of pro- and anti-inflammatory signals is crucial. Above immunostimulation, AMPs also take part in the fine-tuning of the immune response by additionally regulating anti-inflammatory mechanisms. Activation of the complement system, which can be induced in the lung (Varsano et al. 2000), is inhibited by β-defensin 2 (Bhat et al. 2007). β-Defensin 3 has been shown to inhibit ERK and, via downmodulation of TRIF and MyD88, TLR signaling, leading to a decreased production of proinflammatory cytokines in macrophages and dendritic cells (Semple et al. 2010, 2011; Pingel et al. 2008). α-Defensins have an anti-inflammatory effect on macrophages, reducing production of reactive oxygen species and inhibiting the expression of proinflammatory cytokines (Miles et al. 2009). However, compared to PAM3CSK4, another TLR1/2 agonist, β-defensin 3-derived TLR1/2 activation induces a pro- rather than an anti-inflammatory cytokine pattern in monocytes (Funderburg et al. 2011).
Excessive immunostimulation by bacterial PAMPs is considered to be a key event in the pathogenesis of septic shock. Cathelicidin inhibits proinflammatory cytokine release from neutrophils and macrophages upon stimulation with the gram-negative endotoxin LPS (Mookherjee et al. 2006; Alalwani et al. 2010; Scott et al. 2011). In particular, cathelicidin antagonizes LPS by preventing the formation of the LPS/LBP complex, which is required for TLR4-dependent LPS recognition (Scott et al. 2000; Kirikae et al. 1998). Synthetic mimics of AMPs further intercept the classical gram-positive PAMP and TLR2 agonist LTA (Scott et al. 1999a). The role of cathelicidin at the crossroads of inflammation is further underlined by its influence on bacterially stimulated neutrophils: cathelicidin increases their antimicrobial activity and phagocytotic capacity. In cathelicidin-deficient neutrophils, antimicrobial activity is impaired; instead, they react with more production of proinflammatory cytokines (Alalwani et al. 2010). Thus, AMPs may limit the adverse effects of extensive immune cell activation.
On the other hand, AMPs seem to especially promote airway epithelial innate immune mechanisms: cathelicidin leads to a decrease in epithelial permeability, thereby preventing transepithelial invasion of bacterial pathogens (Byfield et al. 2011). It further induces internalization of LPS into airway epithelial cells, which leads to activation of endosomal TLR4 and consequently LPS-induced epithelial cytokine production (Shaykhiev et al. 2010). Moreover, cathelicidin facilitates signaling via TLR3, a receptor for viral dsRNA (Lai et al. 2011). In synergy with other inflammatory mediators and TLR agonists, cathelicidin activates cytokine production in epithelial cells even in low concentrations (Filewod et al. 2009; Nijnik et al. 2012). Cathelicidin is also able to activate NF-κB and induce cytokine production in airway epithelial cells on its own (Pistolic et al. 2009).
The pro- and anti-inflammatory capacities of AMPs in different settings are still incompletely understood and even seem to be self-contradictory to some degree. Anyway, their position within the hierarchically structured immune network of the lung is strategic: at the first line of defense, they ensure an early answer to pathogen exposition, namely, through direct microbicidal activity, PAMP interception, and immunological activation of resident cells. In the next step, they trigger more potent mechanisms of the innate and adaptive immunity while simultaneously contributing to attenuate those mechanisms in order to avoid immunopathology.
In summary, AMPs are key players in the initiation and amplification of the innate immune response and contribute to activation of adaptive immunity. At the same time, they seem to protect the host from overshooting inflammation by coordination of pro- and anti-inflammatory signaling.
3.5.2 Modulation of Lung Injury and Repair
Infection and inflammation leading to epithelial injury are characteristic hallmarks of acute and chronic lung diseases. Termination and resolution of inflammation and maintenance of tissue homeostasis are active and coordinated processes which include the expression of immune cell-derived and epithelial factors such as pro- and anti-inflammatory cytokines, the keratinocyte growth factor (KGF), and the epidermal growth factor (EGF) (Tjabringa et al. 2003; Campbell et al. 2011; Puddicombe et al. 2000; Michelson et al. 1999). AMPs qualify as mediators of wound healing and tissue repair as the expression of AMPs by inflammatory and epithelial cells is increased under infectious and inflammatory conditions and epithelial injury induced by infectious agents leads to an increased risk that pathogens invade the host (Steinstraesser et al. 2008; Lai and Gallo 2009). Indeed, there is strong evidence that AMPs are involved in epithelial repair processes, such as cell proliferation, epithelial regeneration, and reepithelialization (Lai and Gallo 2009; Heilborn et al. 2003; Tokumaru et al. 2005; Carretero et al. 2008; Beisswenger and Bals 2005; Murphy et al. 1993; Aarbiou et al. 2004).
The function of AMPs in the repair of epithelial injury has been particularly studied in wound healing of the skin. Cathelicidin, for instance, induces reepithelialization after skin injury via transactivation of the EGF receptor (Heilborn et al. 2003; Tokumaru et al. 2005; Carretero et al. 2008). Cathelicidin also plays an important role in angiogenesis and thereby contributes to wound repair (Koczulla et al. 2003). As cathelicidins and defensins induce proliferation of diverse airway epithelial cell lines and primary airway epithelial cells in vitro, it is suggested that AMPs also mediate wound healing in the lung via the activation of pro-proliferative signaling cascades (Beisswenger and Bals 2005; Murphy et al. 1993). Cathelicidin activates airway epithelial cell via metalloproteinase-mediated cleavage of membrane-anchored EGF receptor ligands as well as MAP kinase-dependent signaling pathways (Tjabringa et al. 2003) and stimulates airway epithelial wound closure in an EGF receptor-, G protein-coupled receptor-, and MAP kinase-dependent manner (Shaykhiev et al. 2005). A function of cathelicidins in tissue repair is further supported by a mouse study which associates deficiency of the mouse cathelicidin CRAMP with increased lung injury during infection with gram-negative bacteria (Kovach et al. 2012).
Like cathelicidin, α-defensins from human neutrophils induce proliferation of airway epithelial cell lines via activation of MAP kinase signaling cascades (Aarbiou et al. 2002) and enhance wound closure in an EGF-dependent manner (Aarbiou et al. 2004). α-Defensins also increase proliferation of lung fibroblasts and collagen synthesis (Han et al. 2009).
However, in vitro studies also suggest that cathelicidins and defensins induce the release of inflammatory mediators by airway epithelial cells and that AMPs are cytotoxic at higher concentrations (Tjabringa et al. 2003; Shaykhiev et al. 2005; Sakamoto et al. 2005). Therefore, the proinflammatory, cytotoxic, and pro-proliferative functions of AMPs may also have adverse consequences by perpetuating inflammation and potentially contribute to epithelial injury in lung diseases (Shaykhiev et al. 2005; Sakamoto et al. 2005). Moreover, concentrations of AMPs have been found to be increased in severe lung diseases. Plasma concentrations of α-defensins, for instance, are increased in pulmonary fibrosis (Mukae et al. 2002) and β-defensins are increased in bronchoalveolar lavage fluids of patients with diffuse panbronchiolitis and in bronchiolitis obliterans syndrome after lung transplantation (Ross et al. 2004; Hiratsuka et al. 2003). Cathelicidin associates with bronchial inflammation in cystic fibrosis lung disease (Chen et al. 2004).
In summary, AMPs may contribute to tissue regeneration and epithelial regeneration in the lung during infection and inflammation by inducing proliferation of structural cells, but they also potentially contribute to epithelial injury and fibrotic remodeling during airway inflammation when present at high concentrations.
3.6 Clinical Perspectives
3.6.1 Acute Respiratory Tract Infections
Respiratory tract infections are very common. Especially pneumonia is a major cause of global morbidity and mortality in children as well as in adults (Klugman and Garau 2009). Due to their microbicidal properties (“endogenous antibiotics”), the role of AMPs in acute respiratory tract infection has drawn much attention. AMP levels are increased in patients with pneumonia. High levels of β-defensins and cathelicidin can be detected in plasma or sputum of patient with acute pneumonia (Hiratsuka et al. 1998; Herr et al. 2009; Schaller-Bals et al. 2002; Ishimoto et al. 2006).
The important role of AMPs in respiratory tract infection is especially underlined by studies in animal models. Cathelicidin treatment has been shown to reduce proinflammatory cytokine release during MRSA pneumonia and improves the histopathological outcome (Hou et al. 2013). In cathelicidin-deficient mice, the immune response to gram-negative pneumonia with K. pneumoniae is delayed, eventually resulting in more inflammation and increased lung injury (Kovach et al. 2012). During gram-negative pneumonia with P. aeruginosa, overexpression of β-defensin 2 in rats reduces bacterial load in the lung as well as inflammation and lung injury and increases survival rate (Shu et al. 2006; Hu et al. 2010). In β-defensin 1-deficient mice, clearance of H. influenzae in the lung is impaired (Moser et al. 2002).
The effect of AMPs is also modulated by host-microbe interactions. The S. aureus virulence factor staphylokinase acts synergistically with cathelicidin to promote fibrinolysis during mouse pneumonia, which leads to a more invasive infection (Braff et al. 2007). Staphylokinase is also able to inactivate α-defensins (Jin et al. 2004).
While there are natural limitations in evaluation of the in vivo impact of AMPs in human pneumonia, the high AMP levels found in patients with pneumonia together with the studies mentioned above suggest a crucial role of AMPs in the response to lung infection.
3.6.2 Chronic Lung Diseases
Cigarette smoke is the major risk factor for the development of the chronic obstructive pulmonary disease (COPD). COPD is characterized by chronic inflammation of the lung leading to tissue destruction, emphysema, and loss of pulmonary function (Sethi and Murphy 2008; Sethi 2010). Stable COPD patients are frequently colonized with bacterial pathogens (e.g., H. influenzae); recurrent infections of the respiratory tract and chronic bronchitis are common in COPD (Sethi and Murphy 2008; Sethi 2010; Moghaddam et al. 2011; Garmendia et al. 2012). Microbial infections of the lung evoke harmful inflammatory responses that contribute to clinical deterioration of COPD patients (Sethi 2010). Even in healthy individuals, smoking suppresses pulmonary host defense making the lung more susceptible for microbial colonization and infection (Herr et al. 2009; Sethi 2010; Mehta et al. 2008). Ex vivo and in vitro studies showed that the expression of AMPs is inhibited by cigarette smoke and that COPD is associated with skewed AMP expression in the respiratory tract. Analyses of human samples showed that current or former smoking is associated with significantly reduced levels of the β-defensin hBD-2 in pharyngeal washing fluids and sputum from patients with acute pneumonia (Herr et al. 2009). In vitro studies demonstrated that exposure to cigarette smoke and reactive oxygen species inhibit the expression of hBD-2 in respiratory epithelial cells in response to inflammatory mediators or bacteria (Herr et al. 2009). The effects of cigarette smoke on β-defensin expression are likely due to the suppression of cellular signaling cascades by cigarette smoke, such as NF-κB and AP-1 signaling (Pace et al. 2012; Kulkarni et al. 2010; Laan et al. 2004). In currently smoking COPD patients, hBD-2 expression is decreased in central but not in distal airways, inversely correlating with cigarette smoke exposure (Pace et al. 2012). This has been underlined by the finding that hBD-2 mRNA expression in peripheral lung samples has been found to be increased in COPD patients and was associated with higher IL-8 expression and poor lung function (Liao et al. 2012). Cathelicidin has also been shown to be increased in small airways of COPD patients, where it may contribute to airway remodeling (Sun et al. 2014). In addition, a proteomic study showed that the α-defensins are increased in bronchoalveolar lavage fluids of COPD patients (Merkel et al. 2005). In summary, the expression pattern of AMP seems to be deranged in COPD with low antimicrobial activity in the central airways. Altered AMP expression may lead to airway colonization with pathogens in smokers and patient with COPD, providing reservoirs for recurrent infection. Enhanced susceptibility to recurrent respiratory tract infections subsequently leads to chronic inflammation, aberrant expression of AMPs, and disease progression (Herr et al. 2009; Sethi 2010).
Studies also suggest that skewed expression of AMPs and inhibition of the bactericidal activity of AMPs play a role in cystic fibrosis (CF). AMPs are detectable in sputum or bronchoalveolar lavage fluid of CF patients with α-defensins and cathelicidin being present at high levels (Felgentreff et al. 2006; Chen et al. 2004; Soong et al. 1997). Moreover, enhanced levels of cathelicidin correlate with severity of CF lung disease (Chen et al. 2004). β-Defensin levels do not seem to correlate with the severity of inflammation, but decreased levels of hBD-2 are associated with severity of CF (Chen et al. 2004; Dauletbaev et al. 2002; Bals et al. 2001). In vitro, the bactericidal activity of cationic AMPs is salt sensitive. Thus, it is suggested that increased salt concentrations in airway fluids of CF patients contribute to chronic lung infection by inhibiting bactericidal functions of AMPs (Bals et al. 1998a). In line with this hypothesis, bactericidal activity of hBD-1 has been shown to be decreased in airways of CF patients and CF airway fluids fail to kill S. aureus and P. aeruginosa (Goldman et al. 1997; Bals et al. 1999). However, in a human bronchial xenograft model, salt-independent dysfunction of antimicrobial activity was corrected by adenovirus-mediated gene transfer of CFTR (Bals et al. 2001). Thus, the role of altered salt concentrations in airway fluids of CF patients is controversial and yet unknown mechanisms may contribute to the reduced bactericidal activity in the fluids lining the mucosal surfaces of CF patients. Excessive mucus production, for instance, also potentially contributes to impaired bactericidal activities of AMPs in CF patients as mucus interferes with AMPs (Felgentreff et al. 2006).
3.6.3 Lung Cancer
For several tumor types such as ovarian cancer and malignant melanoma, adverse effects of AMPs on tumor development and progression have been shown. In lung cancer, overexpression of different AMPs was found. Cathelicidin is overexpressed in up to 25 % of NSCLC samples (Von Haussen et al. 2008). It is also released by myeloid cells in the tumor microenvironment (Li et al. 2014). Cathelicidin has been shown to transactivate EGFR in airway epithelial cells (Tjabringa et al. 2003). EGFR signaling is one of the critical pathways in lung carcinogenesis (Mitsudomi and Yatabe 2010). Through interaction with the EGFR pathway, cathelicidin promotes tumor growth in vitro and in vivo (Von Haussen et al. 2008), which is further induced by cigarette smoke (Li et al. 2014).
As for β-defensins, hBD-1 and hBD-2 are overexpressed in about 50–60 % of NSCLC tissue samples (Shestakova et al. 2008). In serum of patients with lung cancer, concentrations of hBD-1 and hBD-2 are significantly higher as compared to healthy controls and even patients with pneumonia. Therefore, hBD-1 and hBD-2 have even been proposed as lung tumor markers (Arimura et al. 2004).
AMPs take part in the orchestration of important cellular functions that contribute to oncogenesis and tumor promotion, as they modulate proliferation, cell migration, and angiogenesis. Thus, given their central role in chronic lung disease, AMPs might link chronic airway inflammation to the development of lung cancer.
In contrast to cathelicidin and β-defensins, the α-defensin HNP-1 has been shown to act as an inhibitor of tumor growth. HNP-1 is cytotoxic to tumor cells (Van Wetering et al. 1997; Okrent et al. 1990) and induces apoptosis in lung cancer and other cells (Xu et al. 2008; Lichtenstein 1991). This even led to successful gene therapy approaches with direct intratumoral administration of plasmid DNA encoding HNP-1 in a murine xenograft tumor model (Xu et al. 2008).
3.7 Conclusions
In the human lung, AMPs are abundantly expressed by different cell types, including epithelial and immune cells. The expression of defensins is largely triggered upon stimulation with PAMPs and inflammatory mediators, whereas cathelicidin expression mainly underlies a vitamin D-dependent mechanism. At least in vitro, AMPs are capable of neutralizing a broad array of bacterial, viral, and fungal species, including the most common respiratory pathogens. While their microbicidal impact in vivo is still not completely clear, it is now recognized that the abilities of AMPs within a complex immune network by far exceed antimicrobial activity. AMPs take part in the initiation, amplification, and modulation of lung innate immunity. Remarkably, AMPs seem to be involved in balancing of pro- and anti-inflammatory signaling, thus protecting the host from excessive immunostimulation. Thus, AMPs seem to have an important role in acute lung infection. However, in the context of chronic inflammation and infection-related lung diseases, AMPs can be dysregulated and even act adversely: AMP dysfunction may contribute to the pathogenesis of COPD, asthma, and cystic fibrosis. Furthermore, while AMPs contribute to lung repair after injury, they may also have harmful effects regarding fibrogenesis and lung tumor growth.
References
Aarbiou J, Ertmann M, van Wetering S et al (2002) Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol 72:167–174
Aarbiou J, Verhoosel RM, Van Wetering S et al (2004) Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol 30:193–201
Aerts AM, François IEJA, Cammue BPA, Thevissen K (2008) The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci 65:2069–2079
Agerberth B, Grunewald J, Castaños-Velez E et al (1999) Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med 160:283–290
Akira S (2009) Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B Phys Biol Sci 85:143–156
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Alalwani SM, Sierigk J, Herr C et al (2010) The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol 40:1118–1126
Albanesi C, Fairchild HR, Madonna S et al (2007) IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol 179:984–992
Alekseeva L, Huet D, Féménia F et al (2009) Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 9:33
Arimura Y, Ashitani J, Yanagi S et al (2004) Elevated serum beta-defensins concentrations in patients with lung cancer. Anticancer Res 24:4051–4057
Bals R, Wang X, Wu Z et al (1998a) Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest 102:874–880
Bals R, Wang X, Zasloff M, Wilson JM (1998b) The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 95:9541–9546
Bals R, Weiner DJ, Meegalla RL, Wilson JM (1999) Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest 103:1113–1117
Bals R, Weiner DJ, Meegalla RL et al (2001) Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am J Respir Cell Mol Biol 25:21–25
Beisswenger C, Bals R (2005) Antimicrobial peptides in lung inflammation. Chem Immunol Allergy 86:55–71
Benincasa M, Mattiuzzo M, Herasimenka Y et al (2009) Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J Pept Sci 15:595–600
Bhat S, Song Y-H, Lawyer C, Milner SM (2007) Modulation of the complement system by human beta-defensin 2. J Burns Wounds 5, e10
Biragyn A, Ruffini PA, Leifer CA et al (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025–1029
Boniotto M, Jordan WJ, Eskdale J et al (2006) Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob Agents Chemother 50:1433–1441
Bowdish DME, Davidson DJ, Speert DP, Hancock REW (2004) The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol 172:3758–3765
Bowdish DME, Davidson DJ, Lau YE et al (2005) Impact of LL-37 on anti-infective immunity. J Leukoc Biol 77:451–459. doi:10.1189/jlb.0704380
Braff MH, Jones AL, Skerrett SJ, Rubens CE (2007) Staphylococcus aureus exploits cathelicidin antimicrobial peptides produced during early pneumonia to promote staphylokinase-dependent fibrinolysis. J Infect Dis 195:1365–1372
Byfield FJ, Kowalski M, Cruz K et al (2011) Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J Immunol 187:6402–6409
Campbell EL, Serhan CN, Colgan SP (2011) Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol 187:3475–3481
Carretero M, Escámez MJ, García M et al (2008) In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol 128:223–236
Chen CI-U, Schaller-Bals S, Paul KP et al (2004) Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros 3:45–50
Chen X, Niyonsaba F, Ushio H et al (2005) Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci 40:123–132
Chertov O, Michiel DF, Xu L et al (1996) Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 271:2935–2940
Chong KT, Thangavel RR, Tang X (2008) Enhanced expression of murine beta-defensins (MBD-1, -2,- 3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology 380:136–143
Cowland JB, Johnsen AH, Borregaard N (1995) hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett 368:173–176
Dauletbaev N, Gropp R, Frye M et al (2002) Expression of human beta defensin (HBD-1 and HBD-2) mRNA in nasal epithelia of adult cystic fibrosis patients, healthy individuals, and individuals with acute cold. Respir Int Rev Thorac Dis 69:46–51
Davidson DJ, Currie AJ, Reid GSD et al (2004) The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol 172:1146–1156
Di Nardo A, Vitiello A, Gallo RL (2003) Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol 170:2274–2278
Doss M, White MR, Tecle T, Hartshorn KL (2010) Human defensins and LL-37 in mucosal immunity. J Leukoc Biol 87:79–92
Duits LA, Ravensbergen B, Rademaker M et al (2002) Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology 106:517–525
Duits LA, Nibbering PH, van Strijen E et al (2003) Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol 38:59–64
Felgentreff K, Beisswenger C, Griese M et al (2006) The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 27:3100–3106
Filewod NCJ, Pistolic J, Hancock REW (2009) Low concentrations of LL-37 alter IL-8 production by keratinocytes and bronchial epithelial cells in response to proinflammatory stimuli. FEMS Immunol Med Microbiol 56:233–240
Froy O (2005) Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol 7:1387–1397
Frye M, Bargon J, Dauletbaev N et al (2000) Expression of human alpha-defensin 5 (HD5) mRNA in nasal and bronchial epithelial cells. J Clin Pathol 53:770–773
Funderburg N, Lederman MM, Feng Z et al (2007) Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci U S A 104:18631–18635
Funderburg NT, Jadlowsky JK, Lederman MM et al (2011) The toll-like receptor 1/2 agonists Pam(3) CSK(4) and human β-defensin-3 differentially induce interleukin-10 and nuclear factor-κB signalling patterns in human monocytes. Immunology 134:151–160
Futosi K, Fodor S, Mócsai A (2013) Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17:638–650
Ganz T, Selsted ME, Szklarek D et al (1985) Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 76:1427–1435
García JR, Krause A, Schulz S et al (2001) Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J 15:1819–1821
Garmendia J, Morey P, Bengoechea JA (2012) Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J 39:467–477
Goldman MJ, Anderson GM, Stolzenberg ED et al (1997) Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553–560
Gombart AF, Borregaard N, Koeffler HP (2005) Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077
Han W, Wang W, Mohammed KA, Su Y (2009) Alpha-defensins increase lung fibroblast proliferation and collagen synthesis via the beta-catenin signaling pathway. FEBS J 276:6603–6614
Hancock REW, Scott MG (2000) The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci 97:8856–8861
Hansdottir S, Monick MM, Hinde SL et al (2008) Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181:7090–7099
Harder J, Meyer-Hoffert U, Teran LM et al (2000) Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol 22:714–721
Harder J, Bartels J, Christophers E, Schroder JM (2001) Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem 276:5707–5713
Heilborn JD, Nilsson MF, Kratz G et al (2003) The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 120:379–389
Herr C, Beisswenger C, Hess C et al (2009) Suppression of pulmonary innate host defence in smokers. Thorax 64:144–149
Hertz CJ, Wu Q, Porter EM et al (2003) Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol 171:6820–6826
Hess C, Herr C, Beisswenger C et al (2010) Myeloid RelA regulates pulmonary host defense networks. Eur Respir J 35:343–352
Hiratsuka T, Nakazato M, Date Y et al (1998) Identification of human beta-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem Biophys Res Commun 249:943–947
Hiratsuka T, Mukae H, Iiboshi H et al (2003) Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax 58:425–430
Hou M, Zhang N, Yang J et al (2013) Antimicrobial peptide LL-37 and IDR-1 ameliorate MRSA pneumonia in vivo. Cell Physiol Biochem 32:614–623
Hu Q, Zuo P, Shao B et al (2010) Administration of nonviral gene vector encoding rat beta-defensin-2 ameliorates chronic Pseudomonas aeruginosa lung infection in rats. J Gene Med 12:276–286
Ishimoto H, Mukae H, Date Y et al (2006) Identification of hBD-3 in respiratory tract and serum: the increase in pneumonia. Eur Respir J 27:253–260
Iwase T, Uehara Y, Shinji H et al (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349
Jin T, Bokarewa M, Foster T et al (2004) Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol 172:1169–1176
Kandler K, Shaykhiev R, Kleemann P et al (2006) The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol 18:1729–1736
Kao CY, Chen Y, Zhao YH, Wu R (2003) ORFeome-based search of airway epithelial cell-specific novel human [beta]-defensin genes. Am J Respir Cell Mol Biol 29:71–80
Kao C-Y, Chen Y, Thai P et al (2004) IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 173:3482–3491
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:464
Khine AA, Del Sorbo L, Vaschetto R et al (2006) Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood 107:2936–2942
Kirikae T, Hirata M, Yamasu H et al (1998) Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against murine endotoxemia. Infect Immun 66:1861–1868
Klotman ME, Chang TL (2006) Defensins in innate antiviral immunity. Nat Rev Immunol 6:447–456
Klugman KP, Garau J (2009) A preventable killer: pneumonia. Clin Microbiol Infect 15:989–990
Koczulla R, von Degenfeld G, Kupatt C et al (2003) An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 111:1665–1672
Koeffler HP, Reichel H, Bishop JE, Norman AW (1985) gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun 127:596–603
Kota S, Sabbah A, Chang TH et al (2008) Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J Biol Chem 283:22417–22429
Kovach MA, Standiford TJ (2011) Toll like receptors in diseases of the lung. Int Immunopharmacol 11:1399–1406
Kovach MA, Ballinger MN, Newstead MW et al (2012) Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol 189:304–311
Kulkarni R, Rampersaud R, Aguilar JL et al (2010) Cigarette smoke inhibits airway epithelial cell innate immune responses to bacteria. Infect Immun 78:2146–2152
Kurosaka K, Chen Q, Yarovinsky F et al (2005) Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol 174:6257–6265
Kusagaya H, Fujisawa T, Yamanaka K et al (2014) Toll-like receptor-mediated airway IL-17C enhances epithelial host defense in an autocrine/paracrine manner. Am J Respir Cell Mol Biol 50:30–39
Laan M, Bozinovski S, Anderson GP (2004) Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J Immunol 173:4164–4170
Lai Y, Gallo RL (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30:131–141
Lai Y, Adhikarakunnathu S, Bhardwaj K et al (2011) LL37 and cationic peptides enhance TLR3 signaling by viral double-stranded RNAs. PLoS One 6, e26632
Larrick J, Hirata M, Balint R et al (1995) Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun 63:1291–1297
Li D, Beisswenger C, Herr C et al (2014) Expression of the antimicrobial peptide cathelicidin in myeloid cells is required for lung tumor growth. Oncogene 33:2709–2716
Liao Z, Dong J, Hu X et al (2012) Enhanced expression of human β-defensin 2 in peripheral lungs of patients with chronic obstructive pulmonary disease. Peptides 38:350–356
Lichtenstein A (1991) Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Invest 88:93–100
Liu PT, Stenger S, Li H et al (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773
MacRedmond R, Greene C, Taggart CC et al (2005) Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide. Respir Res 6:116
McCray PB, Bentley L (1997) Human airway epithelia express a beta-defensin. Am J Respir Cell Mol Biol 16:343–349
Mehta H, Nazzal K, Sadikot RT (2008) Cigarette smoking and innate immunity. Inflamm Res 57:497–503
Méndez-Samperio P, Alba L, Trejo A (2007) Mycobacterium bovis-mediated induction of human beta-defensin-2 in epithelial cells is controlled by intracellular calcium and p38MAPK. J Infect 54:469–474
Merkel D, Rist W, Seither P et al (2005) Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 5:2972–2980
Michelson PH, Tigue M, Panos RJ, Sporn PH (1999) Keratinocyte growth factor stimulates bronchial epithelial cell proliferation in vitro and in vivo. Am J Physiol 277:L737–L742
Miles K, Clarke DJ, Lu W et al (2009) Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol 183:2122–2132
Mitsudomi T, Yatabe Y (2010) Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J 277:301–308
Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF (2011) Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int J Chron Obstruct Pulmon Dis 6:113–123
Mookherjee N, Brown KL, Bowdish DME et al (2006) Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol 176:2455–2464
Moser C, Weiner DJ, Lysenko E et al (2002) beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun 70:3068–3072
Mukae H, Iiboshi H, Nakazato M et al (2002) Raised plasma concentrations of alpha-defensins in patients with idiopathic pulmonary fibrosis. Thorax 57:623–628
Murphy CJ, Foster BA, Mannis MJ et al (1993) Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol 155:408–413
Nijnik A, Pistolic J, Filewod NCJ, Hancock REW (2012) Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun 4:377–386
Niyonsaba F (2002) Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol 14:421–426
Niyonsaba F, Someya A, Hirata M et al (2001) Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol 31:1066–1075
Niyonsaba F, Iwabuchi K, Someya A et al (2002) A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20–26
O’Neil DA, Porter EM, Elewaut D et al (1999) Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol 163:6718–6724
Okrent DG, Lichtenstein AK, Ganz T (1990) Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis 141:179–185
Pace E, Ferraro M, Minervini MI et al (2012) Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One 7, e33601
Parker D, Prince A (2011) Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45:189–201
Pingel LC, Kohlgraf KG, Hansen CJ et al (2008) Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol 86:643–649
Pistolic J, Cosseau C, Li Y et al (2009) Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun 1:254–267
Platz J, Beisswenger C, Dalpke A et al (2004) Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol 173:1219–1223
Proud D, Sanders SP, Wiehler S (2004) Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J Immunol 172:4637–4645
Puddicombe SM, Polosa R, Richter A et al (2000) Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 14:1362–1374
Rivas-Santiago B, Schwander SK, Sarabia C et al (2005) Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 73:4505–4511
Rivas-Santiago B, Hernandez-Pando R, Carranza C et al (2008) Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun 76:935–941
Röhrl J, Yang D, Oppenheim JJ, Hehlgans T (2010) Human beta-defensin 2 and 3 and their mouse orthologs induce chemotaxis through interaction with CCR2. J Immunol 184:6688–6694
Ross DJ, Cole AM, Yoshioka D et al (2004) Increased bronchoalveolar lavage human beta-defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation 78:1222–1224
Saiman L, Tabibi S, Starner TD et al (2001) Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. Antimicrob Agents Chemother 45:2838–2844
Sakamoto N, Mukae H, Fujii T et al (2005) Differential effects of alpha- and beta-defensin on cytokine production by cultured human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 288:L508–L513
Schaller-Bals S, Schulze A, Bals R (2002) Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med 165:992–995
Scharf S, Vardarova K, Lang F et al (2010a) Legionella pneumophila induces human beta defensin-3 in pulmonary cells. Respir Res 11:93
Scharf S, Hippenstiel S, Flieger A et al (2010b) Induction of human β-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-κB, and AP-1. Am J Physiol Lung Cell Mol Physiol 298:L687–L695
Scharf S, Zahlten J, Szymanski K et al (2012) Streptococcus pneumoniae induces human β-defensin-2 and -3 in human lung epithelium. Exp Lung Res 38:100–110
Schauber J, Dorschner RA, Coda AB et al (2007a) Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest 117:803–811
Schauber J, Oda Y, Büchau AS et al (2007b) Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824
Schiemann F, Brandt E, Gross R et al (2009) The cathelicidin LL-37 activates human mast cells and is degraded by mast cell tryptase: counter-regulation by CXCL4. J Immunol 183:2223–2231
Scott MG, Gold MR, Hancock RE (1999a) Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect Immun 67:6445–6453
Scott MG, Yan H, Hancock REW (1999b) Biological properties of structurally related alpha -helical cationic antimicrobial peptides. Infect Immun 67:2005–2009
Scott MG, Vreugdenhil AC, Buurman WA et al (2000) Cutting edge: cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol 164:549–553
Scott MG, Davidson DJ, Gold MR et al (2002) The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol 169:3883–3891
Scott A, Weldon S, Buchanan PJ et al (2011) Evaluation of the ability of LL-37 to neutralise LPS in vitro and ex vivo. PLoS One 6, e26525
Seiler F, Hellberg J, Lepper PM et al (2013) FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J Immunol 190:1603–1613
Selsted ME, Ouellette AJ (2005) Mammalian defensins in the antimicrobial immune response. Nat Immunol 6:551–557
Semple F, Webb S, Li H-N et al (2010) Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol 40:1073–1078
Semple F, MacPherson H, Webb S et al (2011) Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur J Immunol 41:3291–3300
Sethi S (2010) Infection as a comorbidity of COPD. Eur Respir J 35:1209–1215
Sethi S, Murphy TF (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 359:2355–2365
Shaykhiev R, Beisswenger C, Kändler K et al (2005) Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol 289:L842–L848
Shaykhiev R, Sierigk J, Herr C et al (2010) The antimicrobial peptide cathelicidin enhances activation of lung epithelial cells by LPS. FASEB J 24:4756–4766
Shestakova T, Zhuravel E, Bolgova L et al (2008) Expression of human beta-defensins-1, 2 and 4 mRNA in human lung tumor tissue: a pilot study. Exp Oncol 30:153–156
Shu Q, Shi Z, Zhao Z et al (2006) Protection against Pseudomonas aeruginosa pneumonia and sepsis-induced lung injury by overexpression of beta-defensin-2 in rats. Shock 26:365–371
Singh PK, Jia HP, Wiles K et al (1998) Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A 95:14961–14966
Soong LB, Ganz T, Ellison A, Caughey GH (1997) Purification and characterization of defensins from cystic fibrosis sputum. Inflamm Res 46:98–102
Soruri A, Grigat J, Forssmann U et al (2007) beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol 37:2474–2486
Steinstraesser L, Koehler T, Jacobsen F et al (2008) Host defense peptides in wound healing. Mol Med 14:528–537
Sun C, Zhu M, Yang Z et al (2014) LL-37 secreted by epithelium promotes fibroblast collagen production: a potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab Invest 94:991–1002
Tjabringa GS, Aarbiou J, Ninaber DK et al (2003) The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 171:6690–6696
Tokumaru S, Sayama K, Shirakata Y et al (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 175:4662–4668
Tripathi S, Tecle T, Verma A et al (2013) The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol 94:40–49
Van Wetering S, Mannesse-Lazeroms SP, Dijkman JH, Hiemstra PS (1997) Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol 62:217–226
Vandamme D, Landuyt B, Luyten W, Schoofs L (2012) A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol 280:22–35
Varsano S, Kaminsky M, Kaiser M, Rashkovsky L (2000) Generation of complement C3 and expression of cell membrane complement inhibitory proteins by human bronchial epithelium cell line. Thorax 55:364–369
Von Haussen J, Koczulla R, Shaykhiev R et al (2008) The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer 59:12–23
Wang X, Zhang Z, Louboutin J-P et al (2003) Airway epithelia regulate expression of human beta-defensin 2 through Toll-like receptor 2. FASEB J 17:1727–1729
Wang T-T, Nestel FP, Bourdeau V et al (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912
Wehkamp J, Harder J, Wehkamp K et al (2004) NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 72:5750–5758
Xiong YQ, Yeaman MR, Bayer AS (1999) In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother 43:1111–1117
Xu N, Wang Y-S, Pan W-B et al (2008) Human alpha-defensin-1 inhibits growth of human lung adenocarcinoma xenograft in nude mice. Mol Cancer Ther 7:1588–1597
Yanagi S, Ashitani J, Ishimoto H et al (2005) Isolation of human beta-defensin-4 in lung tissue and its increase in lower respiratory tract infection. Respir Res 6:130
Yang D, Chertov O, Bykovskaia SN et al (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528
Yang D, Chen Q, Chertov O, Oppenheim JJ (2000a) Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 68:9–14
Yang D, Chen Q, Schmidt AP et al (2000b) LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192:1069–1074
Yang D, Chertov O, Oppenheim JJ (2001) Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J Leukoc Biol 69:691–697
Ye P, Garvey PB, Zhang P et al (2001) Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 25:335–340
Yu J, Mookherjee N, Wee K et al (2007) Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol 179:7684–7691
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Seiler, F., Bals, R., Beisswenger, C. (2016). Function of Antimicrobial Peptides in Lung Innate Immunity. In: Harder, J., Schröder, JM. (eds) Antimicrobial Peptides. Birkhäuser Advances in Infectious Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-24199-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-24199-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24197-5
Online ISBN: 978-3-319-24199-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)