Abstract
The discovery of the SoxB2/Sox21 regulatory element, conserved from basal metazoa to human, opened novel perspectives to study the conservation among distant related genomes. This discovery represents exceptional maintenance of an almost identical enhancer structure controlling a gene that is fundamental for nervous system development. The activity of metazoan SoxB2 enhancers was previously demonstrated in zebrafish embryos by cross-species experiments.
Here we tested the activity of human and amphioxus orthologue cis-regulatory sequences in embryos of the tunicate Ciona intestinalis through a transgenic approach, and found out that SoxB2 enhancers retained their activity in neuronal differentiation even in a non-vertebrate chordate.
This result was unexpected since the conserved SoxB2 enhancer was not found in Ciona in previous studies. Nevertheless, we adopted a different comparative approach and performed a phylogenetic footprinting analysis using two congeneric tunicate species, C. intestinalis and Ciona savignyi, that, in fact, evidenced a conserved SoxB2 3′ element. The discovered element could potentially be the missing orthologous SoxB2 enhancer previously identified in human, zebrafish, and amphioxus.
A detailed search for possible transcription factors revealed the massive presence of Sox, Pou and Fox binding sites as found in other deuterostomes. Nevertheless, whether the conserved SoxB2 element of Ciona possesses a functional ability as gene transcriptional enhancer remains to be demonstrated experimentally.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
One of the most intriguing mechanisms of nervous system (NS) development is related to the neuronal lineage specification, which is of great interest to science and subject of numerous and intensive studies. Nevertheless, we are still far from a complete understanding of these processes. The discovery of an evolutionary conserved non-coding element (CNE) in the animal kingdom by Royo et al. [16] started a discussion about this key aspect of neural state regulation in animal development. It was found to be an example of gene regulatory element conservation in all metazoans, from the cnidarian Nematostella to human. Only the exonic regions of genes were known to have such a degree of conservation among animals so different in body shape and complexity, diverging from a common ancestor around 600 My ago [14].
Royo and colleagues discovered a highly conserved CNE that regulates the SoxB2 gene (SRY-box B), recognizable at the sequence level within metazoans, and explored its functional significance in transphyletic cis-regulatory DNA experiments. The invertebrate SoxB2, and the orthologous gene in vertebrates Sox21, are involved in neuronal development, differentiation and regeneration, indicating that these genes are responsible for the pluripotent features of presumptive neuronal tissues in animal [7, 9, 10, 15, 17–19, 21]. The sequence comparison of SoxB2/Sox21 CNE among human (Homo sapiens), zebrafish (Danio rerio), amphioxus (Branchistoma floridae), acorn worm (Saccoglossus kowalevskii), sea urchin (Strongylocentrotus purpuratus) and cnidarian (Nematostella vectensis) showed an evolutionary conserved region of 200 bp, located at the 3′ of the gene in all analysed loci [16]. Royo and collaborators demonstrated trough transgenic experiments on zebrafish embryos that CNEs from diverse animal genomes were functional regulative elements for different stages of neurogenesis, including patterning and development of the vertebrate forebrain. Similarly, the reporter gene expression driven by human SOX21 CNE and sea urchin SoxB2 CNE was functional in developing the nervous system of Drosophila, despite absence of clear sequence orthology. This was the first study pointing to the fact that the regulatory state recognized by a conserved DNA sequence may have been redeployed at different levels of the developmental regulatory program during evolution of the complex central nervous system (CNS).
A detailed study focused on the regulation of Sox21b (fish ortholog of SoxB2) expression highlighted 19 regulatory DNA elements conserved between vertebrates (human, chicken, mouse, frog, zebrafish and fugu) [13]. Transgenic experiments using conserved fragments from the fugu genome in zebrafish showed that the majority of these CNEs were able to generate tissue-specific expression patterns in the CNS and sensory organs, in agreement with Sox21b expression domains. As expected, one of the enhancers analysed in this study corresponded to the evolutionary conserved element discovered by Royo and colleagues, the CNE17 in the Sox21b locus [13]. Nevertheless, CNE17 and CNE6 were the only enhancer elements able to drive the expression of the reporter gene in the lens, which represents an innovation in vertebrates. A possible explanation for this could be that CNE17, orthologous to the highly conserved metazoan CNE, was co-opted in the fish lineage for the lens expression, as a consequence of the sub-functionalization of the two fish paralogs, Sox21a and Sox21b [10].
An evolutionary puzzling case remained to be solved. As mentioned above, one of SoxB2/Sox21 enhancer was found to be conserved in highly distant related animals and transcriptionally active during CNS development, indicating a key role in nervous system evolution. Nevertheless, in previous studies it was not possible to detect any trace of the SoxB2 enhancer conservation in the lineage of tunicates, the sister group of vertebrates [5] which are considered important model systems for the study of evolution and development in chordates. Tunicates, differently from cephalochordates, are highly diverged from the common chordate ancestor, both morphologically and genetically, and this represents an additional difficulty for evolutionary biologists that take advantage of homologies between body structures and sequence conservation as main principles. Here we tried, therefore, to reveal the potentiality of tunicates in our understanding of deuterostome NS evolution.
The ascidian C. intestinalis represents a very useful animal model to perform in vivo transgenic assays because it possesses most of the molecular pathways and gene repertoire as the rest of chordates. Nevertheless, the Ciona genome shows divergent characteristics that sometimes can represent a limitation to experimental approaches and on the other hand species-specific genomic events, such as gene loss, can be considered an experimental advantage in evolutionary devoted studies.
2 Results
Two main evolutionary questions prompted us to choose C. intestinalis as the model organism for this study, taking into account the advantage of the easy application of transgenesis approaches that are very well established for Ciona.
First, is the ascidian embryonic transcription factors (TF) machinery able to recognize the transcriptional information contained in cross-species enhancers, considering the loss of the evolutionary conserved SoxB2 CNE? Second, could the presence of the conserved SoxB2 CNE be masked at sequence level by the highly divergent genome of ascidians?
To answer these questions, that are interesting per se from an evolutionary point of view, we performed a series of computational and transgenic experiments. To understand whether the regulation of the SoxB2 enhancer is maintained in Ciona, despite the loss of the orthologous region, we carried out transgenic experiments in C. intestinalis, introducing exogenous DNA regulative fragments in developing embryos. More in detail, we used the technique of transgenesis by electroporation of a purified plasmid containing the putative enhancer with a GFP reporter gene into fertilized eggs. In the first series of in vivo experiments we used CNE fragments, amplified by PCR on genomic DNA, corresponding to SoxB2/Sox21 CNEs from different animal models: acorn worm S. kovalewskii (hemichordate), sea urchin S. purpuratus (echinoderm), B. floridae (cephalochordate) and human H. sapiens (vertebrate). These DNA fragments correspond to the enhancers previously used in transgenic experiments on zebrafish by Royo et al. [16].
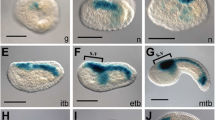
Transgenic experiments using B. floridae and human H. sapiens CNEs gave positive results as shown in Fig. 1. The SoxB2 enhancer from B. floridae was able to drive the expression of the GFP reporter gene in paired tail epidermal neurons in about 70 % of the larvae (red arrows in Fig. 1c, d). This result was confirmed by the human SOXB2 enhancer activity in about 60 % of the larvae (red arrow in Fig. 1f), albeit that we recorded fainter signals. Interestingly, the human DNA construct was also able to drive the GFP expression in another neuronal compartment in the head region (arrowhead in Fig. 1e). In Fig. 1a we present a diagram of the Ciona larval body plan with the central nervous system in grey. Figure 1b is a magnification of the tail region showing in green the paired epidermal neurons, GFP positive with both the human and cephalochordate exogenous DNA constructs.
Transgenic larvae using human and amphioxus cnes driving GFP expression in neural territories. (a) Ciona intestinalis 20 hpf larvae body plan in a schematic representation: a anterior, p posterior, d dorsal, v ventral. The CNS is indicated in grey. (b) Magnification of larval tail. Pairs of caudal epidermal neurons are indicated in green. (c, d) amphioxus SoxB2 CNE drives GFP expression in Ciona ectodermal neurons in the tail (red arrows). (e, f) Human Sox21 CNE resulted active in Ciona ectodermal neurons in the tail (red arrows) and in the head (red arrowhead)
To answer the second evolutionary question we performed phylogenetic footprinting analyses that showed an extremely low degree of conservation between C. intestinalis and other deuterostomal orthologous regions containing the conserved SoxB2 CNE. Nevertheless, to go deeper into this comparative analysis and trying to find the orthologous CNE in tunicates, we compared two congeneric species that diverged 180 My ago [1], with the aim to reveal conserved non-coding regions that were unrecognizable when searched between tunicates and distant related species. We therefore performed a Vista analysis that allowed to discover three main regions highly conserved between C. intestinalis and C. savignyi in the 3′ of SoxB2 (Fig. 2a), that could correspond to the orthologous region previously found conserved in other deuterostomes. A local alignment was performed showing a very high degree of sequence conservation between the two Ciona species, at least 70 % identical in non-coding regions (Fig. 2b). Hence, a detailed in silico analysis was performed using Jaspar software in order to reveal potential transcription factor binding sites (TFBS), and compare them with those predicted in sea urchin, amphioxus and human SoxB2 enhancers [16]. This allowed the identification of a cluster of several Sox and Fox binding sites in the 3′ CNE-1 peak, which was not detected in other 5′ and 3′ CNEs (Figs. 2b and 3a–c).
Phylogenetic footprinting and Ciona’s CNE alignment. (a) Vista analysis between SoxB2 loci of Ciona intestinalis and Ciona savignyi. Dark blue peaks represent conserved non-coding elements between the two species, pink indicates the SoxB2 5′ and 3′ UTRs and blue the exons. (b) Alignment of Ciona’s SoxB2 3′CNE-1. Potential binding sites for Pou, Sox and Fox are highlighted by frames
Diagrams of TF binging sequences conserved in deuterostomes. Ciona’s SoxB2 3′CNE-1 contains Sox and Fox (a, b) and Sox (c) binding sites that are found in orthologous sequences from Ciona savignyi, amphioxus, zebrafish and human. Black background indicates a 100 % match of identity between all species considered
Three short sequence fragments were found to be highly conserved in 3′ CNE-1, which could be the potential binding targets for Sox and Fox (Fig. 3a–c). The level of conservation of the multiple sequence alignment using other chordates (amphioxus, zebrafish and human) was 67 % (Fig. 3a ), 56 % (Fig. 3b) and 63 % (Fig. 3c).
3 Discussion
The regulatory landscape of genes involved in developmental processes is constrained by enhancers that remained conserved during evolution. Therefore, cis-regulatory elements conserved between orthologous genes in vertebrates have been readily recognized in comparative genomic studies as soon as multiple genomes sequencing projects become available. The exceptional case of the discovery of an ancient enhancer retained in metazoans has opened new perspectives in the research field of cis-regulatory elements.
The direct comparison between distantly related animals can be inconclusive when the degree of nucleotide conservation is low, while on the contrary the choice of congeneric species is often fruitless because the high homology becomes uninformative in the search for non-coding active elements. In this perspective, the availability of genomes from numerous metazoan species help greatly in reconstruction of metazoan evolution. We applied a transgenic approach using human SOX21 and amphioxus SoxB2 enhancers exogenously in C. intestinalis embryos and more important we demonstrated that they were functional in pro-neural tissues, as previously demonstrated in a related study on zebrafish and Drosophila embryos. Here we found the putative ancestral enhancer of the SoxB2 gene by comparing two tunicates, which was thought to be lost in such fast evolving genomes. A detailed bioinformatics search in the conserved non-coding regions onCiona’s SoxB2 loci revealed a cluster of four TFBS of the Sox and Fox class in the 3′ CNE-1 (Figs. 2b and 3a–c), who correspond to the SoxB2 CNEs reported by Royo et al. [16].
However recent studies demonstrated that the ancestral regulatory function of SoxB2 CNE is still conserved, despite the lack of sequence similarity among different phyla [6, 11]. Furthermore, similar to our results of Ciona transgenic experiments, the human SOX21 and sea urchin SoxB2 CNEs were demonstrated to be functional in the neuroblasts of the presumptive brain and ventral nerve cord of D. melanogaster embryos [16]. These transgenic approaches highlighted the deep functional conservation of metazoan SoxB2 CNEs in neurogenesis, not only in species possessing high sequence similarity but also in animals showing significantly divergent SoxB2 regulatory elements. Recently a finding was reported of so called FCNEs (Functional Conserved Non-coding Elements) concerning those cis-regulatory elements that, despite a low sequence similarity across distant related species, still keep the ancestral function during developmental processes [20].
The potential transcriptional activity of the SoxB2 CNEs identified in the present study in two Ciona species, despite missing a high degree of sequence similarity with other deuterostomes, remains to be experimentally confirmed in future studies.
4 Materials and Methods
4.1 Animals and Embryos
Adult individuals of C. intestinalis used in this study were collected from the Gulf of Naples (Italy) and kept in tanks at 18 °C until further use. To prevent spontaneous spawning in captivity, ripe animals were exposed to continuous light. Gametes were collected from the gonoducts of several animals and used for in vitro fertilization.
4.2 Comparative Genomics
To obtain DNA sequences for SoxB2 loci, a series of databases was used: ANISEED database (www.aniseed.cnrs.fr/) for C. intestinalis and C. savignyi sequences; SpBase (www.spbase.org) for sea urchin S. purpuratus; JGI (http://genome.jgi-psf.org/Brafl1/Brafl1.home.html) for amphioxus B. floridae, and NCBI (http://www.ncbi.nlm.nih.gov/) for human and acorn worm S. kowalevskii sequences.
4.3 Phylogenetic Footprinting and in Silico Analyses
Genomic sequences from the two congeneric Ciona species, including SoxB2 locus plus 5 kb upstream and 5 kb downstream, were aligned using the AVID software [2]. Sequences were compared using mVISTA ([8]; http://genome.lbl.gov/vista/mvista/submit.shtml ), with the following parameters: 100 bp of fragment length with 70 % of sequence identity.
In order to reveal TFBSs in SoxB2 CNEs, dna sequences were analysed using Jaspar (http://jaspar.genereg.net/), a TF binding profile database [12]. Diagrams of POU, Sox and Fox binding sequences conserved between two Cionas, human and amphioxus were generated using WebLogo software [3].
4.4 Transgenic Experiments
Four CNEs from S. kovalewskii, S. purpuratus, B. floridae and H. sapiens, were amplified by PCR and cloned in the pSP72:CNE:2XGFP:SV40 vector, containing the GFP reporter gene and SV40 polyadenylation sequence. C. intestinalis transgenic embryos were obtained via electroporation experiments, as previously described [4], and observed with confocal microscopy after immunohistochemical detection. Each experiment was performed in triplicate, comparing at least 100 embryos for each single construct. Briefly, eggs were dechorionated, before fertilization to be ready to incorporate the exogenous DNA using a solution containing: 1 % sodium thioglycolate, 0.05 % proteinase E and 1N sodium hydroxide (NaOH), and afterwards washed in filtered sea water (FSW). The 200 μl of dechorionated and fertilized eggs were transferred into Bio-Rad Gene Pulser 0.4 cm cuvettes containing a 0.77 M mannitol solution and 100 μg of the exogenous DNA plasmids, and subsequently electroporated using a Bio-Rad Gene Pulser II™ with the following settings: constant 50 V and 800 μF. Electroporated eggs were transferred into petri dishes with 1 % agarose bottom with FSW and let develop at 18 °C until the desired developmental stage.
4.5 Whole Mount Immunohistochemistry
Embryos were fixed in 4 % formaldehyde during 30 min at room temperature and washed with PBT (PBS 1x, 0.1 % Tween20). Embryos were dehydrated gradually in 70 % ethanol, followed by rehydration in PBS 1x four times. To permeabilize the embryos, they were incubated in PBS containing 0.01 % Triton-100 for 30 min. Embryos were incubated in blocking buffer (PBS 1x, 0.01 % Triton-100, 30 % goat serum) over night. Next, embryos were kept in blocking buffer with 1:300 polyclonal anti-GFP Ab from rabbit (TP401; Torrey Pines Bionabs) and 1:300 monoclonal anti-Acetylated Tubulin (AcTubulin) Ab from mice (T7451; Sigma) for 2 days at 4 °C and subsequently washed with PBT changing the solution every 15 min for 4 h. Then, embryos were incubated with the secondary anti-mouse Alexa 488 Ab or anti-rabbit Alexa 633 Ab in PBT (1:500), over night at 4 °C, then washed in PBT and incubated with DAPI (D9542; Sigma) 1:104 in PBT for 10 min. Laser scanning confocal images were obtained with a Zeiss LSM 510 META confocal microscope.
References
Bernà, L., Alvarez-Valin, F., D’Onofrio, G.: How fast is the sessile Ciona? Comp. Funct. Genomics (2009). 875901
Bray, N., Dubchak, I., Pachter, L.: AVID: a global alignment program. Genome Res. 13, 97–102 (2003)
Crooks, G.E., Hon, G., Chandonia, J.M., Brenner, S.E.: WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004)
D’Aniello, S., D’Aniello, E., Locascio, A., Memoli, A., Corrado, M., Russo, M.T., Aniello, F., Fucci, L., Brown, E.R., Branno, M.: The ascidian homologue of the vertebrate homeobox gene Rx is essential for ocellus development and function. Differentiation 74, 222–234 (2006)
Delsuc, F., Brinkmann, H., Chourrout, D., Philippe, H.: Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006)
Doglio, L., Goode, D.K., Pelleri, M.C., Pauls, S., Frabetti, F., Shimeld, S.M., Vavouri, T., Elgar, G.: Parallel evolution of chordate cis-regulatory code for development. PLoS Genet. 9, e1003904 (2013)
Ferrero, E., Fischer, B., Russell, S.: SoxNeuro orchestrates central nervous system specification and differentiation in Drosophila and is only partially redundant with Dichaete. Genome Biol. 15, R74 (2014)
Frazer, K.A., Pachter, L., Poliakov, A., Rubin, E.M., Dubchak, I.: VISTA: computational tools for comparative genomics. Nucleic Acids Res. 32, W273–W279 (2004)
Kamachi, Y., Kondoh, H.: Sox proteins: regulators of cell fate specification and differentiation. Development 140, 4129–4144 (2013)
Lan, X., Wen, L., Li, K., Liu, X., Luo, B., Chen, F., Xie, D., Kung, H.F.: Comparative analysis of duplicated Sox21 genes in zebrafish. Dev. Growth Differ. 53, 347–356 (2011)
Maeso, I., Irimia, M., Tena, J.J., Casares, F., Gómez-Skarmeta, J.L.: Deep conservation of cis-regulatory elements in metazoans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130020 (2013)
Mathelier, A., Zhao, X., Zhang, A.W., Parcy, F., Worsley-Hunt, R., Arenillas, D.J., Buchman, S., Chen, C.Y., Chou, A., Ienasescu, H., Lim, J., Shyr, C., Tan, G., Zhou, M., Lenhard, B., Sandelin, A., Wasserman, W.W.: JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 42, D142–D147 (2013)
Pauls, S., Smith, S.F., Elgar, G.: Lens development depends on a pair of highly conserved Sox21 regulatory elements. Dev. Biol. 3665, 310–318 (2012)
Putnam, N.H., Srivastava, M., Hellsten, U., Dirks, B., Chapman, J., Salamov, A., Terry, A., Shapiro, H., Lindquist, E., Kapitonov, V.V., Jurka, J., Genikhovich, G., Grigoriev, I.V., Lucas, S.M., Steele, R.E., Finnerty, J.R., Technau, U., Martindale, M.Q., Rokhsar, D.S.: Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007)
Reiprich, S., Wegner, M.: From CNS stem cells to neurons and glia: Sox for everyone. Cell Tissue Res. 359, 111–124 (2014)
Royo, J.L., Maeso, I., Irimia, M., Gao, F., Peter, I.S., Lopes, C.S., D’Aniello, S., Casares, F., Davidson, E.H., Garcia-Fernández, J., Gómez-Skarmeta, J.L.: Transphyletic conservation of developmental regulatory state in animal evolution. Proc. Natl. Acad. Sci. U. S. A. 108, 14186–14191 (2011)
Sandberg, M., Kallstrom, M., Muhr, J.: Sox21 promotes the progression of vertebrate neurogenesis. Nat. Neurosci. 8, 955–1001 (2005)
Taguchi, S., Tagawa, K., Humphreys, T., Satoh, N.: Group B Sox genes that contribute to specification of the vertebrate brain are expressed in the apical organ and ciliary bands of hemichordate larvae. Zoolog. Sci. 19, 57–66 (2002)
Uchikawa, M., Yoshida, M., Iwafuchi-Doi, M., Matsuda, K., Ishida, Y., Takemoto, T., Kondoh, H.: B1 and B2 Sox gene expression during neural plate development in chicken and mouse embryos: universal versus species-dependent features. Dev. Growth. Differ. 53, 761–771 (2011)
Vassalli, Q.A., Anishchenko, E., Caputi, L., Sordino, P., D’Aniello, S., Locascio, A.: Regulatory elements retained during chordate evolution: coming across tunicates. Genesis 53, 66–81 (2015)
Whittington, N., Cunningham, D., Le, T.K., De Maria, D., Silva, E.M.: Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Dev. Biol. 397, 237–247 (2015)
Acknowledgments
The authors are grateful to Maria Ina Arnone, Margherita Branno, Annamaria Locascio, Filomena Ristoratore and Antonietta Spagnuolo for their suggestions about in vivo experiments and for sharing with us DNA plasmids for transgenesis. We thank Mara Francone for technical assistance with DNA maxi-preparation, the Marine Resources for Research Unit of Stazione Zoologica Anton Dohrn for animal fishing and maintenance. Heartfelt gratitude to Rita Marino for her excellent suggestions concerning immunohistochemical experiments. Evgeniya Anishchenko has been supported by a SZN PhD fellowship (2011–2014). This work was supported by a Marie Curie Career Integration Grant (FP7-PEOPLE-2011-CIG, PCIG09-GA-2011-293871) to Salvatore D’Aniello.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Anishchenko, E., D’Aniello, S. (2015). Tunicate Neurogenesis: The Case of the SoxB2 Missing CNE. In: Zazzu, V., Ferraro, M., Guarracino, M. (eds) Mathematical Models in Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-23497-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-23497-7_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23496-0
Online ISBN: 978-3-319-23497-7
eBook Packages: Mathematics and StatisticsMathematics and Statistics (R0)