Abstract
The contents of the retroperitoneum are defined by the boundaries of the potential space behind the posterior abdominal parietal peritoneum and the fascia investing the lumbar musculature. Primary retroperitoneal neoplasms are a rare group of tumors which do not arise from a specific organ but rather originate from tissues or rests of embryonic cells which exist in the retroperitoneum (Rodríguez et al., Arch Esp Urol, 63[1], 13–22, 2010). The most common variety is sarcoma, which accounts for up to 90 % of lesions after lymphoma is excluded. Liposarcomas and leiomyosarcomas are the next most common types, accounting for up to 15 % of tumors. The average age of presentation is during the fifth to seventh decades, and the tumors are often large in size at diagnosis due to the paucity of symptoms associated with growth of retroperitoneal tumors in general. Excluding lymphomas, the most frequent primary retroperitoneal malignancies in decreasing order include liposarcoma, MFH, leiomyosarcoma, rhabdomyosarcoma, and malignant nerve sheath tumors (Nishino et al., Radiographics, 23[1], 45–57, 2003). Both Hodgkin’s and non-Hodgkin’s lymphoma may also occur in the retroperitoneum. Epithelial tumors may rise from the kidney, adrenal gland, and pancreas, and metastatic disease from germ cell tumors, primary carcinomas, or melanomas can also occur. Benign tumors may have neurogenic origins as well (schwannomas, neurofibroma, paraganglioma).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Retroperitoneal biopsy
- Imaging the retroperitoneum
- Biopsy of the retroperitoneum
- Liposarcomas
- Leiomyosarcomas
- Primary retroperitoneal neoplasms
- Sarcoma
- Rhabdomyosarcoma
- Malignant nerve sheath tumor
- Hodgkin’s and non-Hodgkin’s lymphoma of the retroperitoneum
The contents of the retroperitoneum are defined by the boundaries of the potential space behind the posterior abdominal parietal peritoneum and the fascia investing the lumbar musculature. Primary retroperitoneal neoplasms are a rare group of tumors which do not arise from a specific organ but rather originate from tissues or rests of embryonic cells which exist in the retroperitoneum [1]. The most common variety is sarcoma, which accounts for up to 90 % of lesions after lymphoma is excluded. Liposarcomas and leiomyosarcomas are the next most common types, accounting for up to 15 % of tumors. The average age of presentation is during the fifth to seventh decades, and the tumors are often large in size at diagnosis due to the paucity of symptoms associated with growth of retroperitoneal tumors in general. Excluding lymphomas, the most frequent primary retroperitoneal malignancies in decreasing order include liposarcoma, MFH, leiomyosarcoma, rhabdomyosarcoma, and malignant nerve sheath tumors [2]. Both Hodgkin’s and non-Hodgkin’s lymphoma may also occur in the retroperitoneum. Epithelial tumors may rise from the kidney, adrenal gland, and pancreas, and metastatic disease from germ cell tumors, primary carcinomas, or melanomas can also occur. Benign tumors may have neurogenic origins as well (schwannomas, neurofibroma, paraganglioma) [3].
Retroperitoneal tumors are diagnosed at physical examination if they are particularly large, or commonly by imaging when the patient presents with insidious onset of non-localizing symptoms such as lower extremity or genital edema, weight loss, anorexia, urological symptoms, or back pain.
Imaging of Retroperitoneal Tumors
Precise localization and compartmentalization of large masses can be difficult as the size the mass obscures its focus of origin. Displacement of anatomic structures may help to localize the origin. Cross-sectional imaging including MRI and CT provides a complete overview of the peritoneal cavity and retroperitoneal spaces. MRI imaging can exquisitely “characterize” retroperitoneal masses [4], although no specific tumor histology features unique or diagnostic imaging characteristics by virtue of shared tissue components. At the same time, these common tumor tissue components may provide important clues to the origin of large retroperitoneal tumors, including fat signal associated with lipoma, liposarcoma, and teratoma, and myxoid stromal seeding in neurogenic tumors as well as myxoid liposarcomas and malignant fibrous histiocytoma.
A concise review of the imaging of retroperitoneal masses is provided by Rajiah et al. [5]. Additional radiographic signs which may aid in the identification of mass origin include beak sign, embedded organ sign, and embedded organ sign [2]. Nishino et al. [2] also summarize patterns of tumor spread around and between normal structures which may provide clues to retroperitoneal tumor origins, e.g., tumor extension along and around normal structures as a characteristic of tumors of sympathetic ganglia origin [6]. Viable tumor often shows some degree of enhancement after the administration of intravenous contrast material, while necrotic material shows reduced density, hyperintensity on T2W imaging, and absence of contrast enhancement. Fat-containing tumors show high signal intensity on T1-weighted MR imaging, as well as corresponding loss of signal on fat-suppressed image sequences. Myxoid stroma characteristically appears hyperintense on T2W MR imaging and may show delayed enhancement after gadolinium injection [6]. Teratomas may feature fluid attenuation, fat-fluid levels, and calcifications [7]. Neurogenic lesions such as schwannomas typically appear hypointense on T1W and T2W noncontrast imaging and exhibit heterogeneous patterns of contrast enhancement [5]. Malignant fibrous histiocytoma displays heterogeneous signal characteristics on all MR pulse sequences [4].
Benign retroperitoneal masses include lymphangioma, lipoma, myelolipoma, angiomyolipoma, lipoblastoma, hibernoma, nerve sheath tumors, and paraganglioma. Lymphangiomas have a unilocular or multilocular cystic appearance and are diagnosed in infancy, while lipoblastomas typically present in childhood or teenage years. Hibernoma is a rare tumor composed of fetal or brown fat which is most frequently diagnosed in the fourth decade of life. Lipomas rarely occur in the retroperitoneum. These tumors typically grow rather slowly and present as large retroperitoneal masses whose radiographic appearance is characterized by their fat content. These tumors must be distinguished from pelvic lipomatosis as well as liposarcoma; even pathological diagnosis of lipoma should be suspected as under sampled liposarcoma [8].
Myelolipoma is also characterized by an abundance of adipocytes but more commonly arises from the adrenal glands. Extra-adrenal examples are exceedingly uncommon and may be misinterpreted at biopsy [9]. Extrarenal angiomyolipoma is an extremely rare tumor which typically presents as incidental findings during investigations for other purposes, or with abdominal pain and hemorrhagic shock [10].
Paragangliomas, the extra-adrenal equivalent of pheochromocytomas which arise from residual adrenal medullary chromaffin cells, are most commonly found in proximity to the aorta and sympathetic ganglia. Malignant paragangliomas may be difficult to distinguish from benign lesions, with malignancy established by the recognition of local invasion or metastases.
Peripheral nerve sheath tumors comprise another group of benign retroperitoneal neoplasms. The most common peripheral nerve tumor is the schwannoma, which is typically discovered as a large, well-circumscribed mass featuring cystic degeneration.
Lymphoma is the most common form of retroperitoneal malignancy. Non-Hodgkin’s lymphoma tends to involve a larger variety of lymph node groups than Hodgkin’s lymphoma. Malignant lymph nodes may show moderate homogeneous to patchy inhomogeneous enhancement postgadolinium administration. In contrast to metastatic lymphadenopathy, primary lymphoma usually does not demonstrate nodal necrosis. [Retroperitoneal fibrosis after lymphoma therapy may be difficult to distinguish from but fibrosis most likely has low T2W signal]. Retroperitoneal lymphadenopathy is typically seen in other nonneoplastic conditions such as mycobacterium avium-intracellulare infection (MAI) or Castleman’s disease (giant lymph node hyperplasia).
Liposarcoma is the most common malignant primary retroperitoneal neoplasm. Although difficult to distinguish from benign lipoma, the presence of proportionally larger nonadipose components and greater enhancement of septations may be more suggestive of malignancy.
The retroperitoneum is the second most common site for malignant fibrous histiocytoma, the most common type of adult soft tissue sarcoma [11]. This tumor is frequently associated with invasion of adjacent organs, together which large size conveys a poorer prognosis. Calcification has been identified in 7–20 % of lesions [5]. Solid lesions often demonstrate a peripheral nodular enhancement pattern or “pseudocapsule” on precontrast MRI imaging [4].
Germ cell tumors uncommonly originate in the retroperitoneum and are more commonly observed in men [5]. Rajiah et al. [5] further observe that extragonadal germ cell tumors are often observed in the midline between the T6 and S2 vertebrae and that a mass in this location is more suggestive of a primary extragonadal germ cell tumor than metastasis.
Biopsy Indications and Image-Guided Approach
Diagnosis of retroperitoneal tumors often requires tissue sampling; Strauss et al. [3] propose that for patients with (1) retroperitoneal tumors for which diagnosis is uncertain from the radiological appearance, (2) histologies for which neoadjuvant therapy may be appropriate as induction therapy, and preoperative biopsy is “mandatory.” Surgical tissue sampling is invasive and associated with fixed morbidity [12]. In the past, fine needle aspirations were recommended primarily to reduce the risk of hemorrhage or injury to adjacent organs, but the safety and efficacy of cutting needles has been firmly established [13]. Image-guided percutaneous biopsy has been shown to provide satisfactory yield with reduced morbidity and mortality [14]. Tissue sampling is also important to characterize retroperitoneal lesions for therapy planning.
Biopsy needle selection has been based primarily upon efficacy of tissue sampling for lymphoma. Knelson et al. [15] reviewed CT-guided needle biopsy for retroperitoneal lesions, and both the diagnosis and the histological subtyping of lymphoma could be determined in 10 of 11 cases using the 14-gauge Tru-Cut needle, but it was not possible to make the specific diagnosis in any of the lymphoma patients using the 20-gauge Chiba needle. Agid et al. [16] reported that CT-guided core needle biopsies were sufficient to establish a diagnosis in 83 % of the patients with lymphoproliferative disorders and they suggested that it should be used as the first step in the diagnosis of lymphomas. Stattaus et al. [17] reported that the correct lymphoma subtype could be revealed for retroperitoneal masses in 87 % of the patients by using a 16- or 18-gauge core biopsy system with the coaxial technique under CT guidance. In the study of Tomozawa et al. [18], 43 (96 %) of 45 patients had a defined diagnosis with the correct histological subtype determined with an 18-gauge core needle, and subsequent treatment was performed on the basis of the biopsy results. Although needle size will be best determined by suspected histology as well as local expertise, most core needle biopsies performed with 18-guage needles will provide diagnostic material.
Imaging guidance is often provided by static CT imaging, CT fluoroscopy, cone beam CT, or ultrasound, although the specific guidance modality is often dictated by operator preference. The simplest and preferred biopsy path trajectory is often a straight line to the tumor target from the skin entry site in a single axial plane. Off-plane approaches are possible and often aided by combinations of imaging systems and biopsy planning software packages. UItrasound, CT fluoroscopy, and cone beam CT packages which link CT coordinates to the active fluoroscopic image can provide real-time visualization of the biopsy needle in the trajectory to the target lesion. These advantages may shorten procedure time, reduce nontarget punctures, and reduce radiation exposure [19].
Ultrasound guidance enjoys the advantage of avoiding patient exposure to ionizing radiation. Ultrasound images of the retroperitoneum are generated based upon the differential ability of tissue to reflect or transmit sound of frequency between 3.5 and 7 MHz in the clinical realm. Ultrasound permits the operator to monitor needle placement in real-time fashion without ionizing radiation as metallic needles are sonoreflective. In addition, sonography with color Doppler technology can identify significant intra-tumoral vascularity to be avoided by needle puncture. Deep abdominal and pelvic targets are best imaged with a curved array 3.5–5 MHz probe, while more superficial targets can be imaged with improved resolution with 5 MHz and greater linear array probes. Needle guidance can be accomplished “freehand” or with the use of specifically designed needle guides which attach to the transducer. Ultrasound delineation of retroperitoneal masses may be obscured by overlying bowel gas, necessitating placing the patient in a lateral decubitus or prone position for visualization. Yarram et al. observed an ultrasound-guided pelvic mass biopsy success rate of 95.4 % compared with 84.6 % for CT guidance [20].
A pre-procedure CT or MRI scan can provide a basis for biopsy path and target planning. In general, the planned needle biopsy path should exclude the viscera, the pleura, and the visible blood vessels. Other structures to be avoided which are inconstantly imaged or not visualized include the ureters and sciatic and genitofemoral nerves. In some instances, target selection must be refined toward viable regions of tumors as opposed to more necrotic regions. Tumor viability is indirectly evidenced by increased soft tissue density comparable to the muscle as well as observed enhancement following the administration of vascular contrast material (CT or MRI). CT showing low-density tissue in the central portion of the tumor may be related to liquifactive or hemorrhagic necrosis and should be avoided during tissue sampling. Leiomyosarcomas in particular may demonstrate significant zones of necrosis [21]. Hypervascularity may be recognized according to the presence of adjacent discrete blood vessels, increased density following vascular contrast administration (CT), or by noting increased blood flow within portions of the lesion according to color Doppler sonography. Characteristic tissue components not only aid in radiographic diagnosis but also may inform biopsy planning; liposarcomas will have varying amounts of fat, with high-grade liposarcomas having the least amount. Tissue sampling of suspected liposarcoma from areas of increased tissue density may increase diagnostic yield. Myxoid tissue has well-recognized specific MR imaging characteristics [6]. Neurogenic tumors more typically contain myxoid tissue, although liposarcomas and myxoid malignant fibrous histiocytoma may also exhibit myxoid tissue [5].
MRI has been employed both as a diagnostic imaging and an imaging guidance modality. Previous reports have predominantly documented the value of MRI guidance with biopsies of hepatic dome masses, masses visible only at MRI, or in instances where ultrasound is not feasible [22]. Kariniemi et al. (2005) [23] demonstrated high sensitivity and specificity ranging from 71 to 100 % of both aspiration and core biopsies guided by low-field MRI in 31 consecutive patients with liver, lymph node, retroperitoneal, adrenal, and splenic masses for whom ultrasound-guided procedures were not feasible. However, optical tracking was used in this study to determine the skin entrance site as well as the puncture route. MRI-guided procedures require the use of MRI-compatible (nonferromagnetic) needles in order to avoid magnetic susceptibility artifacts.
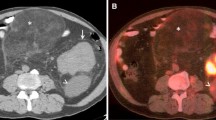
Percutaneous image-guided biopsy for diagnosis and subtyping may be occasionally be limited by the presence of fibrosis, necrosis, and limited ability to specifically target viable tumor cells, but PET imaging have the capability to further refine biopsy target selection based upon tissue viability and exclusion of necrotic tumor. Kitajima et al. (2013) [24] summarize the 18F-FDG-PET/CT findings of patients with retroperitoneal tumors. Absence of FDG uptake may be seen with necrosis, and corresponding areas should be avoided for tissue sampling. With large and/or previously treated lesions, PET scans can identify portions of tumor which are viable and suitable for sampling. Specifically registered PET images can be used to target lesions inconspicuous at nonenhanced CT or malignant portions of lesions which also contain benign tissue such as fat [25]. Care must be exercised, as activity in a suspected lesion shows SUV close to the cutoff value, or alternatively when a lesion shows FDG uptake due to posttreatment inflammation [26].
Additional Imaging Considerations
Image Registration for Biopsy Guidance
When conventional CT is used for biopsy guidance, images are most frequently obtained without administration of intravenous contrast. In some instances, viable or otherwise suitable tumor tissue for biopsy may not be apparent on the nonenhanced CT images. In these instances, nonenhanced images can be manually or automatically registered with imaging obtained with an alternative modality, permitting image-guided biopsy based upon the intra-procedural nonenhanced CT images. Contrast-enhanced CT, MRI, as well as PET-CT can be registered in this fashion and the combined images displayed in such a manner which guides biopsy needle placement.
Lesions which are out of the convenient transaxial plane with reference to the skin entrance site may also be approached for biopsy using image registration with magnetically tracked instruments [27]. This approach relies on the successful and accurate registration of the imaging (often CT) Cartesian coordinate system with a simultaneous magnetic reference field. With this approach, the magnetically tracked biopsy needle position can be displayed using the registered CT dataset. Real-time needle position information is available to the operator without CT or fluoroscopic imaging until final needle position is verified.
Alternatively, an ultrasound probe can be electromagnetically tracked after registration of the magnetic field positional coordinates with a coincidentally or previously obtained CT dataset. In this fashion, although the needle may be visualized with ultrasound, the needle position can be displayed in multiplanar format determined by the tracked ultrasound probe. Under optimal conditions, the severity of the registration error between imaging and magnetic coordinates should be less than 3 mm.
Developments in flat panel detector technology have permitted implementation of cone beam CT mounted on imaging suite fluoroscopic C-arm to deliver first computed rotational 3D digital subtraction angiography and subsequently cone beam computed tomographic imaging (CBCT). Orth et al. (2008) [28] review the technical innovations featured in current clinical CBCT systems. CBCT systems allow automatic registration of three-dimensional CT data with live fluoroscopic imaging, while a software package displays a biopsy trajectory planned with the 3D dataset to be superimposed upon the live fluoroscopic images. Furthermore, pre-procedure contrast-enhanced conventional CT or MRI as well as PET-CT images can be registered with intra-procedural cone beam CT to further refine a biopsy target according to enhancement pattern, FDG-PET avidity, or MRI appearance observed prior to the planned procedure. Cone beam CT systems potentially reduce the amount of contrast material as well as radiation required to complete image-guided biopsies.
Cytogenetics
Molecular and genetic profiling of tumors is likely to play a significantly increased role in the optimization of treatment of tumors in individual patients. Identification of specific mutations and improvements in tissue arrays will contribute to improve more specific diagnoses as well as development of specifically targeted therapeutic agents. Conyers et al. summarized the cytogenetics of liposarcomas [29]. In the past, the pathological differentiation of a benign lipoma from a well-differentiated liposarcoma has been difficult. The oncogene MDM2 is seen in 100 % of well-differentiated and dedifferentiated liposarcomas, for example, and amplification of CDK4 has been described in 90 % of liposarcomas as well. In addition to differentiating lesions based upon biopsy histopathology, these markers may have prognostic value and may predict the risk of transformation.
The role of percutaneous biopsy for the procurement of samples for molecular or genetic profiling in addition to diagnostic histopathology has not been defined. The ability to perform molecular or genetic profiling on small caliber biopsy samples is evolving as advances in tissue array technology and next-generation sequencing are introduced into clinical practice. The efficacy of percutaneous needle biopsy for the molecular profiling of non-small cell lung cancer, for example, has been demonstrated [30]. It seems likely that specimens adequate for molecular profiling could be efficiently and safely obtained percutaneously from retroperitoneal tumors with imaging guidance.
Tips and Tricks
Patient Preparation
Normal status of the retroperitoneal biopsy patient candidate’s coagulation parameters should be confirmed prior to biopsy attempt. The Society of Vascular and Interventional Radiology Standards of Practice Committee has designated retroperitoneal biopsies (excluding renal biopsies) as Category 2 procedures with moderate risk of bleeding [31]. Correction of INR to <1.5 and platelet count >50,000/μL is recommended according to these practice standards.
Although percutaneous core needle biopsy of the retroperitoneum is not often associated with significant pain, individual patients may require conscious sedation for relief of anxiety related to anticipation of pain. Patients receiving conscious sedation should observe an overnight fast prior the procedure. Coagulopathy and severe thrombocytopenia are relative contraindications for percutaneous biopsy, and coagulation parameters should be confirmed and corrected prior to planned biopsy.
Coaxial approach: Use of a coaxial technique allows for diagnostic aspirations to confirm cellularity and cell viability prior to obtaining core biopsies. A coaxial approach for suspected lymphoma also facilitates sampling for flow cytometry studies and core specimens using a single puncture. De Bazelaire et al. [32] described that a coaxial introducer provided with an additional blunt tip stylet allows safe access to difficult-to-reach lymph nodes in the chest, abdomen, and pelvis under CT control.
Biopsy path planning: Biopsies of lesions adjacent to the aorta and vena cava are most often approached posteriorly with the patient prone or in lateral decubitus position in order to avoid bowel loops, large vessels, and viscera such as the pancreas. Large masses can often be approached using an anterolateral trajectory in the supine position. A safe trajectory can often be facilitated by a change in the patient’s position; a bowel loop can be displaced medially or laterally out of a planned needle trajectory by rotating the patient into a lateral decubitus position, for example.
Additional measures to successfully biopsy lesions with limited access include hydrodissection, blunt needle technique, and alternative transgluteal and transosseous approaches in addition to the anterior extraperitoneal approach through the iliopsoas muscle. These approaches are systematically reviewed by Gupta et al. [33]. Hydrodissection with sterile physiological saline may displace an interposed bowel loop, where displacement is by as little as 1 cm which might otherwise facilitate a planned approach. Alternatively, small mesenteric blood vessels can be displaced and biopsy accomplished using a coaxial approach with a blunt tip needle such as the Hawkins ™ blunt needle access system. Alternatively, infrarenal or perivascular lesions which may appear obscured for biopsy purposes in some instances can be accessed using a transvenous approach as described by Maleux et al. [34].
Lesions or lymph nodes in the internal or external iliac chain may be obscured by overlying bowel loops and adjacent vessels. In these cases, a transmuscular biopsy trajectory through the iliopsoas muscle may avoid bowel injury or injury to the external iliac vessels. The transgluteal approach is particularly advantageous for presacral or pararectal masses, although care must be taken to avoid injury to the sciatic nerve by planning the needle trajectory adjacent to the sacrum rather than the posteromedial margin of the iliac bone. In rare instances, access to a biopsy target lesion can be obtained using a transosseous approach through the iliac bone [33].
References
Rodríguez JAV, José M, Moreno D, Navarro HP, Carrión P. Primary retroperitoneal tumors: review of our 10-year case series. Arch Esp Urol. 2010;63(1):13–22.
Nishino M, Hayakawa K, Minami M, Yamamoto A, Ueda H, Takasu K. Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic clues. Radiographics. 2003;23(1):45–57. doi:10.1148/rg.231025037.
Strauss DC, Hayes AJ, Thomas JM. Retroperitoneal tumours: review of management. Ann R Coll Surg Engl. 2011;93:275–80. doi:10.1308/003588411X571944.
Elsayes KM, Staveteig PT, Narra VR, Chen Z-M, Moustafa YL, Brown J. Retroperitoneal masses: magnetic resonance imaging findings with pathologic correlation. Curr Probl Diagn Radiol. 2007;36(June):97–106. doi:10.1067/j.cpradiol.2006.12.003.
Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31:949–76. doi:10.1148/rg.314095132.
Nishimura H, Zhang Y, Ohkuma K, Uchida M, Hayabuchi N, Sun S. MR imaging of soft-tissue masses of the extraperitoneal spaces. Radiographics. 2001;21:1141–54. doi:10.1148/radiographics.21.5.g01se141141.
Lee J, Hiken J, Semelka S. Retroperitoneum. In Lee J, Sagel S, Stanley R, Heiken J editors, Computed tomography with MRI correlation. 3rd ed. 1996. p. 1023–1086.
Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH. Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics. 2009;29(1):261–90. doi:10.1148/rg.291085203.
Butori N, Guy F, Collin F, Benet C, Causeret S, Isambert N. Retroperitoneal extra-adrenal myelolipoma: appearance in CT and MRI. Diagn Interv Imaging. 2012;93(3):204–7. doi:10.1016/j.diii.2011.12.010.
Tsutsumi M, Yamauchi A, Tsukamoto S, Ishikawa S. A case of angiomyolipoma presenting as a huge retroperitoneal mass. Int J Urol : Off J Jpn Urol Assoc. 2001;8(8): 470–1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11555018.
Ahlén J, Enberg U, Larsson C, Larsson O, Frisk T, Brosjö O, Rosen A, Bäckdahl M. Malignant fibrous histiocytoma, aggressive fibromatosis and benign fibrous tumors express mRNA for the metalloproteinase inducer EMMPRIN and the metalloproteinases MMP-2 and MT1-MMP. Sarcoma. 2001;5(3):143–9. doi:10.1080/13577140120048601.
Hopper KD. Percutaneous, radiographically guided biopsy: a history. Radiology. 1995;196(2):329–33. doi:10.1148/radiology.196.2.7617841.
Nolsøe C, Nielsen L, Torp-Pedersen S, Holm HH. Major complications and deaths due to interventional ultrasonography: a review of 8000 cases. J Clin Ultrasound : JCU; 1990;18(3):179–84. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2155937.
Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology. 1993;186(2):515–22. doi:10.1148/radiology.186.2.8421758.
Knelson M, Haaga J, Lazarus H, Ghosh C, Abdul-Karim F, Sorenson K. (1989). Computed tomography-guided retroperitoneal biopsies. J Clin Oncol : Off J Am Soc Clin Oncol. 7(8):1169–73. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2754451.
Agid R, Sklair-Levy M, Bloom AI, Lieberman S, Polliack A, Ben-Yehuda D, Sherman Y, Libson E (2003). CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol. 58(2):143–7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12623044.
Stattaus J, Kalkmann J, Kuehl H, Metz K, Metz KA, Nowrousian MR, Forsting M, Ladd SC. Diagnostic yield of computed tomography-guided coaxial core biopsy of undetermined masses in the free retroperitoneal space: single-center experience. Cardiovasc Interv Radiol. 2008;31:919–25. doi:10.1007/s00270-008-9317-5.
Tomozawa Y, Inaba Y, Yamaura H, Sato Y, Kato M, Kanamoto T, Sakane M. Clinical value of CT-guided needle biopsy for retroperitoneal lesions. Korean J Radiol. 2011;12(3):351–7. doi:10.3348/kjr.2011.12.3.351.
Braak SJ, van Strijen MJL, van Es HW, Nievelstein RAJ, van Heesewijk JPM. Effective dose during needle interventions: cone-beam CT guidance compared with conventional CT guidance. J Vasc Interv Radiol: JVIR. 2011;22(4):455–61. doi:10.1016/j.jvir.2011.02.011.
Yarram SG, Nghiem HV, Higgins E, Fox G, Nan B, Francis IR. Evaluation of imaging-guided core biopsy of pelvic masses. AJR Am J Roentgenol. 2007;188(5):1208–11. doi:10.2214/AJR.05.1393.
Shah J, Kirshenbaum M, Shah K. CT characteristics of primary retroperitoneal tumors and the importance of differentiation from secondary retroperitoneal tumors. Curr Probl Diagn Radiol. 2008;31(17):1–5.
Lu DS, Silverman SG, Raman SS. MR-guided therapy. Applications in the abdomen. Magn Reson Imaging Clin North Am. 1999;7(2):337–48. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10382165.
Kariniemi J, Blanco Sequeiros R, Ojala R, Tervonen O. MRI-guided abdominal biopsy in a 0.23-T open-configuration MRI system. Eur Radiol. 2005;15:1256–62. doi:10.1007/s00330-004-2566-z.
Kitajima K, Kono A, Konishi J, Suenaga Y, Takahashi S, Sugimura K. 18F-FDG-PET/CT findings of retroperitoneal tumors: a pictorial essay. Jpn J Radiol. 2013;31:301–9. doi:10.1007/s11604-013-0192-x.
Tatli S, Gerbaudo VH, Mamede M, Tuncali K, Shyn PB, Silverman SG. Abdominal masses sampled at PET/CT-guided percutaneous biopsy: initial experience with registration of prior PET/CT images. Radiology. 2010;256(1):305–11. doi:10.1148/radiol.10090931.
Kobayashi K, Bhargava P, Raja S, Nasseri F, Al-Balas HA, Smith DD, George SP, Vij MS. Image-guided biopsy: what the interventional radiologist needs to know about PET/CT. Radiographics. 2012;32:1483–501. doi:10.1148/rg.325115159.
Wallace MJ, Gupta S, Hicks ME. Out-of-plane computed-tomography-guided biopsy using a magnetic-field-based navigation system. Cardiovasc interv Radiol. 2006;29(November 2005):108–13. doi:10.1007/s00270-005-0041-0.
Orth RC, Wallace MJ, Kuo MD. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol: JVIR. 2008;19(4):814–20. doi:10.1016/j.jvir.2008.02.002.
Conyers R, Young S, Thomas DM. Liposarcoma: molecular genetics and therapeutics. Sarcoma. 2011;2011:483154. doi:10.1155/2011/483154.
Albanna AS, Kasymjanova G, Robitaille C, Cohen V, Brandao G, Pepe C, Small D, Agulnik J. Comparison of the yield of different diagnostic procedures for cellular differentiation and genetic profiling of non-small-cell lung cancer. J Thorac Oncol: Off Publ Int Assoc Study of Lung Cancer. 2014;9(8):1120–5. doi:10.1097/JTO.0000000000000230.
Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, Saad WA. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–36. doi:10.1016/j.jvir.2012.02.012.
De Bazelaire C, Farges C, Mathieu O, Zagdanski AM, Bourrier P, Frija J, De Kerviler E. Blunt-tip coaxial introducer: a revisited tool for difficult CT-guided biopsy in the chest and abdomen. Am J Roentgenol. 2009;193(August):144–8. doi:10.2214/AJR.08.2125.
Gupta S, Nguyen HL, Morello Jr FA, Ahrar K, Wallace MJ, Madoff DC, Murthy R, Hicks ME. Various approaches for CT-guided percutaneous biopsy of deep pelvic lesions: anatomic and technical considerations. Radiographics. 2004;24:175–89. doi:10.1148/rg.241035063.
Maleux G, Hertogh GD, Lavens M, Oyen R. Transvenous biopsy of retroperitoneal tumoral masses : value of cone-beam CT guidance. J Vasc Interv Radiol. 1830;25(11):1830–2. doi: 10.1016/j.jvir.2014.07.006.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Levy, E. (2016). Retroperitoneal Biopsy: Indications and Imaging Approach. In: Rastinehad, A., Siegel, D., Pinto, P., Wood, B. (eds) Interventional Urology. Springer, Cham. https://doi.org/10.1007/978-3-319-23464-9_29
Download citation
DOI: https://doi.org/10.1007/978-3-319-23464-9_29
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23463-2
Online ISBN: 978-3-319-23464-9
eBook Packages: MedicineMedicine (R0)