Abstract
Lung disease is the most commonly identified clinical manifestation of alpha-1 antitrypsin deficiency (AATD) and is usually characterized by pulmonary emphysema and/or bronchiectasis (Silverman and Sandhaus, N Engl J Med 360(26):2749–57, 2009). It also represents the most common reason that an adult is tested for AATD (The Alpha 1-Antitrypsin Deficiency Registry Study Group, Chest 106(4):1223–32, 1994). This bias toward testing individuals with lung disease that is of unexplained severity or age of onset has led to the potentially mistaken impression that most individuals with AATD suffer from precocious lung disease. In fact, the percentage of individuals with AATD who suffer from pulmonary problems is currently not known, and most collections of individuals diagnosed with this genetic condition reveal an average age not that dissimilar from patients with non-AATD chronic obstructive pulmonary disease (COPD) (Campos et al., Chest 128(3):1179–86, 2005). The importance of diagnosing AATD-related lung disease is centered around family genetic counseling and the availability of specific therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
A deficiency of the circulating glycoprotein, alpha-1 antitrypsin (AAT), can lead to clinically significant lung injury in a proportion of those who inherit this condition. The risk of lung disease is affected by the specific genotype of the SERPINA1 gene that codes for the AAT protein, risk factor exposures, and the individual’s genetic background [1, 2]. Even those with the most commonly identified severely deficient genotype, PiZZ, may lead their entire lives without evidence of clinically significant, AAT deficiency-related disease. The types of lung injury that occur in individuals with AAT deficiency (AATD) are not unique; people with normal AAT genes can develop similar lung diseases with extended exposures to the same risk factors as those that accelerate damage in those with AATD. In some ways, AATD simply magnifies and amplifies the damage done to lung tissue by such risk factors, most prominently tobacco smoke exposure [3].
The two most common pulmonary conditions associated with AATD are pulmonary emphysema and bronchiectasis. The pulmonary emphysema tends to be panlobular in distribution and most prominent in the lower lobes but this is not invariable [4]. Upper zone disease and centrilobular distribution can be seen in AATD-related lung disease but this tends to be less common. Similarly, the bronchiectasis noted in those with AATD does not have unique characteristics except for its ubiquity. In one study [5], approximately 94 % of individuals with AATD had computerized tomography (CT) lung radiology consistent with bronchiectasis. In most cases, this bronchiectasis is asymptomatic, the so-called anatomic bronchiectasis.
Rarer lung conditions have been associated with AATD. Patients with ANCA-positive granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) appear to have a higher prevalence of abnormal AAT genotypes than the general population [6]. There is a biochemical logic to this since the ANCA antigen, proteinase 3, is inhibited by AAT.
It is important to identify individuals with AATD, especially those with lung disease, because progression of disease can be prevented, slowed, or halted by elimination of risk factors [3]. In addition, in many countries, individuals with emphysema due to AATD can receive intravenous infusions of wild-type AAT protein, derived from human plasma, a therapy shown to reduce the rate of emphysema progression. Potential future therapies for AATD-related lung disease include gene therapies, stem cell therapies, small-molecule protease inhibitors, and gene correction. While most of the pulmonary injury associated with bronchiectasis and emphysema is considered irreversible, future therapeutics may reverse or repair these injuries.
History
Lung disease and AATD have been intimately associated since the first description of this genetic condition. In 1963, the publications by Laurell and Eriksson identified a genetic deficiency of the AAT protein and described its association with “familial emphysema” [7]. A mechanism that might link this plasma protein deficiency with the alveolar destruction of pulmonary emphysema was unknown at the time.
At essentially the same time, Gross and associates found that intratracheal instillation of certain proteases produced pulmonary injury in rodents analogous to the histology of human pulmonary emphysema [8]. Further work by a number of investigators found that only proteases able to degrade elastin, a prominent lung connective tissue protein, were capable of inducing pulmonary emphysema in animal models [9, 10]. While a number of different proteases with elastolytic properties had been identified and tested with success, none of these appeared to have access to human lungs. This led to questions about the applicability of these animal models to human disease. Since 1964 was the year of the US Surgeon General’s report linking cigarette smoking to lung diseases including pulmonary emphysema, investigators even evaluated tobacco and tobacco smoke for elastolytic activity, but none was found.
This picture changed in 1968 with Janoff and Sherrer’s first description of a potent elastolytic protease isolated from the human polymorphonuclear neutrophilic leukocyte or neutrophil [11]. This “human neutrophil elastase” (HNE) proved to be quite potent at producing emphysema in a number of different animal species. Soon after the first descriptions of the effects of this elastase, reports appeared documenting that human AAT protein was an exceptionally potent inhibitor of HNE [12, 13]. While AAT is a broad-spectrum serine protease inhibitor (SERPIN), its inhibitory kinetics suggest that its preferred target is HNE. Finally, a potential mechanism for the emphysematous lung injury seen in patients with AATD was emerging.
The proposed mechanism for lung injury in AATD revolved around the protease-antiprotease balance in the lung parenchyma. In individuals with normal levels of AAT protein in the lung, neutrophils perform their function as the major acute inflammatory cells of the body with AAT protein acting as a barrier of sorts to protect normal lung connective tissue, especially elastin, from proteolytic degradation during lung inflammation. In individuals with a hereditary deficiency of AAT, there is insufficient anti-elastase protection and, over time, emphysema can result [14].
Soon after this scenario became accepted, a new wrinkle appeared in the story. Investigators noted that certain products of tobacco combustion were capable of inactivating the anti-elastase properties of AAT [15]. Simply bubbling cigarette smoke through a solution of AAT could completely inactivate elastase inhibition by the AAT protein. The mechanism of this inactivation proved to be oxidation of a methionine residue in the catalytic site of the AAT molecule [16]. The result is that the protease pathogenesis mechanism of pulmonary emphysema was extended to those with normal circulating AAT levels who smoke cigarettes.
Given this unifying theory of emphysema pathogenesis, how can one explain the fact that some with AATD never develop clinically significant lung disease? Individuals with the most commonly identified genotypes associated with AATD have plasma AAT levels that are approximately 10–15 % of the usual levels expected in individuals with the normal, PiMM, AAT genotype. It appears that under most circumstances in individuals with AATD, when there is no increased inflammation in the lungs and there is little oxidative inactivation of the AAT protein bathing the lungs, even this reduced level of AAT is sufficient to protect the lung from significant destruction. Events that can upset this delicate balance would include lung infection and exposure to agents that can increase inflammation or decrease the effectiveness of AAT as an elastase inhibitor, such as cigarette smoke, including secondhand smoke, and occupational exposures.
A group of AAT mutations, known collectively as “null mutations,” lead to the production of no AAT protein [17]. Virtually all individuals identified with two null SERPINA1 genes develop pulmonary emphysema as young adults and, without therapy, their disease progresses rapidly. This suggests that even having a reduced level of AAT protein in the lungs provides significantly more protection than a total absence of AAT protein.
In the early 1980s, armed with this understanding of the role of AAT in lung protection, a group of investigators in the Pulmonary Division of the NIH intramural facility in Bethesda, Maryland, sought to evaluate whether supplementation of the circulating levels of AAT protein might benefit patients with lung disease due to AATD [18]. They developed a methodology for the purification of AAT from the plasma of healthy individuals with normal SERPINA1 genes and infused individuals with AATD with this purified AAT concentrate. Further, they demonstrated that they could raise both the plasma and lung levels of AAT with these infusions. Unfortunately, AAT is cleared relatively rapidly from the circulation with a half-life of less than 1 week, so infusions with multiple grams of this “augmentation therapy” on a weekly schedule were required to maintain levels felt to be adequate. Since this regimen failed to maintain a “normal” trough level through the end of a week, even once a steady-state level had been achieved, a “protective threshold” was defined, based on the evaluation of AAT genotypes that produced mildly decreased levels but seemed to have no or minimal increased risk of lung disease. The regimen of 60 mg/kg of body weight, given by weekly intravenous infusion, was found to keep patients studied above this protective threshold [19].
The US Food and Drug Administration (FDA) approved the marketing of the first AAT augmentation therapy based on this biochemical efficacy in December of 1987. Since it was appreciated that a significant percentage of individuals with AATD would never develop lung disease, it was directed that this therapy should be reserved for individuals with severe AAT deficiency who had documented lung disease. For 15 years, this was the only augmentation therapy product approved in the USA and was widely prescribed to treat AATD-related emphysema. Beginning in 2003, additional products gained marketing approval until, at the time of this writing, there are a total of four such products available in the USA and several products available in other parts of the world. In the USA, each of the three additional augmentation therapy products was approved based on small studies that demonstrated the newer products were not inferior to the original in safety and biochemical efficacy.
Liver injury in AATD is associated with polymerization and retention of AAT protein within hepatocytes (see appropriate chapters in this book). Recent work has demonstrated that these same polymers can be found in blood and within the connective tissue of the pulmonary parenchyma in deficient individuals [20, 21]. In addition, these polymers in the lung appear to have pro-inflammatory properties that may enhance the lung damage associated with AATD.
Clinical Presentation of AATD Lung Disease
Lung disease in AATD is mostly indistinguishable from chronic obstructive pulmonary disease (COPD) in general. Identification of AATD in a given individual depends on a laboratory diagnosis indicating a low level of circulating AAT protein and Pi-type or genotype revealing two abnormal AAT-coding genes [22]. Pi-typing evaluates circulating AAT protein using isoelectric focusing to reveal differences in protein migration patterns due to molecular isoforms. Genotyping evaluates the SERPINA1 gene for known mutations associated with deficiency. In rare cases, it is sometimes necessary to sequence the SERPINA1 genes to identify a rare or previously unknown genotype. To date, more than 400 mutations of the SERPINA1 gene have been reported. A minority of these is associated with a deficiency or dysfunction of circulating AAT protein.
While the definitive diagnosis of AATD is based on laboratory testing, there are clinical indicators that increase the likelihood of its presence. Precocious emphysema or emphysema out of proportion to smoking history often prompts testing for AATD. Similarly, a family history of emphysema, especially with lung disease out of proportion to risk factors like smoking, can be suggestive and a family history of unexplained liver disease may also raise suspicion. It is increasingly common for radiologists to suggest a diagnosis of AATD based on chest CT evaluation showing lower zone, panlobular emphysema, often accompanied by bronchiectasis. Current guidelines and standards suggest testing all individuals diagnosed with COPD for AATD regardless of age and smoking history. Several studies have demonstrated an increased prevalence of undetected AATD (between 0.6 and 3 %) among the general COPD population [23, 24].
Finally, family testing of those identified with AATD will often reveal undetected, and often asymptomatic, AATD in family members. In the future, routine AATD testing of newborns may well be the norm. A focus on risk factor reduction in those identified at an early age may well prevent much of the disease we currently see in this condition.
A retrospective study of a single center’s experience has suggested an increased incidence of lung cancer in individuals carrying at least one AAT deficiency mutation [25]. This work still requires confirmation.
Management of AATD Lung Disease
Once identified, an individual with AATD should be assessed for the conditions associated with this genetic condition including, most prominently, lung and liver disease. Individuals with normal lung function and no evidence of significant destructive lung disease should be educated about AATD and risk factors for disease. Smoking prevention and cessation are among the most important steps to prevent lung disease in AATD (as in many lung conditions). Currently, augmentation therapy with plasma-derived AAT protein is not indicated in those without evidence of destructive lung disease. Careful monitoring of lung function and radiology is suggested.
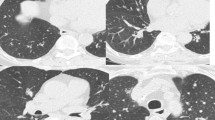
While there are no evidence-based recommendations for the follow-up of healthy individuals with AATD, in general, clinicians treating a large number of such patients do annual or biannual complete pulmonary function testing often accompanied by liver function studies. A baseline high-resolution CT of the chest without contrast can be considered (Fig. 6.1). Interval repetition of the chest CT is not recommended but is reserved for evaluating significant changes in an individual’s respiratory status [26].
Computed tomography of the chest in patients with AAT deficiency shows a broad range of manifestations. AAT deficiency has classically been associated with the development of basilar-predominant panacinar emphysema (Panel a). However, upper-lobe-predominant emphysema (Panel b) and bronchiectasis (Panel c) can also be observed, and sometimes the lungs are normal (Panel d) (reprinted with permission from Ref. [26])
The clinical evaluation and follow-up of individuals with AATD-related lung disease is similar to the monitoring of healthy individuals with AATD. The frequency of evaluation is generally increased, however. Those with destructive lung disease usually have their lung function evaluated semiannually until stable and then are moved to annual visits unless an unexpected change occurs. Generally, those with lung disease have some evaluation of oxygenation included in their workup, such as an arterial blood gas, a 6-min walk with oximetry, or a formal pulmonary exercise study. Depending on risk factors and age, a cardiopulmonary exercise study may be considered. In those requiring supplemental oxygen, it is often reasonable to consider an echocardiogram to evaluate for right-sided heart disease and pulmonary hypertension.
The treatment of lung disease due to AATD broadly follows the treatment recommendations for individuals with non-AATD COPD and/or bronchiectasis. While clinical trials of medications for the treatment of COPD have generally excluded individuals with AATD and none of the usual COPD drugs have been specifically evaluated for effectiveness in AATD-related lung disease, it is generally assumed that AATD-associated lung disease responds to the same medications and other treatments that are used in treating typical, smoking-related COPD. The mainstays of therapy include long-acting inhaled beta-agonists, long-acting inhaled anticholinergics, and long-acting inhaled corticosteroids. These medications are presumed to have the same beneficial effects on pulmonary exacerbation incidence and symptoms as in non-AATD COPD. As with these agents in any patient being treated for COPD, the goal is to use the lowest dose and fewest drugs that achieve the desired benefit.
Pulmonary exacerbations are common in AATD lung disease. Studies have suggested that on average AATD patients with lung disease have just over two exacerbations per year, with subpopulations within these studies that have no exacerbation and others with many more than two each year [27]. This latter group may benefit from the addition of a phosphodiesterase 4 inhibitor or chronic macrolide therapy, again based on studies in non-AATD COPD patients. For individuals with clinically significant bronchiectasis, airway clearance devices and techniques may be beneficial. Antibiotic therapy should be based on sputum culture and sensitivity results in this setting and resistant enteric organisms may colonize AATD patients with long-standing bronchiectasis, just as in this condition in those without AATD.
With respect to the treatment of an exacerbation, logic would suggest that patients with AATD would be at greatest risk of incremental lung destruction during a time of acute lung inflammation. Therefore, aggressive treatment of pulmonary exacerbations is recommended. Empiric therapy with antibiotics is recommended, if no recent sputum culture results are available to guide antibiotic choice. Addition of oral or parenteral corticosteroids may be required for severe exacerbations.
Other treatments initially developed for non-AATD COPD are also employed in the management of AATD lung disease. Pulmonary rehabilitation appears to benefit many with this condition. Immunization against organisms that commonly cause community-acquired pneumonia is recommended for those with AATD lung disease as is annual influenza immunization. Many clinicians recommend immunization against hepatitis A and B virus in order to reduce the risk of liver injury.
The use of AAT augmentation therapy should be considered in any AATD patient with documented emphysema. The goal of augmentation therapy is to prevent or slow the rapid decline in lung function found in individuals with lung disease due to AATD. The appropriate time to start augmentation therapy in an individual with AATD lung disease is an area of some disagreement. The first large study (large by rare disease standards) of patients with AATD was the NIH/NHLBI Registry of Patients with Alpha-1 Antitrypsin Deficiency (AATD) which enrolled 1129 individuals with AATD with a goal of evaluating the natural history of AATD [28]. Enrolled individuals were followed for approximately 5 years (3.5–7 years) with annual or semiannual testing of lung function, chest radiology, and blood work. The study began soon after the marketing approval of the first augmentation therapy product in the USA, and a majority of enrollees were treated with augmentation therapy for all or part of their time in the registry. A post-hoc analysis of the effect of augmentation therapy on mortality and lung function was performed [29]. A significant improvement in survival was noted in those who (1) received augmentation therapy during any of their time in the registry and (2) had an entry forced expiratory volume in 1 s (FEV1) of less than 50 % predicted. In addition, there was a significantly decreased rate of decline of lung function, as assessed by FEV1, in individuals receiving augmentation therapy whose entry FEV1 was between 35 and 49 % predicted compared to those who did not receive augmentation. Studies performed concurrently in Europe, with smaller subject numbers, confirmed many of the findings of the US registry study, although survival improvement could not be documented in those studies [30, 31].
Based on these studies, some clinicians elect to initiate augmentation therapy only once the FEV1 of an affected individual declines to less than 50–60 % predicted. Others note the small number of healthier but declining patients, and thus the lack of power to detect improvement in healthier individuals, in the studies just cited. In addition, it can be pointed out that individuals who have emphysema due to AATD but have an FEV1 greater than 50–60 % of predicted would be asked to wait until additional lung tissue was irreversibly destroyed if the former approach is used to guide the initiation of augmentation therapy. Once the decision to initiate augmentation therapy is made, the dose approved for the currently available therapies remains the same as initially described in the publications from the NIH intramural investigations of the 1980s: 60 mg/kg/week by intravenous infusion. There remains some controversy about whether this is the appropriate dosage and administration interval for all patients. At the time of this writing, there is at least one dose-ranging study enrolling subjects.
What remains controversial still, more than 25 years after the introduction of the first AAT augmentation product, is the measurable clinical benefit of this therapy. At the time of approval of the first product in this class, there were too few identified potential subjects to perform a well-powered, placebo-controlled, long-term prospective clinical efficacy study. Now that detection efforts have identified a sufficient number of potential subjects to perform such studies, the effectiveness of augmentation therapy is so widely accepted, at least within the USA, that a long-term placebo-controlled trial is virtually impossible to enroll because potential subjects fear randomization into the placebo group. Further, a growing number of ethics committees/institutional review boards (IRB) are finding ethical conflicts with studies that intend to enroll AATD patients and randomize some into placebo arms for prolonged periods. Several small, pilot, placebo-controlled prospective studies have been performed [32, 33], and, to date, one well-powered placebo-controlled trial of augmentation therapy has been completed [34]. The most recent studies have used longitudinal measurements of lung CT densitometry as the primary endpoint, since this appears to allow the most direct quantification of lung tissue destruction currently available.
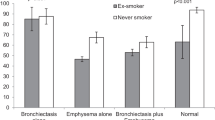
The single well-powered study enrolled about 180 subjects with approximately half randomized to receive augmentation therapy at standard doses and the other half receiving placebo infusions for 2 years. This study demonstrated a significant preservation of lung tissue comparing the treated to the control group. In addition, an extension study allowed treated subjects to continue on therapy for an additional 2 years and rolled the placebo subjects onto treatment for this second 2-year period. The extension study demonstrated that, during the second 2 years of therapy, the rate of loss of lung tissue slowed even further in the group on 4 years of augmentation therapy. See Fig. 6.2. In addition, those initially receiving placebo showed a decrease in the rate of loss of lung tissue once they were started on augmentation therapy for the 2 years of the extension study. An interesting finding in this study was that the trough blood levels of AAT were inversely correlated with the rate of lung tissue loss. See Fig. 6.3.
Effect of augmentation therapy (A1PI) versus placebo on rate of lung density decrease during the double-blind and open-label portions of the trial in all patients. Values on graph are annual rates of decrease calculated from CT densitometry at total lung capacity. A1PI n = 92; placebo n = 85 during double-blind period. A1PI n = 50; placebo n = 47 during open-label period. p = 0.03 during the double-blind portion (reprinted with permission from Ref. [32])
Rates of lung density decrease at total lung capacity versus trough augmentation therapy serum concentrations achieved. Response-exposure curve. Shaded area represents 90 % confidence intervals. A1PI = α1 (reprinted with permission from Ref. [32])
While it is encouraging to see the results of this trial, the use of CT densitometry to quantify lung tissue destruction is not accepted by all. In addition, the clinical significance of the magnitude of benefit demonstrated in these studies remains unclear. Still, in a condition that progresses over decades, even small annual improvements can have large long-term benefits. At this time, it can be said that augmentation is generally well accepted in areas of the world where it is available and a growing number of health systems are approving the administration of this class of product. Augmentation therapy remains the only specific therapy currently available to treat the lung disease of AATD. It should be noted that no benefit of augmentation therapy in granulomatosis with polyangiitis has been demonstrated.
Another powerful treatment for individuals with AATD is education. Education of affected individuals and their family members regarding the mechanisms of disease, risk factors, and treatment can be a powerful therapy to help prevent and treat lung disease in AATD. This has been demonstrated by the publications from the AlphaNet health management program, evaluating the benefits of patient self-management in a large group of lung-affected individuals with AATD [35]. Currently including nearly 5000 individuals, most on augmentation therapy, this program continues to involve those with AATD in their own care and prevention of disease.
An important unanswered question is the risk of lung disease in those who inherit a single abnormal AAT gene. For several common genotypes, there appears to be little evidence of an increase in risk. These include the S, I, P, and F genes when each is inherited with a normal M gene. Two heterozygote combinations deserve special attention: PiMZ and PiMNull. The blood levels of AAT with these latter heterozygotes can approach those seen to lead to increased risk of lung disease in those who are homozygote for a deficient gene and those who inherit a complex heterozygote, such as PiSZ. Studies have suggested that individuals with the PiMZ genotype have an increased risk of lung disease only if they smoke tobacco products with little or no increased risk seen in nonsmokers [36, 37]. It is possible that there is a subgroup of PiMZ individuals with significantly increased risk of lung disease but this has yet to be demonstrated conclusively.
Summary
Lung disease is the most common trigger for testing of individuals for AATD. Evidence suggests that the great majority of individuals with AATD remain undetected [38]. Whether they remain untested because of lack of significant illness or because the diagnosis remains unconsidered even in those with cardinal diseases is not clear. Based on family testing results, it appears likely that many of those with AATD who remain undetected are perfectly healthy. However, studies of those with the diagnosis of COPD suggest that there are a sizable number of individuals with undetected AATD within the COPD population.
Since there is specific therapy for AATD-related COPD that is distinct for that provided to patients with more usual COPD, identifying those with lung disease due to AATD can be clinically important. In addition, identifying an individual with lung disease due to AATD also identifies a family that is potentially at risk.
References
Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246–59.
DeMeo DL, Campbell EJ, Brantly ML, Barker AF, Eden E, McElvaney NG, et al. Heritability of lung function in severe alpha-1 antitrypsin deficiency. Hum Hered. 2009;67(1):38–45.
Mayer AS, Stoller JK, Vedal S, Ruttenber AJ, Strand M, Sandhaus RA, et al. Risk factors for symptom onset in PI*Z alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2006;1(4):485–92.
Dowson LJ, Guest PJ, Hill SL, Holder RL, Stockley RA. High-resolution computed tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health status. Eur Respir J. 2001;17(6):1097–104.
Parr DG, Guest PG, Reynolds JH, Dowson LJ, Stockley RA. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176(12):1215–21.
Elzouki AN, Segelmark M, Wieslander J, Eriksson S. Strong link between the alpha 1-antitrypsin PiZ allele and Wegener’s granulomatosis. J Intern Med. 1994;236(5):543–8.
Laurell C-B, Eriksson S. The electrophoretic alpha-1-globulin pattern of serum in alpha-1 antitrypsin deficiency. Scan J Clin Lab Invest. 1963;15:132–40.
Gross P, Babyak MA, Tolker E, Kaschak M. Enzymatically produced pulmonary emphysema; a preliminary report. J Occup Med. 1964;6:481–4.
Snider GL, Hayes JA, Franzblau C, Kagan HM, Stone PS, Korthy AL. Relationship between elastolytic activity and experimental emphysema-induced properties of papain preparations. Am Rev Respir Dis. 1974;110(3):254–62.
Blackwood CE, Hosannah Y, Perman E, Keller S, Mandl I. Experimental emphysema in rats: elastolytic titer of inducing enzyme as determinant of the response. Proc Soc Exp Biol Med. 1973;144(2):450–4.
Janoff A, Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968;128(5):1137–55.
Janoff A. Inhibition of human granulocyte elastase by serum alpha-1-antitrypsin. Am Rev Respir Dis. 1972;105(1):121–2.
Pannell R, Johnson D, Travis J. Isolation and properties of human plasma alpha-1-proteinase inhibitor. Biochemistry. 1974;13(26):5439–45.
Laurell CB. Is emphysema in alpha 1-antitrypsin deficiency a result of autodigestion? Scand J Clin Lab Invest. 1971;28(1):1–3.
Janoff A, Carp H. Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis. 1977;116(1):65–72.
Janoff A, Carp H, Lee DK, Drew RT. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979;206(4424):1313–4.
Fregonese L, Stolk J, Frants RR, Veldhuisen B. Alpha-1 antitrypsin null mutations and severity of emphysema. Respir Med. 2008;102(6):876–84.
Gadek JE, Klein HG, Holland PV, Crystal RG. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68(5):1158–65.
Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055–62.
Tan L, Dickens JA, Demeo DL, Miranda E, Perez J, Rashid ST, et al. Circulating polymers in alpha1-antitrypsin deficiency. Eur Respir J. 2014;43(5):1501–4.
Parmar JS, Mahadeva R, Reed BJ, Farahi N, Cadwallader KA, Keogan MT, et al. Polymers of alpha(1)-antitrypsin are chemotactic for human neutrophils: a new paradigm for the pathogenesis of emphysema. Am J Respir Cell Mol Biol. 2002;26(6):723–30.
McElvaney NG, Stoller JK, Buist AS, Prakash UB, Brantly ML, Schluchter MD, Alpha 1-Antitrypsin Deficiency Registry Study Group, et al. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute registry of alpha 1-antitrypsin deficiency. Chest. 1997;111(2):394–403.
Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986;89(3):370–3.
Rahaghi FF, Sandhaus RA, Strange C, Hogarth DK, Eden E, Stocks JM, et al. The prevalence of alpha-1 antitrypsin deficiency among patients found to have airflow obstruction. COPD. 2012;9(4):352–8.
Yang P, Wentzlaff KA, Katzmann JA, Marks RS, Allen MS, Lesnick TG, et al. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8(5):461–5.
Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360(26):2749–57.
Campos MA, Alazemi S, Zhang G, Wanner A, Salathe M, Baier H, et al. Exacerbations in subjects with alpha-1 antitrypsin deficiency receiving augmentation therapy. Respir Med. 2009;103(10):1532–9.
The Alpha 1-Antitrypsin Deficiency Registry Study Group. A registry of patients with severe deficiency of alpha 1-antitrypsin. Design and methods. Chest. 1994;106(4):1223–32.
The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49–59.
Seersholm N, Wencker M, Banik N, Viskum K, Dirksen A, Kok-Jensen A, et al. Does alpha1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary alpha1-antitrypsin deficiency? Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL) alpha1-AT study group. Eur Respir J. 1997;10(10):2260–3.
Wencker M, Fuhrmann B, Banik N, Konietzko N. Longitudinal follow-up of patients with alpha(1)-protease inhibitor deficiency before and during therapy with IV alpha(1)-protease inhibitor. Chest. 2001;119(3):737–44.
Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1468–72.
Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136.
Chapman KR, Burdon JGW, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. The Lancet. 2015. doi:10.1016/S0140-6736(15)60860-1
Campos MA, Alazemi S, Zhang G, Wanner A, Sandhaus RA. Effects of a disease management program in individuals with alpha-1 antitrypsin deficiency. COPD. 2009;6(1):31–40.
Hersh CP, Dahl M, Ly NP, Berkey CS, Nordestgaard BG, Silverman EK. Chronic obstructive pulmonary disease in alpha1-antitrypsin PI MZ heterozygotes: a meta-analysis. Thorax. 2004;59(10):843–9.
Molloy K, Hersh CP, Morris VB, Carroll TP, O’Connor CA, Lasky-Su JA, et al. Clarification of the risk of chronic obstructive pulmonary disease in alpha1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189(4):419–27.
Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sandhaus, R.A. (2016). Lung Disease of Alpha-1 Antitrypsin Deficiency. In: Wanner, A., Sandhaus, R. (eds) Alpha-1 Antitrypsin. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-23449-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-23449-6_6
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-23448-9
Online ISBN: 978-3-319-23449-6
eBook Packages: MedicineMedicine (R0)